Abstract
Although application of three-dimensional (3D) printing in oral and maxillofacial surgery (OMFS) was first reported almost 30 years back, reduction in its manufacturing cost and availability of affordable 3D printing devices have popularized its use over the past few years. The 3D-printed objects include anatomical models, occlusal splints, drilling, or cutting guides and patient-specific implants (custom made plates and reconstruction devices). The anatomical model not only assists the surgeon in better understanding of the deformity or pathology but also aids in explaining the same to the patient and relatives. Mock surgery carried out on these models improve precision and thereby reduce the operating time. The guiding splints provide an exact design and fit for the graft, thus replicating form and function of the jawbone. The patient specific implants manufactured through computer-assisted designing help in superior replication of original anatomical form. This paper intends to highlight the current applications of 3D printing in field of maxillofacial surgery in the management of facial deformity, esthetic disturbances, and jaw pathologies. Cases of condylar hyperplasia, jaw tumor, facial asymmetry secondary to joint deformity, apertognathia, and chin augmentation managed with the application of 3D printing have been described in this paper. It also discusses the history, techniques, advantages, limitations, and future scope of 3D printing technology in OMFS.
Keywords: Computer-aided designing, computer-aided manufacturing, jaw deformity, orthognathic surgery, three-dimensional model
INTRODUCTION
The field of oral and maxillofacial surgery (OMFS) has witnessed numerous breakthrough innovations in every aspect of clinical practice ranging from evaluation, diagnosis to treatment of the patients. The last decade, has emphasized on the use of newer diagnostic tools, virtual treatment planning techniques, minimally invasive surgeries, regeneration techniques, and precise reconstruction of hard and soft tissues.[1,2]
Various deformities have plagued the head and neck region, which may either arise due to congenital defect or may be acquired due to ablative jaw surgeries and traumatic injuries.[3,4] Such conditions result in compromised aesthetics as well as function. Technological advances in the past decade have developed techniques of printing three-dimensional (3D) models of the jaw and facial bones, which have equipped the oral and maxillofacial surgeon to understand the complex deformities thoroughly. It has significantly aided in preoperative patient assessment and treatment planning.[5] It has also permitted carrying out mock surgeries, replicating the real-life situation resulting in decreased intraoperative time and precise reconstruction of the tissues. The use of surgical guides have helped in precise placement of osteotomy cuts and preadapt the fixation devices, thus saving intraoperative time and improving surgical outcome.[6] The use of patient specific implants have allowed exact restoration of anatomical defects involving the jaw and facial bones.[7]
3D printing involves fabrication of objects by layering method. In this process different materials such as plastic, ceramics, or metals are sequentially layered based on computer-aided data to build a 3D structure.[8] The 3D printing was 1st described by Charles W. Hull and Raymond S. Freed in the year 1986 and 1st commercially marketed in 1988.[9] Conventionally, 3D printing is utilized in the automobile industry for manufacturing of frameworks for different models and instruments.[10] In the last decade, 3D printing has evolved and has been applied in various fields of medicine and surgery. Applications of 3D printing in medical field includes; treatment planning, prosthesis implant fabrications, and medical training.[11] The 1st use of 3D printing in OMFS was reported by Brix et al.[12] Mankovich et al. further popularized its application in treating patients having craniofacial deformities.[13] They used it to simulate bony anatomy of the cranium using computed tomography (CT) scan.
Because of its advantages, 3D printing has found application in the various fields of dentistry.[14] The basic form of 3D printing have been commonly applied for CT-guided stent fabrication which help in precise drilling and placement of dental implants.[15] The use of 3D-printed models in ablative and orthognathic surgeries for treatment planning and simulation has become popular over the past few years.[16] It can be used in patients with facial bone fractures to prepare diagnostic models and for fabrication of patient specific implants, which can improve the reduction of fractures.[3] In orthognathic surgeries, it can be used for performing mock surgeries and for fabrication of cutting guides and occlusal splints to improve accuracy of outcome.[6] In reconstructive surgeries, it can be used for fabrication of cutting guides, which allow 3D accuracy in reconstruction of defects of face and jaw.[5,10] In cleft lip and palate surgeries, it can be utilized to fabricate the nasoalveolar molding plates.[17] It can also be used in manufacturing facial prosthesis especially the jawbone, temporomandibular joint (TMJ), ear, and eye.[14]
Felid of surgery in general and maxillofacial surgery in particular can significantly benefit from 3D printing.[18] 3D printing can aid in plate bending/contouring, manufacturing bone graft templates, tailoring custom-made implants, designing osteotomy guides, and intraoperative occlusal splints.[19,20] It can also reduce the intraoperative time and enhance preciseness of surgeries. Conventionally, the limitations of 3D printing were cost of manufacturing, time taken in fabrication and strict medical regulation for it use. However, with advent of affordable printers and simplification of manufacturing process, its application in field of maxillofacial surgery is expected to rise exponentially.
