Abstract
Background and Objectives
Although an infectious etiology of Alzheimer disease (AD) has received renewed attention with a particular focus on herpes viruses, the longitudinal effects of symptomatic herpes virus (sHHV) infection on brain structure and cognition remain poorly understood, as does the effect of sHHV on AD/neurodegeneration biomarkers.
Methods
We used a longitudinal, community-based cohort to characterize the association of sHHV diagnoses with changes in 3 T MRI brain volume and cognitive performance. In addition, we related sHHV to cross-sectional differences in plasma biomarkers of AD (β-amyloid [Aβ]42/40), astrogliosis (glial fibrillary acidic protein [GFAP]), and neurodegeneration (neurofilament light [NfL]). Baltimore Longitudinal Study of Aging participants were recruited from the community and assessed with serial brain MRIs and cognitive examinations over an average of 3.4 (SD = 3.2) and 8.6 (SD = 7.7) years, respectively. sHHV classification used International Classification of Diseases, Ninth Revision codes documented at comprehensive health and functional screening evaluations at each study visit. Linear mixed-effects and multivariable linear regression models were used in analyses.
Results
A total of 1,009 participants were included in the primary MRI analysis, 98% of whom were cognitively normal at baseline MRI (mean age = 65.7 years; 54.8% female). Having a sHHV diagnosis (N = 119) was associated with longitudinal reductions in white matter volume (annual additional rate of change −0.34 cm3/y; p = 0.035), particularly in the temporal lobe. However, there was no association between sHHV and changes in total brain, total gray matter, or AD signature region volumes. Among the 119 participants with sHHV, exposure to antiviral treatment attenuated declines in occipital white matter (p = 0.04). Although the sHHV group had higher cognitive scores at baseline, sHHV diagnosis was associated with accelerated longitudinal declines in attention (annual additional rate of change −0.01 Z-score/year; p = 0.008). In addition, sHHV diagnosis was associated with elevated plasma GFAP, but not related to Aβ42/40 and NfL levels.
Discussion
These findings suggest an association of sHHV infection with white matter volume loss, attentional decline, and astrogliosis. Although the findings link sHHV to several neurocognitive features, the results do not support an association between sHHV and AD-specific disease processes.
Age-related cognitive decline remains a prominent public health challenge, with Alzheimer disease (AD) accounting for the majority of dementia diagnoses (i.e., 60%–80%).1 Despite its growing prevalence, the biological mechanisms that trigger and exacerbate AD remain poorly understood. Recent findings indicate the molecular processes underlying AD can begin years or even decades before the onset of behavioral symptomology, whereas epidemiologic, preclinical, and autopsy evidence suggests infectious pathogens may increase dementia risk.2,3 In turn, these findings have prompted researchers to investigate the potential CNS consequences of chronic viral infections.
The possibility of an infectious etiology of AD has received renewed (albeit controversial) enthusiasm, with a particular focus on human herpes viruses (HHVs). Contemporary debate of this hypothesis was spurred in the 1990s on the observation of herpes simplex virus (HSV)-1 DNA in AD brains.4 More recently, studies leveraging large-scale clinical cohorts and electronic health records in Asia (Taiwan and South Korea), Europe (France, Wales, Germany, Scotland, Denmark, and Sweden) and the United States have suggested an association between herpetic infection and increased risk for dementia, with evidence of decreased risk among infected individuals exposed to antiviral medication.5-10 Several functional validation studies in preclinical models have also supported the relationship between HHVs and dementia risk, while neuroimaging evidence indicates the increased dementia risk associated with HHVs may be attributed to compromised white matter.3,11 However, some studies have found conflicting results, and a recent meta-analysis found insufficient evidence to suggest an association between HHVs and the risk for dementia or mild cognitive impairment (MCI).12-14 Furthermore, it is still unknown whether herpetic infections are associated with longitudinal alterations in regional brain volumes or cognitive performance, nor have herpetic infections been associated with validated plasma biomarkers of AD pathology, reactive astrogliosis, and neurodegeneration within a single cohort. Longitudinal studies of this nature have the advantage of capturing the temporal link between herpetic infection and a dynamic set of neurocognitive changes while reducing the influence of reverse causation and residual confounding on study results.
Using data from the Baltimore Longitudinal Study of Aging (BLSA), this study characterized the association between symptomatic HHV (sHHV) diagnoses and longitudinal changes in brain volume and cognitive performance. In addition, analyses examined the association of sHHV with plasma biomarkers of AD (β-amyloid [Aβ]42/40), reactive astrogliosis (glial fibrillary acidic protein [GFAP]), and neurodegeneration (neurofilament light [NfL]). Among a subset of participants with sHHV diagnoses, the association of antiviral treatment with outcome measures was also assessed. Given the reported effects of HHVs on the temporal cortex, previous associations with white matter abnormalities, and evidence for involvement in AD, we hypothesized that sHHV diagnoses are associated with volumetric loss in white matter (particularly in the temporal lobe) and volume loss in a set of gray matter structures known to be vulnerable to AD-related atrophy.15-20 In addition, we hypothesized that sHHV diagnoses are associated with declines in cognitive performance (particularly memory), and greater levels of biomarker-defined amyloidosis, reactive astrogliosis, and neurodegeneration.
