To the Editor:
Although asthma prevalence varies across age, sex, ethnicity, and poverty level, one lesser defined asthma subtype consists of women with asthma onset after menopause (1). There is a clear association between aging and asthma incidence and severity in both men and women (2). However, women over the age of 65 have been shown to have more severe asthma than men of the same age group (3), indicating a distinct role of menopause and sex hormones in asthma pathogenesis. Mechanisms driving menopause-associated asthma are unknown; however, post-menopausal women tend to have worse asthma symptoms (4) and respond poorly to standard treatments (1). One study of menopausal women indicated that forced vital capacity (FVC) declined at a more rapid rate in peri- and post-menopausal women, beyond the expected decline with age (5).
The lack of cellular, molecular, and physiological data relating to asthma in menopausal women exposes a gap in our knowledge pertaining to this subgroup of asthmatics with severe symptoms. Animal modeling thus far has been unable to adequately mimic the decreased lung function associated with human menopause-onset asthma (6–8). More specifically, gonadectomized female mice display decreased airway hyperresponsiveness (AHR) and airway inflammation in response to ovalbumin, which is opposite from what we would expect based on the human condition (7). The goal of this study was to develop a mouse model of the more severe AHR observed in menopausal women by combining two well-established models: 4-Vinylcyclohexene diepoxide (VCD) to induce menopause and house dust mite (HDM) extract to induce asthma.
VCD acts directly, and specifically, on the ovarian follicles, inducing an apoptotic mechanism that mimics follicular atresia as seen in human ovaries while leaving the ovaries intact, allowing them to continue to produce other androgenic hormones (9). To assess the potential toxic and carcinogenic effects of VCD on other tissues, an extensive study examined long term exposure to high levels of VCD in mice and determined no pathological effects on any major tissues (10). By performing the VCD protocol on young, reproductively healthy animals, we remove any effect aging may have on asthma severity, and instead focus on the effects of hormone changes associated with menopause (11).
Experiments were performed in accordance with University of Arizona Institutional Animal Care and Use Committee requirements on 8-week-old female C57BL/6J mice obtained from the Jackson Laboratory. Daily intraperitoneal injections of VCD or sesame oil (vehicle [Veh] control) were given for 20 consecutive days (Figure 1A). Two weeks following the final IP injection, cyclicity checks were performed daily via a vaginal lavage. Vaginal cytology was monitored daily to determine the onset of menopause (ovarian failure), defined as 10 consecutive days of diestrus (11, 12). Representative hematoxylin and eosin-stained images for each experimental and control group demonstrated that VCD treated mice had fewer follicles and exhibited ovarian atrophy (Figure 1B). Mice that received sesame oil maintained healthy ovarian follicle maturation (Figure 1C).
