Abstract
Background and Objectives
Brain lesions are a well-recognized etiology of dystonia. These cases are especially valuable because they offer causal insight into the neuroanatomical substrates of dystonia. To date, knowledge of lesion-induced dystonia comes mainly from isolated case reports or small case series, restricting broader description and analysis.
Methods
Cases of lesion-induced dystonia were first identified from a systematic review of published literature. Latent class analysis then investigated whether patients could be classified into subgroups based on lesion location and body regions affected by dystonia. Regression analyses subsequently investigated whether subgroup membership predicted clinical characteristics of dystonia.
Results
Three hundred fifty-nine published cases were included. Lesions causing dystonia occurred in heterogeneous locations, most commonly in the basal ganglia (46.2%), followed by the thalamus (28.1%), brainstem (22.6%), and white matter (21.2%). The most common form of lesion-induced dystonia was focal dystonia (53.2%), with the hand (49.9%) and arm (44.3%) most commonly affected. Of all cases, 86.6% reported co-occurring neurologic manifestations and 26.1% reported other movement disorders. Latent class analysis identified 3 distinct subgroups of patients: those with predominantly limb dystonias, which were associated with basal ganglia lesions; those with hand dystonia, associated with thalamic lesions; and those with predominantly cervical dystonia, associated with brainstem and cerebellar lesions. Regression demonstrated significant differences between these subgroups on a range of dystonia symptoms, including dystonic tremor, symptom latency, other movement disorders, and dystonia variability.
Discussion
Although dystonia can be induced by lesions to numerous brain regions, there are distinct relationships between lesion locations and dystonic body parts. This suggests that the affected brain networks are different between types of dystonia.
Dystonia is a neurologic disorder involving involuntary muscle contractions, leading to twisting or repetitive movements or abnormal postures.1 Although dystonia is characterized by these specific motor features, a cohesive pathophysiologic model underlying dystonia has yet to be established.2 Traditionally, symptoms have been ascribed to dysfunction of the basal ganglia,3,4 but several studies have now demonstrated the involvement of other key brain regions, such as the cerebellum, thalamus, and somatosensory cortex.5,6 These findings have led to suggestions of a “network” model,7,8 where dystonia might be caused by abnormalities of multiple brain regions. However, such brain regions have yet to be fully elucidated, and there may also be differences in the affected regions between dystonia types.9,10
Although the most dystonia cases are idiopathic,2 acquired dystonia cases due to focal brain lesions are uniquely valuable because they allow for causal links between the damaged brain region and the resultant symptoms.2,5 However, because lesion-induced dystonia is relatively rare, most lesion data come from case reports or case series describing a particular type of dystonia.2,11 To date, there are no studies investigating lesion locations across all types of dystonia. Nor are there formal statistical analyses investigating relationships between lesion locations and dystonia symptoms. As a result, it remains unknown what types of dystonia are most frequently caused by lesions, where these lesions are located, and whether lesions in certain brain regions are associated with certain types of dystonia. To this end, this study sought to collate all reported cases of lesion-induced dystonia, providing a description of clinical features and an in-depth statistical analysis of possible relationships between lesion location, dystonia type, and associated symptoms of dystonia, such as tremor, symptom latency, and co-occurring neurologic manifestations.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This project was deemed exempt from an ethical review by the Deakin University Human Research Ethics Committee because it involved only the use of preexisting, nonidentifiable, or reidentifiable data. The systematic search and reporting of data were conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. See eAppendix 1, links.lww.com/WNL/C258, for the completed PRISMA checklist.12
Case Selection
Patients with lesion-induced dystonia were identified from a systematic search of Pubmed and Embase in May 2020 using the combination of synonyms (and alternative spellings) of the following terms: dystonia, blepharospasm, meige, lesion, stroke, infarct, ischem*, hemorrhag*, tumor, and plaque. The search strategy was developed by D.C. and J.J. The exact search syntax is provided in eAppendix 2, links.lww.com/WNL/C258. Reference lists of included articles were searched for possible cases missed in the initial search.13 Inclusion criteria were a published report describing human patient/s with lesion-induced dystonia, including a neurologic examination where the authors of the report concluded that the dystonia was caused by a lesion of the CNS, and the report included a description of body parts affected. Several reports described torticollis caused by a lesion (e.g., Ref. 14) but did not give an explicit diagnosis of dystonia; therefore, these cases did not meet inclusion criteria. There were no restrictions on study design, including published conference abstracts (returned in Embase searches15), as long as cases were described in sufficient detail to satisfy the inclusion criteria. There were no restrictions as to the type of lesion, how many, or how diffuse. Atrophy was not considered a lesion. Given obvious limitations in patient re-examination based on published reports, we relied on reporting, diagnosis, and assessment of dystonia etiology by the primary authors of the included cases. Exclusion criteria were no lesion (e.g., atrophy16), lesions outside of the CNS (e.g., Ref. 17), and inadequate description of the case to establish the inclusion criterion (e.g., Ref. 18). As the emergence of dystonia may be delayed by months or years after a brain insult,2 we did not apply a strict time limit for the onset of symptoms postlesion.5
Case Description
Data from published reports were independently extracted by D.C. and J.P., and discrepancies were resolved by J.J. Based on the definitions provided in the latest consensus statement on the phenomenology and classification of dystonia,1 we grouped patients' symptoms into the following categories: body distribution (focal, segmental, multifocal, generalized, or hemidystonia), variability (e.g., persistent, action-specific, diurnal, or paroxysmal), associated features (other movement disorders and co-occurring neurologic manifestations), and alleviating maneuvers (sensory tricks). We also classified dystonic tremor (tremor occurring within a dystonic body part) and tremor associated with dystonia (tremor in a nondystonic body part, referred to as “associated tremor” henceforth), according to the recent consensus statement on the classification of tremors.19 If the published report did not use the specific terms to describe symptoms, categorization was performed based on definitions within the aforementioned consensus statements.1,19 For example, if the authors did not explicitly use the term “generalized dystonia,” but described the body parts affected (e.g., Ref. 20), this could be classified based on the definition by Albanese et al.1 (“The trunk and at least 2 other sites are involved”).
