Abstract
Calcitonin gene-related peptide (CGRP) is involved in several of the pathophysiologic processes underpinning migraine attacks. Therapies that target CGRP or its receptor have shown efficacy as preventive or acute treatments for migraine. Two small-molecule CGRP receptor antagonists (rimegepant and ubrogepant) are approved for the acute treatment of migraine, and 4 monoclonal antibodies (eptinezumab, erenumab, fremanezumab, and galcanezumab) are approved for migraine prevention; erenumab targets the canonical CGRP receptor, the others CGRP ligand. CGRP plays a role in gastrointestinal nociception, inflammation, gastric acid secretion, and motility. Nausea and vomiting are among the gastrointestinal symptoms associated with migraine, but individuals with migraine may also experience functional upper and lower gastrointestinal comorbidities, such as gastroesophageal reflux disease, gastroparesis, functional diarrhea or constipation, and irritable bowel syndrome. Although gastrointestinal symptoms in migraine can be treatment-related, they may also be attributable to increased CGRP. In this review, we summarize the epidemiologic evidence for associations between migraine and gastrointestinal disorders, consider the possible physiologic role of CGRP in these associations, and review the clinical occurrence of gastrointestinal events in patients with migraine receiving CGRP-based therapies and other migraine treatments. Because patients with migraine are at an increased risk of comorbid and treatment-related gastrointestinal effects, we also propose a patient-management strategy to mitigate these effects.
Migraine is a chronic neurologic condition characterized by recurrent headaches and associated symptoms typically lasting around 72 hours.1,2 In the US population, government health surveys indicate that approximately 1 in 6 adults (i.e., approximately 32 million) have reported migraine or severe headache,3 and an estimated 8.7 million women and 2.6 million men experience migraines resulting in moderate to severe disability.1 Susceptibility to migraine is multifactorial.4 Besides photophobia and phonophobia,e1 migraine can present with a number of gastrointestinal (GI) symptoms, such as nausea,5 diarrhea, and vomiting, and is associated with GI disorders such as cyclical vomiting syndrome (CVS)6 and irritable bowel syndrome (IBS).e1 There is also evidence that symptoms such as fatigue and insomnia are more common in people with chronic migraine (CM) than in those with episodic migraine (EM).7 The relationship between migraine and GI comorbidities is multifactorial, involving several neuropeptides, proinflammatory molecules, and the gut microbiota, among other factors.8 In this study, we specifically review the role of the neuropeptide calcitonin gene-related peptide (CGRP) on the GI symptoms of migraine.
During a migraine attack, serum CGRP concentration increases and decreases in parallel with headache intensity.9 The relationship between CGRP and migraine has led to the development of CGRP pathway-based therapies, including small-molecule CGRP receptor antagonists (“gepants”) and monoclonal antibodies (mAbs), which bind to either CGRP or its canonical receptor.10 Insights into the mechanistic relationship between migraine and GI comorbidities may be gained from understanding the role of CGRP in the gut. As well as being a mediator of migraine, CGRP is involved in functional aspects of the GI system, including gastric acid secretion, gut motility, inflammation, and nociception.11 Furthermore, CGRP pathway-based therapies may produce GI adverse events (AEs).12 In this review, we review the GI comorbidities associated with migraine, the possible mechanisms underpinning these associations (with a focus on CGRP), and the effect of acute and preventive migraine therapies on GI comorbidities.
Data Sources
This narrative synthesis of evidence was based on literature searches of PubMed tailored by the authors' expert knowledge and opinion, on citations within selected publications, and on resources such as ClinicalTrials.gov, product labels, and the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS). Episodically up to 31 March 2021, PubMed searches were conducted without date restriction using combinations of terms “calcitonin gene-related peptide AND CGRP”, and/or “migraine”, refined by adding gastroenterological terms (“gastro*“, “gastroenterol*“, “gastrointestinal”, “diarrh*“, “constipation”, “vomit*“, etc.) and/or specific drug names (“eptinezumab”, “erenumab”, “fremanezumab”, “galcanezumab”, “olcegepant”, “telcagepant”, “rimegepant”, “ubrogepant”, “atogepant”, “zavegepant”) and/or other drug names or drug classes known to be prescribed in migraine.
Association Between Migraine and GI Comorbidities
Patients with migraine have an increased risk of GI disorders, including CVS,13 gastroesophageal reflux disease (GERD),14 gastroparesis,15 celiac disease (CD),16 and IBS.17 There is also evidence for a stronger relationship between GI comorbidities and CM than with EM. The CM Epidemiology and Outcomes (CaMEO) study of 16,763 respondents found the following rates of GI conditions among patients with CM and EM, respectively: GERD, 24.4% vs 14.3% (p < 0.001); frequent constipation, 14.8% vs 9.0% (p < 0.001); and IBS, 15.5% vs 7.9% (p < 0.001).18
GERD
Gastroesophageal reflux is a normal physiologic event; GERD develops with retrograde flow of stomach acid toward the esophagus, provoking bothersome symptoms (typically heartburn or regurgitation) or structural damage to the esophageal lining (such as erosive esophagitis and stricture).e2 It has a prevalence in adults of approximately 20% in the United States.e2 However, it may be more common in patients with migraine, as suggested by a survey of 1,832 patients with physician-diagnosed migraine, which determined that 22% had physician-diagnosed GERD and that an additional 27% had diagnosed heartburn or other undiagnosed reflux symptoms.14 Triptans were used as first-line treatment in 69% of these patients with migraine; a greater proportion of individuals with undiagnosed GERD or heartburn symptoms (18%) received nonsteroidal anti-inflammatory drugs (NSAIDs) than did those with diagnosed GERD or heartburn (10%) or no GERD or heartburn (12%).