Figure 1.
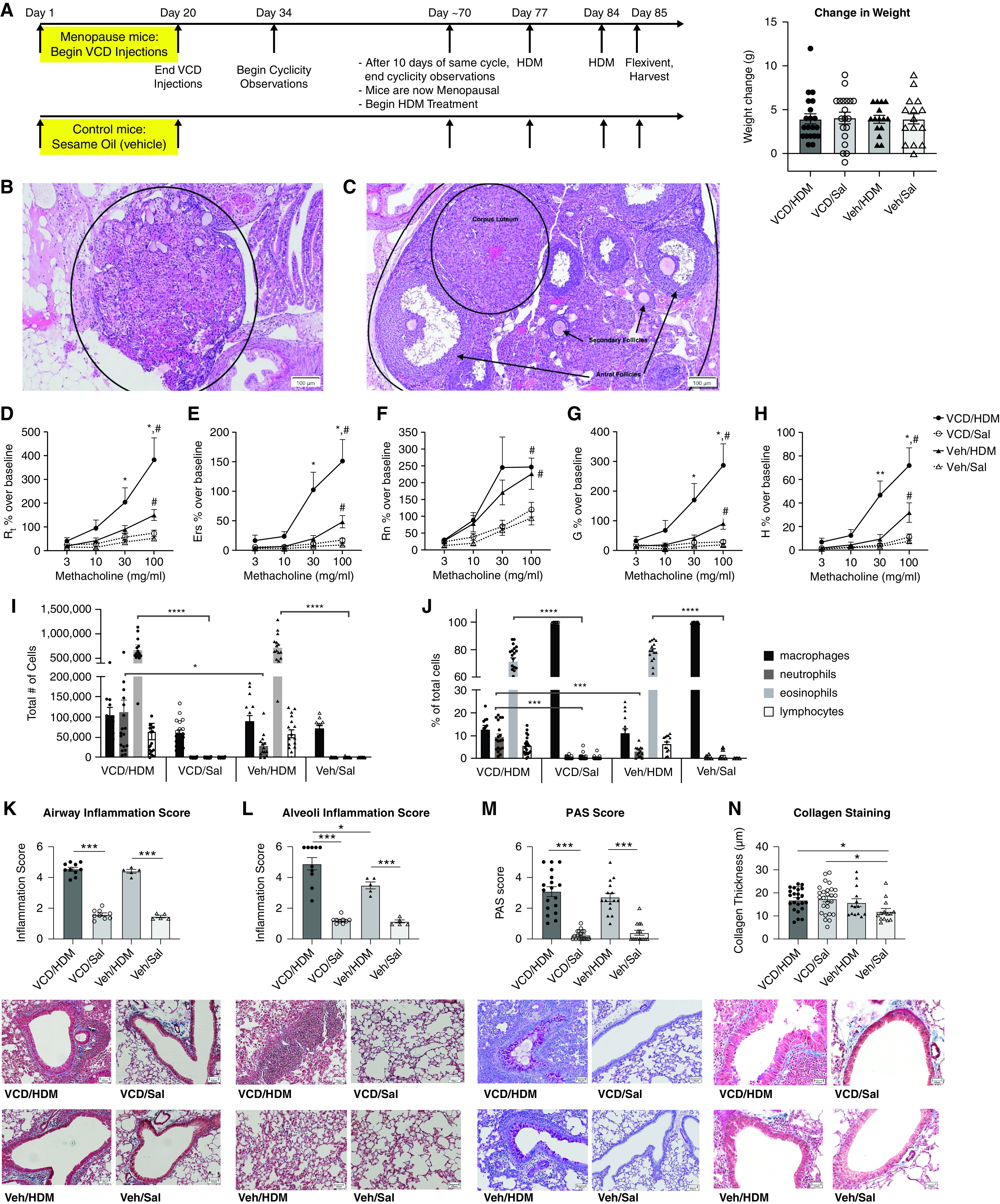
Development of a novel menopause-associated asthma model in mice. (A) Experimental timeline for 4-Vinylcyclohexene diepoxide (VCD) injections and house dust mite (HDM) dosing, with the change in weight over the course of the experiment. (B) Hematoxylin and eosin (H&E) stained menopausal ovaries from VCD injected mice with follicle depletion as compared with (C) healthy ovaries from control mice that display follicles at various stages of development. Scale bar in B, 100 μm. Scale bar in C, 100 μm. (D–H) Lung function assessment in experimental mice. VCD/HDM (n = 20), VCD/Saline (n = 19), Vehicle/HDM (n = 15), and Vehicle/Saline (n = 15) female mice were assessed for pulmonary function 24 hours after the last HDM challenge and parameters are reported as percent over their respective baseline measurement; all HDM treated mice had significantly increased airway hyperresponsiveness (AHR) in all parameters of pulmonary functions assessed at the 100 mg/ml MCH dose compared with saline controls (#P < 0.05). D) Total airway resistance (Rrs): VCD/HDM mice compared with Veh/HDM mice at MCH doses: 30 mg/mL (*P = 0.0426) and 100 mg/mL (*P = 0.0334). E) Total airway elastance (Ers): VCD/HDM mice compared with Veh/HDM mice at MCH doses: 30 mg/mL (*P = 0.0101) and 100 mg/mL (*P = 0.0043). (F) Newtonian resistance (Rn): HDM-treated groups had significant increases in Rn compared with their respective saline controls (#P < 0.05). (G) Tissue damping (G): VCD/HDM mice compared with Veh/HDM mice at MCH doses: 30 mg/mL (*P = 0.0417) and 100 mg/mL (*P = 0.0281). (H) Tissue elastance (H): VCD/HDM mice had significantly higher H compared with Veh/HDM mice at MCH doses: 30 mg/mL (**P = 0.0026) and 100 mg/mL (**P = 0.0057). (I–J) The total number and percentage of inflammatory cells present in the BALF was determined by cytospin and differential counts after H&E stain. *P < 0.05, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA. Lung sections from the right lobe were stained and assessed for inflammation, collagen, and mucin production at 10X microscopy. Masson’s Trichrome stained sections were assessed for (K) inflammation surrounding the large airways on a scale of 1 (no inflammation) to 5 (full inflammation) and (L) alveolar inflammation on a scale of 1 (no inflammation) to 6 (full inflammation). (M) PAS-stained sections were analyzed for mucin production using a scale of 0 (no mucin) to 5 (full mucin production). (N) Masson’s Trichrome stained sections were assessed for collagen (blue) thickness surrounding the large airways using MetaMorph software. *P < 0.05 and ***P < 0.001 by one-way ANOVA for multiple comparisons. Veh = vehicle.