There were many co-occurring neurologic manifestations described in published reports. Although somewhat subjective, we created 4 symptom categories to investigate these in greater detail. The definitions of these categories were (1) corticospinal (motor weakness, Babinski sign, abnormal tendon reflexes, and spasticity), (2) somatosensory (sensory deficits, such as hypoesthesia), (3) posterior fossa (cerebellar, brainstem, and cranial nerve manifestations, such as ataxia or abnormal eye movements), and (4) other (e.g., impaired cognition, abnormal behavior, visual field defects, or any other neurologic manifestation that did not belong to the other categories). See eAppendix 3, links.lww.com/WNL/C258, for categorizations in greater detail. These broad categories were chosen because they covered the predominant manifestations associated with dystonia, and the number of categories was not too great that it would dilute statistical power and preclude quantitative analysis. This was performed by J.P., D.C., and J.J. using judgment on analysis of each case description (rather than the original report the authors having to explicitly use these terms).
In addition, the age, sex, lesion type, lesion and symptom lateralization, body part affected (cervical, blepharospasm, face [excluding blepharospasm], arm, hand, trunk, leg, and foot), lesion location in CNS, symptom latency, and lesion etiology (infarct, tumor, hemorrhage, inflammatory, or other) of each case were extracted and tabulated (eTable 1, links.lww.com/WNL/C258). Inflammatory lesions were defined as those caused by multiple sclerosis, clinically isolated syndrome, neuromyelitis optica spectrum disorder, Behçet disease, or those with unknown etiology with an inflammatory CSF finding or MRI findings which are described as inflammatory, while other lesions were defined as those with, e.g., tuberculoma, subacute combined degeneration, or unknown etiology.
To enable analyses of lesion location, lesions were recorded as occurring within 11 regions of the CNS: basal ganglia, thalamus, brainstem, parietal lobe, white matter, frontal lobe, cerebellum, spinal cord, occipital lobe, temporal lobe, and ventricles. The lesion location was recorded based on the description by the authors of the original published reports. In cases where a figure of the lesion was also supplied, this was evaluated, and additional brain regions were recorded if they were obviously affected by a lesion. This occurred in 5 cases. Although further delineation of lesion locations would have been valuable (e.g., into smaller parts of the brain, such as the globus pallidus, sensory cortex, or into more specific regions of the white matter), this level of detail was not available in all cases and would also have created many more groups/predictors and diluted the statistical power to demonstrate meaningful relationships between lesion location and dystonia types.
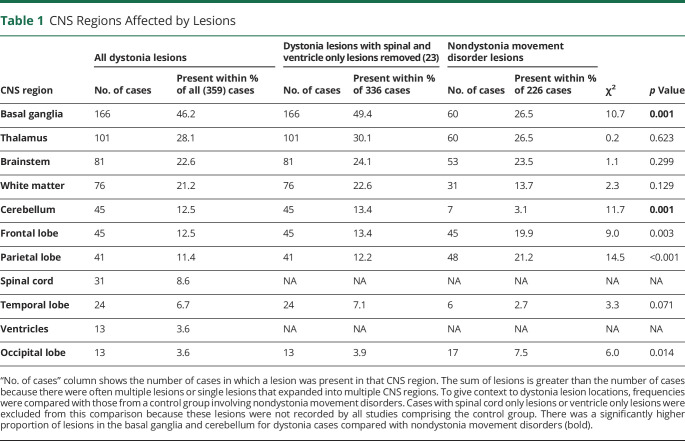
To provide context to lesion locations in dystonia, we also tabulated the frequency of lesions found within each brain region for a cohort of nondystonia movement disorders (eTable 2, links.lww.com/WNL/C258). To do this, we recorded 327 lesion locations (from 226 cases, some cases had multiple lesions) from 8 other available movement disorders. These datasets were derived from “lesion network mapping” studies by the authors of this paper, which involves tracing lesions based on figures in published reports.21 These lesions were combined to create a “nondystonia movement disorders” control group. Disorders included parkinsonism (33 lesion locations), alien limb syndrome (92), akinetic mutism (55), hemichorea-hemiballismus (58), asterixis (30), freezing of gait (15), and Holmes tremor (44).22-27 The frequency of lesions in each brain location was compared between groups (dystonia vs nondystonia movement disorders) using a χ2 test for independence.28
Latent Class Analysis
Latent class analysis (LCA) was then performed on aggregated data. LCA is a mixture modeling statistical technique that can be used to identify classes, or subgroups, of patients based on the clustering of common characteristics or symptoms.29 In this study, we used LCA to identify classes of patients based on their lesion location and where in the body, dystonia symptoms occurred. Two LCAs were run, including (1) lesion locations and body distribution affected (focal, segmental, multifocal, generalized, or hemidystonia) and (2) lesion locations and body parts affected (cervical, blepharospasm, face [excluding blepharospasm], arm, hand, trunk, leg, and foot). The second model was run to investigate whether greater anatomical specificity may allow better grouping of patients into distinct symptom classes. Patients often had lesions in multiple brain locations or dystonia in multiple body parts. In these cases, all lesion locations/body parts were included in the LCA and associated with the other factors. The LCA model fit was evaluated using the Akaike information criterion (AIC), Bayesian information criterion (BIC), entropy, and the Lo-Mendell-Rubin (LMR) and Vuong-LMR likelihood ratio tests.30 Smaller values of AIC and BIC, and larger values of entropy, indicate a better model fit.30 LCAs were run in Mplus software, version 8.4,31 and figures were made in RStudio version 1.3.1093 (PBC: Boston, MA).
