Abstract
Background and Objectives
Identification of an epileptogenic lesion on structural neuroimaging in individuals with focal epilepsy is important for management and treatment planning. The objective of this study was to determine the frequency of MRI-identified potentially epileptogenic structural abnormalities in a large multicenter study of adolescent and adult patients with newly diagnosed focal epilepsy.
Methods
Patients with a new diagnosis of focal epilepsy enrolled in the Human Epilepsy Project observational cohort study underwent 3 T brain MRI using a standardized protocol. Imaging findings were classified as normal, abnormal, or incidental. Abnormal findings were classified as focal or diffuse and as likely epilepsy-related or of unknown relationship to epilepsy. Fisher exact tests were performed to determine whether abnormal imaging or abnormality type was associated with clinical characteristics.
Results
A total of 418 participants were enrolled. Two hundred eighteen participants (59.3%) had no abnormalities detected, 149 (35.6%) had abnormal imaging, and 21 (5.0%) had incidental findings. Seventy-eight participants (18.7%) had abnormalities that were considered epilepsy-related, and 71 (17.0%) had abnormalities of unknown relationship to epilepsy. Older participants were more likely to have imaging abnormalities, while participants with focal and epilepsy-related imaging abnormalities were younger than those without these abnormalities. One hundred thirty-one participants (31.3%) had a family history of epilepsy. Epilepsy-related abnormalities were not associated with participant sex, family history of epilepsy, or seizure type.
Discussion
We found that 1 in 5 patients with newly diagnosed focal epilepsy has an MRI finding that is likely causative and may alter treatment options. An additional 1 in 5 patients has abnormalities of unknown significance. This information is important for patient counseling, prognostication, and management.
Identification of an epileptogenic lesion on structural neuroimaging in individuals with focal epilepsy is important for management and prognostication. The frequency of MRI abnormalities among patients with drug-resistant focal epilepsy has been extensively studied because MRI is typically used in the further investigation of intractable cases.1 By contrast, the frequency of lesions among newly diagnosed patients is less well-studied because MRI is not always obtained at disease onset, and a substantial proportion of patients will respond to treatment with antiseizure medications.2
Estimates of lesion occurrence range from 14% among patients with a first seizure to 84% of surgical candidates at a tertiary care epilepsy center.1,3 One study of patients with newly diagnosed epilepsy found 1.5 T MRI abnormalities in 35.3% of patients, although this study included patients with both focal and generalized epilepsy.4 Small studies using 1.5 T MRI have identified abnormalities in approximately 1 in 4 patients with newly diagnosed focal epilepsy, with 24% of 63 adult patients having abnormalities in 1 study and 26% of 103 adolescent and adult patients in another.5,6 However, a large (N = 993) Australian study using a mix of 1.5 and 3 T MRI scans found potentially epileptogenic abnormalities in 56% of patients with focal epilepsy, and a British study using 3 T MRI found potentially epileptogenic abnormalities in only 18% of patients.7,8
The Human Epilepsy Project (HEP), a multicenter prospective observational study of adolescent and adult patients with a recent diagnosis of focal epilepsy, offers an opportunity to determine the frequency of imaging abnormalities among a large sample of patients with newly diagnosed focal epilepsy. We describe the baseline imaging findings of this cohort and test the association between imaging findings and clinical characteristics.
Methods
We included individuals with newly diagnosed focal epilepsy who were enrolled in the HEP between 2012 and 2017 (N = 418). The HEP (humanepilepsyproject.org) is a prospective multicenter study aimed at gathering information on biomarkers and treatment response in patients with newly diagnosed focal epilepsy. Participants were recruited from 34 sites: 28 sites in North America, 4 in Australia, and 2 in Europe (eFigure 1, links.lww.com/WNL/C269). Participants were eligible for enrollment if they were between ages 12 and 60 years, had 2 confirmed spontaneous seizures in the 12 months preceding enrollment, had not received treatment or had been treated with antiseizure medications for fewer than 4 months preceding enrollment, and had either (1) a definitive clinical history of recurrent seizures with focal onset, (2) an ictal or interictal EEG showing a focal abnormality, or (3) a focal lesion on MRI. Patients were ineligible for enrollment if they had generalized or mixed epilepsy, a history of intracranial bleeding, recent traumatic brain injury, moderate or greater developmental delay before seizure onset, or significant medical, psychiatric, or progressive neurologic comorbidities.
