Abstract
Ameloblastic fibrosarcoma (AFS) is an extremely rare malignant odontogenic tumor. It is composed of benign odontogenic epithelium, resembling that of ameloblastoma, and a mesenchymal part exhibiting features of fibrosarcoma. The development of this lesion in the jaws is either de novo or from preexisting ameloblastic fibroma which has been well documented. The most commonly affected site within the jaw is the posterior mandible. These tumors show local aggressiveness and a high tendency to recur. We present a case of a 33-year-old female patient with swelling of the right posterior mandible for 2 months and progressive paresthesia of the same region for the past 6 months. Patient's history revealed undergoing surgical enucleation for ameloblastic fibroma before a year in the same region as current swelling. Examination of the swelling revealed an ulceroprolifeartive Growth of 6 × 4.5 cm extending from premolar to molar region. Primary investigation involved biopsy of the swelling, which was reported as sarcoma for which resection of the right hemimandible and selective neck dissection was performed. Following surgery, the final histopathology report of the resected specimen was reported to be AFS. One year after the surgical procedure, the patient is clinically and radiologically disease-free. Considering the aggressive nature of these tumors, it is vital to give an accurate diagnosis through biopsy, which is considered as gold standard diagnostic evidence, so that the surgeon plans the appropriate therapeutic decision. Knowledge of this rare entity and its histologic features as opposed to the more common benign counterparts such as ameloblastoma or ameloblastic fibroma is crucial as the latter involves a conservative treatment approach while the former can only be treated through aggressive resections.
Keywords: Ameloblastic fibroma, ameloblastic fibrosarcoma, mandible
INTRODUCTION
Odontogenic tumors (OTs) are a rare group of heterogeneous tumors which are derived from epithelial, ectomesenchymal and mesenchymal elements of dental apparatus. Malignant OTs are classified as odontogenic carcinomas and odontogenic sarcomas.[1] Ameloblastic fibrosarcoma (AFS) was first described in 1887 by Heath et al., a malignant OT composed of a benign epithelium and a malignant mesenchymal component.[2] The World Health Organization (WHO) defined AFS as “a neoplasm with a similar structure to ameloblastic fibroma, but in which the ectomesenchymal component shows the features of a sarcoma.”[3]
If the mesenchymal stroma of an ameloblastic fibroma is affected, the recurrent tumor may transform into AFS.[4] However, AFS can also originate de novo.[5] Muller et al. reported that 44% of AFS had a previous diagnosis of AF.[6] According to the literature to date, the most of the AFS cases are located in the mandible.[7] This low-grade sarcoma also has a slightly male preponderance with a mean age of 27.3 years.[8] In this case report, a case of AFS arising from a preexisting AF is described.
CASE REPORT
A 33-year-old woman presented to our department with a 2-month-old history of the right posterior mandibular swelling. Patient reported a gradual increase of swelling's size which led to difficulty in mastication and deglutition. Patient's medical history revealed undergoing surgical enucleation for ameloblastic fibroma in the same region of current swelling in the mandible, operated elsewhere before a year which was evidential through all the previous operative records. Patients also reported progressive paraesthesia of the right mandible for the past 1 year.
On intraoral examination, an ulceroproliferative growth of 6 cm × 4.5 cm extending from premolar to retromolar region in anteroposterior and buccolingual direction respectively [Figure 1]. On palpation, the growth is soft with well-defined borders, tender on palpation, and bleeds.
Figure 1.
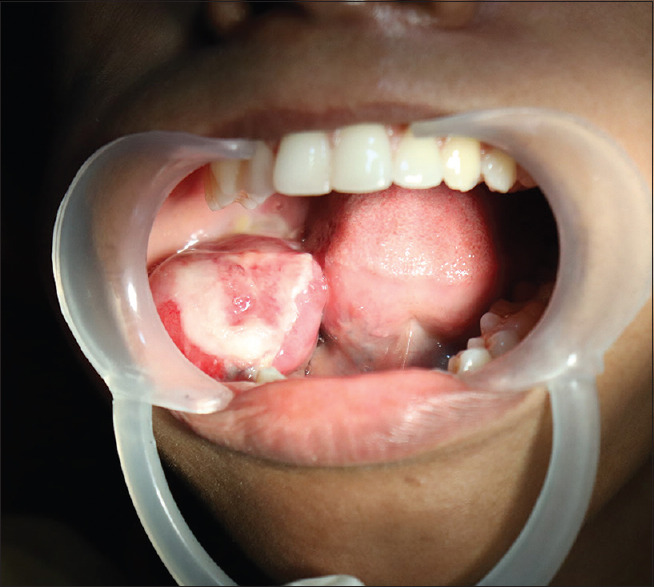
Proliferative lesion of the right mandible involving ramus, the body of the mandible, retromolar trigone, and pterygomandibular raphe
The CT scan revealed a well-defined, multilocular, and osteolytic expansile lesion involving the right ramus and the body of the mandible with the involvement of retromolar trigone, pterygomandibular raphe and gingivobuccal sulcus [Figure 2]. Level I and II lymph nodes of the right side were also positive. Clinical impression of ameloblastoma or ameloblastic fibroma was made and an incisional biopsy was performed.