Methods
Study Sample
This study used data from the BLSA, an ongoing longitudinal study in Baltimore, MD, designed to assess physical and cognitive measures in a cohort of community-dwelling volunteers. Participants received comprehensive health and functional screening evaluations at each study visit and were free of major chronic diseases as well as cognitive and functional impairment at the time of enrollment. In accordance with institutional review board–approved protocols, evaluations at study visits were completed by licensed healthcare professionals (e.g., nurse practitioner and medical doctor). A detailed description of the BLSA study design and its procedures have been published elsewhere.21
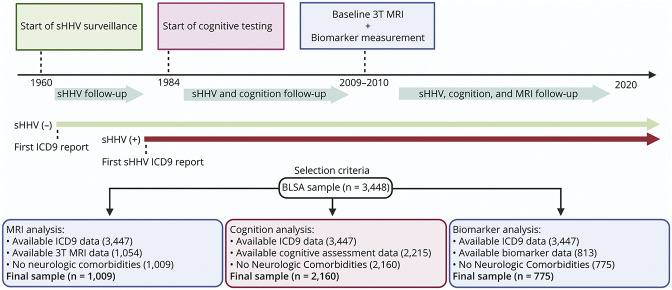
Primary analyses evaluated the association of sHHV diagnoses with regional brain volumes obtained from 3 T MRI. Secondary analyses evaluated the association of sHHV with performance across 5 cognitive domains and plasma biomarkers. As illustrated in Figure 1, comprehensive cognitive assessments were initiated between 1984 and 1993. Repeated 3 T MRIs were initiated in 2009–2010. Plasma biomarkers were measured at the time of the initial 3 T MRI and, for a subset of participants, at the time of a first PET scan as part of a separate study. Study visits occurred biannually until 2005, then every 1–4 years depending on age (age <60 years, every 4 years; age 60–79 years, every 2 years; and age ≥80 years, every year). Participants entered the study at different times and thus varied about follow-up times due to BLSA's continuous enrollment. As displayed in Figure 1, participants were selected if they had MRI, cognitive, or plasma biomarker data, International Classification of Diseases, Ninth Revision (ICD-9) codes, and did not display the existence of significant health conditions that could affect brain structure or function (e.g., stroke, seizures, and brain surgery). For sHHV+, the baseline visit for MRI or cognitive assessment for each participant was the earliest visit at which the presence of sHHV diagnosis was documented. For sHHV-, the baseline visit for MRI or cognitive assessment for each participant was the earliest visit at which the absence of sHHV diagnosis was documented.
Figure 1. Study Design and Participant Selection.
BLSA = Baltimore Longitudinal Study of Aging; ICD-9 = International Classification of Diseases, Ninth Revision; sHHV = symptomatic human herpes virus.
Standard Protocol Approvals, Registrations, and Patient Consents
The BLSA protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Science, NIH (03AG0325), and this study has been approved by its ethical standards committee. All participants gave written informed consent before participation, and deidentified BLSA data were used for analyses.
sHHV Diagnoses
Participants were categorized according to the presence (+) or absence (−) of sHHV diagnoses using ICD-9 codes corresponding to herpetic diagnoses documented in participant medical history reports collected at comprehensive health and functional screening evaluations at each study visit beginning as early as 1960 (varicella-zoster virus [VZV]: ICD-9 codes 053.0X:53.9X; HSV1 or HSV2: ICD-9 codes 054.1X:54.XX). Thus, participants with documented codes that corresponded to chicken pox (i.e., 52.9) were not classified as sHHV in this study. Due to the lack of serologic testing for HHVs in the BLSA, the specificity of virus documentation could not be independently verified. In addition, the specificity of HSV1 and HSV2 could not be distinguished because participant history reports often used similar ICD-9 codes for both diagnoses. Such classification parallels methodology used in recent large-scale studies, whereby the diagnosed sample primarily represents HHV-infected participants with clinical symptoms severe enough to report during patient-health care provider consultations.5-7,9 Such categorization also reflects the potential mechanisms by which HHVs may ultimately contribute to dementia risk, whereby the recurrent activation and virial replication typically associated with symptom presentation (as well as the collateral consequences on host cells and tissues associated with this reactivation) may over time contribute to aberrant biological processes in the CNS.3,22 In addition, given that HHV prevalence is high (>90%) and routine serologic assays can vary with the degree of viral reactivation at the time of assessment, the inclusion of titer measurements may have enabled the exclusion of seronegative participants but would have unlikely enhanced the operationalized construct of sHHV diagnoses.23-27
Antiviral Treatment
Antiviral treatment was defined as the use of one of the following agents documented in participant medical history reports collected at any visit: acyclovir, cidofovir, famciclovir, ganciclovir, valaciclovir, valganciclovir, brivudine, tromantadine, idoxuridine, and penciclovir. Antiviral treatment alone was not considered sufficient to support sHHV diagnostic classification.
3 T MRI
T1-weighted magnetization-prepared rapid gradient echo scans were acquired on a 3 T Philips Achieva (repetition time = 6.8 milliseconds, echo time TE = 3.2 milliseconds, flip angle = 8°, image matrix = 256 × 256, 170 slices, pixel size = 1 × 1 mm, and slice thickness = 1.2 mm). We applied a validated, multiatlas label fusion approach specifically designed to achieve a consistent parcellation of brain anatomy in longitudinal MRI studies using T1-weighted sequences. By combining different atlases, warping algorithms, standardizing parameters, and using a consensus labeling approach to fuse these labels into a final segmentation, the multiatlas region segmentation utilizing ensembles (MUSE) anatomic labeling method generates an ensemble of labeled atlases in target image space; a detailed description of the MUSE method has been previously published.28 Primary analyses examined total brain, total gray matter, and total white matter volumes, as well as AD signature region volume: the combined volume of hippocampus, parahippocampal gyrus, entorhinal cortex, posterior cingulate gyrus, precuneus, and cuneus regions of interest (ROIs), similar to previous investigations.29 Secondary analyses examined lobar ROIs if associations with total gray matter or white matter volume met statistical significance.
