Abstract
Lactoferrin (Lf), an iron-sequestering glycoprotein, predominates in mucosal secretions, where the level of free extracellular iron (10−18 M) is not sufficient for bacterial growth. This represents a mechanism of resistance to bacterial infections by prevention of colonization of the host by pathogens. In this study we were able to show that Streptococcus pneumoniae specifically recognizes and binds the iron carrier protein human Lf (hLf). Pretreatment of pneumococci with proteases reduced hLf binding significantly, indicating that the hLf receptor is proteinaceous. Binding assays performed with 63 clinical isolates belonging to different serotypes showed that 88% of the tested isolates interacted with hLf. Scatchard analysis showed the existence of two hLf-binding proteins with dissociation constants of 5.7 × 10−8 and 2.74 × 10−7 M. The receptors were purified by affinity chromatography, and internal sequence analysis revealed that one of the S. pneumoniae proteins was homologous to pneumococcal surface protein A (PspA). The function of PspA as an hLf-binding protein was confirmed by the ability of purified PspA to bind hLf and to competitively inhibit hLf binding to pneumococci. S. pneumoniae may use the hLf-PspA interaction to overcome the iron limitation at mucosal surfaces, and this might represent a potential virulence mechanism.
Streptococcus pneumoniae is one of the most important microorganisms infecting humans. Pneumococci colonize the upper respiratory tract and are one of the major causes of bacterial pneumonia, meningitis, bacteremia, and otitis media. Despite the availability of antibiotics, mortality and morbidity rates remain high, especially in high-risk groups such as infants, the elderly, and immunocompromised individuals (7). The mechanism of iron acquisition on mucosal surfaces by pneumococci, a prerequisite for multiplication, is unknown. Bacterial pathogenesis is a complex process and depends largely on the efficiency with which pathogens gain access to host niches. For pathogens requiring the uptake of essential exogenous nutrients, colonization of the host is the most critical point in the process of infection. Iron is essential for bacterial growth; however, as the majority of iron within the host is complexed to proteins, free iron is present at only very low levels (∼10−18 M) at mucosal surfaces (3). Most of the intracellular iron is bound to ferritin or held as a component of heme compounds. In extracellular spaces, iron is associated with iron transport proteins referred to as siderophilins, which possess a high affinity for iron(III). The siderophilins lactoferrin (Lf) and transferrin are monomeric glycoproteins which are important in the pathogenesis of infectious bacteria. Whereas transferrin is predominant in serum and lymphatic fluids, Lf is the major iron-binding protein in mucosal secretions and phagocytic cells (1). In response to iron limitation, bacterial pathogens have developed diverse strategies to acquire iron from the host. Many pathogens acquire iron by synthesis and secretion of low-molecular-weight and high-affinity iron chelator compounds called siderophores (5, 20). In contrast, non-siderophore-producing bacteria are capable of iron scavenging from iron-containing transport proteins or heme-containing molecules. Our knowledge of the biochemistry and genetics of such receptor-mediated processes is based mainly on studies of gram-negative pathogens (8). Uptake of iron from iron transport proteins, however, has also been demonstrated for gram-positive pathogens (10, 16, 18). Little is known about the mechanisms of iron acquisition by S. pneumoniae. Recently, Tai et al. (21) identified a hemin-binding polypeptide and described the use of hemin as an iron source for pneumococcal growth. It was also reported that pneumococci do not produce siderophores. In this report, we describe the characterization of a specific interaction of S. pneumoniae with human Lf (hLf) and the identification of the pneumococcal surface protein A as a specific receptor for hLf.
MATERIALS AND METHODS
Binding of 125I-labelled hLf to S. pneumoniae.
Binding assays with pneumococcal cells were performed as described by Hammerschmidt et al. (9) with 125I-labelled hLf and human transferrin (Sigma). In competitive binding experiments, the binding of 13.8 ng of labelled hLf to 5 × 108 pneumococcal cells (type 3 strain NCTC 7978) was determined in the presence of various concentrations of unlabelled hLf. The data for the equilibrium binding were plotted as described by Scatchard (19).
Western blot analysis.