Class Membership Predicting Dystonia Symptoms
The LCA of body part provided the best fit (compared with the LCA of body distribution—see results), classifying patients into 3 distinct classes/subgroups. Therefore, we then performed a series of logistic regression analyses to investigate whether class membership could predict case characteristics/symptoms classified as per recent guidelines.1 (see above). These characteristics/symptoms included patient sex, symptom latency, lesion type, dystonia variability, tremor (dystonic and associated), other movement disorders, co-occurring neurologic factors, lesion lateralization, and presence of a sensory trick. We refrained from categorizing symptom laterality for cervical dystonia cases given the complexity of neck muscle anatomy.32 Therefore, symptom lateralization was excluded from regression analysis because the results would be biased by the number of cervical dystonia cases within each class. The dependent variable was the percentage of cases within each class that reported the characteristic/symptom, with regression comparing these percentages between classes. For example, 21% of cases in class 3 possessed a tumor, which was a significantly higher percentage than class 1 (7%). Given that the “hand” and “limb” dystonia classes both contained a high percentage of hand dystonia cases, we ran a specific logistic regression analysis comparing the number of action-specific hand dystonia cases between these classes. Finally, we investigated possible differences between the classes in patient age (continuous variable) using linear regression. All regression analyses were performed using StataSE software (version 15.1, StataCorp LLC: College Station, TX).
Data Availability
Data used for analyses were extracted from published reports and are available in eTable 1, links.lww.com/WNL/C258. Analytic code, or any other deidentified data not published within this article is available to any qualified investigator on request by email to the corresponding author.
Results
Case Description
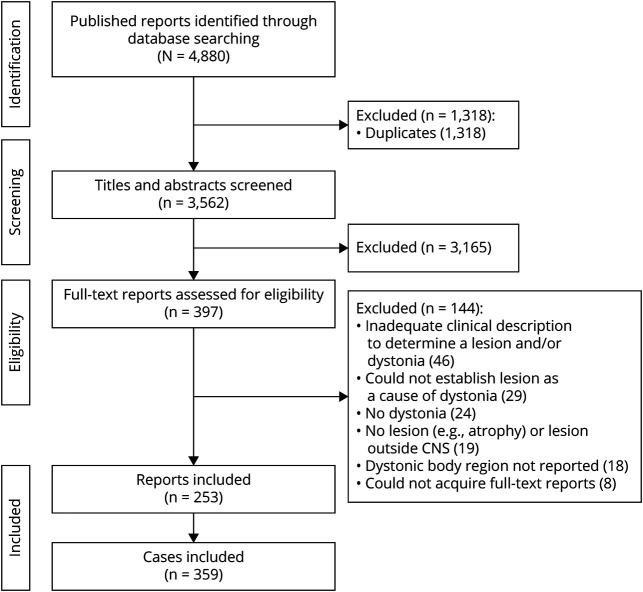
In total, 359 cases of lesion-induced dystonia, extracted from 253 published reports, were included for analysis (Figure 1). Most of these publications were single-case studies; however, lesion-induced dystonia was also reported in other study designs, such as case series (e.g., Ref. 33) and case-control studies (e.g., Ref. 34). A detailed description of each case is presented in eTable 1, links.lww.com/WNL/C258.
Figure 1. PRISMA Flowchart.
Systematic search process. Flowchart adapted from the work of Liberati et al. (2009). There were more cases than published reports because some reports contained multiple cases.
Cases consisted of 171 men and 176 women (12 cases NR), with a mean age of 40.0 years; SD = 20.9. Infarct (142 cases) was the most common cause of lesions, while there were 50 cases with hemorrhage, 46 with a tumor, and 40 with inflammatory lesions. 81 cases had other lesions, such as tuberculoma,35 osmotic demyelination,36 and striatal necrosis.37 One hundred six lesions were localized to the right hemisphere, 98 to the left, and there were 152 bilateral lesions (3 NR). Lesions causing dystonia occurred in a range of CNS regions. The most common region affected was the basal ganglia (46.2%, Table 1). Two hundred eighty-two cases (78.6%) had a lesion in either basal ganglia, thalamus, or brainstem. Three hundred thirty-five cases (93.3%) had a lesion involving any subcortical brain regions (i.e., 24 cases had lesions that only affected the cortex). Table 1 shows a significantly higher proportion of lesions in the basal ganglia and cerebellum for dystonia cases compared with the nondystonia movement disorder cohort.
Table 1.
CNS Regions Affected by Lesions
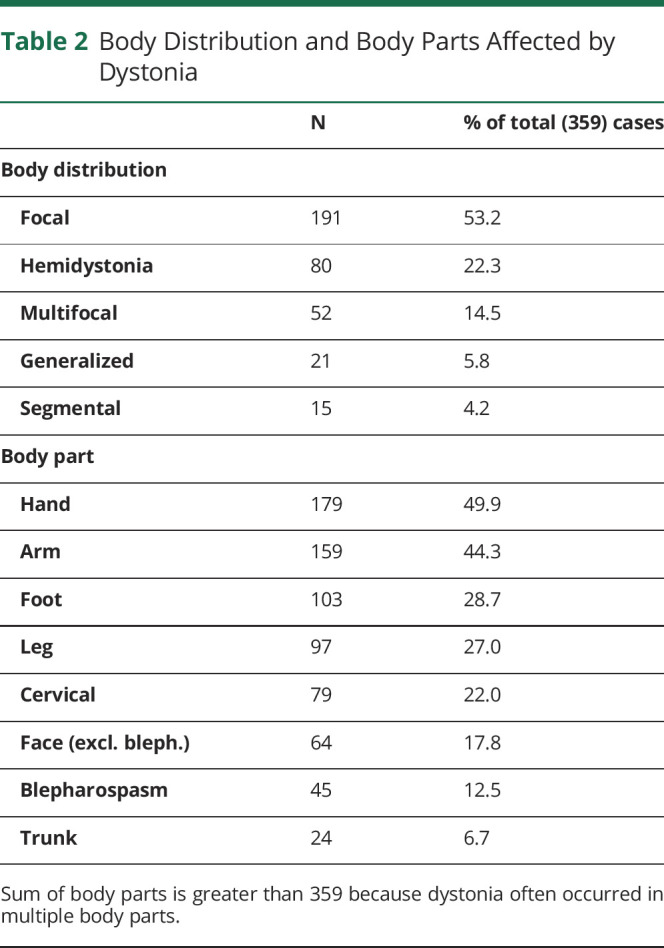
Dystonia cases were most often focal, while the hand was the most common body part affected by dystonia (Table 2). As described above, symptom laterality was not categorized for cervical cases (79) given the complexity of neck muscle anatomy.32 Of the remaining cases without cervical dystonia (280), dystonia symptoms were localized to the left side of the body in 102 cases (36.4%), bilateral in 99 cases (35.4%), and to the right in 75 cases (26.8%) (NR = 4). 112 of these 280 had bilateral lesions, 69 of whom involved bilateral dystonia, 25 had left side dystonia only, and 17 had right side dystonia only (NR = 1). Thirty-two of the 280 cases involved “paradoxical lateralization” involving dystonia ipsilateral (including bilateral dystonia) to unilateral lesion/s in the cerebrum or brainstem, or dystonia contralateral to unilateral lesion/s in the cerebellum. Fourteen of these 32 cases (43.8%) were infarcts, 8 were hemorrhages (25%), and 7 were other lesions (21.8%). Twelve of the 32 (37.5%) cases involved a thalamus lesion, 9 (28.1%) involved a brainstem lesion, and 6 (18.8%) involved a basal ganglia lesion.