Cognitive Domains
Performance was assessed in composite scores across 5 cognitive domains, as described previously,30 with individual task components in each domain chosen based on previous BLSA procedures. In brief, scores from individual cognitive tasks were standardized (converted to a z score using the baseline mean and SD) and averaged within each cognitive domain. As certain cognitive tasks were initiated in the BLSA at different periods according to protocol changes, composite scores for participants at each visit were computed from those tasks available at the time of clinical assessment. Attention was assessed using Trail Making Test Part A and the Digit Span Forward subset of the Wechsler Adult Intelligence Scale-Revised. Executive function was assessed using Trail Making Test Part B and the Digit Span Backward subset of the Wechsler Adult Intelligence Scale-Revised. Verbal fluency was assessed using Verbal Fluency-Letters (F, A, S) and Verbal Fluency-Categories (fruits, animals, vegetables). Verbal memory was assessed using immediate (sum of 5 learning trials) and long-delay free recall from the California Verbal Learning Test. Visuospatial ability was assessed using a modified version of the Educational Testing Service Card Rotations Test and 2 clock-drawing tests (CDTs), where participants were asked to draw the hands and face of clocks indicating 3:25 and 11:10. Here, a composite score was calculated using the mean of the standardized z scores from the Card Rotations Test and the mean of the CDTs.
Plasma Biomarkers
Participants with 3 T MRI data and available plasma specimens were selected for biomarker measurement. Plasma biomarkers were measured from blood collected at the time of the baseline 3T MRI and, for a subset of participants, at the time of a first PET scan as part of a separate study. Blood was stored at −80°C using standardized protocols until the day of analysis. Aβ40, Aβ42, NfL, and GFAP concentrations were measured in EDTA plasma using the single-molecule array (Simoa) neurology 4-Plex E assay on the Simoa HD-X instrument (Quanterix Corp., Billerica, MA). Assays were run in duplicate and values averaged. Intra-assay coefficients of variation were 2.8, 1.9, 5.1, and 5.0 for Aβ40, Aβ42, NfL, and GFAP, respectively. Values for NfL and GFAP were log2 transformed to correct for skewness. Biomarker values were standardized, and at a threshold of 5 SDs, no outliers were detected.
Covariates
Baseline age, sex (male/female), race (White/non‐White; see Table 1 for non‐White categories), and education level (years) were defined based on participant reports. APOEε4 carrier status (0 ε4 alleles/≥1 ε4 alleles/unknown) was defined by PCR with restriction isotyping using the Type IIP enzyme Hhai or the Taqman method, as described previously.31 The estimated glomerular filtration rate (eGFR)-creatinine was defined at the time of biomarker measurement using the Chronic Kidney Disease-Epidemiology Collaboration criteria.32 Chronic comorbid conditions that represent potential confounders were defined using a comorbidity index calculated as the sum (score range: 0–8; converted to a percentage to account for missing data) of 8 comorbid conditions: obesity, hypertension, diabetes, cancer, ischemic heart disease, chronic heart failure, chronic kidney disease, and chronic obstructive pulmonary disease.33
Statistical Analyses
Linear mixed-effects models were used to examine associations of sHHV diagnoses with baseline and longitudinal rates of change in measures of brain volume and cognition. All models also included the following fixed effects as covariates: baseline age, sex, race, education, APOEε4, comorbidity index, and the interactions of age, sex, race, education, APOEε4, and comorbidity index with time. Analyses of brain volumes also adjusted for baseline intracranial volume defined at age 70. The main fixed effects of interest were sHHV, time (time from baseline visit), and sHHV × time. From this model specification, the fixed effect of sHHV estimated the cross-sectional association between sHHV and baseline brain volume and cognition, sHHV × time estimated the effect of sHHV on brain volume and cognition rates of change, and time estimated the average rate of change when all the predictors that interact with time are equal to zero. Random effects of intercept and time with unstructured covariance were included to account for the within-subject correlation of the repeated assessments. Separate linear mixed-effects models incorporated 2-way and 3-way interaction terms (sHHV × moderator, sHHV × moderator × time) to examine whether sex, APOEε4 status, and age modified the association of sHHV with brain volume and cognition both at baseline and longitudinally. Independent samples t tests followed by multivariable linear regression analysis adjusting for covariates listed above were used to examine differences in plasma biomarkers by sHHV diagnoses. Analyses of plasma biomarkers also adjusted for eGFR-creatinine. Separate linear regression models incorporated 2-way interaction terms (sHHV × moderator) to examine whether sex, APOEε4 status, and age modified the association of sHHV with biomarkers. Among a subset of sHHV+ participants, the association of antiviral treatment on outcome measures was also assessed using similar models. Sensitivity analyses were performed to examine the effect of excluding participants on cognitive impairment (i.e., dementia, MCI, and impaired but not MCI) at baseline (procedures for determining cognitive status are detailed elsewhere34). Post hoc analyses were performed to examine the relationship between VZV-infected sHHV+ status and each neurocognitive outcome; limitations in sample size prohibited subgroup analyses of HSV-1/2-infected sHHV+ participants. Statistical significance was defined at 2-sided p < 0.05. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC). Figures were generated in R version 3.4.0 (R Foundation, Vienna, Austria). Data from the BLSA are available on request from the BLSA website (blsa.nih.gov). All requests are reviewed by the BLSA Data Sharing Proposal Review Committee.
Data Availability
Anonymized data not published within this article may be shared on request from qualified investigators for purposes of replicating procedures and findings.