This article intends to highlight the use of 3D-printed anatomical models, customized prosthesis and implantable devices in the field of OMFS. A case series is presented describing the utility of 3D printing. Its use in the treatment of jaw deformities secondary to condylar hyperplasia is presented, where the anatomical model assisted in evaluation of the facial deformity and explain the extent of the skeletal problem to the patient. It also permitted to carry mock surgery to evaluate the proposed treatment outcome and was used to prebend and adapt the miniplates. The use of 3D models for detailed study of jaw pathology, patient education, shaping and designing of bone graft and prebending of plates is described for a case of large giant cell lesion of mandible in a 15-year-old female patient. The application of 3D models in correction of facial asymmetry secondary to joint deformity and apertognathia is also reported. Utility of 3D printing for fabrication of patient specific implant for chin augmentation in a female patient with deficiency of mandible symphysis is also reported. Institutional Ethical approval and patient consent were obtained for the presented cases.
CASE REPORTS
Case 1 (unilateral condylar hyperplasia)
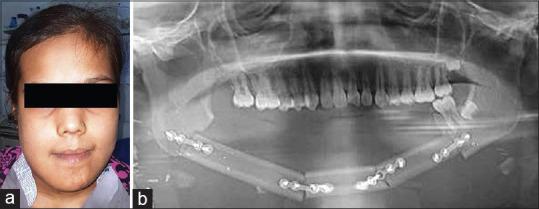
A 24 years old male patient reported with a chief complaint of facial asymmetry, which was evident to him since the past 3 years. Asymmetry was slowly progressing initially but had stabilized over the past 1 year. The patient also complained of difficulty in mastication. On extraoral examination, chin was deviated to the left side [Figure 1a] Intraoral examination revealed the mandibular dental midline was shifted to the left side with posterior cross bite and occlusal cant. Routine radiographic evaluation showed that the right condyle was longer than the left. CT scan confirmed the radiographic findings of right condylar enlargement, increase in length of right mandibular rami with deviation to chin to the right side [Figure 1b and c]. Based on the clinical and radiographic findings, the diagnosis of right condylar hyperplasia was made. Bone scan was performed to study the activity of right condyle, which showed no significant finding.
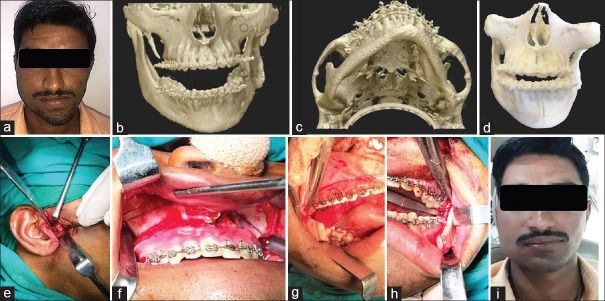
Figure 1.
Preoperative photograph showing facial asymmetry with chin deviation (a), Computed tomography scan of deformed mandible (b and c), Three-dimensional printed anatomical model used for patient education, preoperative assessment, and mock surgery (d), Intraoperative steps involving high condylar shave (e), Lefort I osteotomy with right maxillary impaction (f), and sagittal split ramus osteotomy with differential mandibular setback (g and h), Postoperative photograph showing facial symmetry with correction of chin deviation (i)
A 3D-printed anatomical model of facial skeleton was prepared based on the data available from the CT scan [Figure 1d]. Anatomical model was used to evaluate the facial deformity and explain the extent of the skeletal problem to the patient. Treatment plan for the management of right condylar hyperplasia with associated skeletal and occlusal discrepancy included; high condylar shave on right side to remove the hyperplastic area, Lefort I osteotomy with unilateral (right) maxillary impaction (5 mm) to correct the occlusal cant and sagittal split ramus osteotomy (SSRO) with differential mandibular setback to correct the chin deviation. Mock surgery was performed as per the treatment plan and the outcome was evaluated. The 3D model was used to prebend and adapts the miniplates after the osteotomy cuts were designed and the desired movements were achieved. These plates were used as patient specific implants intraoperatively. The surgical procedure was carried out as planned and executed during the mock surgery [Figure 1e-h]. The patient had uneventful postoperative healing and was kept on periodic follow-up visits. At 2 years' postoperative period, the corrected facial symmetry was stable with no occlusal discrepancy [Figure 1i].
