Abstract
Background and Objectives
Focal cortical dysplasia (FCD) is the most common cause of surgically remediable epilepsy in children. Little is known about the risk factors for the timing and development of pharmacoresistance in this population. This study sought to evaluate the prevalence and risk factors for pharmacoresistance in pediatric FCD-related epilepsy.
Methods
In this retrospective single-center cohort design, patients were identified from search of centralized radiology report database and a central epilepsy surgical database. Inclusion criteria consisted of 3T MRI-confirmed FCD from January, 2011, to January, 2020; ages 0 days to 22 years at MRI; and at least 18 months of documented follow-up after MRI, unless had single seizure or incidentally discovered FCD. Records were excluded if there was dual pathology (except for mesial temporal sclerosis), hemimegalencephaly, or tuberous sclerosis complex present in imaging or history.
Results
One hundred forty-three patients with confirmed FCD met the inclusion criteria. One hundred twenty-four children had epilepsy (87% of patients with FCD) with median age at seizure onset 2.7 years (IQR 0.75–6 years, range 0–17 years). Twelve children (8.5%) had a single lifetime seizure (provoked or unprovoked) or recurrent provoked seizures. Seven children (4.9%) had incidental FCD. Ninety-two patients (74%) of those with epilepsy met criteria for pharmacoresistance. Of children with epilepsy of all types, 93 children (75%) were seizure-free at the last visit; 82 patients underwent epilepsy surgery, of whom 59 (72%) achieved seizure freedom. Seven percent (9/124) achieved seizure freedom with a second ASM and 5.6% (7/124) with a third or more ASMs. Failure of only 1 antiseizure medication is associated with enormous increased incidence and earlier development of pharmacoresistance (OR 346; 95% CI 19.6–6,100); Cox regression showed FCD lobar location, pathologic subtype, and age at seizure onset are not.
Discussion
Failure of 1 antiseizure medication is associated with substantial risk of pharmacoresistance. These data support an operational redefinition of pharmacoresistance, for surgical planning, in FCD-related epilepsy to the failure of 1 antiseizure medication and support early, potentially curative surgery to improve outcomes in this patient population.
Focal cortical dysplasia (FCD) is the most common cause of surgically remediable pharmacoresistant epilepsy in children.1,2 Surgery can lead to seizure-free outcome3-5 even with reoperation.6 The prevalence of FCD in focal epilepsy is estimated to be 5%–25%.7 Despite its prevalence, the natural history of FCD-related epilepsy remains relatively unknown. There have been variable findings about the age of epilepsy onset in FCD-related epilepsy. Studies report onset in the neonatal period,8 early childhood (61% before 5 years), and adolescence (93% before 16 years), but there can also be adult-onset epilepsy.7,9,10 A retrospective study of 97 children with MRI-identified FCD over a 9-year period found an estimated epilepsy prevalence of 71% with 33% prevalence of pharmacoresistance in the MRI-identified population.11 This group found earlier age of epilepsy onset in those patients with pharmacoresistant epilepsy.
There are inconsistent reports in clinical, radiologic, and pathologic associations with FCD-related epilepsy onset. FCD cortical lobar location has variable association with age at onset in different studies: 1 study found no difference in age at onset in extratemporal, temporal, and multilobar dysplasias,9 whereas a retrospective cohort found a trend that frontal FCDs are associated with younger epilepsy onset and temporal FCD are associated with older epilepsy onset.12 Lesions in primary sensory areas are associated with younger epilepsy onset and lesions in association cortex with older onset.12,13 Pathology is inconsistently associated with age at onset. Some studies report those FCDs with cytoarchitectural abnormalities have younger age at onset compared with those FCDs without cytoarchitectural abnormalities,9,14 whereas others have found no clear correlation between pathology subtype and age at onset.12,15
Because surgery is effective in the treatment of FCD-related epilepsy and the typical minimum threshold for surgical consideration is the development of pharmacoresistance, we designed this study (1) to evaluate the natural history of epilepsy and development of pharmacoresistance in a large, single-center FCD cohort and (2) to evaluate the prevalence and risk factors of pharmacoresistance in this population. We hypothesized that earlier age at onset poses greater risk of development of pharmacoresistant FCD-related epilepsy. We also hypothesized that the failure of 1 antiseizure medication is highly likely to lead to pharmacoresistance. Finally, we hypothesized that there is a subpopulation of children with FCD without epilepsy and/or a population with pharmacosensitive epilepsy.