Demographic information including sex, age at seizure onset, and race was collected for each participant. Participants' clinical history was reviewed by the HEP clinical core committee to ascertain a definitive diagnosis of focal epilepsy. Seizures were classified as focal without impairment of awareness (including seizures with observable motor or autonomic phenomena and seizures with subjective sensory and psychic phenomena), focal with impairment of awareness, focal with evolution to bilateral tonic-clonic, or unclassifiable.
All patients enrolled in the HEP underwent 3T MRI seizure protocol studies. The image acquisition protocol included a whole brain T1-weighted MRI with a 1-mm3 voxel size and a fluid-attenuated inversion recovery (FLAIR) acquisition (either a 1-mm isotropic whole brain 3D acquisition or multislice FLAIR acquisitions in coronal and axial planes).
Image acquisitions were derived from the Alzheimer's Disease Neuroimaging Initiative (ADNI) GO 3T MRI protocols with the modification that T1-weighted MRI scans were obtained with a 1-mm isotropic voxel size rather than 1 × 1 × 1.2 mm as per the ADNI protocol.9 After implementation of image acquisition protocols at each site with a specific make and model of MRI scanner, the protocol sheets were returned to the HEP MRI core and then distributed to each subsequent site with the same make and model of the MRI scanner. Image acquisition protocols were therefore largely standardized across sites for the HEP study, with some variability in FLAIR acquisitions. Occasionally, additional sequences such as gradient recalled echo acquisitions were obtained at individual sites.
Images were visually reviewed by specialists with substantial experience with epilepsy neuroimaging (R.K, R.C.K., G.D.C., G.J.). Image reviewers were aware of each participant's age and the diagnosis of focal epilepsy, but no other clinical information. Inter-rater agreement for the initial review was fair (Cohen κ = 0.37). This was primarily attributable to disagreements about findings of unknown significance, which tended to require consensus review to determine whether they should be classified as abnormal or incidental. Divergent assessments were resolved by reevaluation and consensus.
Imaging features for each study participant were classified following the National Institute of Neurological Disorders and Stroke Common Data Elements for epilepsy neuroimaging.10 Participant neuroanatomy was classified as normal, abnormal, or incidental, and description of the abnormality type for abnormal scans was obtained. Imaging abnormalities were classified as focal or diffuse. All diffuse brain abnormalities were classified as having an unknown relationship to epilepsy. Focal abnormalities were classified as likely epilepsy-related or with unknown relationship to epilepsy. All participants with at least 1 epilepsy-related abnormality were classified as epilepsy-related in the statistical analysis, even if they also had focal abnormalities with unknown relationship to epilepsy or diffuse abnormalities.
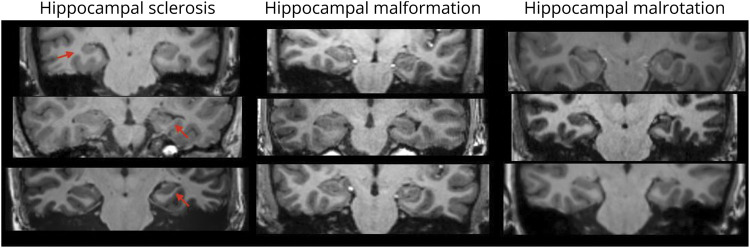
Epilepsy-related abnormalities were those in which the relationship between imaging features and epileptogenicity has been well established by previous research, such as hippocampal sclerosis, malformations of cortical development, and foreign tissue lesions (i.e., suspected tumors).11 Findings with an unknown relationship to epilepsy included diffuse brain atrophy and enlarged ventricles, diffuse white matter changes, and malrotated hippocampi. In these cases, although the brain appears abnormal relative to healthy controls of a similar age, the relationship between the specific abnormality and the underlying epilepsy has not been definitively established.12-15 Hippocampal abnormalities were classified as hippocampal sclerosis if there was atrophy, signal change, and loss of internal architecture, as malformations if there was only loss of internal architecture and change in the hippocampal morphology without atrophy, and as malrotations if both size and internal architecture were maintained (Figure 1).
Figure 1. Examples of Hippocampal Imaging Abnormalities.