Case 2 (large central giant cell lesion of mandible)
A 15-year-old girl was referred to oral surgery clinic for the management of a rapidly growing expansile lesion involving the mandible. On examination, there was gross facial asymmetry involving the anterior region of mandible [Figure 2a]. Intraoral examination revealed expansion of buccal and lingual plates of mandible with obliteration of anterior lingual vestibule [Figure 2b]. The mucosa overlying the swelling was normal. Panoramic radiograph revealed a multilocular radiolucent lesion extending from tooth 35 to tooth 46 [Figure 2c]. The overall clinical and imaging characteristics were suggestive of a locally aggressive intraosseous pathology. Aspiration from the lesion was negative. Incisional biopsy was performed under local anesthesia. Histopathological assessment provided a confirmatory diagnosis of central giant cell tumor of mandible. Due to the large size of lesion and young age of the patient, conservative management using intralesional injections of steroid (triamcinolone acetate 1 ml/cm of the lesion every week) were started. However, at 2nd week follow-up, there was further increase in size of the lesion [Figure 2d]. After discussion with the patient and her parents, decision of surgical resection of the lesion followed by reconstruction was made. The options of reconstructions included; use of reconstruction plate, avascular bone graft (fibula/iliac bone), and fibula graft with microvascular anastomosis. Due to the large size of the surgical defect and young age of the patient, free fibula was chosen for reconstruction.
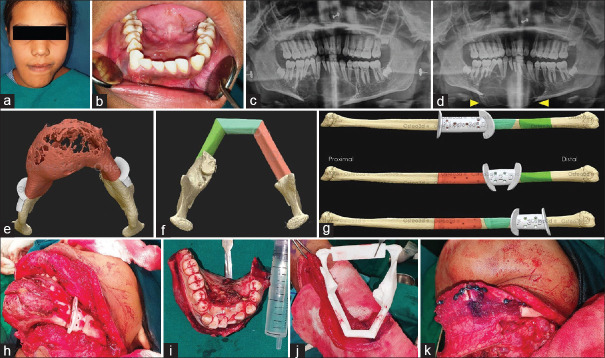
Figure 2.
Photographs showing; swelling over chin (a), intraoral view of swelling obliterating the lingual vestibule (b), OPG showing osteolytic lesion (c), OPG taken 2 weeks after intralesional steroid therapy showing increase in the size of the lesion (arrow head) (d), software generated three-dimensional anatomical model with cutting guides (e), software designed shaping of fibula (f), cutting guide for fibula (g), intraoperative use of the surgical guide (h), excised lesion (i), three-dimensional model to shape fibula to match the neo-mandible (j) and fibula graft fixed using pre-adapted manipulates (k)
Virtual planning of the procedure was carried out before surgery. High-resolution CT scans (0.50 mm cuts) of mandible and fibula was submitted for processing to the manufacturer. Software-based 3D anatomic model of the mandible was generated and simulation of mandibular osteotomies was done. The 3D manufacturer worked in tandem with the surgeon for confirmation and evaluation of the osteotomy lines. Cutting guides on mandible were designed to assist in precise intraoperative placement of osteotomy [Figure 2e]. The 3D imaging of fibula was processed. Shaping and fitting of fibula were planned by superimposition on the preoperative image of the mandible, to match its lower border [Figure 2f]. Areas where destruction of lower border was present, mirroring tools were used for correction. Osteotomy cuts on fibula were designed as per the measurements and cutting guides were prepared [Figure 2g]. The Titanium miniplates were prebent using the mandible model as a template and these plates were used as patient-specific implants. The surgical procedure was carried as planned under general anesthesia. The surgical guide helped in exact placement of the osteotomy cuts over the mandible and precisely excising the lesion in toto [Figure 2h and i]. The fibula was shaped and matched to form the neo-mandible, using the surgical guide and 3D model. It was fixed using preadapted manipulates saving intraoperative time [Figure 2j and k]. Patient had uneventful postoperative recovery. She was regularly recalled and showed acceptable facial contour and function. At 1-year follow-up, the patient showed good healing with no clinical and on radiological evidence of recurrence [Figure 3a and b].
Figure 3.
Postoperative clinical photograph (a) and OPG (b) showing acceptable facial contour and fibula in situ with no evidence of recurrence
Case 3 (unilateral fibrous ankyloses of temporomandibular joint)
A 22-year-old male presented to oral surgery clinic with chief complaint of deformed facial appearance. The deformity was present since the past 10 years. The patient gave a history of fall leading to facial injury 12 years back, for which he received no treatment. On clinical examination, gross facial asymmetry was seen, with deviation of chin to the left side [Figure 4a]. On intraoral examination, inter-incisal opening of 30 mm was present with deviation of mandible toward the left on opening. Significant amount of occlusal cant was seen. Panoramic radiograph revealed deformed mushroom-shaped left mandibular condylar head with reduced joint space when compared to the right side. CT scan was confirmatory of the clinical and radiological findings [Figure 4b-d]. Based on clinical and imaging findings, the diagnosis of unilateral fibrous ankylosis of the left side of TMJ was made.