Methods
This is an institutional review board-approved retrospective cohort design study from Children's National Hospital (CNH) in Washington, DC. Patients were identified from an electronic medical record search of centralized radiology database (Nuance mPower) containing all imaging reports for the hospital system for the term “FCD” and from a central epilepsy surgical database. We included patients with 3T MRI-confirmed FCD who were treated at CNH from January, 2011, to January, 2020, with ages 0 day to 22 years. MRIs were all reported by board-certified pediatric neuroradiologists (including M.T.W.) and independently reviewed by N.T.C. Clinical notes from Cerner PowerChart were reviewed by P.C. and N.T.C. to determine whether the patient met inclusion criteria. Children were included if they had at least 18 months of documented follow-up from MRI identification of FCD, unless they had single seizure or incidentally discovered FCD in which case these patients were all included. Children were excluded if they had dual pathology (except for mesial temporal sclerosis), hemimegalencephaly, or tuberous sclerosis complex. The following data were abstracted manually from clinical notes into a database: age at seizure onset; demographic data; epilepsy diagnosis; date of MRI; MRI result; dates the first, second, and third ASMs were prescribed; reason for ASM discontinuation (intolerance vs inefficacy); seizure types; duration of follow-up; and if applicable, epilepsy surgery date/side/procedure/pathologic diagnosis and Engel outcome.
Definitions and Outcomes
Pharmacoresistant epilepsy (PRE) was defined by either starting a third antiseizure medication (ASM) (because of inefficacy of 2 prior adequately dosed ASM, not including intolerance because of adverse reaction following the ILAE criteria16) or having undergone epilepsy surgery (in cases where failure of 2 ASMs documented but specific date of third ASM initiation could not be found). Time to PRE (in years) was defined as the date the first ASM was prescribed subtracted from either the date the third ASM was prescribed or the date of initial epilepsy surgery. Pharmacosensitive epilepsy (PSE) was defined as drug-sensitive (epilepsy with seizures controlled on 1 or 2 appropriately dosed ASM). Any patient with epilepsy who was not seizure-free at the last follow-up but who had not yet met the criteria for pharmacoresistance (either on 1 inadequately dosed ASM or 2 ASMs with 1 inadequately dosed ASM) was included in the PSE group for purposes of analysis. Patients with single lifetime unprovoked or recurrent provoked seizures (e.g., febrile seizures) and those children with incidentally discovered FCD were also included.
Data Analysis
Survival analysis was conducted to test the hypothesis that failure of 1 ASM leads to earlier development of PRE. Kaplan-Meier survival curves were constructed. Survival time was defined as the date of last follow-up minus the age at seizure onset. Multivariate regression using Cox proportional hazards model was performed to evaluate the hazard rates contributing to development of PRE for clinical and imaging variables. These variables were selected because they are demographic or there are disparate reports in the literature of their effect on epilepsy risk: sex, FCD lobar location, pathology, and age at seizure onset. Failure of 1 ASM could not be included because it is impossible to develop PRE without failure of 1 ASM, so there are no observations in this category. FCD lobar location was defined as frontal, occipital, parietal, temporal, or other. The FCD lobar location variable violated the assumptions of proportional hazards and was included as a stratification variable. FCD pathology was defined as type I, type II, or type III, or unknown. Age at seizure onset was transformed to square root of age at seizure onset to maintain the assumption of proportional hazards because age at seizure onset as a continuous variable violated the assumption.
The chi-square test was used for categorical comparison (failure of 1 ASM with PRE or PSE). Analysis of Variance (ANOVA) was used for interval and normal comparisons (age of epilepsy onset vs PRE or PSE). The Kruskal-Wallis test was used for ordinal comparison (Engel outcome vs lobar distribution). OR was calculated with 95% CI. Significance threshold was p < 0.05 for all testing. Statistical analysis was performed using R17 and Jamovi.18
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Children's National Institutional Review Board (Protocol 00012692) as exempt.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
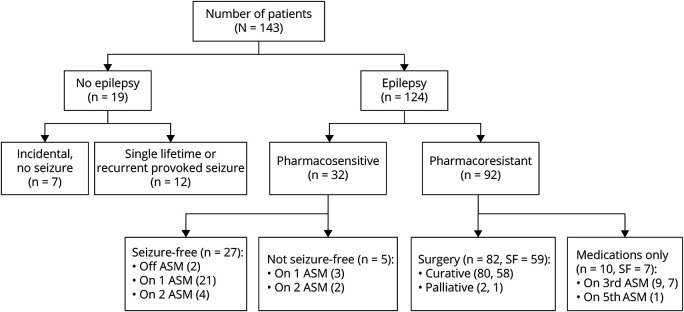
We included 143 children (41% female) meeting inclusion criteria in our analysis (Figure 1). Median duration of follow-up from MRI was 50.6 months (interquartile range [IQR] 31.6–82.2 months). One hundred twenty-four children (87%) had epilepsy. Median age at seizure onset was 2.7 years (IQR 0.75–6 years, range 0–17 years). Twelve children (8.5%) had single lifetime seizure (provoked or unprovoked) or recurrent provoked seizures with median 15.8 months (IQR 4.1–57.2 months) of follow-up. Seven children (4.9%) had incidental FCD with median 25.4 months (IQR 11.8–47.2 months) of follow-up. A total of 92 patients (74%) of those with epilepsy met criteria for PRE. Of children with epilepsy of all types, 93 children (75%) were seizure-free at the last visit. Eighty-two patients (57.7%, all with PRE) underwent epilepsy surgery, of which 59 (72%) were seizure-free at the last visit. Of the 42 children with epilepsy who did not undergo surgery, 34 (81%) were seizure-free at the last visit.