Figure 2.
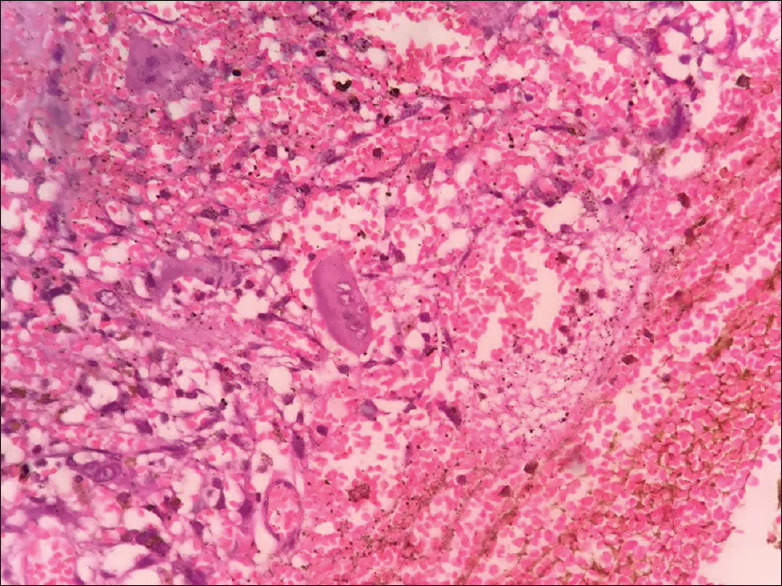
Histopathology showing tumor giant cells with pleomorphism
The initial histopathological results were suggestive of spindle cell carcinoma and fibrosarcoma. Positron emission tomography-computed tomography scans were consistent with biopsy-proven lytic lesion of the right ramus and body of the mandible [Figure 3]. Patient underwent right hemimandibulectomy with adequate margins, selective neck dissection of Level I and II and a reconstruction plate is fixed.
Figure 3.
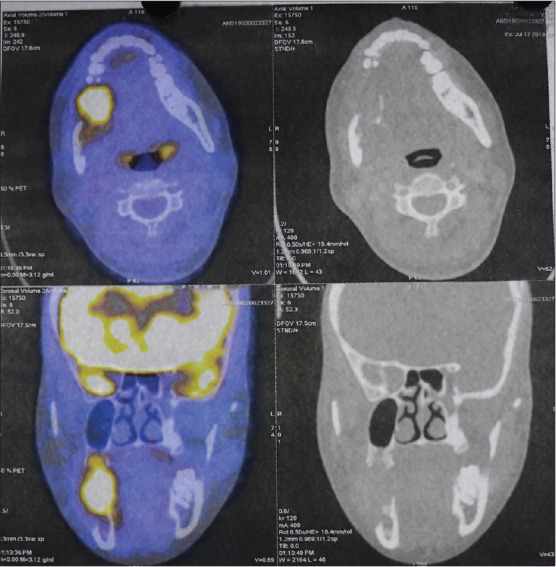
Positron emission tomography-computed tomography scan showing malignant lytic lesion of the right mandible
The histopathologic analysis showed highly cellular connective tissue stroma showing pleomorphic cells of variable size and shape. The ovoid to spindle-shaped tumor cells exhibiting vesicular nuclei and prominent nucleoli. Nuclei exhibiting varying degrees of hyperchromatism and pleomorphism along with numerous mitotic figures were seen. A few tumor giant cells along with clustering of pleomorphic cells in a streaming pattern were also evident. There were distinct areas of ameloblastic fibroma without malignant changes.
Few ameloblastic follicles were evident among the highly cellular and pleomorphic connective tissue stroma. The follicles showed peripherally arranged tall columnar cells with reversal of polarity including few follicles showing central stellate reticulum like areas. There was evidence of an area of transition of primitive mesenchyme to pleomorphic stroma. There was also a presence of tumor cells infiltrating the skeletal muscle. There were areas of round cells in the connective tissue stroma.