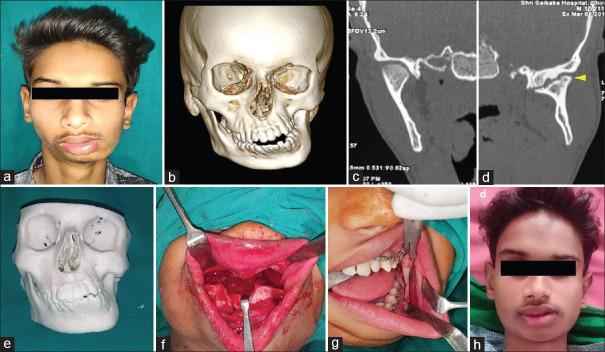
Figure 4.
Photographs showing; gross facial asymmetry (a), three-dimensional computed tomography image of mandible (b), coronal section of computed tomography normal right (c) and deformed mushroom shaped left mandibular condyle head with reduced joint space (d), three-dimensional printed study model (e), surgical steps Lefort 1 with down fracture of maxilla (f), SSRO of mandible (g), and postoperative view showing correction of facial deformity (h)
The patient was informed about the nature of deformity. Treatment plan for the case included TMJ surgery with either high condylar shaving or inter-positional arthroplasty, followed by corrective jaw osteotomy procedure for the management of the facial asymmetry. However, due to lack of any functional disturbance and adequate mouth opening, the patient was unwilling for TMJ surgery and only desired for the correction of facial deformity. The data of facial skeleton CT scan were submitted to the manufacturer and 3D-printed study model was prepared [Figure 4e]. The 3D model was used to study the deformity of the jaw and provided a useful aid to explain the same to the patient. The model was used to plan the osteotomy designs for correction of the facial deformity. The surgical plan included; SSRO with unilateral advancement and inferior positioning of mandible on left side, Lefort 1 osteotomy and correction of occlusal cant and extended lateral sliding genioplasty. Mock surgery was performed on the 3D model to simulate the surgical steps and to evaluate the outcome. The surgical plan as described was executed under general anesthesia [Figure 4f and g]. The postoperative period was uneventful and the patient was kept on regular follow-up visit. At last recall visit 2 years after the surgery, the patient showed marked improvement in facial appearance with correction of the facial deformity [Figure 4h].
Case 4 (apertognathia)
A 21-year-old young autistic male patient was referred to oral surgery clinic with chief complaint of inability to close the mouth completely and difficulty in chewing food. On clinical examination, severe incompetent lips with increased lower facial height were noted. Significant anterior open bite with anterior facial divergence was seen [Figure 5a and b]. The routine panoramic radiograph and lateral cephalogram were done to study the skeletal discrepancy [Figure 5c]. Burstone, Jarabak, and Steiner analysis was done as a part of preoperative orthodontic planning. Based on preoperative evaluation, the treatment options included; single jaw surgery involving Lefort 1 osteotomy and posterior maxillary impaction or bilateral SSRO with counterclockwise rotation of mandible for correction of the anterior open bite. However, due large anterior open bite the risk of unstable result and relapse was high. Subsequently, treatment plan of bi-jaw surgery with Lefort 1 osteotomy (with posterior maxillary superior positioning) and SSRO with mandibular setback along with advancement genioplasty was formulated and discussed with patient and his parents.
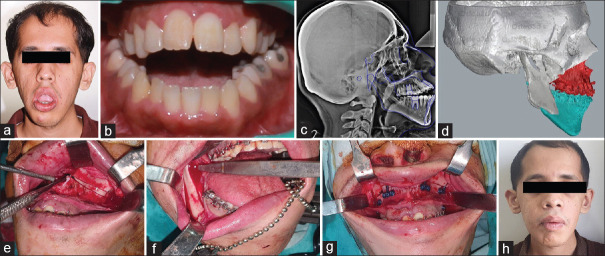
Figure 5.
Photographs showing; severely incompetent lips with increased lower facial height (a), anterior open bite (b), lateral cephalogram to study the skeletal discrepancy (c), virtual surgical planning and placement of computer guided osteotomy cuts (d), intraoperative steps showing Lefort 1 osteotomy (e), osteotomy cuts for SSRO (f), fixation of maxilla segment using preadapted plates (g) and postoperative photograph with correction of the deformity (h)
The patient underwent routine presurgical orthodontic treatment for alignment of arches and decompensation of occlusion. After completion of presurgical orthodontic treatment, 3D-printed models were obtained from CT data of the patient. Virtual surgical planning was done. Computer-guided osteotomy cuts were placed as per the planned surgery [Figure 5d]. The segments were moved in premeasured desired direction to access the treatment outcome. Occlusal splint and surgical cutting guides were 3D printed. The 3D anatomical model was used for preoperative assessment and patient education. Surgical steps were practiced on the anatomical model. Patient was taken for the planned procedure under general anesthesia [Figure 5e-g]. The osteotomies were executed in both jaws as per the preoperative planning. The 3D-printed surgical guides and occlusal splints aided in precise movements of the osteotomized segments intraoperatively. The prebent plates were used, saving intraoperative time. The jaw segments were fixed in its new place and the skeletal deformity was corrected. The patient had uneventful postoperative period. At 2 years' recall visit, the patient showed stable result with correction of the anterior open-bite deformity [Figure 5h].