Figure 1. Patient Flowchart.
Flowchart demonstrating breakdown of patients into groups. SF (seizure-free) indicates the number of patients achieving seizure freedom in that group. Of note, for the PSE group, 9 patients were seizure-free at the last follow-up (5 were on second ASM as monotherapy; 4 were on second ASM as combination therapy). ASM = antiseizure medication; PSE = pharmacosensitive epilepsy.
Median age at surgery was 9.48 years (IQR 5.42–14.36 years). Median duration of postoperative follow-up was 43.8 months (IQR 14.7–72.9 months). Eighty patients had resective (n = 79) or ablative surgery (n = 1); 2 patients had neurostimulators placed (1 vagus nerve stimulator, 1 responsive neurostimulator). Pathology was available for 78 patients (55% of cohort): type I (n = 22), type II (n = 50), and type III (n = 6). Engel outcome was Class I (n = 59, 72%), Class II (n = 9, 11%), Class III (n = 12, 15%), and Class IV (n = 2, 2%).
Survival Analysis
Failure of 1 ASM was associated with an increased risk of pharmacoresistance (log rank, p < 0.0001) (Figure 2).
Figure 2. Kaplan-Meier Curve of Development of Pharmacoresistance by Failure of 1 Antiseizure Medication.
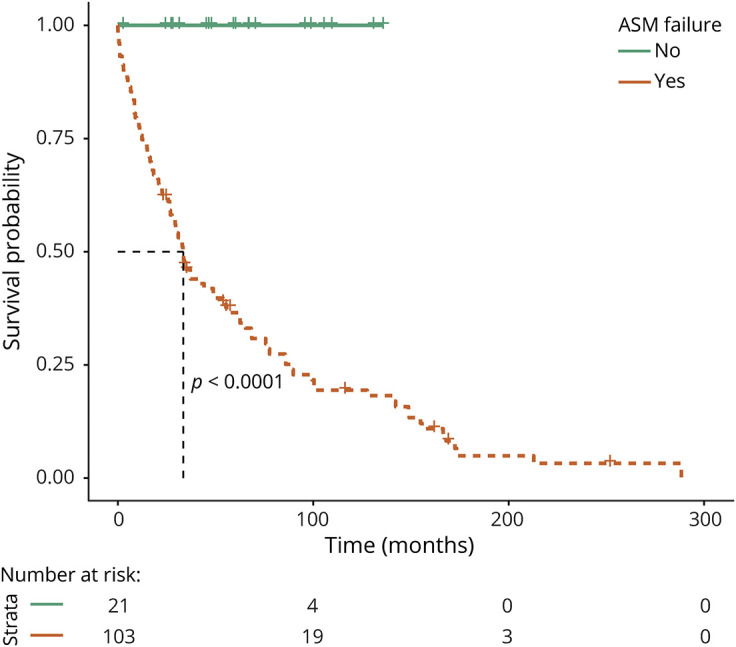
Orange line shows those patients with epilepsy and failure of 1 antiseizure medication. Green line shows those patients with pharmacosensitive epilepsy on 1 antiseizure medication.
Multivariate Analysis
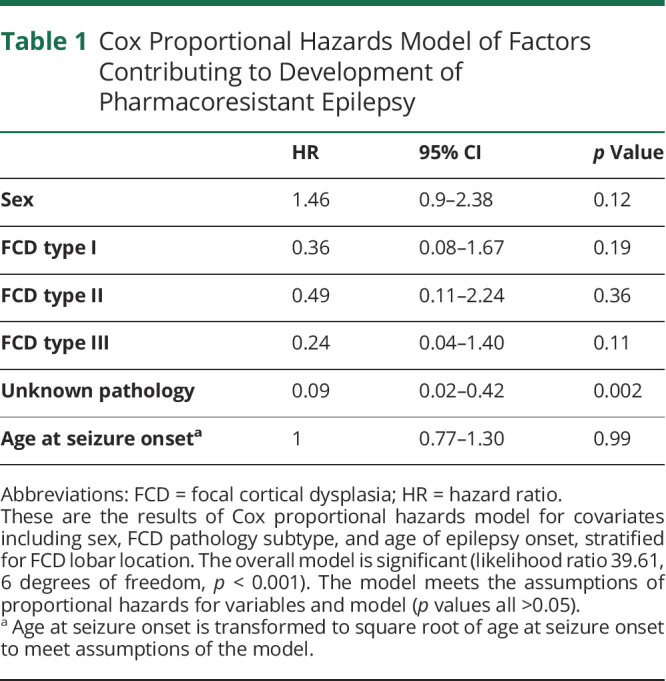
Cox proportional hazards regression was performed to compare the relative hazards of clinical and imaging variables on the development of PRE (Table 1). The Cox model showed that while stratifying for cortical lobar location; sex; FCD pathologic subtypes I, II, or III; and age at seizure onset do not contribute significantly to the risk of developing PRE.