Results
sHHV Infection and Brain Volume Trajectory
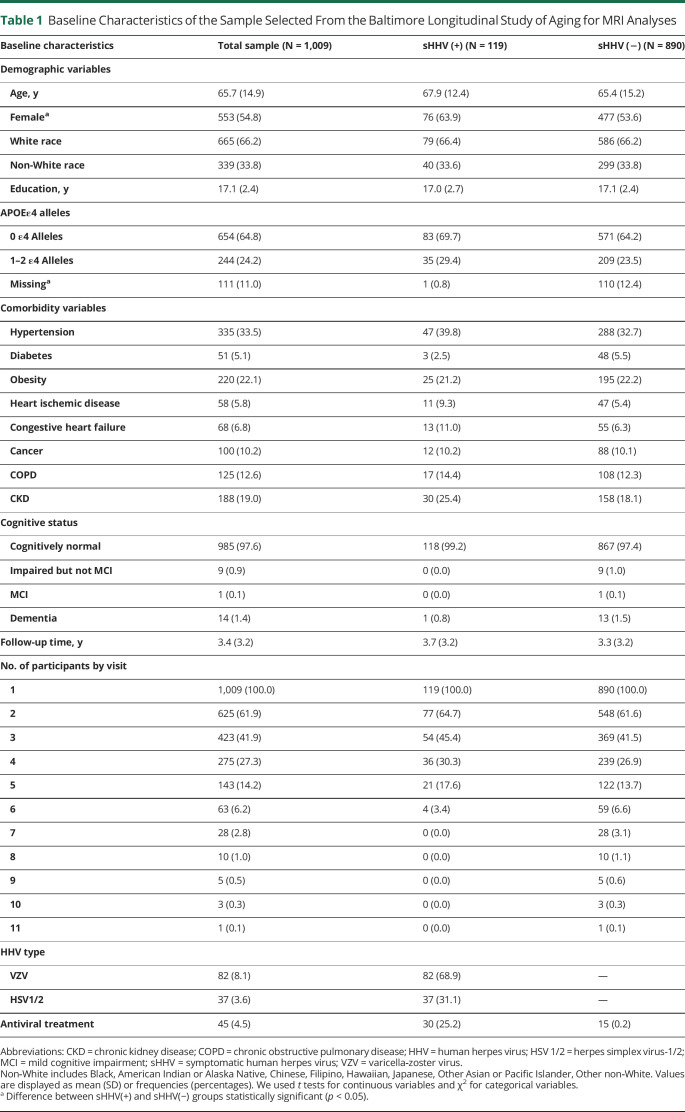
A total of 1,009 participants were included in primary MRI analyses (Table 1; Figure 1). The average follow-up time for the overall sample was 3.4 years (median 3.2; interquartile range [IQR] 0.0–6.2). Participants had an average of 2.4 (SD = 1.5) MRI scans (range 1–11). The average time between the initial date of documented sHHV diagnoses and the first 3T MRI was 16.8 years (median 15.0; IQR 4.0–26.0; min/max 0.0/41.5). Among the 119 sHHV+ participants, 68.9% (n = 82) reported a history of VZV and 31.1% (n = 37) maintained a history of HSV1 or HSV2; 25.2% (n = 30) maintained a history of antiviral treatment.
Table 1.
Baseline Characteristics of the Sample Selected From the Baltimore Longitudinal Study of Aging for MRI Analyses
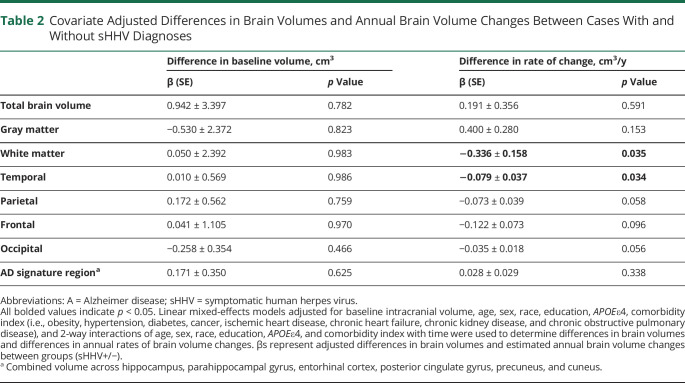
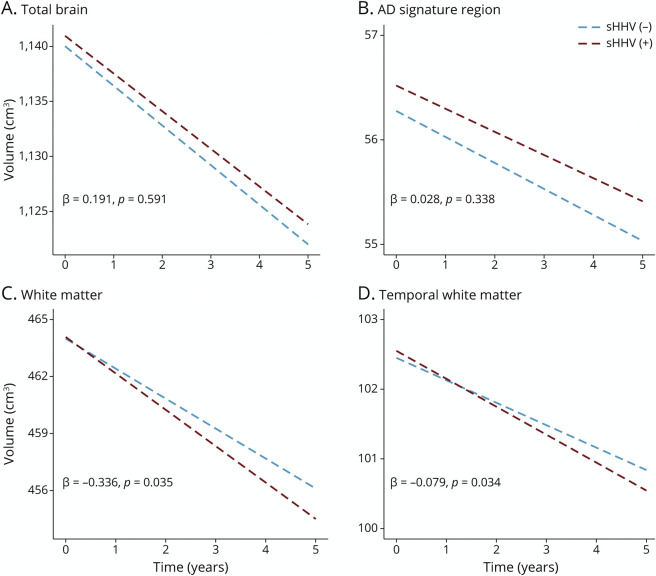
The associations of sHHV diagnoses with baseline brain volume and longitudinal brain volume changes are provided in Table 2. Baseline brain volumes did not significantly differ by sHHV status. However, sHHV was associated with accelerated longitudinal declines in white matter volume (β = −0.336, SE = 0.158, p = 0.035; Figure 2C). Follow-up analyses indicated that the most prominent sHHV-associated white matter loss was in the temporal lobe (β = −0.079, SE = 0.037, p = 0.034; Figure 2D). Although not statistically significant, similar patterns of volume loss were observed across other white matter regions (Table 2). We found no association of sHHV with longitudinal changes in total brain, total gray, or AD signature region volumes. Results were similar when analyses were restricted to participants cognitively normal at the time of baseline MRI (eTable 1, links.lww.com/WNL/C255). Unadjusted analyses are provided in eTable 2; fully adjusted models are provided in eTable 3. In addition, there was no evidence for effect modification by sex, APOEε4 status, or age, and results were consistent when analyses were restricted to VZV-infected sHHV+ participants. Among sHHV+ participants, exposure to antiviral treatment was associated with slower declines in occipital white matter volume (β = 0.097, SE = 0.046, p = 0.036; eTable 4; unadjusted analyses are provided in eTable 5). For descriptive purposes, we also provided the association of sHHV with all defined MRI ROIs in eTable 6.