Case 5: (chin augmentation using patient specific polyether ether ketone implant)
A 26-year-old female reported to oral surgery clinic with the chief complaint of facial asymmetry involving the chin region. Deficiency in the width of mandible in the left symphysis region was noted on extraoral examination. CT scan with 3D reconstruction was done for evaluation of the deformity, which was suggestive of anterior mandible asymmetry. The asymmetry was localized due to mandibular deficiency over the left parasymphysis region. The treatment options for correction of the skeletal deformity included; lateral sliding genioplasty, augmentation with alloplast/prosthesis and autogenous bone graft. On consultation with patient and her parents, choice of augmentation with patient specific 3D-printed implant using polyether ether ketone (PEEK) was made. Computer-guided analysis of deformity was done. Lateral augmentation of left symphysis with overlying soft tissue reconstruction was carried out [Figure 6a and b]. Once the reconstruction was found to be satisfactory, the PEEK implant was 3D printed. Surgical augmentation of deficient parasymphysis region with implant was done using intraoral labial vestibular approach. The mucoperiosteal flap was raised over the left mandible anterior region. Mental foramen was identified and the mental nerve was dissected and retracted from the surgical field. The patient specific implant was then placed over the lateral aspect of mandible to restore the contour defect. The implant was stabilized using two 2 mm × 8 mm titanium screws [Figure 6c and d]. The patient had uneventful postoperative period and was kept on periodic recall visits. The patient showed adequate facial symmetry with restoration of the contour defect of anterior mandible at 1-year follow-up visit.
Figure 6.
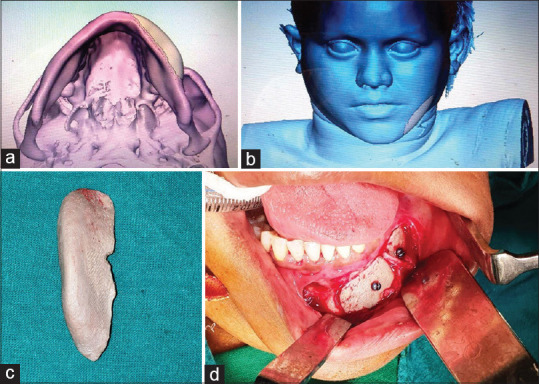
Computer-guided lateral augmentation of left symphysis (a) with overlying soft-tissue envelop (b), Patient-specific implant made from three-dimensional printing of PEEK (c) used for skeletal augmentation in parasymphysis region (d)
DISCUSSION
The reconstructive surgery should not only aim to restore of the preoperative form and function, but also reduce the intraoperative time and postoperative morbidity. Due to the complex anatomy of maxillofacial region, correction of complex developmental/acquired deformities and defects from ablative surgeries becomes a challenging task. In the past few decades advanced imaging modalities, sophisticated instrumentation, advances in anesthesia and refined surgical techniques have resulted in better surgical outcome with lesser morbidity. 3D printing in OMFS has further improved preoperative assessment, intraoperative execution, and postoperative outcome of surgical procedures.[5,7,16,18]
Evolution of three-dimensional printing
3D printing is an additive technology in which life like objects are built by successively adding material in thin layers, using computer aided design.[8] The first reported commercially used 3D printer was based on the technique called stereolithography (SLA). It was described by Charles Hull and Raymond S. Freed in the year 1986.[9] Since 1990 when the first publication on potential use of 3D printing for the preparation of anatomic models in maxillofacial surgery was done by Mankovich et al., its application has significantly gained popularity.[13] Unlike medical device, which has to follow strict government regulations for manufacturing, 3D-printed anatomical models (which are not supposed to come in contact with human body) do not require to follow those norms. Along with this, availability of low-cost printers in the market over the last few years have led to the reduction in cost and increase in the use of 3D printing. Literature review showed a sudden increase in use of these devices in OMFS since 2008, coinciding with arrival of low-cost 3D printers in the market.[21] Unlike 3D-printed anatomical models, 3D-printed occlusal splints, surgical guides, and patient-specific implants have to follow regulatory guidelines similar to medical devices.