Nausea and Vomiting
Nausea and vomiting are among the symptoms associated with migraine.e1 Among patients with migraine, 60%–95% develop nausea and 50%–62% develop vomiting during attacks.19 Among 6,488 respondents with EM who completed the 2009 American Migraine Prevalence and Prevention survey, approximately half (49.5%) reported high-frequency nausea (i.e., at least half the time) with headache.20 In addition, a retrospective database analysis of 835 patients with CM found that 77.6% and 40.9% of patients experienced nausea and vomiting, respectively.21 A retrospective analysis of 1,025 patients with migraine found that headache intensity correlated significantly with nausea and vomiting, as well as with other symptoms associated with migraine.22 Regarding the possible pathophysiology of nausea and vomiting in migraine, ascending axonal projections of trigeminovascular neurons of the spinal trigeminal nucleus (SpV) transmit monosynaptic nociceptive signals to the basal ganglia nuclei, brain stem nuclei, and hypothalamic nuclei. These projections may be critical for the initiation of nausea and vomiting as well as other headache-associated symptoms.23 In approximately one-quarter of people, nausea can occur as a premonitory symptom, independent of pain and trigeminal activation. Comparison of PET scans of individuals with or without nausea whose migraine was induced by nitroglycerin found brain regions (including the periaqueductal gray within the brain stem nuclei) that were only activated among those with nausea during the premonitory phase.24
Cyclical Vomiting Syndrome
A chronic disorder of the foregut (the section of the intestine that ends where the bile duct enters the duodenum),e3 CVS, is characterized by recurrent episodes of severe nausea and frequent vomiting.13 It is associated with autonomic dysfunction and has a strong association with migraine.13 CVS is commonly treated with medications used for migraine treatment.e4 It affects girls more than boys, typically beginning before age 5 years, and it may resolve during adolescence but persists into adulthood in some individuals; most of those affected are predicted to develop migraines.25 Migraine and/or a family history of migraine have been reported in 24%–70% of adults with CVS.26 A multivariate analysis in a population of hospitalized adults comprising 20,952 with CVS and 44,262 without CVS also identified significant associations between CVS and several GI disorders (gastroparesis, GERD, and IBS), as well as with migraine and autonomic dysfunction.27 CVS has different phases of presentation (prodrome, vomiting, recovery, and interictal period), and the results of studies have suggested that autonomic neuropathy involving the sympathetic nervous system may underlie its pathogenesis.28
Abdominal Migraine
Abdominal migraine is usually recognized in childhood and is characterized by recurrent attacks of moderate to severe midline abdominal pain, associated with vasomotor symptoms, nausea, and vomiting, without headache.e1,e5 It is most commonly observed in children between ages 5 and 9 years and is rarely seen in adults. For approximately two-thirds of children, abdominal migraine resolves by their late teenage years, and 50%–70% of these individuals go on to develop migraine headaches.e6 Episodes of abdominal pain last between 2 and 72 hours, separated by symptom-free periods.e1
Gastroparesis
Gastroparesis is a sensorimotor disorder affecting the foregut characterized by nausea, vomiting, and abdominal pain. A useful way to distinguish nausea and vomiting related to gastroparesis from that which may be present during migraines is the relationship to meals (with gastroparesis being associated with feeding difficulties postprandially). A diagnosis of gastroparesis generally requires gastric scintigraphy with a standardized meal to document emptying delays at 4 hours.e7 Gastric emptying is mediated by the autonomic nervous system and evidence suggests that migraine attacks are associated with delayed gastric emptying.15 Gastric scintigraphy determined that the average time to half-emptying of the stomach after a standard meal was 149.9 minutes in 9 patients experiencing a migraine attack, compared with 111.8 minutes among 10 healthy controls.e8 Thus, rates of absorption of oral migraine treatments tend to be slower during attacks than in migraine-free periods, which can affect the treatment response.15
Celiac Disease and Nonceliac Gluten Sensitivity
CD is an immunologic GI disorder that occurs in approximately 1% of individuals and is caused by ingestion of gluten, a protein found in barley, rye, and wheat. CD can be associated with multiple GI symptoms including abdominal discomfort, bloating, and diarrhea and is generally diagnosed with a mucosal biopsy of the duodenum during an esophagogastroduodenoscopy with assessment of tissue transglutaminase immunoglobulin A (IgA) antibodies. It is also associated with certain human leukocyte antigen (HLA)–DQ haplotypes.e7,e9 In a preliminary case-control study, 4.4% of patients with migraine (n = 90) and 0.4% of blood-donor controls (n = 236) had CD,16 and another case-control study found a higher prevalence of migraine disorder (based on ID migraine diagnostic tool criteria)29 in patients with CD than in controls (21% vs 6%; p < 0.0001). The likelihood of migraine disorder was 3.79-fold greater in patients with CD than in controls (odds ratio [OR]: 3.79; 95% CI: 1.78–8.10; p = 0.0006).30 Nonceliac gluten sensitivity (NCGS) is also triggered by gluten ingestion and is associated with similar GI symptoms as those observed in individuals with CD.e10 NCGS is diagnosed in individuals experiencing gluten sensitivity, in whom CD, food allergies, and other GI diseases have been ruled out. In Western populations, NCGS has a prevalence of 0.6%–10.6%. NCGS has been associated with a 9.53-fold increase in migraines compared with controls (OR: 9.53, 95% CI: 3.24–28.09; p < 0.0001).30 Studies have shown total headache resolution in up to three-quarters of pediatric patients with CD and reduced frequency and intensity of migraine in adults with CD and in those with NCGS, after adopting a gluten-free diet.31,32,e10
Functional Diarrhea
Functional diarrhea has a reported prevalence in the general population in the range 1.5%–17% and is characterized by recurrent passage of loose or watery stools; it can be associated with abdominal pain or bloating as seen in IBS.e11 Several mechanisms seem to contribute to functional diarrhea, including altered GI motility, brain-gut disturbances, genetics, environmental factors, prior infections, and psychosocial factors.e11 Functional diarrhea is among the symptoms of altered autonomic function in migrainee12 and can be a premonitory symptom. Functional diarrhea had a prevalence of 28.2% in a study of 1,025 consecutive patients with migraine; however, its occurrence did not correlate with headache intensity.22 Functional diarrhea is also often associated with therapy in migraine; a registry analysis of nearly 150,000 patients found that 10.4% of those receiving opioids experienced diarrhea.33 Functional diarrhea is also associated with certain migraine preventive therapies, such as magnesiume13 and the anticonvulsant topiramate.34
Functional Constipation
Functional constipation is a functional disorder of the hindgut (the section of the gut commencing at the junction of the right and middle thirds of the transverse colon)e3 defined by a reduction in bowel movement frequency and may be primary or secondary to an underlying disorder. Like functional diarrhea, it can be associated with IBS if there is a component of abdominal pain or bloating.e11 A systematic review found the median global rate of functional constipation to be 16%.35 A population-based survey of 645 participants demonstrated that the cumulative incidence of new-onset chronic constipation over a median of 12 years increased with advancing age among men and was more prevalent in women than in men among those younger than 50 years at baseline.36 In a study of GI disorders among 1,574 patients referred for treatment at an obesity clinic, migraine was diagnosed in 181 patients (11.5%). An adjusted multivariate regression analysis determined that individuals with migraine were approximately 4 times more likely to have functional constipation than controls (OR: 3.96; 95% CI: 2.25–6.99).17 The same study also found an increased likelihood of dyspepsia, heartburn, and IBS in the migraine group.17
Irritable Bowel Syndrome