Table 2.
Body Distribution and Body Parts Affected by Dystonia
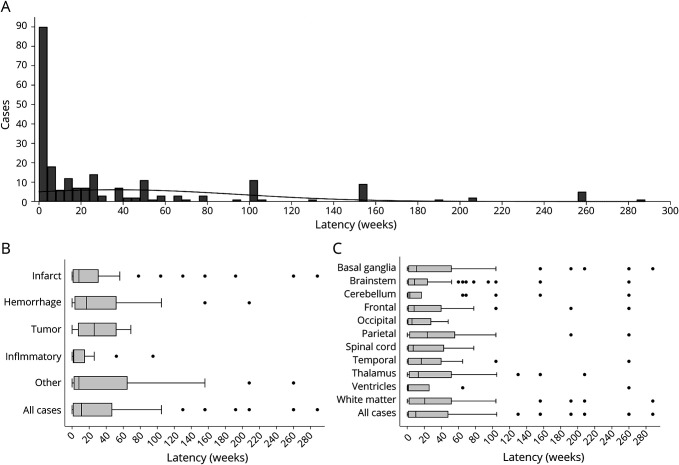
As per the definition of dystonia “variability,”1 dystonia was most often persistent (262 of 359 cases, 73.0%), followed by paroxysmal (53, 14.8%), action-specific (21, 5.9%), and diurnal (10, 2.8%) (NR = 13). 222 cases provided the latency of onset of dystonia symptoms after the occurrence of the lesion. The median latency was 11 weeks, while 61 cases (27.4%) had a latency of ≤1 week, 43 cases (19.3%) had a latency of >1 year, and the maximum reported latency was approximately 5.5 years34 (Figure 2A). There was large variability in symptom latency within individual lesion etiologies, but symptom latency appeared to be shortest for inflammatory lesions (Figure 2B). However, this may also be driven by delays in identifying the lesion. For example, patients are likely to present for medical care urgently for symptoms caused by a stroke, but other lesions, such as multiple sclerosis lesions, may be detected after a longer delay.
Figure 2. Dystonia Symptom Latency.
Histogram (A) shows the number of weeks after the lesion that dystonia symptoms first arose for all cases with latency data. Each bar represents 4 weeks. Figure B shows latency data by lesion etiology. Figure C shows latency by lesion location. Boxes represent 25th, 50th, and 75th percentiles, and dots show values falling outside whiskers (1.5 × interquartile range).
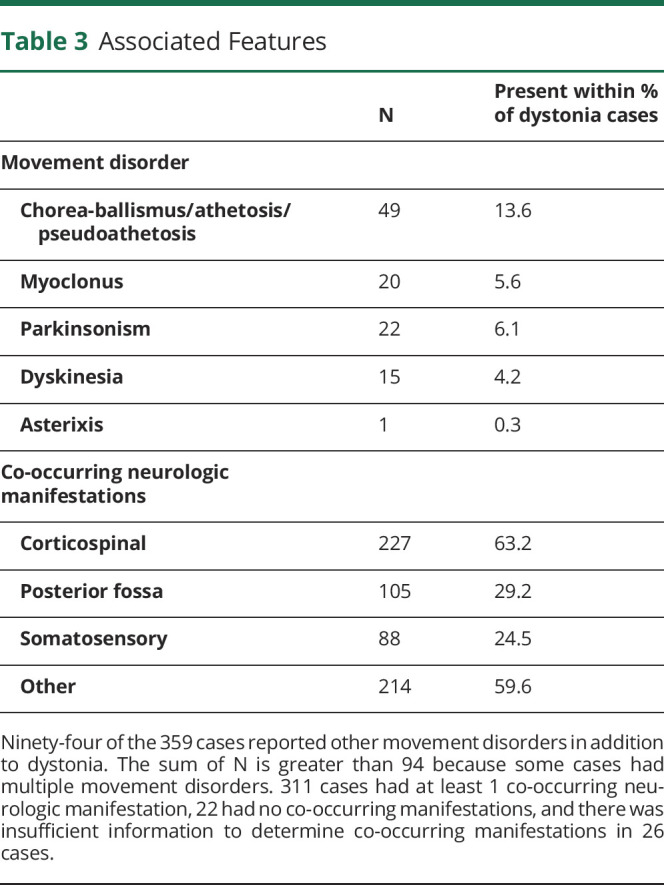
Regarding associated features,1 94 cases (26.1%) reported an additional movement disorder in addition to dystonia (i.e., “combined” dystonia) (Table 3). Co-occurring neurologic manifestations were reported in 311 cases (86.6%) (NR = 26) (Table 3). This left 22 cases (6.1%) without co-occurring neurologic manifestations (i.e., dystonia only). Of these, basal ganglia (12 cases), white matter (6), and thalamus lesions (5) were most common, and most of these patients had focal dystonia (14 cases). Hand dystonia was the most commonly affected body part (8 of the 22 cases), followed by cervical (7) and leg dystonia (6).