Table 2.
Covariate Adjusted Differences in Brain Volumes and Annual Brain Volume Changes Between Cases With and Without sHHV Diagnoses
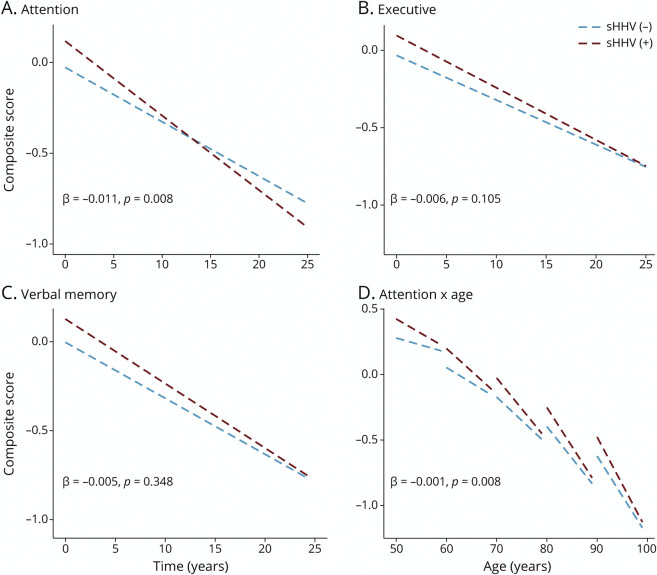
Figure 2. Estimated Changes in Total Brain, AD Signature Region, White Matter and Temporal White Matter Volumes (cm3) Associated With sHHV Diagnoses.
Estimated volumes derived from linear mixed-effects models were adjusted for baseline intracranial volume, age, sex, race, education, APOEε4, comorbidity index (i.e., obesity, hypertension, diabetes, cancer, ischemic heart disease, chronic heart failure, chronic kidney disease, and chronic obstructive pulmonary disease), and 2-way interactions of age, sex, race, education, APOEε4, and comorbidity index with time. βs and corresponding p values represent adjusted differences in estimated annual brain volume changes between groups (sHHV+/−). AD = Alzheimer disease; sHHV = symptomatic human herpes virus.
sHHV Infection and Cognitive Trajectory
Next, we examined the association of sHHV diagnoses with subsequent changes in performance across 5 cognitive domains. A total of 2,160 participants were included in this analysis (Figure 1). Participant characteristics are provided in eTable 7 (links.lww.com/WNL/C255). The average cognitive follow-up time was 8.6 years (median 7.0; IQR 1.9–14.5); participants had an average of 4.2 cognitive assessments (range 1–23). The average time between the date of documented sHHV diagnoses and the first cognitive assessment (defined by the verbal memory domain) was 9.3 years (median 3.5; IQR 1.0–16.0; min/max: 0.0/33.8).
sHHV was associated with better performance on measures of attention, executive function, and verbal memory at baseline (Figure 3, A–C; eTable 8, links.lww.com/WNL/C255). Despite having better cognition cross-sectionally, sHHV+ participants demonstrated greater longitudinal declines in attention (β = −0.011, SE = 0.004, p = 0.008; Figure 3A). Baseline age modified the association between sHHV and change in attention, whereby sHHV-associated declines in attention were stronger in younger participants (3-way interaction: β = −0.001, SE < 0.000, p = 0.008; Figure 3D). sHHV was not significantly associated with longitudinal changes in other areas of cognition (eTable 8). Unadjusted analyses are provided in eTable 9; fully adjusted models are provided in eTable 10. The results were consistent when analyses were restricted to VZV-infected sHHV+ participants. APOEε4 status modified the association of sHHV with declines in the verbal fluency domain (β = 0.019, SE = 0.010, p = 0.043), whereby sHHV-associated declines were greater in APOEε4-negative participants. However, the sHHV− verbal fluency associations were not significant in stratified analyses. The sHHV-cognition results were similar when analyses were restricted to participants cognitively normal at the time of baseline cognitive assessment (eTable 11). Among sHHV+ participants, exposure to antiviral treatment was associated with higher verbal fluency abilities at baseline (β = 0.197, SE = 0.093, p = 0.036); however, antiviral treatment was not related to changes in other cognitive domains (eTable 12; unadjusted analyses are provided in eTable 13).
Figure 3. Estimated Changes in Attention, Executive Function, Verbal Memory, and Attention (as a Function of Age) Composite Scores Associated With sHHV Diagnoses.
Estimated scores from linear mixed-effects models were adjusted for age, sex, race, education, APOEε4, comorbidity index (i.e., obesity, hypertension, diabetes, cancer, ischemic heart disease, chronic heart failure, chronic kidney disease, and chronic obstructive pulmonary disease), and 2-way interactions of age, sex, race, education, APOEε4, and comorbidity index with time. βs and corresponding p values represent adjusted differences in estimated annual composite score changes between groups (sHHV+/−). sHHV = symptomatic human herpes virus.
sHHV Infection and Plasma Biomarkers
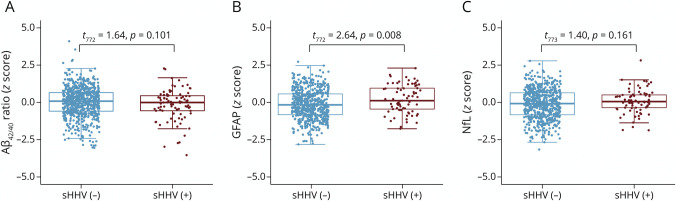
Finally, we examined the cross-sectional association of sHHV diagnoses with plasma biomarkers of amyloid pathology (Aβ42/40), reactive astrogliosis (GFAP), and neurodegeneration (NfL). A total of 775 participants were included in this analysis (Figure 1). Participant characteristics are provided in eTable 14 (links.lww.com/WNL/C255). The average time between the date of documented sHHV diagnoses and biomarker assessment was 10.7 years (median 10.2; IQR 2.0–15.0; min/max: 0.0/41.5).