Technique of three-dimensional printing
The concept behind 3D printing is to capture the anatomical scans using imaging techniques such as magnetic resonance imaging and CT scans.[22] These images are then saved in a standard digital imaging and communications in medicine format. Subsequently with the help of computer-aided design (CAD) software, a virtual 3D prototype is created with standard tessellation language format to allow 3D printing and deposition of the material layer by layer to achieve the final structure. Printing of the final anatomical structure can be achieved by various techniques including; SLA, fused deposition modeling, inkjet bioprinting, extrusion bioprinting, selective laser sintering, and laser-assisted bioprinting.[23,24] The choice of a technique depends on the required application. In the final step, the 3D print goes through minor modifications to obtain the final printed object.[25]
Use of three-dimensional printing in maxillofacial surgery
One of the most traditional and common application of 3D printing in dentistry and OMFS is the use of surgical guides in implant surgeries.[15] These guides are designed to aid in correctly angulating the drills and subsequently placing the dental implants as per preoperative plan.[15] 3D-printed device/object can be broadly classified into five categories, based on its use in OMFS; 3D anatomic models, surgical guides (cutting/drilling/positioning), occlusal splints, patient specific implants (osteosynthesis plates, skeletal reconstruction), and facial prosthesis. A systematic review in 2017, identified 297 articles (2,889 patients) describing use of 3D-printed devices in OMFS.[21] The most commonly printed objects included surgical guides (59%), anatomic models (34%), and patient specific implants (23%), followed by occlusal splints (8%) and prosthesis (4%).[21]
In the present case series, 3D-printed anatomical models were used in four cases (case 1, 2, 3 and 4). Obtaining the accurate anatomical models help in thorough understanding of the defects and help in restoration of postoperative symmetry and form. It helps the clinician to understand the complex anatomical structures such as orbit, maxilla, and mandible accurately and assists the surgeon in minimizing the intraoperative time and morbidity. In the present case series, the 3D anatomical models helped in preoperative assessment and patient motivation. They were used to practice the procedure preoperatively (mock surgery) and for prebending of bone plates for fixation of osteotomized jaw bone. In the cases presented, surgical cutting guide was used in one case (case 2) for shaping and contouring of the vascular fibula graft for mandibular reconstruction. Recently conducted comparative clinical study showed that 3D printing technology allowed better functional restoration of mandible in comparison to the traditional method.[26] The use of anatomical models of mandible and donor graft reduced the operating time and allowed better shape of lower third of the face. Surgical guides have also been used in orthognathic surgery for correct placement of the osteotomies (cutting guides), insertion of screws at predefined sites on the model (drilling guide) and in final positioning and fixation of the osteotomized bone according to preoperative plan (positioning guide).[27]
Computer-aided simulation significantly increases the efficiency and accuracy of correction of the dentofacial deformities by orthognathic surgery. Virtual surgical planning and 3D-printed anatomical model was used in patient with apertognathia (case no. 4) for presurgical work-up. Virtual planning combined with 3D models provided preoperative insight for the planned surgery. It is also used for fabrication of cutting guides and splints which decrease the intraoperative time and minimizes surgical inaccuracies.[28]
The use of patient-specific implants and prosthesis has been frequently used for temperomandibular joint (TMJ) replacement. Mandibular printed implants are only second to TMJ prosthesis in frequency of use in maxillofacial reconstruction surgery.[29] Manufacturing and processing of these implants have to follow strict guidelines as compared to 3D-printed anatomical models, as these are implanted within the human body. Various materials have been used depending on area of application. Jawbone and joint replacement implants are usually made of high grade metal meant for medical use, including titanium, molybdenum, and cobalt-chrome alloys.[29] Recently PEEK which is a polyaromatic linear polymer has emerged as a upcoming material for medical implants due to its high strength, stiffness, durability, and biocompatibility.[30] Conventionally, it is used in aerospace and automotive industries. In medicine, PEEK was originally used for orthopedic reconstructions. Its use in maxillofacial reconstruction is relatively new. By using CAD and manufacturing, patient-specific implant was printed from PEEK for the management of chin defect in one patient (case no. 5), in the present case series. Patient-specific implants made from PEEK have been reportedly used in maxillofacial surgery for malar augmentation, restoration of craniotomy defects, jaw reconstruction, and subperiosteal implants.[31,32]
Current limitation for use of three-dimensional printing