Serial review of the specimens was done. It was positive for vimentin for connective tissue tumor, negative for cytokeratin (epithelium), S100 (to rule out neural component), rule to smooth muscle tumors, desmin (to rule out rhabdomyosarcoma), FLI-1 (for Ewing's sarcoma), and CD 45 (for lymphoma) to confirm the sarcomatous areas. Tumor cells in the sarcomatous areas were positive for vimentin and showed diffuse nuclear mitosis. Final histopathology results reflected as AFS low index for Ki-67. Essentially, the conclusion of AFS came with a diagnosis of exclusion. Patient is evidentially disease free for 1 year postsurgery and is under further follow-up.
DISCUSSION
AFS is a rare neoplasm, in which the clinical and pathological distinction from other neoplasms is essential for appropriate care. Terms such as “ameloblastic dentinosarcoma” and “ameloblastic odontosarcoma” have been used to describe these types of neoplasms depending on the presence of dentin or enamel, as some authors consider these tumors as histopathological variants of the same neoplasm. However, recently in the WHO “classification” of OTs, ameloblastic odontosarcoma and dentinosarcoma are listed separately from AFS. AFS occurs within a wide age range from 3 to 89 years. Only 103 cases of AFS have been reported in the existing literature out of which 71 were primary lesions and 25 were secondary lesions occurring after the treatment of an AF. Central AF and AFS were more prevalent in men than in women. Peripheral AF is more prevalent in men. AFS is more often presented as a multilocular radiolucency. As patients with AFS presented at a higher mean age when compared to those with AF, together with the fact that >50% of reported cases had histological documentation of AF in the same site[5,9] it was hypothesized that there is a stepwise malignant transformation.[8]
While cases of AFS have been observed as arising de novo,[6,10,11] several authors have also demonstrated an ameloblastic fibroma or an ameloblastic fibro-odontoma to be the pre-existing lesion of AFS.[12,13,14,15,16]
In malignant transformation, ameloblastic fibroma was found to be associated with oncogenic aberrations in tumor-related genes, where the unequivocal proliferation of the mesenchymal component within the tumor results in loss of the epithelial component, which is a usual presentation of sarcomatous changes, associated with AF.[17,18]
In reviewing the literature, many instances show that the epithelial component seems to disappear as malignant transformation progresses so that eventually all traces of the odontogenic origin may be lost and the histopathologic features become those of the central odonto sarcoma of the jaws.[6,11,16]
The microscopic features of AFS are of narrow cords and islands of essentially nonneoplastic odontogenic epithelium with palisading peripheral cells embedded in a relatively cellular stroma, the malignant fibroblasts being bizarre and pleomorphic with hyperchromatic nuclei and numerous atypical mitotic figures.[3,19]
In the differential diagnosis of AFS, especially where odontogenic epithelium is absent, dermatofibrosarcoma protuberans, fibrosarcomas, and other spindle cell sarcomas should be ruled out because the mesenchymal cells could be arranged in bundles simulating a herringbone, cartwheel, or storiform pattern. Cytokeratin immunostaining may be helpful in identifying epithelial nests and thus excluding the pure sarcomas.
The initial variance in histopathology results between fibrosarcoma and spindle carcinoma could be due to the presence of spindle cells and sarcomatous changes. Spindle cell carcinoma is excluded as there are no malignant epithelial components. In our case, we arrived at the conclusion of AFS with exclusion of other sarcomas as well as the epithelial tumors.
The epithelial component is not commonly associated with AF or AFS growth; however, evidence of epithelial proliferative activity has been described in AFS.[20,21,22] In our case, marked hyperplasia of ameloblastic-like cells was observed; however, low Ki-67 index was found. The presence of differences in proliferative potential between epithelial and mesenchymal AFS components is relevant for the differentiation of AFS from odontogenic carcinosarcoma, in which the epithelial and mesenchymal components are similarly proliferative and anaplastic.[23,24,25]
Any interference in the mechanism of cell growth can cause abnormal growth or cancer. The cell cycle is controlled by cyclin-dependent kinases and their inhibitors (CDK and CDKIS). These proteins have been used to monitor their biological behavior and prognosis for different neoplasia. p53 was the most studied tumor suppression protein, which binds DNA and activates expression of several genes that culminates with inhibition of cell cycle. When malignant transformation about AF was studied, it has been found that their transformation is linked to the evolution of oncogenic aberrations in TP53. These alterations have been shown by loss of heterozygosity and immunohistochemistry studies.[8]
Other important regulatory cell cycle proteins are p63 and p16. p63 protein has been recognized as a member of the p53 family and is also responsible for cell cycle control. p16 is a tumor suppressor gene protein, which is a CDKI that regulates the G1-S phase of the cell cycle. Studies regarding p63 and p16 on the pathogenesis of AFS are apparently elusive.[26,27]
There was no differentiation change in the epithelium in AFS, although cell proliferation is up-regulated in the sarcomatous component. The malignant sarcomatous component showed positivity only for vimentin, demonstrating no apparent transdifferentiation signal. More studies based on a comparative approach are required to improve the significance of the present findings on the pathogenesis and progression of AFS.