Table 1.
Cox Proportional Hazards Model of Factors Contributing to Development of Pharmacoresistant Epilepsy
Pharmacoresistant vs Pharmacosensitive Epilepsy
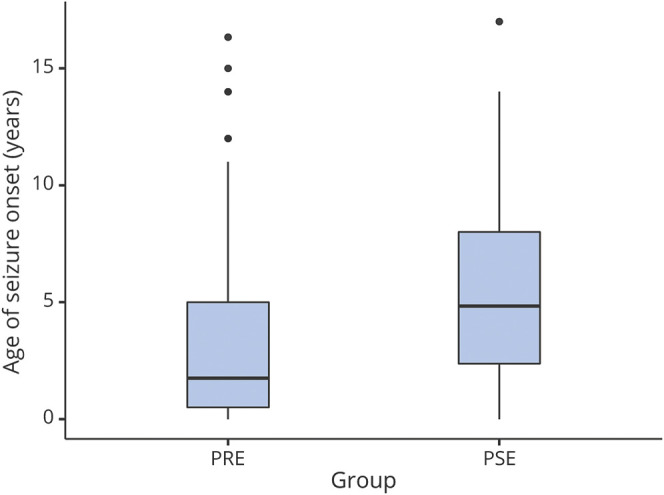
Of the 124 children with epilepsy, 92 were pharmacoresistant (74%) and 32 were pharmacosensitive (26%). Median time to PRE was 29.7 months (IQR 11.1–70.3 months, eFigure 1, links.lww.com/WNL/C254). Of the 32 with PSE, 31 (97%) had focal unaware epilepsy and 1 patient (3%) had focal aware epilepsy with median 43.1 months (IQR 27.6–55.6 months) of follow-up. No patients had EEG or clinical features of a self-limited epilepsy syndrome. Sixty-six of the 92 children (80%) with PRE (59 of whom had surgery, 7 on medications alone) were seizure-free at the last documented follow-up. Twenty-seven of the 32 children (84%) with PSE were seizure-free at the last follow-up. Of those with PSE, 2 were seizure-free off ASM, 21 were seizure-free on 1 ASM at the last visit (for 5 patients this was the second ASM tried), 4 were seizure-free with a second ASM at the last visit, 3 had ongoing seizures with 1 inadequately dosed ASM, and 2 had ongoing seizures with second ASM (with 1 inadequately dosed ASM). Of those with PSE, 6 patients were on a second ASM(s) at the last visit (4 were seizure-free); 26 patients were on 1 ASM at the last visit (23 were seizure-free). Therefore, only 7% (9/124) of those with epilepsy achieved seizure freedom with the second ASM. Only 5.6% (7/124) of patients with epilepsy achieved seizure freedom with the third or more ASMs. Figure 3 shows that children with PRE had a younger age of epilepsy onset (median 3.42 years, IQR 1.17–5.67 years) compared with those with pharmacosensitive epilepsy (4.83 years, IQR 2.82–7.64 years) (ANOVA, p = 0.011). There was no difference of lobar distribution between pharmacoresistance and PSE. There was no difference in surgical outcome by lobar distribution (Kruskal-Wallis, p = 0.49).
Figure 3. Boxplot of Age of Seizure Onset (Years) by Pharmacoresistant (PRE) vs Pharmacosensitive Epilepsy (PSE).