A functional disorder of the hindgut with increased prevalence in women, IBS presents with recurrent episodes of abdominal pain related to defecation, associated with a change in stool frequency or in stool form. Two primary forms of IBS exist: IBS with diarrhea (IBS-D), characterized by recurrent or chronic diarrhea, and IBS with constipation (IBS-C), characterized by abdominal pain or discomfort associated with constipation. Some patients experience IBS with mixed bowel habits.e11 A retrospective case-control study of national registry data compared 14,117 patients with newly diagnosed migraine with a randomly selected group of 56,468 migraine-free individuals. An adjusted proportional hazards model demonstrated that the cumulative incidence of IBS in the migraine cohort was almost twice that in the comparison cohort (73.87 vs 30.14 cases per 10,000 person-years). Moreover, patients with migraine had a greater incidence of IBS than migraine-free individuals during the follow-up years (p < 0.0001).37 Similarly, a retrospective cross-sectional survey of 1,112 consecutive hospital patients found a significantly greater frequency of IBS at baseline in the cohort with migraine (n = 287) than in an age-matched and sex-matched migraine-free cohort (n = 287; 27.5% vs 16.7%, respectively; p = 0.003).38
Given that migraine is a common, chronic health condition, a spurious association between migraine and common GI symptoms cannot be completely ruled out, particularly because there is a lack of long-term, longitudinal studies, which are warranted. Nonetheless, a close relationship between GI symptoms and migraine has been reported in multiple retrospective analyses of data from large registries, suggesting that the gut-brain axis may play a role in migraine pathophysiology. Several mechanisms have been proposed to explain this link.4, e14
Disease Mechanisms That Might Support the Association Between Migraine, GI Comorbidities, and CGRP
Functional and motility disorders may manifest differently in the foregut (e.g., nausea and vomiting) and hindgut (e.g., diarrhea and constipation), but both types of disorders stem from regional modulation of the enteric nervous system by afferent and efferent autonomic stimuli (Figure 1A).11,e15,e16 The commonality of innervation in different regions of the gut and the role of CGRP in GI function may explain why migraine is associated with a range of GI symptoms and comorbidities. Several pathophysiologic mechanisms have been suggested to account for GI symptoms associated with migraine: autonomic nervous system dysfunction linked to nausea, reflux, and constipation;e17 immunologic and inflammatory processes linked to IBS;e18 nausea and vomiting linked to allergen activation of trigeminal afferent nerves through release of inflammatory mediators;e19 mitochondrial dysfunction contributing to nervous system dysfunction39; and hormonal mechanisms linked to IBS.40
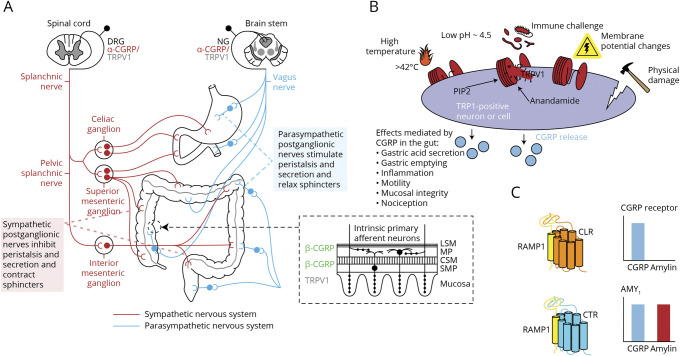
Figure 1. CGRP Activity in the GI Tract and Downstream Effects.
(A) Autonomic innervation of the gastrointestinal tract (Adapted from Snell. Clinical Neuroanatomy, 7th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010,e15 and adapted and reprinted by permission from Springer Nature: Capsaicin Receptor as Target of Calcitonin Gene-Related Peptide in the Gut by Evangelista11 S, 2014). (B) TRPV1 activation and CGRP response (Reproduced from Assas et al. Calcitonin gene-related peptide is a key neurotransmitter in the neuroimmune axis. Front Neurosci 2014; 8[23]: 469–497).e23 (C) CGRP binding dependent on CGRP receptor subunit composition. The bar charts indicate relative ligand activity when binding human receptors. (Adapted and reprinted by permission from Springer Nature: Neurology and Therapy “Hypervigilance, Allostatic Load, and Migraine Prevention: Antibodies to CGRP or Receptor,” Blumenfeld et al., 2021)e24 Abbreviations: AMY1 = amylin receptor 1; CGRP = calcitonin gene-related peptide; CLR = calcitonin-like receptor; CSM = circular smooth muscle; CTR = calcitonin receptor; DRG = dorsal root ganglion; LSM = longitudinal smooth muscle; MP = myenteric plexus; NG = nodose ganglion; PIP2 = phosphatidylinositol 4,5-bisphosphate; SMP = submucosal plexus; RAMP = receptor activity modifying protein; RAMP1 = RAMP type 1; TRPV1 = transient receptor potential vanilloid 1.
Evidence from animal studies suggests a role for CGRP in maintaining the mucosal integrity of the GI tract. A rat model of ischemic GI injury demonstrated that CGRP may participate in modulating intestinal blood flow, sensorimotor activity, and tissue oxygenation.e20 CGRP also plays a role in the gut in gastric acid secretion, inflammation, motility, and nociception (Figure 1B).11 In mouse studies, when the gut is infected by Salmonella, CGRP and other neuropeptides have been shown to influence host gut defenses: nociceptors regulate the production of CGRP and other neuropeptides, which modulates the density of microfold cells and segmented filamentous bacteria levels to protect against the infection.e21-e25 Regarding gut motility, CGRP was involved in regulating gastric emptying and modulating GI tract function, diminishing contractions in the rat colon and reducing food intake.12
CGRP activates both the canonical CGRP receptor and amylin receptor 1 (AMY1; Figure 1C).e26 In a study of gastric emptying regulation, 19 healthy volunteers infused with the amylin analog pramlintide demonstrated delayed gastric emptying, but the small bowel and colonic transit were unaffected.e27 While pramlintide is a nonselective agonist at all 3 amylin receptors,41 this result suggested a potential contribution of AMY1 to gastric mobility, which can also be activated by CGRP. It should be noted, however, that little is known of the role of CGRP binding to the AMY1 receptor. Autonomic dysfunction associated with migraine and GERD may relate to the overlap between the symptomatology of these 2 conditions, and a study found that gastroparesis may play a key role in GERD.14 Conditions such as IBS and CVS consist of symptoms such as vomiting and diarrhea that can heavily overlap with those of migraine. In addition, extensive GI symptoms were reported in humans after infusion with CGRP. Thirty healthy volunteers pretreated with sumatriptan or placebo received a 2-hour infusion of CGRP 1.5 µg/minute, 27/29 of them (93%) reported GI symptoms, the most common of which were stomach rumbling, stomach pain, nausea, an urge to defecate, and defecation. GI symptoms did not seem to be antagonized by sumatriptan, given that there were no differences in GI symptoms between the 2 treatment groups.e28
There are 2 isoforms of CGRP: α-CGRP and β-CGRP. These differ by only 3 amino acids in humans, and no meaningful pharmacologic differences between them have been demonstrated.42,e29,e30 α-CGRP is the main form in the peripheral and central nervous systems, and β-CGRP is mostly found in the enteric nervous system.42 Anti-CGRP receptor antibodies prevent CGRP from binding to its cognate receptor and have also been reported to prevent CGRP and amylin action at AMY1,43 although the pharmacology of the recombinantly expressed CGRP and AMY1 receptors that were used in the latter study differs from that reported elsewhere.44,45,e26 The anti-CGRP antibodies block the binding of α-CGRP and β-CGRP to both the CGRP receptor and AMY1,44,46, but do not prevent amylin from acting at AMY1.43 The CGRP receptor antagonists olcegepant, telcagepant, rimegepant, and ubrogepant also bind to AMY1 but with up to 100-fold lower affinity than to the CGRP receptor.44,e26,e31,e32 It has been postulated that differences in effects on motility observed among CGRP-based therapies may involve the ability of the anti-CGRP ligand antibody to inhibit the effects of CGRP at both receptor types.47 CGRP-induced diarrhea in mice was blocked by prophylactic administration of an anti-CGRP antibody and was attenuated by administration of olcegepant.48 An anti-CGRP receptor mAb and acute dosing with telcagepant also significantly inhibited GI transit in the large intestine of transgenic mice (expressing human receptor activity modifying protein 1 [RAMP1], the receptor subunit common to the CGRP receptor, and the related AMY1); however, no significant effect was seen with a mAb targeting CGRP.47
Given the commonality of innervation and the role of CGRP in GI function, it is reasonable to postulate that therapeutic modulation of CGRP in migraine might prove useful in the management of functional and GI motility disorders; this warrants further study given the limited existing treatment options.