Table 3.
Associated Features
Tremor was present in 74 cases (20.6%) (NR = 8), with 59 (16.4%) of these cases involving tremor to a dystonic body part (dystonic tremor) and 30 (8.4%) cases involving tremor to non-dystonic body parts (associated tremor). This percentage is lower than tremor (any type) rates previously reported in idiopathic dystonia (ranging between 27% and 53%38-40). However, this may also be due to underreporting of tremor in the published reports on which our prevalence rates rely. Symptom relief by sensory tricks was reported in 19 cases (5.3%), 12 of whom involved cervical dystonia and 5 involved face dystonia.
Latent Class Analysis—Lesion Location and Dystonic Body Regions
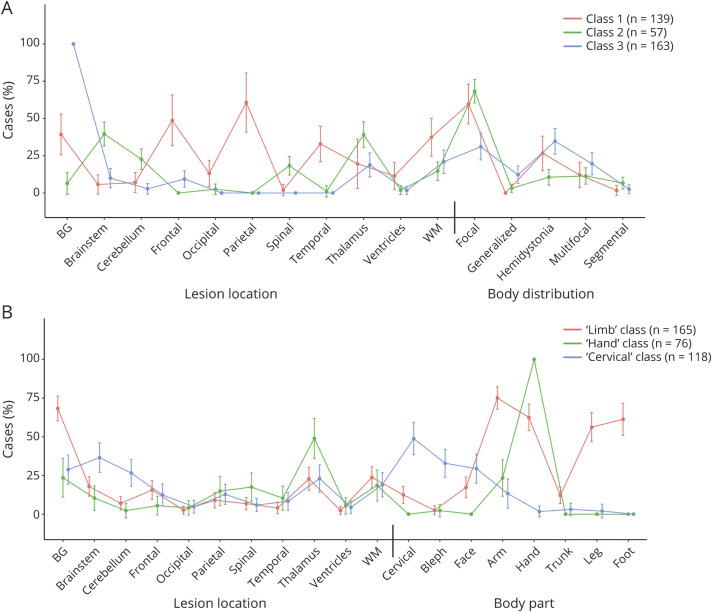
We first investigated whether cases could be classified based on lesion location and dystonia body distribution.1 An LCA with 3 classes provided the best fit (see eAppendix 4, links.lww.com/WNL/C258, for class selection process). However, this model showed high overlap of the 3 classes within the body distribution categories (Figure 3A), demonstrating that the LCA did not classify patients into distinct body distribution subgroups.
Figure 3. Lesion Location and Dystonic Body Region Latent Class Analyses (LCAs).
LCA investigated whether cases could be classified based on lesion location and body distribution (A), as defined by Albanese et al. (2013), or body part (B). There was a relatively high overlap of the classes within the body distribution categories (A), suggesting a limited ability of the model to classify patients into distinct subgroups. The body part LCA demonstrated clearer separation of classes (B), showing that a high percentage of cases fell within one of the 3 dystonia subgroups: (1) limb, (2) hand, or (3) cervical dystonia. Each of these subgroups showed specific relationships to the brain regions affected by lesions. For example, 75% of the limb class had arm dystonia and 68% of these cases had a BG lesion. Significant differences between classes within each category can be seen by nonoverlapping confidence intervals. BG = basal ganglia; WM = white matter.
We then investigated whether classifying cases based on individual body parts affected by dystonia provided a better solution. An LCA with 3 classes provided the best fit (eAppendix 4, links.lww.com/WNL/C258). In contrast to the body distribution LCA, this model identified subgroups with distinct lesion locations and dystonic body parts. Class 1 (n = 165) was characterized by a high percentage of basal ganglia lesions (68%), coupled with a high percentage of arm (75%), hand (63%), leg (56%), and foot (61%) dystonia cases. Class 2 (n = 76) was characterized by a relatively high percentage of thalamus lesions (49%), coupled with a high percentage of hand dystonia cases (100%). Class 3 (n = 118) was characterized by a relatively high percentage of brainstem (37%) and cerebellum (27%) lesions (i.e., a significantly higher number of lesions in these locations than the other 2 classes) coupled with a relatively high percentage of cervical dystonia (49%) and blepharospasm (33%) cases. Thus, henceforth, we operationalize these classes as “limb,” “hand,” and “cervical” dystonia classes, respectively (however, as per the percentages above, it is important to note that not all of these cases in these groups had this type of dystonia). This model shows specific relationships between the lesion locations and the body parts affected by dystonia. See eTable 3, links.lww.com/WNL/C258, for percentages and confidence intervals. The results were very similar when controlling for lesion type in both LCAs (eFigure 1, links.lww.com/WNL/C258).