CONCLUSION
The data compiled to demonstrate that AFS is a low-grade mesenchymal odontogenic malignant neoplasia with a predilection for the posterior mandible, occurring mainly in the third decade of life. The histopathological features of AFS suggest that this entity has pathogenetic relationships with AFDS and AFOS. There was some suggestive morphological and behavioral evidence of similarities among these lesions. Initial treatment includes excisional surgery combined with chemotherapy and radiotherapy. Surgery should include wide excision with negative margins. Cervical lymph node dissection is usually not necessary, because the sarcoma extends through the vascular channels and lymphatic metastasis is absent. In the present case, we decided to perform a lymph node dissection as the MRI indicated the presence of lymphadenopathy. Neoadjuvant and/or adjuvant chemotherapy are indicated. Adjuvant radiotherapy with a total dose of 50–60 Gy is used to decrease the risk of postoperative recurrence. Recurrence rate for AFS is 23.9%–37%, hence the need for radical treatment and regular follow-up. This case reinforces the necessity of treating AFS with an aggressive surgical approach, with need for other complementary therapies. Evaluation of the growth potential in AF and related lesions could therefore be of help in understanding the tumor aggressiveness and in selecting appropriate surgical procedures.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Barnes l. International agency for research on cancer. Pathology and genetics of head and neck tumours. 2005:283–327. [Google Scholar]
- 2.Dallera P, Bertoni F, Marchetti C, Bacchini P, Campobassi A. Ameloblastic fibrosarcoma of the jaw: Report of five cases. J Craniomaxillofac Surg. 1994;22:349–54. doi: 10.1016/s1010-5182(05)80116-7. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi K, Murakami R, Fujii T, Hirano A. Malignant transformation of ameloblastic fibroma to ameloblastic fibrosarcoma: Case report and review of the literature. J Craniomaxillofac Surg. 2005;33:352–5. doi: 10.1016/j.jcms.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Pindborg JJ. Ameloblastic sarcoma in the maxilla. Report of a case. Cancer. 1960;13:917–20. doi: 10.1002/1097-0142(196009/10)13:5<917::aid-cncr2820130509>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Zabolinejad N, Hiradfar M, Anvari K, Razavi AS. Ameloblastic fibrosarcoma of the maxillary sinus in an infant: A case report with long-term follow-up. J Pediatr Surg. 2008;43:e5–8. doi: 10.1016/j.jpedsurg.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 6.Peychl L, Sazama L. Adamantinosarcoma of the maxilla.(Report of a case) Neoplasma. 1971;18:403–6. [PubMed] [Google Scholar]
- 7.Yamaguchi S, Nagasawa H, Suzuki T, Fujii E. Sarcomas of the oral and maxillofacial region: A review of 32 cases in 25 years. Clin Oral. 2004;8:52–5. doi: 10.1007/s00784-003-0233-4. [DOI] [PubMed] [Google Scholar]
- 8.Williams MD, Hanna EY, El-Naggar AK. Anaplastic ameloblastic fibrosarcoma arising from recurrent ameloblastic fibroma: Restricted molecular abnormalities of certain genes to the malignant transformation. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007;104:72–5. doi: 10.1016/j.tripleo.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Lai J, Blanas N, Higgins K, Klieb H. Ameloblastic fibrosarcoma: Report of a case, study of immunophenotype, and comprehensive review of the literature. J Oral Maxillofac Surg. 2012;70:2007–12. doi: 10.1016/j.joms.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Cataldo E, Nathanson N, Shklar G. Ameloblastic sarcoma of the mandible. Oral Surg Oral Med Oral Pathol. 1963;16:953–7. doi: 10.1016/0030-4220(63)90197-x. [DOI] [PubMed] [Google Scholar]
- 11.Hatzifotiadis D, Economou A. Ameloblastic sarcoma in the maxilla a case report. J Maxillofac Surg. 1973;1:62–4. doi: 10.1016/s0301-0503(73)80014-1. [DOI] [PubMed] [Google Scholar]