Potential Associations Between CGRP-Based Migraine Treatments and GI Sensorimotor Effects
Currently, within CGRP-based therapies, 4 mAbs, targeting the CGRP ligand (eptinezumab, fremanezumab, and galcanezumab) or the CGRP receptor (erenumab), are approved by the FDA for preventive migraine treatment, and 2 gepants are approved for acute treatment (rimegepant and ubrogepant). Atogepant and rimegepant are being evaluated for migraine prevention, and zavegepant (administered nasally) is in phase 2/3 development for acute migraine treatment.49,e33 First-generation gepants have had development suspended (olcegepant, BI 44370) or have been withdrawn (telcagepant, MK3207) owing to concerns including hepatotoxicity.50,51 Although GI symptoms during the studies of these agents qualify as AEs, the presence of these symptoms in the absence of treatment suggests potential avenues of CGRP-mediated modulation of the GI tract transit and functions which may warrant further investigation.
CGRP-Based mAbs
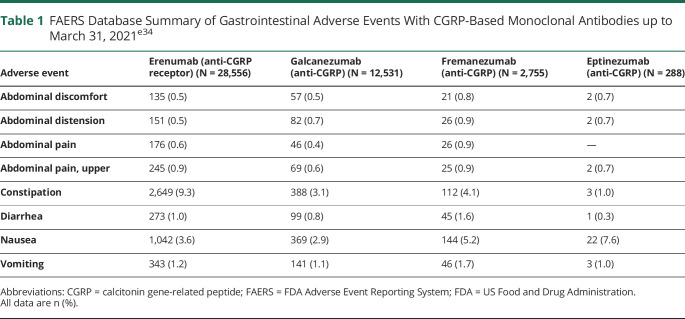
Based on data from the FAERS, it is estimated that 17% of patients treated with mAbs targeting CGRP or its receptor develop GI-related AEs, although rates seen in clinical trials are lower. The FAERS database summarizes the incidence of AE case reports by drug.e34 It should be noted, however, that FAERS represents only part of the FDA postmarket surveillance data, and it has limitations as a surveillance system, including potential submission of incomplete, inaccurate, untimely, and unverified information.e34 Underreporting of events, lack of validation for association of a reported AE with a monotherapy or comorbid illnesses, and lack of information regarding frequency of use of medications can also occur.52,e35
FAERS is a voluntary reporting system and, although the absolute number of AEs is reported, the total number of patients exposed to the drug remains unknown.53 Thus, FAERS data cannot quantify the incidence of AEs or be used to compare event rates across products. Nonetheless, FAERS represents what is, at present, the most comprehensive repository of postmarketing safety data. As an indication of real-world rates, proportions of GI AEs among cases reported to FAERS for CGRP-based therapeutic mAbs up to March 31, 2021 were as follows: eptinezumab, 13.5% (39 of 288 cases); erenumab, 16.4% (4,684 of 28,556 cases); fremanezumab, 15.6% (430 of 2,755 cases); and galcanezumab, 9.6% (1,206 of 12,531 cases).e34 Profiles of GI events were similar across the 3 CGRP-targeting mAbs (eptinezumab, fremanezumab, galcanezumab) for which FAERS data were available, and indicated that nausea and constipation were the GI events most often reported, followed by vomiting, diarrhea, and abdominal symptoms (Table 1).e34
Table 1.
FAERS Database Summary of Gastrointestinal Adverse Events With CGRP-Based Monoclonal Antibodies up to March 31, 2021e34
Constipation was identified as a potential adverse drug reaction with erenumab based on premarketing clinical trials, in which AEs of constipation were mild to moderate in severity, and none of the events were serious.54,e36,e37 In postmarketing settings, AEs of serious constipation, including cases in which surgery was necessary, were received and submitted to the FDA. Based on the postmarketing data, the FDA requested an update to the erenumab US prescribing information (issued in 2020) warning of constipation with serious complications.e38 An integrated safety analysis of 4 double-blind, randomized erenumab trials and their extensions found an exposure-adjusted AE rate of constipation of 7.0 per 100 patient-years (vs 3.8 per 100 patient-years for placebo). Constipation events were mild to moderate in severity, no serious AEs were reported, and no pattern of GI history was evident among individuals who developed constipation while on study.54
In phase 3 clinical studies, constipation was reported in 1.0%–3.0% of patients treated with erenumab54,e38 and in 1.0%–1.5% of those given galcanezumab.55 Published results from eptinezumab and fremanezumab phase 3 trials do not mention constipation.56,e39,e40,e41 In real-world studies, however, constipation has been reported with a higher prevalence (14%–43%).57,e42,e43,e44,e45, Thus, in a retrospective cohort study in the United States involving 241 individuals who had taken erenumab, data on AEs were collected as part of a structured clinical interview, which included an open-ended question followed by reviewing a checklist of possible AEs. Constipation was the most common AE, affecting 43% of patients. AEs were more common in this real-world population than in clinical trials, a discrepancy that the authors attributed to systematic differences between clinical trial participants and patients who received the treatment in clinical practice. Nonetheless, nearly 70% of patients stated that the benefits of erenumab outweighed any drawbacks.57 In another retrospective, exploratory, observational study in the United States, which included patients with numerous comorbidities (including IBS) who had previously tried an average of 11.2 medications, new or worsened constipation with erenumab was reported by 17 of 72 participants (24%).e43
A prospective, single-center, real-world audit in patients with CM (with or without medication overuse) refractory to established preventive medications was conducted in the United Kingdom. Patients received monthly erenumab for 6 months, and constipation was reported in 20%, 11%, and 5% of patients at months 1, 3, and 6, respectively.e44 In another observational study in all patients with migraine treated with erenumab during 2019 in the Abruzzo region of central Italy (n = 89; 6-month follow-up), constipation was reported in 13.5% of patients.e45 The European label for erenumab classifies constipation as a common event (incidence from ≥1/100 to <1/10 treated patients),e46 as does the label for galcanezumab;e47 however, constipation is not listed as an AE in the United States prescribing information for the anti-CGRP mAbs (galcanezumab,e48 fremanezumab,e49 and eptinezumabe50) or in the European label for fremanzezumab.e51
In agreement with FAERS data, nausea was also reported in most clinical trials of the CGRP-based mAbs, with vomiting and diarrhea occurring relatively infrequently,56,e39,e40 although there is little evidence for event frequencies being greater than those in the respective control groups. None of these 3 AEs are noted in the US prescribing information or European labels for any of the CGRP-based mAbs.