Class Membership and Dystonia Symptoms
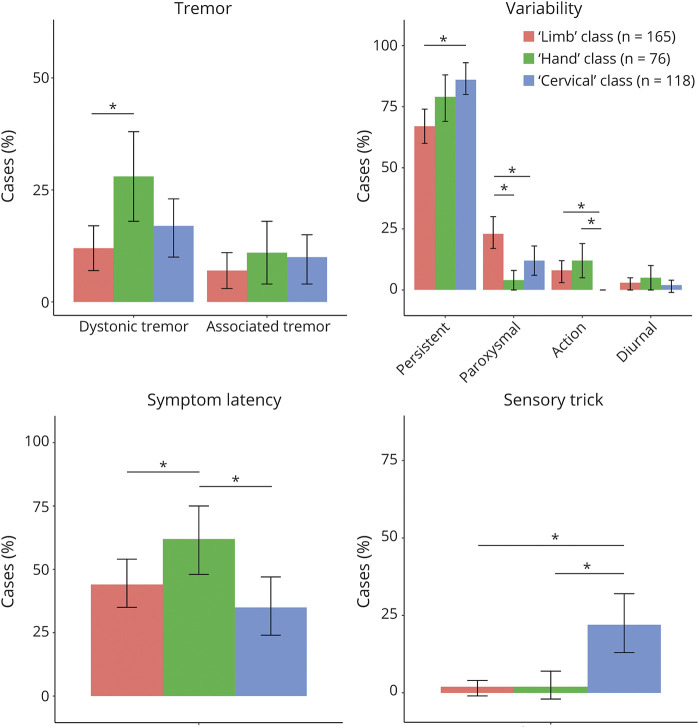
Logistic and linear regression was then used to investigate whether class membership ("limb," "cervical," and "hand dystonia" classes) (independent variables) could predict other dystonia symptoms or case/lesion characteristics (dependent variables). Differences between the 3 classes in other dystonia symptoms are shown in Figure 4. In relation to case and lesion characteristics, there were significant differences in mean age (cervical class = 46 years, hand = 44 years, limb = 33 years), the presence of other movement disorders (present in 44% of hand class cases, 27% of limb cases, and 15% of cervical cases), co-occurring neurologic manifestations (somatosensory manifestations present in 54% of hand cases, 21% of limb cases, and 15% of cervical cases; posterior fossa manifestations present in 42% of cervical cases, 31% of hand cases, and 25% of limb cases; and other manifestations present in 75% of cervical cases, 64% of limb cases, and 49% of hand cases), lesion type (tumors present in 21% of cervical cases, 12% of hand cases, and 7% of limb cases, and other lesions present in 32% of limb cases, 17% of cervical cases, and 12% of hand cases), and lesion lateralization (right hemisphere lesions present in 39% of hand cases, 30% of limb cases, and 23% of cervical cases, and bilateral lesions present in 46% of cervical cases, 45% of limb cases, and 32% of hand cases). p-values for all pairwise comparisons are provided in eTable 4, links.lww.com/WNL/C258.
Figure 4. Class Membership and Dystonia Symptoms.
Logistic and linear regression was used to investigate whether class membership (limb, hand, and cervical dystonia classes) could predict dystonia symptoms or case characteristics. Symptom latency panel shows the percentage of cases above the median latency (11 week). * = p < 0.05.
Additional analyses showed that there was no difference in the number of hand dystonia cases that were action-specific in the hand dystonia class and the limb dystonia class (p = 0.832). Finally, of the 179 cases of hand dystonia (overall, as distinct from the hand dystonia class), 25 (13.9%) had action-specific hand dystonia. In this study, the percentage of patients with thalamic lesions did not differ between patients with action-specific (7/25, 28.0%) hand dystonia and those without action-specific hand dystonia (53/154, 33.4%), χ2 = 0.39; p = 0.529.
Discussion
This study provides a comprehensive description and analysis of previously published cases of lesion-induced dystonia. Lesions causing dystonia occurred in numerous brain regions. The basal ganglia was the most common lesion location, yet the majority of lesions were still located outside the basal ganglia. LCA demonstrated distinct subgroups based on lesion location and dystonic body part, and significant differences between these subgroups in dystonia symptoms, such as the presence of a sensory trick. These findings suggest that while damage to several brain regions can cause dystonia, there are specific relationships between lesion locations and dystonic body parts.
Lesions were found across many regions of the CNS, including the basal ganglia, cerebellum, brainstem, white matter, spinal cord, and cortex. This finding seems to disagree with the traditional view of dystonia being solely a disorder of the basal ganglia.41 However, while dystonia can be caused by damage to various brain regions, most cases seem to be induced by lesions to a smaller number of key structures, of which the basal ganglia was the most commonly affected. In addition, there were a significantly higher number of basal ganglia and cerebellum lesions in the dystonia cases compared with the “nondystonia movement disorders” cases. This suggests a unique role for these 2 structures in dystonia, in agreement with prior hypotheses of the neurobiological mechanisms of dystonia.7,42 However, these findings do not discount the role of other regions in dystonia, but they do suggest that lesions in other brain regions are more likely to also be associated with nondystonia movement disorders.
Given that CNS lesions can cause symptoms that are nearly identical to those observed in idiopathic dystonia,2 the present findings raise a number of possibilities about the neuropathologic mechanisms underlying dystonia. First, some authors have suggested a “motor network” model, involving a broad brain network with key nodes in the basal ganglia, cerebellum, thalamus, brainstem, and cortex. Based on this model, dystonia may be caused by dysfunction of one, or multiple, nodes in the network, or the connections between them.8,43 This model could explain the observation that lesions can occur in various brain regions, which can then modulate neural activity of the broader network. However, it is also possible that there is not one common motor network for dystonia, but that there may be several brain networks that can each produce dystonia.8 If this theory were true, our findings would suggest a cervical dystonia network centered on the brainstem and cerebellum, with less involvement of the thalamus and basal ganglia. In contrast, there may be a limb dystonia network with the key node/s located in the basal ganglia, with less brainstem and cerebellar involvement. Although these may involve distinct brain networks, it is possible that their disruption could trigger common pathologic mechanisms leading to dystonia. However, while the clinical phenotypes of lesion-induced and idiopathic dystonia are similar, it is not known if the underlying mechanisms are the same. A recent study demonstrated that networks affected by lesions causing cervical dystonia were also abnormal in idiopathic dystonia, suggesting the shared neural substrate across etiologies.5 Neuroimaging studies in idiopathic dystonias have also shown abnormalities in multiple brain regions that are also commonly affected by lesions causing dystonia.44,45 However, the results from these neuroimaging studies are heterogeneous, and direct comparisons between different types of dystonia are scarce, precluding definitive conclusions about this issue.
This study performed formal quantitative analysis to demonstrate specific relationships between lesion locations and the body parts affected by dystonia. This largely agrees with previous case series on lesion-induced dystonia.2,11,43 Liuzzi et al.11 showed that upper limb dystonias had a higher number of thalamic (39) and basal ganglia (17) lesions in comparison with brainstem (4) and cerebellar (1) lesions. Furthermore, LeDoux et al.2 showed that of 25 cervical dystonia cases, only 7 cases had basal ganglia (6) or thalamus (1) lesions, while the remaining cases involved lesions of the cerebellum, brainstem, or spinal cord (and frontal lobe—1 lesion). In addition, we recently demonstrated that all 25 lesion locations causing cervical dystonia were functionally connected to the cerebellum.5 However, lesion connectivity has yet to be investigated in other types of dystonia, so these could also demonstrate similar patterns of connectivity.