Proteins from whole-cell lysates of S. pneumoniae were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis by the method described by Laemmli (11) and subsequently transferred to a nylon membrane (Immobilon-P; Millipore) by using a semidry blotting system (Bio-Rad). Membranes were blocked by incubation in 10 mM phosphate-buffered saline (PBS) containing 10% skim milk. To identify the putative hLf-binding component, membranes were probed with either radiolabelled hLf or nonradioactive hLf in conjunction with anti-hLf antibodies (Sigma). 125I-labelled hLf was added to a final concentration of 55 ng ml−1 in PBS–0.05% Tween 20 and incubated at room temperature for 2 h. After four washes with PBS, the membranes were exposed overnight to X-ray film. The binding of nonlabelled hLf to pneumococci was assayed in immunoblots. Treatment of pneumococcal cells with proteolytic enzymes was performed as described previously (9). The pretreated pneumococcal cells were used in binding experiments as well as in Western blot analysis.
Purification and structural analysis of the pneumococcal Lf-binding protein.
The pneumococcal hLf-binding protein from strain NCTC 7978 (type 3) was purified by affinity chromatography. hLf (10 mg) was coupled to CNBr-activated Sepharose 4B (Pharmacia Biotech Products) according to the manufacturer’s instructions. For preparation of cell wall proteins, cells were harvested, washed twice in 0.02 M PBS, and resuspended in PBS containing 1 mM phenylmethylsulfonyl fluoride. Cell lysis was carried out by two passages through a French pressure cell with a calculated internal cell pressure of 17,000 lb/in2. Protoplasts were removed by centrifugation for 10 min at 3,000 × g. The membrane fraction was collected by centrifugation at 19,000 × g for 50 min and resuspended in 0.1 M potassium phosphate buffer (pH 7.2) containing 150 mM KCl, 10 mM EDTA, and 20% glycerol. To separate peripheral membrane proteins, the membrane fraction was incubated for 30 min with different concentrations (5, 10, and 20 mM) of the detergent CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} at 28°C. After centrifugation for 30 min at 19,000 × g, the supernatants were collected, combined, and dialyzed against 0.02 M PBS. All fractions were tested for their capacity to bind hLf by Western blot analysis. To obtain primary structural information on the pneumococcal Lf-binding protein, the purified concentrated proteins were separated by SDS-polyacrylamide gel electrophoresis on a 6% polyacrylamide gel. The proteins were subsequently transferred to a nylon membrane (Immobilon-P; Millipore). The pneumococcal hLf-binding protein band was identified by Western blotting, and the corresponding band was excised from the Coomassie blue-stained gel and used for internal peptide sequencing.
Expression cloning and sequencing.
Primers incorporating an in-frame BamHI restriction site at the 5′ end and a HindIII site at the 3′ end were designed from the pspA sequence (GenBank accession no. M74122) in order to amplify PspA by PCR. Amplified fragments were digested with BamHI and HindIII and ligated into similarly digested pQE30 vector DNA (Qiagen, Hilden, Germany). Oligonucleotides SH33 (5′-GGATCCGAAGAAGAATCTCCCGTAGCC-3′) and SH34 (5′-AAGCTTTTTGGTGCAGGAGCTGGTTTTTC-3′), as well as SH33 and PRP2 (5′-AAGCTTATTAACTGCTTTCTTAAGGTC-3′), were used to amplify the 5′ end of pspA from nucleotide 80 to 1005 and also from nucleotide 80 to 799, resulting in pQP1 and pQP2, respectively. PspA His-tagged fusion proteins were purified by chromatography on Ni-nitrilotriacetic acid resins according to the protocols of the manufacturer (Qiagen). Nucleotide sequence determination was by ABI PRISM dye terminator cycle sequencing (Perkin-Elmer).
RESULTS
Binding of hLf to S. pneumoniae.