Once mice were deemed menopausal, HDM sensitization and challenge were administered as previously described on Days 0, 7, and 14 (13). On Day 15, airway function tests were performed on a flexiVent with increasing concentrations of aerosolized methacholine (MCH), after which BAL fluid (BALF), blood, lung tissue, and ovarian tissue were collected. Both asthmatic groups (VCD/HDM and Veh/HDM) had significant increases across all lung function parameters at the 100 mg/mL MCH dose compared with their respective nonasthmatic controls (VCD/Saline [Sal] and Veh/Sal) (Figures 1D–1H). The menopausal asthmatic (VCD/HDM) mice had significantly higher total airways resistance (Rrs) tissue damping (G), elastance of the respiratory system (Ers), and tissue elastance (H) compared with the nonmenopausal asthmatic (Veh/HDM) mice challenged with 30 mg/mL and 100 mg/mL of MCH. However, Newtonian resistance (Rn) was unchanged in this comparison. These lung function data suggest that changes in the peripheral airways, as opposed to the conducting airways, drive increased responses to MCH in menopausal asthmatic mice.
Although both VCD/HDM and Veh/HDM-treated mice had significantly increased eosinophil and neutrophil numbers compared with their respective controls, VCD/HDM-treated mice had significantly more neutrophils in the BALF compared with the Veh/HDM group (Figures 1I and 1J). Experimental groups that were sensitized with HDM (VCD/HDM and Veh/HDM) had significantly more inflammatory cells surrounding their large airways than their respective saline controls. However, the VCD/HDM group and Veh/HDM groups did not differ from one another (Figure 1K). Similarly, both HDM-sensitized groups had significantly more inflammatory cells within the distal airways and alveolar spaces compared with their respective controls. In this case, the VCD/HDM group had significantly more alveolar inflammation compared with the Veh/HDM group (Figure 1L).
Both asthmatic groups (VCD/HDM and Veh/HDM) had significantly higher mucin production compared with their respective controls, which was independent of menopause status (Figure 1M). In contrast, both menopausal groups (VCD/HDM and VCD/Sal) had significantly increased extracellular matrix production, indicated by collagen staining around large airways, compared with Veh/Sal. The Veh/HDM group, while displaying a trending increase in collagen production, was not significantly higher than Veh/Sal (i.e., Veh/HDM, Veh/Sal; Figure 1N).