No group differences were observed in the Aβ42/40 ratio or NfL in independent sample t tests or in adjusted models (Figure 4; eTables 15 and 16, links.lww.com/WNL/C255). However, sHHV+ participants had elevated GFAP compared with sHHV− participants (t772 = 2.64, p = 0.008; Figure 4B). A similar trend, albeit nonsignificant, was observed in adjusted models (β = 0.178, SE = 0.093, p = 0.057; eTable 16). Fully adjusted models are provided in eTable 17. When analyses were restricted to cognitively normal individuals, sHHV was again significantly associated with elevated GFAP (β = 0.220, SE = 0.098, p = 0.026; eTable 18). There was no evidence for effect modification by sex, APOEε4 status, or age, and the results were consistent when analyses were restricted to VZV-infected sHHV+ participants. Among sHHV+ participants, exposure to antiviral treatment was not associated with plasma biomarker levels (eTable 19).
Figure 4. Plasma Biomarkers (Standardized Values) Associated With sHHV Diagnoses.
Results derived from independent samples t tests. Aβ42/40 = amyloid-β42/40 ratio; GFAP = glial fibrillary acidic protein; NfL = neurofilament light; sHHV = symptomatic human herpes virus.
Discussion
This study demonstrates that sHHV diagnosis is associated with steeper volumetric declines in white matter, particularly in the temporal lobe, while exposure to antiviral treatment among sHHV+ participants is associated with slower declines in occipital white matter volume, suggesting a protective effect. sHHV was also associated with greater longitudinal reductions in attention performance, despite sHHV+ participants showing elevated cognitive performance at baseline. In addition, sHHV+ participants, particularly those who are cognitively normal, maintain elevated plasma levels of GFAP, an indicator of reactive astrogliosis. The current results provide novel insights regarding the association of herpetic viruses with changes in brain structure and cognition and highlight the potential contribution of sHHVs to neurocognitive changes in older adults.
Our findings, which suggest sHHV is associated with white matter volume loss, complement a recent study which reported elevated white matter microstructural alterations in participants who were seropositive for HHV.11 Such deleterious effects on white matter might otherwise be expected, provided that herpetic infection and associated inflammation can compromise white matter integrity in the CNS.15-17 Similarly, the current findings of temporal lobe-specific reductions in white matter volume might be expected, given the reported deleterious effects of HHVs on the temporal cortex.18-20 Among sHHV+ participants, exposure to antiviral treatment was associated with slower declines in occipital white matter volume, an opposite pattern than the association observed as a function of sHHV diagnoses. We did not observe evidence of cross-sectional or longitudinal alterations in gray matter, total brain, or AD signature region volumes as a function of sHHV. A cross-sectional study of patients with AD has demonstrated a positive relationship between HSV-1 serum titers and regional gray matter volumes; however, the directionality of such results may have been dependent on the type of immunoglobulin used to assess herpetic titers.35,36
The association between sHHV and declines on measures of attention is consistent with a number of retrospective observational studies which show increased risk for dementia in HHV seropositive individuals.5-10 The sHHV-associated declines in attention align with our findings of reduced white matter volume associated with sHHV, given previous evidence of impaired attentional capacities and processing speeds among individuals with compromised white matter.37-39 However, the current findings should be considered in the context of several analyses that have found no relationship between HHV seropositivity and dementia risk.12,13 The present results are also challenged by a recent Mendelian randomization study that did not support a causal relationship between HSV infection and general cognitive function.14 The association of sHHV with elevated cognitive performance at baseline was surprising and may be a result of residual confounding, biases in the study design, or perhaps protective effects of HHV-induced immune activation.40-42 This latter interpretation is supported by evidence of elevated HSV-1 titers in healthy controls and patients with MCI compared with AD cases.35,43
This study also examined the link between sHHV and changes in molecular neurobiology using a panel of validated, ultrasensitive plasma biomarkers. We found that sHHV+ participants maintain elevated plasma GFAP but did not differ from sHHV− participants on measures of the Aβ42/40 ratio or NfL. Several studies have previously reported no differences in plasma Aβ42/40 ratios linked herpetic infection.44,45 However, a prior investigation reported increased serum levels of NfL in VZV-infected patients compared with healthy controls.46 Increased GFAP in CSF has been associated with nonencephalitic sHHV diagnoses.47,48 In this study, we show that increased plasma GFAP is also associated with herpetic infections. Elevations in this marker of reactive astrogliosis may be attributed to astrocyte-mediated neuroinflammation precipitated by sHHV infection. Measures of reactive astrogliosis have been implicated in the initial stages of AD progression before the manifestation of other pathologic hallmarks and clinical symptoms.49,50 The idea that plasma GFAP is elevated in the asymptomatic phase of neurodegenerative processes49 is consistent with our observation of a GFAP-sHHV relationship only in the subset of cognitively normal individuals. In the context of the present MRI and cognitive analyses, the observed elevations in GFAP among sHHV+ participants could indicate HHV-induced neuroinflammation mediated by astrocytes that contributes to white matter volume loss and domain-specific cognitive decline. However, the absence of an association between sHHV and Aβ42/40 ratio, AD signature region volume, and verbal memory performance suggest sHHV may not be related to AD-specific disease processes.