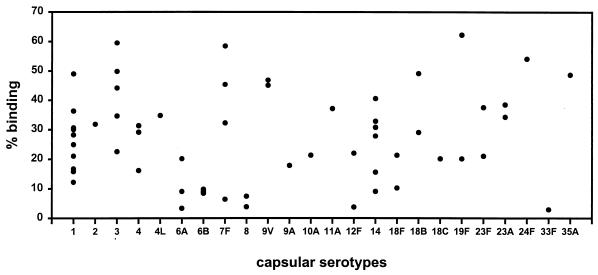
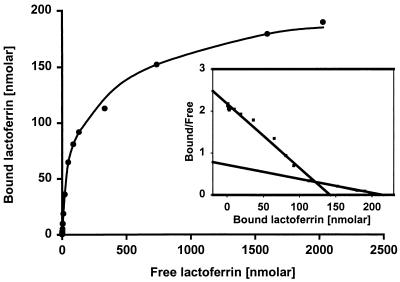
Binding of 125I-labelled hLf to S. pneumoniae was tested with 63 strains belonging to 24 different serotypes. Of these, 88% of the strains bound hLf and 43% of the strains showed a binding of greater than 30% of the radiolabelled hLf (Fig. 1). Binding of less than 5% was considered negative. All strains, including those negative for hLf binding, were collected at late log phase and did not show any autolysis. Competitive inhibition binding experiments using hLf as both radioligand and competitor resulted in a saturation curve. A concentration of ∼300 nM unlabelled hLf caused 50% blocking of binding of 125I-labelled hLf. Plotting the data as described by Scatchard (19) yielded two linear plots, suggesting that two bacterial components on the surface contribute to the binding of hLf. Dissociation constants of 5.7 × 10−8 and 2.74 × 10−7 M were obtained for the high- and low-affinity binding, respectively (Fig. 2). Inhibition experiments carried out with unlabelled bovine Lf and human transferrin as competitors, using a type 3 strain (NCTC 7978), did not influence the specificity of hLf binding (data not shown). In contrast to hLf binding, pneumococci of different capsular serotypes did not bind 125I-labelled human transferrin (data not shown). Proteolytic treatment of pneumococci with trypsin and pronase E resulted in complete loss of binding activity, indicating that the hLf-binding components are proteins.
FIG. 1.
Binding of 125I-labelled hLf to pneumococcal strains belonging to 24 different serotypes, expressed as a percentage of the total radioactivity bound to the cells. Each point represents the mean binding (from triplicate determinations) to a strain. Binding of less than 5% was considered background. A total of 63 strains were tested. Strains were obtained from culture collections (American Type Culture Collection or National Collection of Type Cultures) (n = 5), and serotyped clinical isolates were from the Statens Serum Institute, Copenhagen, Denmark (n = 12), and the Institute of Medical Microbiology, Düsseldorf, Germany (n = 46).
FIG. 2.
Binding of 125I-labelled hLf to S. pneumoniae in the presence of various concentrations of unlabelled hLf. Equilibrium binding of hLf to pneumococcal strain type 3 (NCTC 7978) with 23.6 ng of 125I-labelled hLf and increasing concentrations of unlabelled hLf is shown. The data were also plotted as described by Scatchard (19) (inset). Values shown are the means of triplicate determinations.
Western blot analysis.
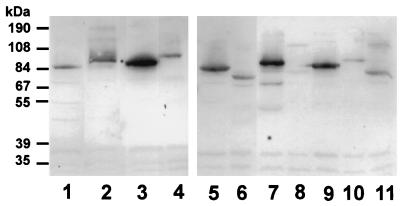
The molecular masses of the putative hLf-binding components of 66 pneumococcal strains were estimated from Western blots by using radiolabelled 125I-hLf. The results indicated that 87.9% of the pneumococci bind hLf by components with apparent molecular masses of between 67 and 104 kDa (Fig. 3). Pretreatment of the pneumococci with proteolytic enzymes such as proteinase K, pronase E, and trypsin abolished the binding of hLf, confirming the proteinaceous nature of the bacterial receptor for hLf (data not shown).
FIG. 3.
Western blot analysis of 125I-labelled hLf binding to pneumococci. The autoradiograph shows the hLf-binding patterns of 10 different serotypes of S. pneumoniae. Lanes: 1, type 3 (NCTC 7978); 2, type 2 (ATCC 11733); 3, type 3; 4, type 4L; 5, type 6A; 6, type 6B; 7, type 9V; 8, type 12F; 9, type 18C; 10, type 19F; and 11, type 23F.
Purification of the pneumococcal hLf-binding protein.