Cytokines IL-4 and IL-5 were significantly increased in BALF from both HDM-treated groups compared with their respective saline controls (Table 1); however, there were no differences between the VCD/HDM and Veh/HDM groups. IL-6 and KC (keratinocyte-derived cytokine) were significantly higher in lung tissue lysates in the VCD/HDM group compared with Veh/HDM and VCD/Sal control groups (Table 1). IL-17F was significantly elevated in the VCD/HDM and the VCD/Sal group compared with the Veh/Saline control. Taken together, these data suggest that an elevated T-helper (Th)-17 response in the menopausal asthmatic mice may contribute to the increased AHR and tissue remodeling in this model, although further investigation is required.
Table 1.
Cytokine Assessment in BALF and Lung Tissue Lysate
| Cytokine | VCD/HDM | VCD/Saline | Vehicle/HDM | Vehicle/Saline | |
|---|---|---|---|---|---|
| IgE (ng/mL + SEM) | 597.1 ± 110.3 *(versus VCD/Sal) | 36.3 ± 22.6 | 920 ± 236.20 ***(versus Veh/Sal) | 5.2 ± 3.7 | Serum |
| IL-4 (pg/mL + SEM) | 65.1 ± 8.4 **(versus VCD/Sal) | 32.8 ± 3.14 | 54.27 ± 6.3 *(versus Veh/Sal) | 29.2 ± 3.7 | BALF
|
| IL-5 (pg/mL + SEM) | 194.1 ± 28.7 ****(versus VCD/Sal) | 16.0 ± 3.9 | 134.1 ± 26.0 ***(versus Veh/Sal) | 8.6 ± 3.0 | |
| IL-13 (pg/mL + SEM) | 117.3 ± 16.2 | 83.0 ± 8.9 | 93.0 ± 14.5 | 74.9 ± 12.0 | |
| IL-4 (pg/mL + SEM) | 23.62 ± 5.96 *(versus VCD/Sal) | 4.45 ± 0.41 | 17.55 ± 7.73 | 3.35 ± 0.42 | Tissue
|
| IL-5 (pg/mL + SEM) | 214.0 ± 54.78 | 116.4 ± 6.04 | 194.6 ± 74.36 | 120.1 ± 59.2 | |
| IL-13 (pg/mL + SEM) | 155.0 ± 27.36 | 156.3 ± 19.11 | 153.0 ± 22.27 | 173.8 ± 19.16 | |
| IL-17A (pg/mL + SEM) | 2962 ± 1479 | 146.5 ± 19.4 | 1963 ± 1076 | 125.7 ± 17.44 | |
| IL-17F (pg/mL + SEM) | 1321 ± 94.99 *(versus Veh/Sal) | 1360 ± 195.7 *(versus Veh/Sal) | 1176 ± 275.5 | 457.5 ± 54.73 | |
| IL-22 (pg/mL + SEM) | 162.9 ± 55.56 | 57.62 ± 8.66 | 106.0 ± 47.28 | 61.28 ± 15.67 | |
| IL-6 (pg/mL + SEM) | 69.12 ± 4.86 *(versus Veh/HDM) | 46.7 ± 2.59 | 53.2 ± 3.14 | 52.25 ± 5.09 | |
| KC (pg/mL + SEM) | 136.9 ± 11.14 ***(versus Veh/HDM) | 57.92 ± 3.14 | 86.61 ± 5.67 | 74.7 ± 9.29 |
Cytokines were assessed in BAL fluid and lung lysates (tissue) by single or multiplex immunoassay kits; IgE was detected in serum by ELISA. Values shown are mean +/−SEM, n = minimum of 5 per group. Statistical analysis performed using one-way ANOVA for multiple comparisons.
P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Recent studies have shown that menopausal asthmatic women do not respond well to therapies targeting Th-2 inflammatory pathways, such as inhaled corticosteroids (1, 14), which may indicate an underlying non–Th-2 inflammation. Although we detected elevated Th-2 cytokines in our menopause asthma model, they were not significantly higher than those in the nonmenopausal asthma model. In fact, our data also suggests non–Th-2 inflammation is driving the augmented AHR in the mice and that factors such as KC, IL-6, and Th-17 related factors may be more at play; the contribution of which warrants further investigation.