Our study had several limitations. First, information obtained from medical history reports taken at study visits was used to identify HHV diagnoses; therefore, inaccurate reporting or undiagnosed sHHV may have led to misclassifications, a bias that would likely push results toward the null. Second, the absence of a clear relationship between outcomes and AD-specific disease processes may be due to the limited observational time window of this cohort or other idiosyncratic effects of the current analyses (e.g., the imaging measures of atrophy used in this study may not have been sensitive enough to detect gray matter changes compared with other techniques, such as voxel-based morphometry). Third, the BLSA recruitment procedures favor healthy adults who have survived to old age. As such, study-specific inclusion criteria may influence the generalizability of results. Replication of the analyses in a population-based sample will strengthen the external validity of current findings.42 Fourth, our study lacked comprehensive assessment of host immune processes. As the reactivation of HHVs can be significantly influenced by the immune status of the host, biological characteristics and genetic variants that confer a host immune response that is permissive to HHV reactivation, rather than sHHV per se, may be a primary contributor to the adverse neurocognitive outcomes in this study. Finally, the current results may be biased due to residual confounding from unmeasured variables jointly associated with sHHV and outcomes of interest. Although analyses were adjusted for potential confounders, including demographic variables, APOEε4 status, and medical comorbidities, it is possible that unmeasured variables, subclinical disease, or features of the study design may account for some sHHV-outcome relationships.
Despite these limitations, this study indicates that sHHV among a population of community-dwelling, older adults is associated with steeper declines in white matter volume, particularly in the temporal lobe, and in attention, a cognitive domain that is particularly vulnerable to disruptions in white matter integrity. Furthermore, these results provide evidence for a link between sHHV and GFAP, a marker of reactive astrogliosis. These findings highlight the neurobiological correlates of herpetic infection in older adults and suggest potential pathways by which herpetic infection may influence dementia risk.
Acknowledgment
The authors thank the BLSA participants and staff for their participation and continued dedication.
Glossary
- AD
Alzheimer disease
- Aβ
β-amyloid
- BLSA
Baltimore Longitudinal Study of Aging
- CDT
clock-drawing test
- eGFR
estimated glomerular filtration rate
- GFAP
glial fibrillary acidic protein
- HHV
human herpes virus
- HSV
herpes simplex virus
- ICD-9
International Classification of Diseases, Ninth Revision
- IQR
interquartile range
- MCI
mild cognitive impairment
- MUSE
multiatlas region segmentation utilizing ensembles
- NfL
neurofilament light
- ROI
region of interest
- sHHV
symptomatic HHV
- VZV
varicella-zoster virus
Appendix. Authors
Study Funding
This study was supported by the National Institute on Aging (NIA/NIH) Intramural Research Program and in part by Grant No. RF1-AG054409 to C. Davatzikos.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2020;16(3):391-460. [Google Scholar]
- 2.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duggan MR, Torkzaban B, Ahooyi TM, Khalili K. Potential role for herpesviruses in Alzheimer's disease. J Alzheimers Dis. 2020;78(3):855-869. [DOI] [PubMed] [Google Scholar]
- 4.Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet. 1997;349(9047):241-244. [DOI] [PubMed] [Google Scholar]
- 5.Lindman KL, Hemmingsson E-S, Weidung B, et al. Herpesvirus infections, antiviral treatment, and the risk of dementia—a registry-based cohort study in Sweden. Alzheimers Dement. 2021;7(1):e12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnier C, Janbek J, Williams L, et al. Antiherpetic medication and incident dementia: observational cohort studies in four countries. Eur J Neurol. 2021;28(6):1840-1848. [DOI] [PubMed] [Google Scholar]
- 7.Bae S, Yun SC, Kim MC, et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: a population-based cohort study. Eur Arch Psychiatry Clin Neurosci. 2021;271(5):987-997. [DOI] [PubMed] [Google Scholar]
- 8.Readhead B, Haure-Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99(1):64-82.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng NS, Chung CH, Lin FH, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex virus infections—a nationwide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15(2):417-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letenneur L, Pérès K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS One. 2008;3(11):e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linard M, Baillet M, Letenneur L, et al. Herpes simplex virus, early neuroimaging markers and incidence of Alzheimer's disease. Transl Psychiatry. 2021;11(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allnutt MA, Johnson K, Bennett DA, et al. Human herpesvirus 6 detection in Alzheimer's disease cases and controls across multiple cohorts. Neuron. 2020;105(6):1027-1035.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren-Gash C, Forbes HJ, Williamson E, et al. Human herpesvirus infections and dementia or mild cognitive impairment: a systematic review and meta-analysis. Sci Rep. 2019;9(1):4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwok MK, Schooling CM. Herpes simplex virus and Alzheimer's disease: a Mendelian randomization study. Neurobiol Aging. 2021;99:101.e111-101.e113. [DOI] [PubMed] [Google Scholar]
- 15.Boukhvalova MS, Mortensen E, Mbaye A, Lopez D, Kastrukoff L, Blanco JCG. Herpes simplex virus 1 induces brain inflammation and multifocal demyelination in the cotton rat Sigmodon hispidus. J Virol. 2019;94:e01161-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bello-Morales R, Andreu S, López-Guerrero JA. The role of herpes simplex virus type 1 infection in demyelination of the central nervous system. Int J Mol Sci. 2020;21(14):5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin JR, Suzuki S, deF Webster H. Central nervous system demyelination in herpes simplex virus type 2 infection. In: Crescenzi GS, editor. A Multidisciplinary Approach to Myelin Diseases. Springer US, 1987:329-340. [Google Scholar]