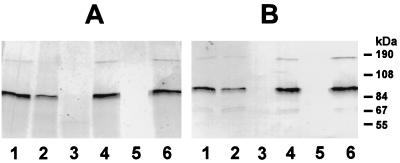
In order to purify the hLf-binding protein from S. pneumoniae, the membranes of a type 3 strain (NCTC 7978) were separated from the cytosolic compartments, and the membranes were subsequently treated with CHAPS to isolate the membrane proteins. All fractions were tested by Western blot analysis with hLf (data not shown). The results showed that the supernatant of the CHAPS-treated membranes contained the hLf-binding protein. These results, together with the results of the binding experiments, suggest that the pneumococcal hLf-binding protein of S. pneumoniae is exposed to the bacterial surface. The fraction containing solubilized membrane proteins was then applied to hLf immobilized on CNBr-activated Sepharose. The membranes prior to CHAPS treatment, and the flowthrough and eluate from the affinity chromatography step, were examined for binding of hLf. Furthermore, the number of proteins eluted by affinity chromatography was verified by Coomassie blue staining of an identical SDS-polyacrylamide gel (data not shown). The results indicated a prominent protein migrating at approximately 87 kDa, corresponding to the identified hLf-binding protein in the crude cell extract and membrane fraction of the type 3 strain (NCTC 7978), and another protein of approximately 180 kDa (Fig. 4). Following purification of the hLf receptor by hLf affinity chromatography, the S. pneumoniae receptor was treated with proteases. Both trypsin and pronase E abolished binding of hLf to the protein purified by affinity chromatography. By contrast, treatment with neuraminidase had no effect on binding capacity (Fig. 4).
FIG. 4.
Analysis of hLf binding and protease sensitivity of the purified hLf-binding receptor from S. pneumoniae. The purified pneumococcal hLf-binding receptor was treated with proteases and neuraminidase. (A) Silver-stained samples; (B) immunoblot analysis with hLf. Lanes: 1, untreated purified hLf protein; 2, 30-min incubation at 37°C without enzyme; 3, trypsin-treated sample; 4, 15-min incubation at 37°C without enzyme; 5, pronase E-treated sample; 6, neuraminidase-treated sample.
Identification and expression of the pneumococcal Lf receptor.
The sequence analysis of an internal peptide of the purified hLf-binding protein revealed a high similarity to pneumococcal surface protein A (PspA) (23). Different fragments of the 5′ end of the pspA gene were amplified by PCR with oligonucleotides derived from the pspA sequence of S. pneumoniae Rx1 (accession no. M74122) (23) and cloned into expression vector pQE30. The different purified His-tagged fusion proteins of PspA and one recombinant protein from another pneumococcal choline-binding protein, SpsA, were tested for the binding of hLf. Western blot analysis indicated that of the tested recombinant proteins, only PspA specifically bound hLf, whereas the secretory immunoglobulin A-binding protein, SpsA, showed no ability to bind hLf (Fig. 5). Binding of hLf was observed only in two recombinant clones harboring plasmids encoding N-terminal domains of PspA protein. The first of these clones, pQP1, expressed amino acids 32 to 370 of PspA, which include the proline-rich domain, whereas the second clone, pQP2, expressed amino acids 32 to 289 and did not include the proline region. This suggests that the binding domain of PspA is located in the N-terminal region of the protein between amino acids 32 and 289 according to the sequence deposited in the database and that hLf binding is not dependent on the presence of either the proline-rich or choline-binding region. Comparison of the mature N-terminal PspA amino acid sequences from S. pneumoniae type 3 (NCTC 7978) and Rx1 (23) revealed an identity of 96.5% (Fig. 6). The 13 amino acid substitutions that are apparent between amino acids 138 and 179 do not affect the binding of hLf, since a recombinant Escherichia coli clone expressing the PspA of S. pneumoniae ATCC 11733 (type 2), which is identical to the Rx1 PspA (23), reacted with hLf.
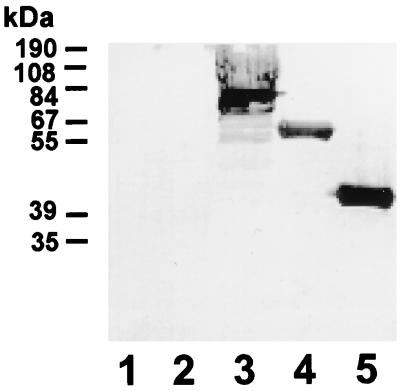
FIG. 5.
Specific binding of hLf to PspA. Immunoblot analysis with hLf, indicating specific binding of hLf to the pneumococcal surface protein A (PspA), is shown. Lanes: 1, secretory immunoglobulin A-binding protein SpsA (control choline-binding protein); 2, E. coli M15(pREP4); 3, S. pneumoniae type 3 (NCTC 7978); 4, PspA residues 32 to 390 (pQP1); 5, PspA residues 32 to 289 (pQP2).