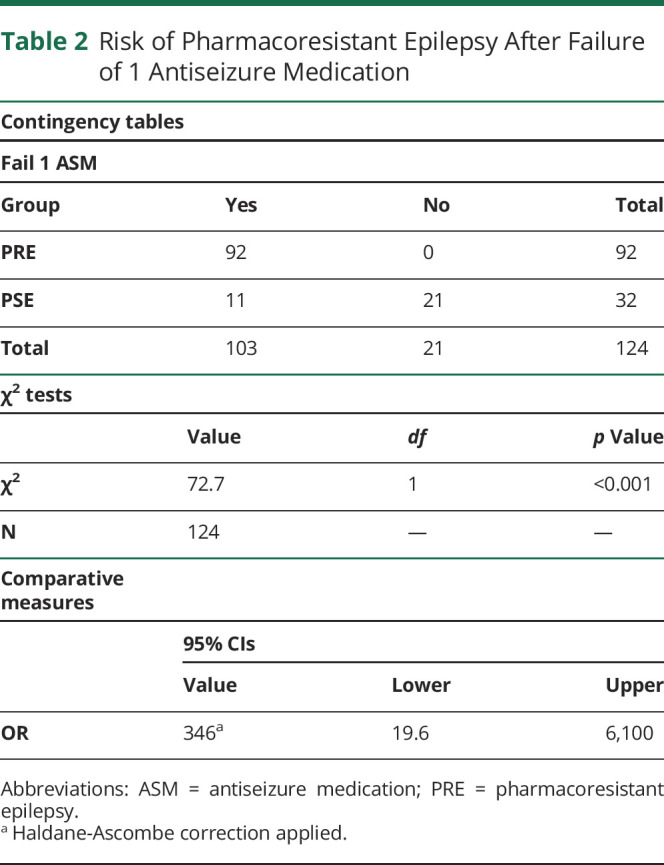
Failure of 1 ASM was associated with high likelihood of developing PRE (p < 0.001; OR 346; 95% CI 19.6–6,100) (Table 2). Eleven (34%) of those with PSE failed to achieve seizure control with 1 ASM (χ2, p < 0.001)—9 were seizure-free and 2 had ongoing seizures at the last follow-up. One hundred three patients failed to achieve seizure control with 1 ASM of which only 15% (n = 16) were seizure-free without surgery at the last documented follow-up (2 or more ASMs).
Table 2.
Risk of Pharmacoresistant Epilepsy After Failure of 1 Antiseizure Medication
Discussion
This large retrospective cohort study examined the prevalence and risk factors associated with the development of pharmacoresistance in children with FCD-related epilepsy. We showed that failure of seizure control with only 1 ASM is associated with marked increased risk, and earlier onset, of pharmacoresistance. We also investigated factors previously evaluated but inconsistently reported in other studies. We did not find that FCD lobar location, pathologic subtype, or age at seizure onset are associated with pharmacoresistance in FCD-related epilepsy.
A comparable 9-year, retrospective cohort epilepsy prevalence study was performed at Cincinnati Children's. Of 97 patients with MRI-identified FCD from a radiology database, there was an estimated prevalence of epilepsy of 71%; 33% developed PRE.11 The authors reported earlier seizure onset in those with PRE compared with those with PSE, but age at seizure onset and age of pharmacoresistance were not reported. Only 22 patients had epilepsy surgery, of which 21 had FCD pathology. The authors found a large subpopulation (29% of the cohort) did not have epilepsy. We found that 19% of our cohort did not develop epilepsy during the period of follow-up included in this study. Our method enhanced this approach by identifying all FCD in the radiology database for our center plus from the epilepsy surgical database. Our method also tracked the age at onset of pharmacoresistance allowing for better analysis of risk correlation. Furthermore, we tracked timing of medication sequences allowing for analysis of medication failure. We confirm the finding that earlier age at seizure onset is associated with an increased risk of pharmacoresistance and demonstrate that earlier seizure onset is associated with earlier onset of pharmacoresistance.
A retrospective combined database and regional network survey at the Great Ormond Street Hospital (GOSH) identified 139 patients with MRI-confirmed FCD with a 94.2% rate of pharmacoresistance.19 They reported a 59.8% Engel I outcome in 92 patients who had 1-year postoperative follow-up. This study focused on factors associated with Engel outcome and found that concordance between FCD MRI location and ictal EEG, as well as older age at seizure onset were associated with Engel I outcome. Duration from diagnosis to surgery, surgery type, number of ASMs, and histology were not associated with Engel I outcome. The group did not evaluate risk factors specific to pharmacoresistance and exhibits a strong selection bias for referral to a tertiary care epilepsy center. Other studies including older populations and longer duration epilepsy observe a decrease in seizure freedom with delays in epilepsy surgery.13,20-22
In this study, we report on the implication of failure of 1 ASM in the FCD-related epilepsy population. We find that failure of 1 ASM is associated with an enormous (OR = 346) increased risk and earlier onset of pharmacoresistance. The current ILAE definition of pharmacoresistance from the ILAE Commission on Therapeutic Strategies is “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.”16 This is based largely on observational cohorts of low likelihood of seizure freedom after failure of 2 ASM trials.23 In adults, there is an 11.6% chance of seizure freedom after failure of 1 ASM and only 1%–4% chance of seizure freedom after failure of 2 ASMs.23,24 In children, there are reports of a 19% chance of seizure freedom from second ASM after failure of 1 ASM and only 9% on any additional ASMs.25 However, only 8.6% of children with PRE and an abnormal MRI achieve seizure control without surgery.26 We find there is only a 7% confirmed chance for seizure freedom with a second ASM. Epilepsy surgery is a potentially curative option for children with FCD-related epilepsy and can lessen the morbidity and mortality associated with PRE. Our cohort (and others5,19,27,28) exhibits a high seizure-free outcome (Engel I, 72%) with a duration of follow-up of 43.8 months. These data suggest an operational redefinition of pharmacoresistance in the FCD-related epilepsy population, for surgical decision-making, as failure of 1 antiseizure medication and, in turn, advocate for earlier consideration of potentially curative surgery. This would need to be evaluated in a prospective, multicenter study.