Linear regression was performed to analyze the association between abnormal imaging and participant age and between abnormality type and participant age. Fisher exact tests were performed between abnormal imaging and participant sex, race, seizure type, and family history of epilepsy and between abnormality type and participant sex, race, seizure type, and family history of epilepsy.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board at the participating institutions. Written informed consent was obtained from all participants.
Data Availability
Anonymized data not published within this article will be made available on request to any qualified investigator.
Results
Participants
A total of 418 participants completed a good-quality standardized HEP MRI. Demographic and clinical characteristics are shown in Table 1. One participant had imaging performed at age 11 years (before HEP enrollment). Four participants were older than age 60 years at the time of enrollment because of protocol exceptions at 2 participating sites due to low enrollment; these participants were between ages 63 and 65 years.
Table 1.
Demographic and Clinical Characteristics of Human Epilepsy Project Participants
Imaging Findings
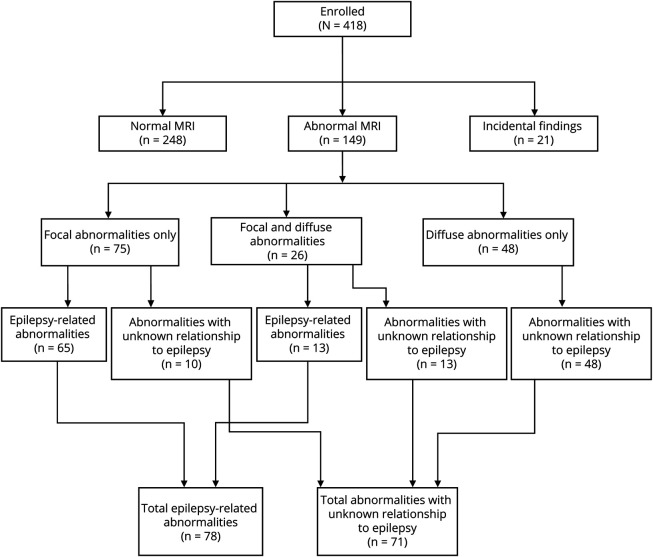
One hundred forty-nine of 418 participants (35.6%) had abnormal imaging, and 21/418 participants (5.0%) had findings considered to be incidental to the diagnosis of epilepsy. Two hundred forty-eight of 418 participants (59.3%) had no abnormal findings detected (Figure 2). Among the participants with abnormal imaging, 75/149 (50.3%) had exclusively focal abnormalities, 48/149 (32.2%) had diffuse abnormalities, and 26/149 (17.4%) had both focal and diffuse abnormalities. Specific abnormalities are shown in Table 2. Seventy-eight of 418 participants (18.7% of the overall sample) had abnormalities that were considered epilepsy-related. Seventy-one of 418 participants (17.0% of the overall sample) had abnormalities in which the relationship to epilepsy is unknown.
Figure 2. MRI Findings of Human Epilepsy Project Participants.
Table 2.
MRI Abnormalities Among Human Epilepsy Project Participants
We found a relationship between age and MRI abnormalities, with an increased likelihood of observing abnormalities in older participants (p = 1.5 × 10−9). This finding remained if participants with foreign tissue lesions were excluded from the analysis (p = 1.47 × 10−9). Participants with focal abnormalities were 4.6 years older than participants with normal imaging (p = 3.9 × 10−3). Participants with enlarged ventricles and diffuse atrophy were 10.4 years older (p = 1.6 × 10−9), and participants with both diffuse and focal abnormalities were 11.4 years older (p = 4.9 × 10−5). For participants with abnormal imaging, participants with epilepsy-related abnormalities were 8.8 years younger than participants with abnormalities with an unclear relationship to the underlying epilepsy (p = 7.2 × 10−5).
Seventy-three of 165 male participants (44.2%) and 76/253 female participants (30.0%) had imaging abnormalities (p = 0.001; note N = 21 participants with incidental findings were excluded from this statistical analysis). Forty-six of 165 male participants (27.9%) and 51/253 female participants (20.2%) had focal abnormalities (p = 0.076). Forty-five of 165 male participants (27.3%), and 32/253 female participants (12.6%) had diffuse abnormalities (p = 0.0003). Among participants with imaging abnormalities, 37/76 female participants (48.7%) and 34/73 male participants (46.6%) had epilepsy-related abnormalities (p = 0.87).