FIG. 6.
Alignment of the mature N-terminal regions of the PspA protein sequence under accession no. M74122 (PspA) (23) and PspA from strain NCTC 7978. Amino acids that differ are boxed. Comparison of the sequences revealed an identity of 96.5%.
Competitive inhibition of hLf binding by PspA.
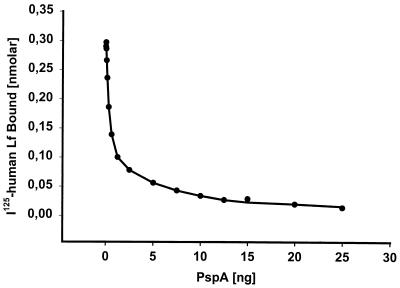
A competitive inhibition assay was performed with 125I-labelled hLf in the presence of different concentrations of the purified 32.1-kDa N-terminal region of the PspA type 25 protein from S. pneumoniae ATCC 11733 (serotype 2) containing the hLf-binding domain. Despite the presence of a second putative hLf-binding protein as revealed by Scatchard analysis, PspA could completely inhibit the binding of the radiolabelled hLf to pneumococci (Fig. 7). These results suggested that PspA is the major pneumococcal receptor for hLf.
FIG. 7.
Competitive inhibition of hLf binding to S. pneumoniae serotype 3 (NCTC 7978) by a purified type 25 PspA cloned from S. pneumoniae ATCC 11733 (serotype 2). The binding assay was performed with 125I-labelled hLf in the presence of increasing concentrations of the 32.1-kDa purified PspA protein (QP2). Values shown are the means of triplicate determinations.
DISCUSSION
S. pneumoniae infections are initiated at the mucosal surface of the respiratory tract, causing a variety of diseases such as otitis media, pneumonia, bacteremia, and meningitis. However, although the sequestering of host iron is essential for bacterial growth and multiplication on mucosal surfaces and therefore constitutes a prerequisite for infections (3), the mechanisms used by pneumococci to sequester iron remain unclear. In this study we demonstrated that 88% of the tested strains (mainly clinical isolates) bound hLf specifically. The characterization of the Lf-binding protein and its amino acid sequence analysis revealed that the pneumococcal surface protein A, PspA, is the binding component. Among the pneumococcal surface proteins, PspA has been studied in detail. Although the importance of PspA as a virulence factor has been well established, the function of PspA in virulence is not yet known. Structurally, PspA is composed of different distinct regions, such as the C-terminal repeats for membrane anchoring, a proline-rich region, and a highly charged N-terminal region which is variable in PspA proteins from different serotypes (23). Western blot analysis indicated that the hLf-binding domain is located in the N-terminal region, which has no homology with the functional parts of the other pneumococcal choline-binding proteins autolysin (6) and SpsA (9), both of which also contribute to virulence of S. pneumoniae. PspA is a serologically highly variable protein (4) capable of eliciting protection against pneumococcal challenge in mice (14). Protection has also been shown to be elicited after oral immunization with Salmonella as a carrier (17) and after DNA immunization (15). The variation in the molecular mass observed for the hLf receptor is in accordance with the reported high variability of PspA. Despite this variability, PspA has been shown to elicit cross protection against different serotypes (14). The facts that protection-eliciting epitopes are located in the N-terminal part of PspA (13) and that different serotypes among clinical isolates bound hLf specifically suggested the occurrence of conserved epitopes in PspA. This might explain the elicitation of cross protection against challenge with heterologous serotypes. The high-molecular-mass protein of approximately 180 kDa demonstrated after purification was previously shown to be a putative dimer generated from the PspA monomer (22). Binding experiments showed that S. pneumoniae distinguishes between hLf and bovine Lf, indicating species specificity, an observation which was also reported for hLf receptors of other human pathogens (8). In addition, human transferrin was unable to block binding of 125I-labelled hLf to the pneumococcal hLf receptor. After identification of PspA as the pneumococcal receptor for hLf, specificity was confirmed by the fact that purified PspA binds Lf and can also competitively inhibit the binding of hLf to pneumococcal cells. Although Scatchard analysis showed the existence of another hLf-binding component, PspA represents the major pneumococcal hLf-binding protein. The presence of two Lf receptors constituting a receptor complex is a typical feature of Lf binders in gram-negative as well as in gram-positive pathogens (8, 16). hLf, an iron-binding, acute-phase protein secreted by inflammatory cells, is bactericidal to many microorganisms (3). Nevertheless, many pathogens acquire host iron in a siderophore-independent pathway by binding Lf and are able to use Lf-bound iron for growth (2). In a previous study it was reported that pneumococci do not produce siderophores and that hemin is able to restore growth of pneumococci under iron-limiting conditions. The hLf receptor PspA is expressed among all clinically important serotypes (4); however, 12% of the tested strains did not express a functional hLf receptor, suggesting either that other iron sources are available in vivo or that their regulation of expression in vitro is different from that in vivo, as already described for other pathogens (12). In conclusion, hLf has been shown to bind specifically to pneumococci, and furthermore, our results attribute for the first time a potential function to the promising vaccine candidate PspA, namely, iron acquisition at the mucosal surface.