Gepants
Relatively few AEs have been recorded through FAERS for the gepants, although these drugs have been available for a shorter time than mAbs. As of March 31, 2021, nausea (55 [12.4%]) and vomiting (21 [4.7%]) associated with ubrogepant were reported among 106 GI cases (total cases, 443); 175 of 943 cases with rimegepant were GI events, with nausea (106 [11.2%]) and vomiting (27 [2.9%]) most common. Only 1 event (non-GI) was reported for the unauthorized drug atogepant.e34 The US prescribing information for rimegepant and ubrogepant list nausea as the most common AE,e52,e53 which reflects the GI AEs reported in clinical trials.58-60,e54 The results from the phase 2/3 placebo-controlled study of atogepant for migraine prevention in patients with EM show that constipation and nausea are among the most common AEs, with frequencies that seem to correlate with dosage.60
GI AEs of Other Migraine Treatments and Concomitant Therapies
Different classes of small-molecule drugs used in acute or preventive migraine treatment can be associated with GI AEs. Among acute treatments for migraine, opioids can cause “opioid-induced bowel dysfunction,” leading to abdominal cramping, bloating, constipation, diarrhea, dry mouth, gastroparesis, GERD, nausea, spasm, and vomiting, and which can affect the entire GI tract.e55,e56 Constipation is the most common AE, reported in 22%–81% of patients.e55 In a registry analysis of long-term treatment patterns and acute medication use among 147,832 individuals with migraine, 77.4% received opioids. Among opioid users, 16.6% reported nausea/vomiting, 12.2% reported constipation, and 10.4% reported diarrhea.33
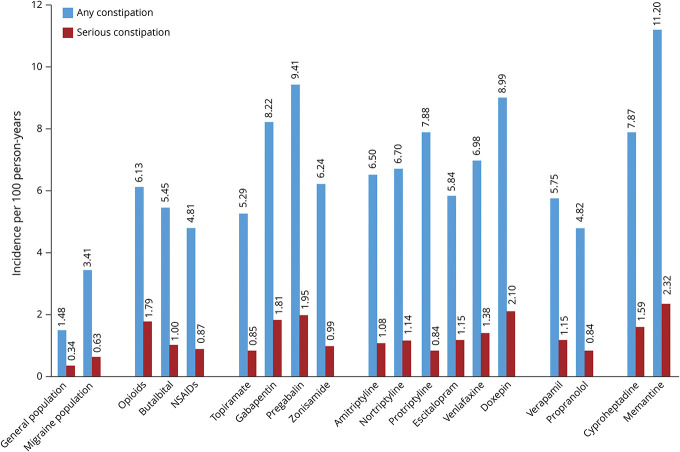
NSAIDs also cause significant GI-related AEs, which can affect the entire GI tract,e57 including peptic ulcer development (complicated by bleeding, obstruction, and perforation) as well as conditions such as NSAID-induced enteropathy.e58,e59 A retrospective cohort study of MarketScan Research Databases in 584,475 patients with migraine estimated incidences per 100 person-years of 3.41 (95% CI: 3.39–3.44) for any constipation and 0.63 (95% CI: 0.62–0.64) for serious constipation.e60 The incidence of constipation increased with age and with the number of comorbidities and was greater in women than in men. The incidence of any, or of serious, constipation was also generally at least two-fold greater in patients starting treatment with acute and preventive small-molecule drugs used commonly in migraine than in the overall migraine population (Figure 2).e60
Figure 2. Incidence of Constipation per 100 Person-Years in Patients Starting Acute and Preventive Migraine Treatments.
The incidence of any or serious constipation was generally at least two-fold higher in patients with migraine beginning treatment with various acute and preventive medications than in all patients with migraine. Data derived from MarketScan® Research Databases; figure reproduced with permission from Amgen. e60
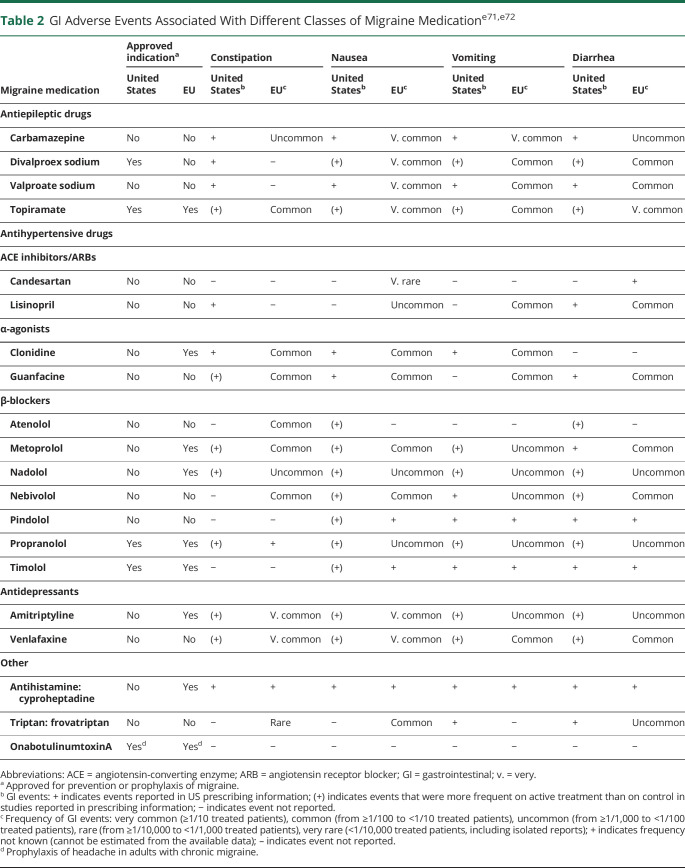
A summary of GI AEs with drugs prescribed for migraine prevention is shown in Table 2. Among the antiepilepsy drugs, only topiramate and divalproex sodium are currently indicated for migraine prophylaxis, and divalproex sodium is more commonly associated than topiramate with a range of GI AEs, including constipation. The reported AE profiles are not from patients with migraine because antiepilepsy drugs are used off-label. Drug-related associations between GI comorbidities and migraine are seen across drug classes.e61,e62 Off-label use of drugs is common in the prevention of migraine; for example, antidepressants such as amitriptyline show good evidence of benefit.e63 In the European label for amitriptyline, GI disorders including constipation, dry mouth, and nausea are noted as being very common (>1/10 treated patients).e64 Of note, low-dose amitriptyline has been used extensively off-label as a treatment for certain functional GI disorders, such as IBS.e65 The antidepressant nortriptyline is also associated with constipation.e66 In a prospective trial of 75 patients with migraine treated with topiramate, amitriptyline, or a combination of both drugs, constipation was reported in 45.5% of those in the amitriptyline group; fewer AEs were seen in the topiramate and combination groups, and no GI AEs were noted.e62 The antihypertensive therapies candesartan and lisinopril also show good evidence of benefit in migraine;e63 the European label for lisinopril cites diarrhea and vomiting as common AEs (from ≥1/100 to <1/10 treated patients).e67 Other medications such as the calcium channel blocker verapamil are used off-label for migraine prevention and are associated with constipation,e66 and GI AEs are reported with β-blockers used for migraine prophylaxis, although they seem to be more frequent with atenolol (constipation and GI disturbances) and metoprolol (abdominal pain, constipation, diarrhea, and nausea) and less common with nadolol and propranolol (Table 2).