Although eosinophils were highly elevated in both asthmatic groups, neutrophilic inflammation in the airways was significantly higher in the menopausal asthmatic group compared with the nonmenopausal asthmatic group. Taken with the cytokine data, this suggests mixed granulocyte and Th-17 inflammatory responses may be involved in the enhanced AHR in the menopausal asthmatic mice. Moreover, these data indicate that menopause itself may upregulate IL-17F signaling in the absence of an asthma challenge, which could contribute to airway remodeling (15). This is in line with the collagen deposition observed in tissue sections where the menopause groups had more collagen deposition independent of asthma status (Figure 1N).
By combining the VCD and HDM models, we were able to induce a severe AHR phenotype in menopausal asthmatic mice, which appears to be driven by changes in distal airways, including tissue inflammation and collagen production. To our knowledge, this is the first instance of enhanced AHR and decreased lung function in an animal model of menopausal asthma (6, 7). This model resulted in a robust inflammatory infiltrate in the alveolar spaces of the menopausal asthmatic mice, with almost complete occlusion of the alveoli in some regions. This level of inflammation, in combination with the enhanced collagen deposition in the small airways that we observed, could prevent gas exchange, and contribute to the changes in airway function.
In conclusion, we believe our newly developed model of menopause associated asthma more closely mimics the severe asthmatic phenotype described in menopausal women (11) and provides a novel tool to better investigate mechanistically and translationally, the factors driving disease onset to develop better therapeutic strategies for this subset of patients with asthma.
Acknowledgments
Acknowledgment
The authors acknowledge Megan A. Sylvester for technical assistance and support.
Footnotes
Supported by National Institutes of Health grants HL142769, HL131834, HL149744, and 5T32AG058503-02.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Balzano G, Fuschillo S, De Angelis E, Gaudiosi C, Mancini A, Caputi M. Persistent airway inflammation and high exacerbation rate in asthma that starts at menopause. Monaldi Arch Chest Dis . 2007;67:135–141. doi: 10.4081/monaldi.2007.484. [DOI] [PubMed] [Google Scholar]
- 2. Braman SS. Asthma in the elderly. Clin Geriatr Med . 2017;33:523–537. doi: 10.1016/j.cger.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 3. Baptist AP, Hamad A, Patel MR. Special challenges in treatment and self-management of older women with asthma. Ann Allergy Asthma Immunol . 2014;113:125–130. doi: 10.1016/j.anai.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balzano G, Fuschillo S, Melillo G, Bonini S. Asthma and sex hormones. Allergy . 2001;56:13–20. doi: 10.1034/j.1398-9995.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- 5. Triebner K, Matulonga B, Johannessen A, Suske S, Benediktsdóttir B, Demoly P, et al. Menopause is associated with accelerated lung function decline. Am J Respir Crit Care Med . 2017;195:1058–1065. doi: 10.1164/rccm.201605-0968OC. [DOI] [PubMed] [Google Scholar]
- 6. Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, et al. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med . 2007;175:126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy . 2007;37:459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- 8. Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol . 2018;120:488–494. doi: 10.1016/j.anai.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diaz Brinton R. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology . 2012;153:3571–3578. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res . 2008;23:1296–1303. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brooks HL, Pollow DP, Hoyer PB. The VCD mouse model of menopause and perimenopause for the study of sex differences in cardiovascular disease and the metabolic syndrome. Physiology (Bethesda) . 2016;31:250–257. doi: 10.1152/physiol.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pollow DP, Jr, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol . 2015;309:R1546–R1552. doi: 10.1152/ajpregu.00170.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Addison KJ, Morse J, Robichaud A, Daines MO, Ledford JG. A novel in vivo system to test bronchodilators. J Infect Pulm Dis . 2017;3 doi: 10.16966/2470-3176.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Expert Panel Report 3: Guidelines for the Dianosis and Management of Asthma. National Asthma Education and Prevention Program; 2007. [Google Scholar]
- 15. Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol . 2013;25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