This study highlights the importance of early imaging with epilepsy protocols to identify high-risk children.29 Despite the similarities between Cincinnati Children's and Children's National Hospital regarding catchment and similar multilevel service (primary, secondary, tertiary care), the Cincinnati group only found a prevalence of 33% of pharmacoresistance. However, 32 of the 69 patients (46.4%) with epilepsy in this study had PRE. In addition, the authors report uncertainty in the radiologic FCD diagnosis in 72% of the cohort (70 patients had either probable (n = 60) or possible (n = 10) FCD; only 28% (n = 27) had definite FCD per their evaluation criteria). It is possible that this uncertainty, combined with the lack of surgical pathology for confirmation, led to the inclusion of falsely positive patients with FCD in the pharmacosensitive or nonepilepsy patients, thus reducing the reported prevalence of pharmacoresistance. It is of interest that 51% (49/97) of the MRIs in the Cincinnati study were performed at 1.5 Tesla. It is also possible that this study had false negatives (e.g., those with epilepsy missed by the lower-resolution 1.5 Tesla scanner), which would also lower the reported pharmacoresistance rate. In the recent Multi-centre Epilepsy Lesion Detection (MELD) study of 580 patients with FCD, epilepsy onset was typically before age 10 years, but 51% of patients waited more than 10 years before having the first MRI scan and undergoing presurgical evaluation.13 Early imaging with an epilepsy protocol MRI30 is crucial for early detection and accurate diagnosis of FCD.31,32 Early epilepsy surgery is associated with improved cognitive and developmental outcomes33,34 and with higher likelihood of seizure freedom.35-38 At our center, we perform 3T epilepsy-protocol MRI at seizure onset, thus increasing the likelihood of identifying children with FCD who may do well. There is a caveat that some FCDs may be difficult to visualize whether the MRI is obtained before age 24 months due to myelination maturation; studies may need to be repeated after age 24 months if seizures persist.39
Our study confirms the existing findings that demonstrate the benefit of surgery in FCD-related epilepsy and augments this literature by determining when pharmacoresistance is met and providing evidence for the redefinition of pharmacoresistance in FCD-related epilepsy. Engel I outcome (seizure freedom) is found in 56.6%–72%.40-42 Similarly, we found a 72% seizure-free outcome after surgery with 43.8 months of follow-up.
This study has several limitations. The retrospective nature of the project allows us to estimate the prevalence of pharmacoresistance in our selected sample but does not allow for population-level natural history data to be determined. Similar to the Cincinnati group, we find a fair portion of the population has PSE or no epilepsy. MRI-identified lesions may rarely represent other etiologies. We also have a high percentage (55%) of patients with available confirmed FCD pathology, thus improving the reliability of our findings. Although we found no 1 specific pathologic subtype to be associated with pharmacoresistance, this must be interpreted in the context that pathology was not available for patients with PSE and without surgery. There may be a referral bias because of the single-center nature of our method. However, similar to Cincinnati Children's and unlike GOSH, Children's National Hospital is a large pediatric hospital with wide regional primary, secondary, and tertiary care capabilities. We aimed to overcome sources of bias by identifying all patients with FCD through the system's searchable database and to include any epilepsy surgical patients. Although there is potential, this study may underestimate the rate of seizure-free patients with FCD because there are patients with FCD without seizure who have not been identified; our method, to the best of our ability, has identified all known radiologic patients with FCD (even those without seizures). While one-third of our patients either did not meet the criteria for epilepsy or were controlled on 1 medication, we do not know the long-term outcomes, and there is potential for prolonged honeymoon periods. Individual practitioner decision-making, such as when to initiate medication changes, could affect the time to pharmacoresistance as defined in this study. Because onset of intractable epilepsy may occur in adulthood, it is possible that some of our patients, in later life, may develop a more severe epilepsy.