One hundred thirty-one of 418 participants (31%) had a family history of epilepsy. Among the patients with a family history of epilepsy, 49/131 (37.4%) had imaging abnormalities, 33/131 (25.2%) had focal abnormalities, 21/131 (16.0%) had diffuse abnormalities, and 30/131 (22.9%) had epilepsy-related abnormalities. Family history of epilepsy was not associated with imaging abnormalities (p = 0.65), focal abnormalities (p = 0.62), diffuse abnormalities (p = 0.49), or epilepsy-related abnormalities (p = 0.22).
Sixty-four of 418 participants (15.3%) were between ages 11 and 18 years. Among these pediatric patients, 26/64 (40.6%) had a family history of epilepsy. Pediatric patients were not more likely than adult patients to have a family history of epilepsy (p = 0.076).
Seizure type was not associated with imaging abnormalities (p = 0.72) or epilepsy-related abnormalities (p = 0.85). (Note participants with incidental imaging findings (N = 21) and unclassified (N = 11) or uncollected (N = 19) seizure types were excluded from these statistical tests.) The association between participant race and imaging abnormalities could not be analyzed because of low numbers of non-White participants.
Discussion
We report the MRI findings from a prospective sample of 418 adolescent and adult patients with newly diagnosed focal epilepsy ranging in age from 11 to 65 years. We used a standardized 3T acquisition protocol with T1 and FLAIR sequences across 34 sites. We found a lesion with an established relationship to focal epilepsy in 18.7% of participants. An additional 17% of participants had a lesion with an unknown relationship to epilepsy.
In 1 previous retrospective study of standardized 3 T MRI in 120 patients with new focal epilepsy (age range 19–69 years), the authors8 found potentially epileptogenic lesions in 18% of patients. Our findings provide further evidence that approximately 1 in 5 adolescent and adult patients with a new diagnosis of epilepsy will have a potentially etiologic lesion identified on 3 T MRI.
By contrast, the authors of another study7 found that 155/275 (56%) of patients presenting to a first-seizure clinic who met criteria for a diagnosis of focal epilepsy had a potentially epileptogenic lesion. We suspect that their frequency of epileptogenic lesions, which is much higher than the frequency in our study, is largely because of age differences between the 2 study populations. The mean patient age in their sample was 42.2 years, and the maximum age was 94.3 years, both of which are higher than the mean of 33.5 years and maximum of 65 years in the HEP study. Older patients with epilepsy are more likely to have imaging abnormalities, with 1 study finding epileptogenic lesions in 67% of newly diagnosed epilepsy patients older than 60 years.16 In addition, in their study, 49 participants (27% of those with potentially epileptogenic lesions) had poststroke gliosis or encephalomalacia, while a focal infarct was only present in 1 patient in our sample. We suspect that the higher prevalence of potentially epileptogenic lesions among their focal epilepsy cohort may be at least partially attributable to poststroke focal epilepsy, which is one of the most common causes of focal epilepsy in older patients.17
The proportion of participants with hippocampal sclerosis (11 cases overall or 2.6% of the study population) was low. Previous studies of patients with newly diagnosed focal epilepsy have found comparably low rates of 1.5%–3.9%, although 1 study found hippocampal sclerosis in 10% of patients.4-6 The low incidence in our study may be due to the exclusion of children younger than 11 years; 1 study of patients with temporal lobe epilepsy due to mesial temporal sclerosis found that 39.8% presented before age 10 years.18 The relationship between hippocampal sclerosis and temporal lobe seizures remains controversial and is likely multifactorial, with heterogeneity among patients.
Magnet strength, imaging protocols, and reviewer experience can all result in underappreciation of MRI abnormalities. However, patients in this study had 3 T MRI scans interpreted by specialists experienced in epilepsy imaging.
Patients with epilepsy-related abnormalities were significantly younger than patients with incidental or diffuse abnormalities. This is an expected finding because diffuse abnormalities such as diffuse atrophy, ventricular enlargement, and microvascular white matter changes are more common in older patients.