ACKNOWLEDGMENTS
We thank M. Tillig for excellent technical assistance and J. Henrichsen, Statens Serum Institute, Copenhagen, Denmark, and G. Zysk, Medical Microbiology, Düsseldorf, Germany, for providing clinical isolates of S. pneumoniae. We are also grateful to M. Kieß (GBF) for internal peptide sequence analysis and to R. Towers for critical reading of the manuscript.
REFERENCES
- 1.Aisen P, Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972;257:314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- 2.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 4.Crain M J, Waltman W D, Turner J S, Yother J, Talkington D F, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia P, Garcia J L, Garcia E, Lopez R. Nucleotide sequence and expression of the pneumococcal autolysin gene in Escherichia coli from its own promotor. Gene. 1986;43:265–272. doi: 10.1016/0378-1119(86)90215-5. [DOI] [PubMed] [Google Scholar]
- 7.Gray B M, Converse III G M, Dillon H C., Jr Serotypes of Streptococcus pneumoniae causing disease. J Infect Dis. 1979;140:979–983. doi: 10.1093/infdis/140.6.979. [DOI] [PubMed] [Google Scholar]
- 8.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. TIM. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 9.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang M, MacLachlan P R, Babiuk L A, Potter A A. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. A bovine lactoferrin-binding protein of Streptococcus uberis: identification of the protein and characterization of the gene, abstr. B-503; p. 115. [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 14.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular type. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDaniel L S, Loechel F, Benedict C, Greenway T, Briles D E, Conry R M, Curiel D T. Immunization with a plasmid expressing pneumococcal surface protein A (PspA) can elicit protection against fatal infection with Streptococcus pneumoniae. Gene Ther. 1997;4:375–377. doi: 10.1038/sj.gt.3300401. [DOI] [PubMed] [Google Scholar]
- 16.Naidu A S, Andersson M, Forsgren A. Identification of a human lactoferrin-binding protein in Staphylococcus aureus. J Med Microbiol. 1992;36:177–183. doi: 10.1099/00222615-36-3-177. [DOI] [PubMed] [Google Scholar]
- 17.Nayak A R, Tinge S A, Tart R C, McDaniel L S, Briles D E, Curtiss R., III A live recombinant avirulent oral Salmonella vaccine expressing pneumococcal surface protein A induces protective responses against Streptococcus pneumoniae. Infect Immun. 1998;66:3744–3751. doi: 10.1128/iai.66.8.3744-3751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rainard P. Binding of bovine lactoferrin to Streptococcus agalactiae. FEMS Microbiol Lett. 1992;98:235–240. doi: 10.1016/0378-1097(92)90162-h. [DOI] [PubMed] [Google Scholar]
- 19.Scatchard G. The attraction of proteins for small molecules and ions. Ann N Y Acad Sci. 1949;51:660–672. [Google Scholar]
- 20.Schneider R, Hantke K. Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system. Mol Microbiol. 1993;8:111–121. doi: 10.1111/j.1365-2958.1993.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 21.Tai S S, Lee C-J, Winter R E. Hemin utilization is related to virulence of Streptococcus pneumoniae. Infect Immun. 1993;61:5401–5405. doi: 10.1128/iai.61.12.5401-5405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talkington D F, Voellinger D C, McDaniel L S, Briles D E. Analysis of pneumococcal PspA microheterogeneity in SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb Pathog. 1992;13:343–355. doi: 10.1016/0882-4010(92)90078-3. [DOI] [PubMed] [Google Scholar]
- 23.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]