Our study provides a granular evaluation at the prevalence of and specific risk factors for the development of pharmacoresistance in FCD-related epilepsy. There is a nearly 90% prevalence of epilepsy over this 9-year period, of which 3 quarters are pharmacoresistant. We find that failure of 1 antiseizure medication is associated with a marked increase in the development of pharmacoresistance. We find no clear relationship between FCD lobar location, pathologic subtype, or age at seizure onset and the development of pharmacoresistance. Despite the high rate of pharmacoresistance, epilepsy surgery led to a 72% Engel I outcome in this cohort. Our data support the operational redefinition of pharmacoresistance in children with FCD-related epilepsy to the failure of 1 antiseizure medication, thus supporting earlier access to surgery and the prospect for an epilepsy cure.
Glossary
- ASM
antiseizure medication
- CNH
Children's National Hospital
- FCD
focal cortical dysplasia
- GOSH
Great Ormond Street Hospital
- IQR
interquartile range
- PRE
pharmacoresistant epilepsy
- PSE
pharmacosensitive epilepsy
Appendix. Authors
Study Funding
N.T. Cohen is supported by the Pediatric Epilepsy Research Foundation/Child Neurology Foundation Shields Research Grant, the Children's National Research Institute Chief Research Officer Award. This work was also supported by DC-IDDRC NICHD NIH P50 HD105328. This publication was supported by Award Number UL1TR001876 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the NIH.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Harvey AS, Cross JH, Shinnar S, Mathern GW, Mathern BW, ILAE Pediatric Epilepsy Surgery Survey Taskforce. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49(1):146-155. [DOI] [PubMed] [Google Scholar]
- 2.Diaz RJ, Sherman EMS, Hader WJ. Surgical treatment of intractable epilepsy associated with focal cortical dysplasia. Neurosurg Focus. 2008;25(3):E6. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD. Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg. 2004;101(1):55-65. [DOI] [PubMed] [Google Scholar]
- 4.Krsek P, Pieper T, Karlmeier A, et al. Different presurgical characteristics and seizure outcomes in children with focal cortical dysplasia type I or II. Epilepsia. 2009;50(1):125-137. [DOI] [PubMed] [Google Scholar]
- 5.Kloss S, Pieper T, Pannek H, Holthausen H, Tuxhorn I. Epilepsy surgery in children with focal cortical dysplasia (FCD): results of long-term seizure outcome. Neuropediatrics. 2002;33(1):21-26. [DOI] [PubMed] [Google Scholar]
- 6.Cohen NT, Ziobro JM, Depositario-Cabacar DF, et al. Measure thrice, cut twice: on the benefit of reoperation for failed pediatric epilepsy surgery. Epilepsy Res. 2020;161:106289. [DOI] [PubMed] [Google Scholar]
- 7.Bast T, Ramantani G, Seitz A, Rating D. Focal cortical dysplasia: prevalence, clinical presentation and epilepsy in children and adults. Acta Neurol Scand. 2006;113(2):72-81. [DOI] [PubMed] [Google Scholar]
- 8.Lortie A, Plouin P, Chiron C, Delalande O, Dulac O. Characteristics of epilepsy in focal cortical dysplasia in infancy. Epilepsy Res. 2002;51(1-2):133-145. [DOI] [PubMed] [Google Scholar]
- 9.Fauser S, Huppertz HJ, Bast T, et al. Clinical characteristics in focal cortical dysplasia: a retrospective evaluation in a series of 120 patients. Brain. 2006;129(pt 7):1907-1916. [DOI] [PubMed] [Google Scholar]
- 10.Sisodiya SM, Fauser S, Cross JH, Thom M. Focal cortical dysplasia type II: biological features and clinical perspectives. Lancet Neurol. 2009;8(9):830-843. [DOI] [PubMed] [Google Scholar]
- 11.Maynard LM, Leach JL, Horn PS, et al. Epilepsy prevalence and severity predictors in MRI-identified focal cortical dysplasia. Epilepsy Res. 2017;132:41-49. [DOI] [PubMed] [Google Scholar]
- 12.Wiwattanadittakul N, Suwannachote S, You X, et al. Spatiotemporal distribution and age of seizure onset in a pediatric epilepsy surgery cohort with cortical dysplasia. Epilepsy Res. 2021;172:106598. [DOI] [PubMed] [Google Scholar]
- 13.Wagstyl K, Whitaker K, Raznahan A, et al. Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: a MELD study. Epilepsia. 2022;63(1):61-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widdess-Walsh P, Kellinghaus C, Jeha L, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: correlation with pathological subtypes. Epilepsy Res. 2005;67(1-2):25-33. [DOI] [PubMed] [Google Scholar]
- 15.Rácz A, Müller AM, Schwerdt J, Becker A, Vatter H, Elger CE. Age at epilepsy onset in patients with focal cortical dysplasias, gangliogliomas and dysembryoplastic neuroepithelial tumours. Seizure. 2018;58:82-89. [DOI] [PubMed] [Google Scholar]
- 16.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077. [DOI] [PubMed] [Google Scholar]
- 17.The R Project for Statistical Computing. Accessed October 1, 2021. r-project.org/.