- 18.Kennedy PG. Viral encephalitis: causes, differential diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75(suppl 1):i10-i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steel AJ, Eslick GD. Herpes viruses increase the risk of Alzheimer's disease: a meta-analysis. J Alzheimers Dis. 2015;47(2):351-364. [DOI] [PubMed] [Google Scholar]
- 20.Barnett EM, Jacobsen G, Evans G, Cassell M, Perlman S. Herpes simplex encephalitis in the temporal cortex and limbic system after trigeminal nerve inoculation. J Infect Dis. 1994;169(4):782-786. [DOI] [PubMed] [Google Scholar]
- 21.Shock NW. Normal Human Aging: The Baltimore Longitudinal Study of Aging. US Department of Health and Human Services, National Public Health Service, 1984. [Google Scholar]
- 22.Lovheim H, Gilthorpe J, Adolfsson R, Nilsson LG, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer's disease. Alzheimers Dement. 2015;11(6):593-599. [DOI] [PubMed] [Google Scholar]
- 23.Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(suppl 1):S111-S118. [DOI] [PubMed] [Google Scholar]
- 24.Olsson J, Kok E, Adolfsson R, Lövheim H, Elgh F. Herpes virus seroepidemiology in the adult Swedish population. Immun Ageing. 2017;14(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dasgupta G, Chentoufi AA, Kalantari M, et al. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol. 2012;86(8):4358-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68(13):1069-1073. [DOI] [PubMed] [Google Scholar]
- 27.Doll JR, Sawtell NM. Analysis of herpes simplex virus reactivation in explant reveals a method-dependent difference in measured timing of reactivation. J Virol. 2017;91(16):e00848-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doshi J, Erus G, Ou Y, et al. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graff-Radford J, Simino J, Kantarci K, et al. Neuroimaging correlates of cerebral microbleeds: the ARIC study (Atherosclerosis Risk in Communities). Stroke. 2017;48(11):2964-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varadaraj V, Munoz B, Deal JA, et al. Association of vision impairment with cognitive decline across multiple domains in older adults. JAMA Netw Open. 2021;4(7):e2117416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafer AT, Beason-Held L, An Y, et al. Default mode network connectivity and cognition in the aging brain: the effects of age, sex, and APOE genotype. Neurobiol Aging. 2021;104:10-23. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seliger SL, Wendell CR, Waldstein SR, Ferrucci L, Zonderman AB. Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. Am J Nephrol. 2015;41(4-5):305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease. Neurology. 2000;54:2072-2077. [DOI] [PubMed] [Google Scholar]
- 35.Mancuso R, Cabinio M, Agostini S, Baglio F, Clerici M. HSV-1-specific IgG(3) titers correlate with brain cortical thinning in individuals with mild cognitive impairment and Alzheimer's disease. Vaccines (Basel). 2020;8(2):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancuso R, Baglio F, Cabinio M, et al. Titers of herpes simplex virus type 1 antibodies positively correlate with grey matter volumes in Alzheimer's disease. J Alzheimers Dis. 2014;38(4):741-745. [DOI] [PubMed] [Google Scholar]
- 37.Atwi S, Metcalfe AWS, Robertson AD, Rezmovitz J, Anderson ND, MacIntosh BJ. Attention-related brain activation is altered in older adults with white matter hyperintensities using multi-echo fMRI. Front Neurosci. 2018;12:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedden T, Van Dijk KRA, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cereb Cortex. 2011;22(5):1038-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling RA, Guttmann CRG, Hohol MJ, et al. Regional magnetic resonance imaging lesion burden and cognitive function in multiple sclerosis—a longitudinal study. Arch Neurol. 2001;58(1):115-121. [DOI] [PubMed] [Google Scholar]
- 40.Fan Z, Brooks DJ, Okello A, Edison P. An early and late peak in microglial activation in Alzheimer's disease trajectory. Brain. 2017;140(3):792-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banack HR, Kaufman JS, Wactawski-Wende J, Troen BR, Stovitz SD. Investigating and remediating selection bias in geriatrics research: the selection bias toolkit. J Am Geriatr Soc. 2019;67(9):1970-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi N, Nagata T, Shinagawa S, et al. Increase in the IgG avidity index due to herpes simplex virus type 1 reactivation and its relationship with cognitive function in amnestic mild cognitive impairment and Alzheimer's disease. Biochem Biophys Res Commun. 2013;430(3):907-911. [DOI] [PubMed] [Google Scholar]
- 44.Féart C, Helmer C, Fleury H, et al. Association between IgM anti-herpes simplex virus and plasma amyloid-beta levels. PLoS One. 2011;6(12):e29480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopatko Lindman K, Weidung B, Olsson J, et al. Plasma amyloid-β in relation to antibodies against herpes simplex virus, cytomegalovirus, and Chlamydophila pneumoniae. J Alzheimers Dis Rep. 2021;5(1):229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyrberg T, Nilsson S, Blennow K, Zetterberg H, Grahn A. Serum and cerebrospinal fluid neurofilament light chain in patients with central nervous system infections caused by varicella-zoster virus. J Neurovirol. 2020;26(5):719-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grahn A, Hagberg L, Nilsson S, Blennow K, Zetterberg H, Studahl M. Cerebrospinal fluid biomarkers in patients with varicella-zoster virus CNS infections. J Neurol. 2013;260(7):1813-1821. [DOI] [PubMed] [Google Scholar]
- 48.Shinomoto M, Kasai T, Tatebe H, et al. Cerebral spinal fluid biomarker profiles in CNS infection associated with HSV and VZV mimic patterns in Alzheimer's disease. Transl Neurodegener. 2021;10(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedet AL, Milà-Alomà M, Vrillon A, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, et al. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer's disease. Brain. 2016;139(Pt 3):922-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article may be shared on request from qualified investigators for purposes of replicating procedures and findings.