Interestingly, LCA was less able to classify patients into specific body distribution categories. This demonstrates that lesion locations were more correlated with the individual body parts affected than the pattern of body parts affected. Therefore, although different body distributions (e.g., focal and segmental) could involve damage to similar brain regions, this seems to be more dependent on the individual body parts involved between these cases, rather than the body distribution categories that they are assigned. These findings raise the question as to whether our body part classifications (limb, cervical, and hand) could be useful in clinical practice. For instance, our data-driven approach demonstrated distinct neuropathologic mechanisms between the subgroups that predicted differences in dystonia symptoms. However, it is important to note that these body part subgroups were defined only on their relationship to lesion location, rather than on a holistic evaluation of the clinical characteristics of patients. Body distribution categories are defined on the study of clinical characteristics by the most recent consensus statement1 and, therefore, should continue to serve as the most useful resource in classifying individual dystonia patients.
A number of limitations should be acknowledged. First, although we conducted a systematic search, we cannot exclude publication bias, given that lesions in CNS locations previously linked to dystonia may be more likely to be reported.5 Second, rather than direct observation, we relied on the judgment of the original publication authors in the reporting, diagnosis, and assessment of dystonia etiology. It is possible that relevant information regarding patient symptoms was not fully reported in some studies. Next, it is difficult to prove a causal relationship between a lesion and a clinical disorder, especially given then long symptom latency associated with dystonia.8,46 Therefore, it is possible that the causal link from the lesion to dystonia in some of the included cases may not be correct, adding noise to our analyses. Furthermore, our cases often involved multiple lesions; thus, it is unclear whether dystonia was caused by one lesion in particular or a combination of lesions. Next, for our “control” group of nondystonia movement disorders, this cohort consisted of only cases that were suitable for “lesion network mapping”21 (i.e., included a drawable image of the lesion). Thus, we cannot rule out bias in the types of cases that were reported in such a manner in these primary studies and those where only a clinical description was given (as with some cases in the dystonia cohort). Finally, we grouped brain lesions into categories, such as the basal ganglia and brainstem, to allow for statistical power in our analyses and provide clinically useful lesion location information. However, it is possible that lesions to specific substructures may influence dystonia manifestation in different ways, but this was beyond the scope of this study.
This is the first study of lesion-induced dystonia to systematically collate and analyze cases across all types of dystonia. We have identified 3 specific dystonia subgroups based on the lesion location and the affected body part, and demonstrated differences between these subgroups in a range of dystonia symptoms, such as dystonic tremor, symptom latency, and other movement disorders. These findings provide clues as to the brain regions driving dystonia symptoms and suggest that these regions may be different between subtypes of patients.
Acknowledgment
The authors thank Louisa Sher from Deakin University Library for checking our search terms and strategy.
Glossary
- AIC
Akaike information criterion
- BIC
Bayesian information criterion
- LCA
latent class analysis
- LMR
Lo-Mendell-Rubin
Appendix. Authors
Footnotes
Editorial, page 777
CME Course: NPub.org/cmelist
Study Funding
H.A. Jinnah was supported by grants from the US government including NIH/NIHDS NS065701, NS116025, NS119831, and NIH/NCATS TR001456; M.D. Fox was supported by the Sidney R. Baer Foundation, the Nancy Lurie Marks Foundation, the Mather's Foundation, the Ellison/Baszucki Foundation, the Kaye Family Research Endowment, and NIH grants R21 MH126271, R56 AG069086, R01MH113929, R01MH115949, and R01AG060987; J. Pullinen was supported by The Finnish Parkinson Foundation; J. Joutsa was supported by the Academy of Finland (295580), the Instrumentarium Research Foundation, the Finnish Medical Foundation, The Finnish Foundation for Alcohol Studies, Turku University Hospital (ERVA funding), was supported by a private donation to the University of Turku, and has received a lecturer honorarium from Lundbeck.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeDoux MS, Brady KA. Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord. 2003;18(1):60-69. [DOI] [PubMed] [Google Scholar]
- 3.Galardi G, Perani D, Grassi F, et al. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand. 1996;94(3):172-176. [DOI] [PubMed] [Google Scholar]
- 4.Naumann M, Pirker W, Reiners K, Lange KW, Becker G, Brücke T. Imaging the pre-and postsynaptic side of striatal dopaminergic synapses in idiopathic cervical dystonia: a SPECT STUDY Using [123I] epidepride and [123I] β-CIT. Mov Disord. 1998;13(2):319-323. [DOI] [PubMed] [Google Scholar]
- 5.Corp DT, Joutsa J, Darby RR, et al. Network localization of cervical dystonia based on causal brain lesions. Brain. 2019;142(6):1660-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opavský R, Hluštík P, Otruba P, Kaňovský P. Somatosensory cortical activation in cervical dystonia and its modulation with botulinum toxin: an fMRI study. Int J Neurosci. 2012;122(1):45-52. [DOI] [PubMed] [Google Scholar]
- 7.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131(pt 9):2499-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jinnah HA, Neychev V, Hess EJ. The anatomical basis for dystonia: the motor network model. Tremor Other Hyperkinet Mov (N Y). 2017;7:506-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morishita T, Foote KD, Haq IU, Zeilman P, Jacobson CE, Okun MS. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor. Stereotact Funct Neurosurg. 2010;88(2):98-104. [DOI] [PubMed] [Google Scholar]
- 10.Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42(2):185-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liuzzi D, Gigante AF, Leo A, Defazio G. The anatomical basis of upper limb dystonia: lesson from secondary cases. Neurol Sci. 2016;37(9):1393-1398. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kombogiorgas D, Hussain I, Sgouros S. Atlanto-axial rotatory fixation caused by spontaneous intracerebral haemorrhage in a child. Childs Nerv Syst. 2006;22(9):1182-1186. doi: 10.1007/s00381-006-0053-3. [DOI] [PubMed] [Google Scholar]
- 15.EMBASE content: list of conferences covered in Embase. Accessed January 31, 2022. elsevier.com/solutions/embase-biomedical-research/embase-coverage-and-content.