Table 2.
GI Adverse Events Associated With Different Classes of Migraine Medicatione71,e72
Implications for Physicians Managing Patients With Migraine
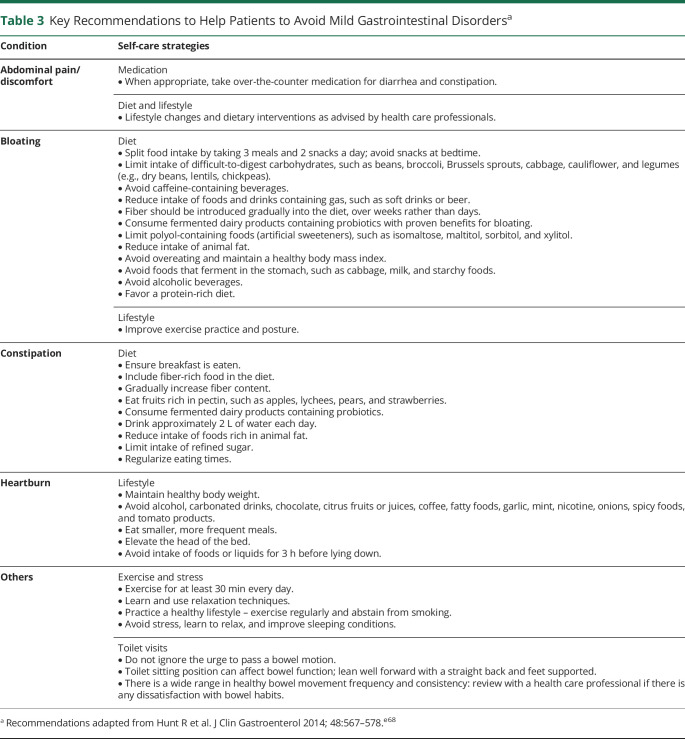
Given that individuals receiving migraine therapies are at risk of developing GI AEs, it is important to consider how both physicians and patients can best mitigate these effects. From the patient's perspective, counseling about possible GI outcomes is important, and some self-care strategies are listed in Table 3;e68 any changes or restrictions in diet should only be introduced in consultation with a health care provider. Accurate medical and treatment history taken by physicians is essential when initiating migraine treatment. A full history should also be noted if GI symptoms are subsequently reported by the patient. Questions for the clinician to consider include the following:
Does the patient have a preexisting GI disorder?
What are the patient's baseline bowel habits, and did these habits change after treatment initiation?
Does the patient have underlying risk factors for GI disorders other than migraine?
Is a particular class of migraine treatment likely to increase the risk of GI AEs or exacerbate an existing GI disorder?
What is the best route of administration of migraine medications, in light of the patient's GI symptoms?
Is the patient receiving an acute therapy (especially an over-the-counter medication) that can confound or exacerbate GI events of preventive medications?
Is the patient taking other nonmigraine medications associated with GI AEs?
Table 3.
Key Recommendations to Help Patients to Avoid Mild Gastrointestinal Disordersa
These factors should be considered when choosing a preventive medication, alongside the indication and established AE profile of the treatment. GI disorders associated with migraine treatments are generally mild and transient, but if they are severe, the patient should be referred to a GI specialist. Guidance on when a GI specialist should be consulted is available.e68
It is possible that magnesium or other preventive therapies concomitantly administered with a mAb may reduce constipation and increase migraine prophylaxis. Studies are needed to test this hypothesis. If NSAIDs are used during acute treatment for migraine, the minimal dose necessary should be used to prevent gastric side effects and nephrotoxicity.e69 Acid suppression in the form of histamine-2 antagonism or proton pump inhibition may be considered, especially for those at risk of GI bleeding.e70
Conclusions
Migraine is associated with several functional and motility disorders of the GI system. The role of CGRP in migraine and the effect of CGRP on different regions of the gut may explain these clinical associations and the finding that CGRP-based therapeutic antagonism in migraine can lead to GI AEs. Ongoing AE monitoring in real-world studies is important to ensure the full AE profiles of new treatments are adequately captured.
Acknowledgment
The authors are grateful to Amy Filby (Oxford PharmaGenesis Ltd) for providing editorial support. These services were paid for by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA).
Glossary
- AE
adverse event
- AMY1
amylin receptor 1
- CaMEO
CM Epidemiology and Outcomes
- CD
celiac disease
- CGRP
calcitonin gene-related peptide
- CM
chronic migraine
- CVS
cyclical vomiting syndrome
- EM
episodic migraine
- FAERS
FDA Adverse Event Reporting System
- FDA
US Food and Drug Administration
- GERD
gastroesophageal reflux disease
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- mAb
monoclonal antibody
- NSAID
nonsteroidal anti-inflammatory drug
- OR
odds ratio
- RAMP1
receptor activity modifying protein 1
Appendix. Authors
Study Funding
This review was supported by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA).