- 12.Cina MT, Dahlin DC, Gores RJ. Ameloblastic sarcoma. Oral Surg Oral Med Oral Pathol. 1962;15:696–700. doi: 10.1016/0030-4220(62)90252-9. [DOI] [PubMed] [Google Scholar]
- 13.Howell RM, Jefferson Burkes E. Malignant transformation of ameloblastic fibro-odontoma to ameloblastic fibrosarcoma. Oral Surg Oral Med Oral Pathol. 1977;43:391–401. doi: 10.1016/0030-4220(77)90326-7. [DOI] [PubMed] [Google Scholar]
- 14.Reichart PA, Zobl H. Transformation of ameloblastic fibroma to fibrosarcoma. Int J Oral Surg. 1978;7:503–7. doi: 10.1016/s0300-9785(78)80045-3. [DOI] [PubMed] [Google Scholar]
- 15.Takeda Y, Kaneko R, Suzuki A. Ameloblastic fibrosarcoma in the maxilla, malignant transformation of ameloblastic fibroma. Virchows Arch A Pathol Anat Histopathol. 1984;404:253–63. doi: 10.1007/BF00694891. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein G, Parker FP, Fitz Slaughter Hugh G. Ameloblastic sarcoma. Pathogenesis and treatment with chemotherapy. Cancer. 1976;37:1673–8. doi: 10.1002/1097-0142(197604)37:4<1673::aid-cncr2820370410>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Arab Oghli A, Scuto I, Ziegler C, Flechtenmacher C, Hofele C. A large ameloblastic fibro-odontoma of the right mandible. Med Oral Patol Oral Cir Bucal. 2007;12:34–7. [PubMed] [Google Scholar]
- 18.Muller S, Parker DC, Kapadia SB, Budnick SD. Ameloblastic fibrosarcoma of the jaws: A clinicopathologic and DNA analysis of five cases and review of the literature with discussion of its relationship to ameloblastic. Oral Surg Oral. 1995;79:469–77. doi: 10.1016/s1079-2104(05)80130-1. [DOI] [PubMed] [Google Scholar]
- 19.Mosqueda Taylor A, Meneses García A, Ruíz Godoy Rivera LM, Suárez Roa M de L, Luna Ortiz K. Malignant odontogenic tumors. A retrospective and collaborative study of seven cases. Med Oral. 2003;8:110–21. [PubMed] [Google Scholar]
- 20.Huguet P, Castellví J, Avila M, Alejo M, Autonell F, Basas C, et al. Ameloblastic fibrosarcoma: Report of a case. Immunohistochemical study and review of the literature. Med Oral. 2001;6:173–9. [PubMed] [Google Scholar]
- 21.Pontes HA, Pontes FS, Silva BS, Cury SE, Fonseca FP, Salim RA, et al. Immunoexpression of Ki67, proliferative cell nuclear antigen, and Bcl-2 proteins in a case of ameloblastic fibrosarcoma. Ann Diagn Pathol. 2010;14:447–52. doi: 10.1016/j.anndiagpath.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Hu YY, Deng MH, Yuan LL, Niu YM. Ameloblastic fibrosarcoma of the mandible: A case report and mini review. Exp Ther Med. 2014;8:1463–6. doi: 10.3892/etm.2014.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkel M, Ghalibafian M, Radner H, Reichert TE, Fischer B, Wagner W. Ameloblastic fibrosarcoma or odontogenic carcinosarcoma: A matter of classification? Oral Oncol. 2004;40:444–9. doi: 10.1016/j.oraloncology.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 24.DeLair D, Bejarano PA, Peleg M, El-Mofty SK. Ameloblastic carcinosarcoma of the mandible arising in ameloblastic fibroma: A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:516–20. doi: 10.1016/j.tripleo.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Chikosi R, Segall N, Augusto P, Freedman P. Odontogenic carcinosarcoma: Case report and literature review. J Oral Maxillofac Surg. 2011;69:1501–7. doi: 10.1016/j.joms.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 26.Takeda Y. Ameloblastic fibroma and related lesions: Current pathologic concept. Oral Oncol. 1999;35:535–40. doi: 10.1016/s1368-8375(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 27.Gilani SM, Raza A, Al-Khafaji BM. Ameloblastic fibrosarcoma: A rare malignant odontogenic tumor. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:53–6. doi: 10.1016/j.anorl.2013.03.001. [DOI] [PubMed] [Google Scholar]