While male patients were more likely to have abnormal imaging findings overall, this difference was attributable to an increased prevalence of diffuse abnormalities, rather than a higher prevalence of potentially epileptogenic lesions. There was no association between sex and epilepsy-related abnormalities. This differs from a previous small study showing that while focal epilepsy was equally prevalent in both sexes, lesional focal epilepsy was more common in male patients.19 Sex differences in the structural and molecular properties of epileptogenic brain tissue remain an active area of research.20,21
Nearly one-third of patients had a family history of epilepsy, which is much higher than previously reported estimates of around 10%.22,23 Pediatric patients were no more likely than adult patients to have a family history of epilepsy. There was no association between family history of epilepsy and epilepsy-related abnormalities. This may reflect a sampling bias if patients with familial focal epilepsies, which are often nonlesional, were more likely to enroll in the study.24,25 It may also be related to the upper age limit for enrollment in the HEP because the exclusion of older patients with acquired epilepsy etiologies such as strokes or tumors may have resulted in disproportionately high representation of patients with genetic epilepsies. In either case, our findings underscore the significance of a genetic load on patients with focal epilepsy.26
There was no association between seizure type and epilepsy-related imaging abnormalities. While focal evolving to bilateral tonic-clonic seizures are more disabling and more distressing for patients, our results suggest that they are not a predictor of underlying structural lesions.
The primary limitation of this study is that it was not a population-based study, and recruitment resources varied among sites. Thus, the study sample may not be a representative sample of patients with focal epilepsy and may not be generalizable to all patients. Female participants were overrepresented, making up 60% of the study sample, while non-White participants were underrepresented, which prevented us from analyzing the association between imaging abnormalities and race or ethnicity.
Another limitation is the presence of some variability in the FLAIR sequence acquisition because not all participating institutions had scanners with 1-mm isotropic FLAIR voxel capability. This may have limited the sensitivity to detect small lesions.
Our results suggest that approximately 1 in 5 patients with focal epilepsy have an MRI-identifiable epilepsy-related imaging abnormality at the time of diagnosis, while another 1 in 5 have an abnormality with an unknown relationship to epilepsy. Hippocampal sclerosis was an uncommon finding in this study. A family history of epilepsy was common in both adolescent and adult patients. Younger patients were more likely to have epilepsy-related imaging abnormalities, while older patients were more likely to have incidental abnormalities or those with an unknown relationship to epilepsy. While most adolescent and adult patients with newly diagnosed focal epilepsy will have normal neuroimaging, many patients will have epilepsy-related or potentially epilepsy-related lesions, potentially altering prognostication and management.
Glossary
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- FLAIR
fluid-attenuated inversion recovery
- HEP
Human Epilepsy Project
Appendix 1. Authors
Appendix 2. Coinvestigators

Study Funding
No targeted funding reported. Creation of Human Epilepsy Project (HEP) was sponsored by the Epilepsy Study Consortium. Funding for HEP was provided by industry, philanthropy, and foundations (UCB Pharma, Eisai, Pfizer, Lundbeck, Sunovion, the Andrews Foundation, the Vogelstein Foundation, Finding a Cure for Epilepsy and Seizures [FACES], and Friends of Faces). The funders of HEP had no role in the design or conduct of this study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol . 2009;66(12):1491-1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med . 2000;342(5):314-319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 3.King MA, Newton MR, Jackson GD, et al. Epileptology of the first-seizure presentation: a clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet . 1998;352(9133):1007-1111. doi: 10.1016/S0140-6736(98)03543-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu RSN, Lemieux L, Bell GS, et al. The structural consequences of newly diagnosed seizures. Ann Neurol . 2002;52(5):573-580. doi: 10.1002/ana.10338. [DOI] [PubMed] [Google Scholar]
- 5.Van Paesschen W, Duncan JS, Stevens JM, et al. Etiology and early prognosis of newly diagnosed partial seizures in adults. Neurology . 1997;49(3):753-757. doi: 10.1212/01.WNL.0000148643.36513.2A. [DOI] [PubMed] [Google Scholar]