Disclosure
J. Ailani reports receiving honoraria for independent consulting from Abbvie, Aeon, Amgen, Axsome, Biohaven, Eli Lilly, GlaxoSmithKline, Impel, Lundbeck, Medscape, Satsuma, Teva, Theranica and Vorso; receiving honoraria for participating in speaker bureaux for Allergan/Abbvie, Amgen, Biohaven, Eli Lilly, Lundbeck and Teva; receiving research grants (no personal compensation) from Allergan/Abbvie, American Migraine Foundation, Biohaven, Eli Lilly, Satsuma and Zosano; receiving honoraria for editorial services from Current Pain and Headache Reports, Infomedica, NeurologyLive and SELF; and holding stock in CtrM. E.A. Kaiser reports royalties from patents with Alder Biopharmaceuticals (now Lundbeck) and research grants from Amgen and NIH. P.G. Mathew reports serving as a consultant for Allergan, Amgen, Biohaven, Impel, Lilly, Revance, Satsuma, Supernus, Takeda, and Theranica. P. McAllister reports consultancy or advisory board compensation from Abbvie, Amgen, Biohaven, Lilly, Lundbeck, Teva and Revance, and research support from Amgen, Biohaven, Lilly, Teva, Aeon, Revance, Genentech and Eisai. A.F. Russo is a consultant for Allergan, Amgen, Lilly, Lundbeck, Novartis, Pharmnovo, and Schedule One Therapeutics, and reports research grants from DoD, Lundbeck, MRF, NIH, and VA. C.D. Vélez receives funding from the Cystic Fibrosis Foundation and sits as a subspecialty representative for the Association of Migraine Disorders. A. Abdrabboh is an employee of Novartis Pharmaceuticals Corporation. C. Xu and S. Rasmussen are employees of Amgen Neuroscience. A. Pozo Ramajo is an employee of Oxford PharmaGenesis. S.J. Tepper reports grants for research (no personal compensation) from Allergan, Amgen, ElectroCore, Eli Lilly, Lundbeck, Neurolief, Novartis, Satsuma, Zosano; consultancy and/or advisory boards fees from Aeon, Align Strategies, Allergan/AbbVie, Alphasights, Amgen, Aperture Venture Partners, Aralez Pharmaceuticals Canada, Axsome Therapeutics, Becker Pharmaceutical Consulting, BioDelivery Sciences International, Biohaven, ClearView Healthcare Partners, CoolTech, CRG, Currax, Decision Resources, DeepBench, DRG, Eli Lilly, Equinox, ExpertConnect, GLG, Guidepoint Global, Healthcare Consultancy Group, Health Science Communications, HMP Communications, Impel, Interactive Forums, Krog and Partners, Lundbeck, M3 Global Research, Magellan Rx Management, Medicxi, Navigant Consulting, Neurolief, Nordic BioTech, Novartis, Palion Medical, Pulmatrix, Reckner Healthcare, Relevale, SAI MedPartners, Satsuma, Slingshot Insights, Spherix Global Insights, Sudler and Hennessey, Synapse Medical Communications, Teva, Theranica, Thought Leader Select, Trinity Partners, Unity HA, XOC, Zosano; salary from American Headache Society, Dartmouth-Hitchcock Medical Center, and Thomas Jefferson University; and CME fees from American Academy of Neurology, American Headache Society, Catamount Medical Education, Cleveland Clinic Foundation, Diamond Headache Clinic, Elsevier, Forefront Collaborative, Hamilton General Hospital, Haymarket Medical Education, Headache Cooperative of New England, Henry Ford Hospital, Inova, Medical Education Speakers Network, Medical Learning Institute Peerview, Miller Medical Communications, North American Center for CME, Physicians' Education Resource, PlatformQ Education, Rockpointe, ScientiaCME, and WebMD/Medscape. Go to Neurology.org/N for full disclosures.
References
- 1.Younger DS. Epidemiology of migraine. Neurol Clin. 2016;34(4):849-861. [DOI] [PubMed] [Google Scholar]
- 2.Vecsei L, Szok D, Nyari A, Tajti J. Treating status migrainosus in the emergency setting: what is the best strategy?. Expert Opin Pharmacother. 2018;19(14):1523-1531. [DOI] [PubMed] [Google Scholar]
- 3.Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from Government health studies. Headache. 2018;58(4):496-505. [DOI] [PubMed] [Google Scholar]
- 4.Camara-Lemarroy CR, Rodriguez-Gutierrez R, Monreal-Robles R, Marfil-Rivera A. Gastrointestinal disorders associated with migraine: a comprehensive review. World J Gastroenterol. 2016;22(36):8149-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munjal S, Singh P, Reed ML, et al. Most bothersome symptom in persons with migraine: results from the migraine in America symptoms and treatment (MAST) study. Headache. 2020;60(2):416-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasler WL, Levinthal DJ, Tarbell SE, et al. Cyclic vomiting syndrome: pathophysiology, comorbidities, and future research directions. Neurogastroenterol Motil. 2019;31(suppl 2):e13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maizels M, Burchette R. Somatic symptoms in headache patients: the influence of headache diagnosis, frequency, and comorbidity. Headache. 2004;44(10):983-993. [DOI] [PubMed] [Google Scholar]
- 8.Arzani M, Jahromi SR, Ghorbani Z, et al. ; School of Advanced Studies of the European Headache Federation EHF-SAS. Gut-brain axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia. 2005;25(3):179-183. [DOI] [PubMed] [Google Scholar]
- 10.De Matteis E, Guglielmetti M, Ornello R, Spuntarelli V, Martelletti P, Sacco S. Targeting CGRP for migraine treatment: mechanisms, antibodies, small molecules, perspectives. Expert Rev Neurother. 2020;20(6):627-641. [DOI] [PubMed] [Google Scholar]
- 11.Evangelista S. Capsaicin receptor as target of calcitonin gene-related peptide in the gut. Prog Drug Res. 2014;68:259-276. [DOI] [PubMed] [Google Scholar]
- 12.Haanes KA, Edvinsson L, Sams A. Understanding side-effects of anti-CGRP and anti-CGRP receptor antibodies. J Headache Pain. 2020;21(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu ES, Priyadharsini S S Y, Venkatesan T. Migraine, cyclic vomiting syndrome, and other gastrointestinal disorders. Curr Treat Options Gastroenterol. 2018;16(4):511-527. [DOI] [PubMed] [Google Scholar]
- 14.Katic BJ, Golden W, Cady RK, Hu XH. GERD prevalence in migraine patients and the implication for acute migraine treatment. J Headache Pain. 2009;10(1):35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkman HP. Migraine and gastroparesis from a gastroenterologist's perspective. Headache. 2013;53(suppl 1):4-10. [DOI] [PubMed] [Google Scholar]
- 16.Gabrielli M, Cremonini F, Fiore G, et al. Association between migraine and Celiac disease: results from a preliminary case-control and therapeutic study. Am J Gastroenterol. 2003;98(3):625-629. [DOI] [PubMed] [Google Scholar]
- 17.Martami F, Ghorbani Z, Abolhasani M, et al. Comorbidity of gastrointestinal disorders, migraine, and tension-type headache: a cross-sectional study in Iran. Neurol Sci. 2018;39(1):63-70. [DOI] [PubMed] [Google Scholar]
- 18.Lipton RB, Martin VT, Reed ML, et al. . Medical comorbidities of migraine: results from the chronic migraine Epidemiology and outcomes (CaMEO) study. Cephalalgia. 2017;37(1 suppl):1-378. (Abstract OC–EP–001).28880583 [Google Scholar]