- 16.Barontini F, Marconi GP, Arnetoli G. Sporadic multi-system atrophy with early onset and rapid fatal outcome (atypical O.P.C.A.?). Case report. Riv Patol Nerv Ment. 1983;104(6):243-254. [PubMed] [Google Scholar]
- 17.Scherokman B, Husain F, Cuetter A, Jabbari B, Maniglia E. Peripheral dystonia. Arch Neurol. 1986;43(8):830-832. [DOI] [PubMed] [Google Scholar]
- 18.Debenedictis CN, Allen JC, Kodsi SR. Brainstem tumor presenting with tearing, photophobia, and torticollis. J AAPOS. 2010;14(4):369-370. doi: 10.1016/j.jaapos.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia KP, Bain P, Bajaj N, et al. , Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus statement on the classification of tremors. From the task force on tremor of the international Parkinson and movement disorder society. Mov Disord. 2018;33(1):75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salerno SM, Kurlan R, Joy SE, Shoulson I. Dystonia in central pontine myelinolysis without evidence of extrapontine myelinolysis. J Neurol Neurosurg Psychiatry. 1993;56(11):1221-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379(23):2237-2245. doi: 10.1056/NEJMra1706158. [DOI] [PubMed] [Google Scholar]
- 22.Joutsa J, Horn A, Hsu J, Fox MD. Localizing Parkinsonism based on focal brain lesions. Brain. 2018;141(8):2445-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci U S A. 2018;115(42):10792-10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017;81(1):129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joutsa J, Shih LC, Fox MD. Mapping Holmes' tremor circuit using the human brain connectome. Ann Neurol. 2019;86(6):812-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology. 2016;86(23):2187-2195. doi: 10.1212/wnl.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JS. Asterixis after unilateral stroke: lesion location of 30 patients. Neurology. 2001;56(4):533-536. [DOI] [PubMed] [Google Scholar]
- 28.McHugh ML. The chi-square test of independence. Biochemia Med. 2013;23(2):143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conley S. Symptom cluster research with biomarkers and genetics using latent class analysis. West J Nurs Res. 2017;39(12):1639-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berlin KS, Williams NA, Parra GR. An introduction to latent variable mixture modeling (part 1): overview and cross-sectional latent class and latent profile analyses. J Pediatr Psychol. 2014;39(2):174-187. [DOI] [PubMed] [Google Scholar]
- 31.Muthén LK, Muthén B. Mplus User's Guide: Statistical Analysis With Latent Variables, User's Guide. Muthén & Muthén; 2017. [Google Scholar]
- 32.Krauss JK. Deep brain stimulation for cervical dystonia. J Neurol Neurosurg Psychiatry. 2003;74(11):1598-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batla A, Sanchez MC, Erro R, et al. The role of cerebellum in patients with late onset cervical/segmental dystonia? Evidence from the clinic. Parkinsonism Relat Disord. 2015;21(11):1317-1322. doi: 10.1016/j.parkreldis.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Trompetto C, Avanzino L, Marinelli L, et al. Corticospinal excitability in patients with secondary dystonia due to focal lesions of the basal ganglia and thalamus. Clin Neurophysiol. 2012;123(4):808-814. doi: 10.1016/j.clinph.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Alarcón F, Tolosa E, Muñoz E. Focal limb dystonia in a patient with a cerebellar mass. Arch Neurol. 2001;58(7):1125-1127. [DOI] [PubMed] [Google Scholar]
- 36.Chuang YC, Chang CS, Hsu SP, Lin TK, Lui CC. Osmotic demyelination syndrome with two-phase movement disorders: case report. Changgeng Yi Xue Za Zhi. 1998;21(4):526-530. [PubMed] [Google Scholar]
- 37.Kawano H, Takeuchi Y, Misawa A, Sawada T, Imahori Y. Putaminal necrosis presenting with hemidystonia. Pediatr Neurol. 2000;22(3):222-224. [DOI] [PubMed] [Google Scholar]
- 38.Molho ES, Feustel PJ, Factor SA. Clinical comparison of tardive and idiopathic cervical dystonia. Mov Disord. 1998;13(3):486-489. [DOI] [PubMed] [Google Scholar]
- 39.Jankovic J, Ford J. Blepharospasm and orofacial-cervical dystonia: clinical and pharmacological findings in 100 patients. Ann Neurol. 1983;13(4):402-411. [DOI] [PubMed] [Google Scholar]
- 40.Shaikh AG, Beylergil SB, Scorr L, et al. Dystonia and tremor: a cross-sectional study of the dystonia coalition cohort. Neurology. 2021;96(4):e563-e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(pt 7):1195-1212. [DOI] [PubMed] [Google Scholar]
- 42.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia the basal ganglia and the cerebellum? Neurology. 2006;67(10):1740-1741. [DOI] [PubMed] [Google Scholar]
- 43.Khooshnoodi MA, Factor SA, Jinnah HA. Secondary blepharospasm associated with structural lesions of the brain. J Neurol Sci. 2013;331(1-2):98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prudente CN, Stilla R, Singh S, et al. A functional magnetic resonance imaging study of head movements in cervical dystonia. Front Neurol. 2016;7:201. doi: 10.3389/fneur.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norris SA, Morris AE, Campbell MC, et al. Regional, not global, functional connectivity contributes to isolated focal dystonia. Neurology. 2020;95(16):e2246-e2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez V, Le Bars E, Cif L, et al. The reorganization of motor network in hemidystonia from the perspective of deep brain stimulation. Brain Imaging Behav. 2015;9(2):223-235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used for analyses were extracted from published reports and are available in eTable 1, links.lww.com/WNL/C258. Analytic code, or any other deidentified data not published within this article is available to any qualified investigator on request by email to the corresponding author.