The major limitation for the application of this 3D technology in routine clinical practice is the cost involved.[33] However, improvement in software development and manufacturing over the past few years have led to reduction in cost of manufacturing. It is expected that the cost will continue to go down as the number of manufactures go up. Another drawback of 3D printing is time taken for manufacturing. Based on its manufacturing, the 3D print can be hospital based or industry based (professional). Although simple anatomical model can be printed in health-care facility, more complex implants such as custom made plates and reconstruction models are more demanding and required professional manufacturing units, increasing its cost and time for fabrication.[34] Need of advanced computer-based skills and regulatory limitations are other hindrances in its development. The accuracy of the 3D-printed models and precision in replicating the complex maxillofacial structures are other challenges for manufacturers. Although 3D-printed patient specific implants have advantages of exact anatomic fit and reduced surgical time, complication such as implant rejection, loosening, and infection have been infrequently reported.[33,34] Most of the evidence of use of 3D-printed objects is through case report and series. In terms of scientific study, there is a need to perform more randomized controlled trials to prove its superiority over the conventional surgical approaches.[34]
Future application
3D printing in maxillofacial surgery has a promising role in future, beyond its current use in preparation of study models, surgical planning, training, use of guide splints, and manufacturing custom fit implants. Higher resolution of printing, faster manufacturing time, and reduced costs would significantly popularize its application. There is a need to be able to print materials with greater biocompatibility to reduce risk of infection and graft rejection. Materials used for patient specific implants should match the flexibility and stiffness of normal bone. Manufacturing of 3D-printed scaffold with internal channel networks for cell proliferation and eventually bone in-growth will further improve the outcome.[35]
CONCLUSION
3D printing has an emerging role in maxillofacial surgery. The current applications include manufacturing of anatomical models, surgical guides/splints, patient specific implants, and prosthesis. 3D printing allows for better preoperative evaluation of facial defect/deformity. It is a useful tool for teaching purpose and for patient education. It allows better preoperative planning and training of the procedure, thus reducing surgical time and postoperative morbidity and in turn improving the surgical outcomes. The major disadvantages of 3D printing are the cost involved and time required for the manufacturing, which can be significantly reduced with the ever-improving technology and increased incorporation of advanced materials in day-to-day clinical practice.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tatullo M, Marrelli M, Paduano F. The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. Int J Med Sci. 2015;12:72–7. doi: 10.7150/ijms.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Yadav N, Singh S, Chauhan N. Minimally invasive (endoscopic-computer assisted) surgery: Technique and review. Ann Maxillofac Surg. 2016;6:159–64. doi: 10.4103/2231-0746.200348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranganath K, Hemanth Kumar HR. The correction of post-traumatic pan facial residual deformity. J Maxillofac Oral Surg. 2011;10:20–4. doi: 10.1007/s12663-010-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrom T, Bast F, Knipping S. Partial mandibulectomy without bony reconstruction in patients with oropharyngeal or mouth cancer. Contemp Oncol (Pozn) 2019;23:146–50. doi: 10.5114/wo.2019.87575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong N, Zhao X. 3D printing for clinical application in otorhinolaryngology. Eur Arch Otorhinolaryngol. 2017;274:4079–89. doi: 10.1007/s00405-017-4743-0. [DOI] [PubMed] [Google Scholar]
- 6.Scolozzi P. Computer-aided design and computer-aided modeling (CAD/CAM) generated surgical splints, cutting guides and custom-made implants: Which indications in orthognathic surgery? Rev Stomatol Chir Maxillofac Chir Orale. 2015;116:343–9. doi: 10.1016/j.revsto.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Stoor P, Suomalainen A, Mesimäki K, Kontio R. Rapid prototyped patient specific guiding implants in critical mandibular reconstruction. J Craniomaxillofac Surg. 2017;45:63–70. doi: 10.1016/j.jcms.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Canstein C, Cachot P, Faust A, Stalder A, Bock J, Frydrychowicz A, et al. 3D MR flow analysis in realistic rapid-prototyping model systems of the thoracic aorta: Comparison with in vivo data and computational fluid dynamics in identical vessel geometries. Magn Reson Med. 2008;59:535–46. doi: 10.1002/mrm.21331. [DOI] [PubMed] [Google Scholar]
- 9.Chua CK, Leong KF, Lim CS. Rapid Prototyping: Principles and Applications. 2nd ed. Vol. 2. Singapore: World Scientific; 2003. [Google Scholar]
- 10.Cunningham LL, Jr, Madsen MJ, Peterson G. Stereolithographic modeling technology applied to tumor resection. J Oral Maxillofac Surg. 2005;63:873–8. doi: 10.1016/j.joms.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Hoy MB. 3D printing: Making things at the library. Med Ref Serv Q. 2013;32:94–9. doi: 10.1080/02763869.2013.749139. [DOI] [PubMed] [Google Scholar]