- 6.Salmenpera T, Kononen M, Roberts N, et al. Hippocampal damage in newly diagnosed focal epilepsy: a prospective MRI study. Neurology. 2005;64(1):62-68. doi: 10.1212/01/WNL.0000148643.36513.2A. [DOI] [PubMed] [Google Scholar]
- 7.Hakami T, McIntosh A, Todaro M, et al. MRI-identified pathology in adults with new-onset seizures. Neurology . 2013;81(10):920-927. doi: 10.1212/WNL.0b013e3182a35193. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths PD, Coley SC, Connolly DJA, et al. MR imaging of patients with localisation-related seizures: initial experience at 3.0T and relevance to the NICE guidelines. Clin Radiol . 2005;60(10):1090-1099. doi: 10.1016/j.crad.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6(3):212-220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loring DW, Lowenstein DH, Barbaro NM, et al. Common data elements in epilepsy research: development and implementation of the NINDS epilepsy CDE Project. Epilepsia . 2011;52(6):1186-1191. doi: 10.1111/j.1528-1167.2011.03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumcke I. Neuropathology of focal epilepsies: a critical review. Epilepsy Behav. 2009;15(1):34-39. doi: 10.1016/j.yebeh.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Caciagli L, Bernasconi A, Wiebe S, et al. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology . 2017;89(5):506-516. doi: 10.1212/WNL.0000000000004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson EL, Krauss GL, Lee AK, et al. Association between white matter hyperintensities, cortical volumes, and late-onset epilepsy. Neurology . 2019;92:e988-e995. doi: 10.1212/WNL.0000000000007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai M, Vaughan DN, Perchyonok Y, et al. Hippocampal malrotation is an anatomic variant and has no clinical significance in MRI-negative temporal lobe epilepsy. Epilepsia . 2016;57(10):1719-1728. doi: 10.1111/epi.13505. [DOI] [PubMed] [Google Scholar]
- 15.Fu T, Ho C, Lin C, et al. Hippocampal malrotation: a genetic developmental anomaly related to epilepsy? Brain Sci . 2021;11(4)463. doi: 10.3390/brainsci11040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arabi M, Dirani M, Hourani R, et al. Frequency and stratification of epileptogenic lesions in elderly with new onset seizures. Front Neurol . 2018;9:995. doi: 10.3389/fneuro.2018.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71(6):576-586. doi: 10.4065/71.6.576. [DOI] [PubMed] [Google Scholar]
- 18.Asadi-Pooya AA, Sperling MR. Age at onset in patients with medically refractory temporal lobe epilepsy and mesial temporal sclerosis: impact on clinical manifestations and postsurgical outcome. Seizure . 2015;30:42-45. doi: 10.1016/j.seizure.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Christensen J, Juel Kjeldsen M, Andersen H, et al. Gender differences in epilepsy. Epilepsia . 2005;46(6):956-960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- 20.Savic I, Engel J. Structural and functional correlates of epileptogenesis—does gender matter? Neurolbiol Dis . 2014;70:69-73. doi: 10.1016/j.nbd.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy DS, Thompson W, Calderara G. Molecular mechanisms of sex differences in epilepsy and seizure susceptibility in chemical genetic, and acquired epileptogenesis. Neurosci Lett . 2021;750:135753. doi: 10.1016/j.neulet.2021.135753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callenbach PMC, Geerts AT, Arts WFM, et al. Familial occurrence of epilepsy in children with newly diagnosed multiple seizures: Dutch study of epilepsy in childhood. Epilepsia . 1998;39(3):331-336. doi: 10.1111/j.1528-1157.1998.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi Al, Viaggi S, Chiossi E; LICE Episcreen Group. Family study of epilepsy in first degree relatives: data from the Italian Episcreen Study. Seizure . 2003;12:203-210. doi: 10.1016/s1059-1311(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 24.Crompton DE, Scheffer IE, Taylor I, et al. Familial mesial temporal lobe epilepsy: a benign epilepsy syndrome showing complex inheritance. Brain . 2010;133(11):3221-3231. doi: 10.1093/brain/awq251. [DOI] [PubMed] [Google Scholar]
- 25.Perucca P. Genetics of focal epilepsies: what do we know and where are we heading? Epilepsy Curr . 2018;18(6):356-362. doi: 10.5698/1535-7597.18.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epi25 Collaborative. Ultra-rare genetic variation in the epilepsies: a whole-exome sequencing study of 17,606 individuals. Am J Hum Genet . 2019;105(2):267-282. doi: 10.1016/j.ajhg.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available on request to any qualified investigator.