- 19.Lainez MJ, Garcia-Casado A, Gascon F. Optimal management of severe nausea and vomiting in migraine: improving patient outcomes. Patient Relat Outcome Meas. 2013;4:61-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton RB, Buse DC, Saiers J, Fanning KM, Serrano D, Reed ML. Frequency and burden of headache-related nausea: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53(1):93-103. [DOI] [PubMed] [Google Scholar]
- 21.Yalin OO, Uluduz D, Ozge A, Sungur MA, Selekler M, Siva A. Phenotypic features of chronic migraine. J Headache Pain. 2016;17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia. 2006;26(5):548-553. [DOI] [PubMed] [Google Scholar]
- 23.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniyar FH, Sprenger T, Schankin C, Goadsby PJ. The origin of nausea in migraine - a PET study. J Headache Pain. 2014;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li BUK. Managing cyclic vomiting syndrome in children: beyond the guidelines. Eur J Pediatr. 2018;177(10):1435-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abell TL, Adams KA, Boles RG, et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil. 2008;20(4):269-284. [DOI] [PubMed] [Google Scholar]
- 27.Bhandari S, Venkatesan T. Clinical characteristics, comorbidities and hospital outcomes in hospitalizations with cyclic vomiting syndrome: a nationwide analysis. Dig Dis Sci. 2017;62(8):2035-2044. [DOI] [PubMed] [Google Scholar]
- 28.Kaul A, Kaul KK. Cyclic vomiting syndrome: a functional disorder. Pediatr Gastroenterol Hepatol Nutr. 2015;18(4):224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipton RB, Dodick D, Sadovsky R, et al. ; ID Migraine validation study. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;61(3):375-382. [DOI] [PubMed] [Google Scholar]
- 30.Dimitrova AK, Ungaro RC, Lebwohl B, et al. Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache. 2013;53(2):344-355. [DOI] [PubMed] [Google Scholar]
- 31.Burk K, Farecki ML, Lamprecht G, et al. Neurological symptoms in patients with biopsy proven celiac disease. Mov Disord. 2009;24(16):2358-2362. [DOI] [PubMed] [Google Scholar]
- 32.Parisi P, Pietropaoli N, Ferretti A, et al. Role of the gluten-free diet on neurological-EEG findings and sleep disordered breathing in children with celiac disease. Seizure. 2015;25:181-183. [DOI] [PubMed] [Google Scholar]
- 33.Bonafede M, Wilson K, Xue F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. Cephalalgia. 2019;39(9):1086-1098. [DOI] [PubMed] [Google Scholar]
- 34.Brandes JL. Practical use of topiramate for migraine prevention. Headache. 2005;45(suppl 1):S66-S73. [DOI] [PubMed] [Google Scholar]
- 35.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011;25(1):3-18. [DOI] [PubMed] [Google Scholar]
- 36.Choung RS, Locke GR III, Schleck CD, Zinsmeister AR, Talley NJ. Cumulative incidence of chronic constipation: a population‐based study 1988-2003. Aliment Pharmacol Ther. 2007;26(11-12):1521-1528. [DOI] [PubMed] [Google Scholar]
- 37.Lau CI, Lin CC, Chen WH, Wang HC, Kao CH. Association between migraine and irritable bowel syndrome: a population-based retrospective cohort study. Eur J Neurol. 2014;21(9):1198-1204. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Yu S, Li H, et al. Clinical features and risk factors for irritable bowel syndrome in migraine patients. Pak J Med Sci. 2017;33(3):720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JW, Cho YS, Lee SY, et al. Concomitant functional gastrointestinal symptoms influence psychological status in Korean migraine patients. Gut Liver. 2013;7(6):668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson WC, Sullivan SN, Corke M, Rush D. Globus and headache: common symptoms of the irritable bowel syndrome. Can Med Assoc J. 1978;118(4):387-388. [PMC free article] [PubMed] [Google Scholar]
- 41.Gingell JJ, Burns ER, Hay DL. Activity of pramlintide, rat and human amylin but not Aβ1-42 at human amylin receptors. Endocrinology. 2014;155(1):21-26. [DOI] [PubMed] [Google Scholar]
- 42.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 2013;53(8):1230-1244. [DOI] [PubMed] [Google Scholar]
- 43.Bhakta M, Vuong T, Taura T, Wilson DS, Stratton JR, Mackenzie KD. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia. 2021;41(5):499-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan KS, Siow A, Hay DL, Walker CS. Antagonism of CGRP signaling by rimegepant at two receptors. Front Pharmacol. 2020;11:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54(2):233-246. [DOI] [PubMed] [Google Scholar]
- 46.Deen M, Correnti E, Kamm K, et al. ; European Headache Federation School of Advanced Studies EHF-SAS. Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain. 2017;18(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson K, Li X, Li B. Characterization of transit times in the large intestine of mice following treatment with a CGRP antibody, CGRP receptor antibody and a small molecule CGRP receptor antagonist. Neurology. 2020;94:P14-P005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser EA, Rea BJ, Kuburas A, et al. Anti-CGRP antibodies block CGRP-induced diarrhea in mice. Neuropeptides. 2017;64:95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercer SE, Chaturvedula PV, Conway CM, et al. Azepino-indazoles as calcitonin gene-related peptide (CGRP) receptor antagonists. Bioorg Med Chem Lett. 2021;31:127624. [DOI] [PubMed] [Google Scholar]
- 50.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338-350. [DOI] [PubMed] [Google Scholar]
- 51.Mathew PG, Klein BC. Getting to the heart of the matter: migraine, triptans, DHE, ditans, CGRP antibodies, first/second-generation gepants, and cardiovascular risk. Headache. 2019;59(8):1421-1426. [DOI] [PubMed] [Google Scholar]
- 52.Chedid V, Vijayvargiya P, Camilleri M. Advantages and limitations of the Federal Adverse Events Reporting System in assessing adverse event reporting for eluxadoline. Clin Gastroenterol Hepatol. 2018;16(3):336-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Zhang X, Tong J, et al. Comparing drug safety of hepatitis C therapies using post-market data. BMC Med Inform Decis Mak. 2019;19(suppl 4):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashina M, Kudrow D, Reuter U, et al. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: a pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia. 2019;39(14):1798-1808. [DOI] [PubMed] [Google Scholar]
- 55.Bangs ME, Kudrow D, Wang S, et al. Safety and tolerability of monthly galcanezumab injections in patients with migraine: integrated results from migraine clinical studies. BMC Neurol. 2020;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanaan S, Hettie G, Loder E, Burch R. Real-world effectiveness and tolerability of erenumab: a retrospective cohort study. Cephalalgia. 2020;40(13):1511-1522. [DOI] [PubMed] [Google Scholar]
- 58.Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737-745. [DOI] [PubMed] [Google Scholar]
- 59.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230-2241. [DOI] [PubMed] [Google Scholar]
- 60.Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19(9):727-737. [DOI] [PubMed] [Google Scholar]
- References e1-e72 are available at links.lww.com/WNL/C347.