- 12.Brix F, Hebbinghaus D, Meyer W. Method and device for the model construction in the context of orthopedic and traumatological operations planning. Rötgen Praxis. 1985;38:290–2. [Google Scholar]
- 13.Mankovich NJ, Cheeseman AM, Stoker NG. The display of three-dimensional anatomy with stereolithographic models. J Digit Imaging. 1990;3:200–3. doi: 10.1007/BF03167610. [DOI] [PubMed] [Google Scholar]
- 14.Dawood A, Marti Marti B, Sauret-Jackson V, Darwood A. 3D printing in dentistry. Br Dent J. 2015;219:521–9. doi: 10.1038/sj.bdj.2015.914. [DOI] [PubMed] [Google Scholar]
- 15.Kalman L. 3D printing of a novel dental implant abutment. J Dent Res Dent Clin Dent Prospects. 2018;12:299–303. doi: 10.15171/joddd.2018.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun PY. The application of three-dimensional printing techniques in the field of oral and maxillofacial surgery. J Korean Assoc Oral Maxillofac Surg. 2015;41:169–70. doi: 10.5125/jkaoms.2015.41.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritschl LM, Rau A, Güll FD, diBora B, Wolff KD, Schönberger M, et al. Pitfalls and solutions in virtual design of nasoalveolar molding plates by using CAD/CAM technology – A preliminary clinical study. J Craniomaxillofac Surg. 2016;44:453–9. doi: 10.1016/j.jcms.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Mehra P, Miner J, D'Innocenzo R, Nadershah M. Use of 3-d stereolithographic models in oral and maxillofacial surgery. J Maxillofac Oral Surg. 2011;10:6–13. doi: 10.1007/s12663-011-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eppley BL, Sadove AM. Computer-generated patient models for reconstruction of cranial and facial deformities. J Craniofac Surg. 1998;9:548–56. doi: 10.1097/00001665-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Gerstle TL, Ibrahim AM, Kim PS, Lee BT, Lin SJ. A plastic surgery application in evolution: Three-dimensional printing. Plast Reconstr Surg. 2014;133:446–51. doi: 10.1097/01.prs.0000436844.92623.d3. [DOI] [PubMed] [Google Scholar]
- 21.Louvrier A, Marty P, Barrabé A, Euvrard E, Chatelain B, Weber E, et al. How useful is 3D printing in maxillofacial surgery? J Stomatol Oral Maxillofac Surg. 2017;118:206–12. doi: 10.1016/j.jormas.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Aldaadaa A, Owji N, Knowles J. Three-dimensional printing in maxillofacial surgery: Hype versus reality. J Tissue Eng. 2018;9:2041731418770909. doi: 10.1177/2041731418770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: A review of the methods and applications. Curr Probl Diagn Radiol. 2016;45:2–9. doi: 10.1067/j.cpradiol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: Review of medical applications. Int J Comput Assist Radiol Surg. 2010;5:335–41. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 25.Frame M, Huntley JS. Rapid prototyping in orthopaedic surgery: A user's guide. ScientificWorldJournal. 2012;2012:838575. doi: 10.1100/2012/838575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacek B, Maciej P, Tomasz P, Agata B, Wiesław K, Radosław W, et al. 3D printed models in mandibular reconstruction with bony free flaps. J Mater Sci Mater Med. 2018;29:23. doi: 10.1007/s10856-018-6029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Shen S, Jiang W, Li J, Jiang T, Xia JJ, et al. A new approach of splint-less orthognathic surgery using a personalized orthognathic surgical guide system: A preliminary study. Int J Oral Maxillofac Surg. 2017;46:1298–305. doi: 10.1016/j.ijom.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrell BB, Franco PB, Tucker MR. Virtual surgical planning in orthognathic surgery. Oral Maxillofac Surg Clin North Am. 2014;26:459–73. doi: 10.1016/j.coms.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Mercuri LG, Wolford LM, Sanders B, White RD, Hurder A, Henderson W. Custom CAD/CAM total temporomandibular joint reconstruction system: Preliminary multicenter report. J Oral Maxillofac Surg. 1995;53:106–15. doi: 10.1016/0278-2391(95)90381-x. [DOI] [PubMed] [Google Scholar]
- 30.Hussain RN, Clark M, Berry-Brincat A. The use of a polyetheretherketone (PEEK) implant to reconstruct the Midface region. Ophthalmic Plast Reconstr Surg. 2016;32:e151–e153. doi: 10.1097/IOP.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 31.Lethaus B, Safi Y, ter Laak-Poort M, Kloss-Brandstätter A, Banki F, Robbenmenke C, et al. Cranioplasty with customized titanium and PEEK implants in a mechanical stress model. J Neurotrauma. 2012;29:1077–83. doi: 10.1089/neu.2011.1794. [DOI] [PubMed] [Google Scholar]
- 32.Mounir M, Atef M, Abou-Elfetouh A, Hakam MM. Titanium and polyether ether ketone (PEEK) patient-specific sub-periosteal implants: Two novel approaches for rehabilitation of the severely atrophic anterior maxillary ridge. Int J Oral Maxillofac Surg. 2018;47:658–64. doi: 10.1016/j.ijom.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed Eng Online. 2016;15:115. doi: 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonkergouw J, van de Vijfeijken SE, Nout E, Theys T, Van de Casteele E, Folkersma H, et al. J outcome in patient-specific PEEK cranioplasty: A two-center cohort study of 40 implants. Craniomaxillofac Surg. 2016;44:1266–72. doi: 10.1016/j.jcms.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]