- 18.Jamovi. Accessed October 1, 2021.jamovi.org/.
- 19.Zvi IB, Enright N, D'Arco F, et al. Children with seizures and radiological diagnosis of focal cortical dysplasia: can drug-resistant epilepsy be predicted earlier? Epileptic Disord. 2022;24(1):111-122. [DOI] [PubMed] [Google Scholar]
- 20.Bjellvi J, Olsson I, Malmgren K, Wilbe Ramsay K. Epilepsy duration and seizure outcome in epilepsy surgery: a systematic review and meta-analysis. Neurology. 2019;93(2):e159-e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simasathien T, Vadera S, Najm I, Gupta A, Bingaman W, Jehi L. Improved outcomes with earlier surgery for intractable frontal lobe epilepsy. Ann Neurol. 2013;73(5):646-654. [DOI] [PubMed] [Google Scholar]
- 22.Lamberink HJ, Boshuisen K, van Rijen PC, Gosselaar PH, Braun KPJ. Changing profiles of pediatric epilepsy surgery candidates over time: a nationwide single-center experience from 1990 to 2011. Epilepsia. 2015;56(5):717-725. [DOI] [PubMed] [Google Scholar]
- 23.Kwan P, Brodie MJ. Early identification of refractory epilepsy. New Engl J Med. 2000;342(5):314-319. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arts WFM, Brouwer OF, Peters ACB, et al. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127(pt 8):1774-1784. [DOI] [PubMed] [Google Scholar]
- 26.Wirrell EC, Wong-Kisiel LCL, Mandrekar J, Nickels KC. What predicts enduring intractability in children who appear medically intractable in the first 2 years after diagnosis? Epilepsia. 2013;54(6):1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rácz A, Becker AJ, Quesada CM, et al. Post-surgical outcome and its determining factors in patients operated on with focal cortical dysplasia type II-A retrospective monocenter study. Front Neurol. 2021;12:666056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fauser S, Schulze-Bonhage A, Honegger J, et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004;127(pt 11):2406-2418. [DOI] [PubMed] [Google Scholar]
- 29.Coryell J, Gaillard WD, Shellhaas RA, et al. Neuroimaging of early life epilepsy. Pediatrics. 2018;142(3):e20180672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernasconi A, Cendes F, Theodore WH, et al. Recommendations for the use of structural magnetic resonance imaging in the care of patients with epilepsy: a consensus report from the International League against Epilepsy Neuroimaging Task Force. Epilepsia. 2019;60(6):1054-1068. [DOI] [PubMed] [Google Scholar]
- 31.Knake S, Triantafyllou C, Wald LL, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005;65(7):1026-1031. [DOI] [PubMed] [Google Scholar]
- 32.Mellerio C, Labeyrie MA, Chassoux F, et al. 3T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia. 2014;55(1):117-122. [DOI] [PubMed] [Google Scholar]
- 33.Braun KPJ, Cross JH. Pediatric epilepsy surgery: the earlier the better. Expert Rev Neurotherapeutics. 2018;18(4):261-263. [DOI] [PubMed] [Google Scholar]
- 34.Braun KPJ. Influence of epilepsy surgery on developmental outcomes in children. Eur J Paediatric Neurol. 2020;24:40-42. [DOI] [PubMed] [Google Scholar]
- 35.Adler S, Wagstyl K, Gunny R, et al. Novel surface features for automated detection of focal cortical dysplasias in paediatric epilepsy. NeuroImage Clin. 2017;14:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. New Engl J Med. 2017;377(17):1639-1647. [DOI] [PubMed] [Google Scholar]
- 37.Lamberink HJ, Otte WM, Blümcke I, Braun KPJ. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19(9):748-757. [DOI] [PubMed] [Google Scholar]
- 38.Boshuisen K, Arzimanoglou A, Cross JH, et al. , TimeToStop Study Group. Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol. 2012;11(9):784-791. [DOI] [PubMed] [Google Scholar]
- 39.Gaillard WD, Chiron C, Cross JH, et al. , ILAE, Committee for Neuroimaging, Subcommittee for Pediatric. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50(9):2147-2153. [DOI] [PubMed] [Google Scholar]
- 40.Rowland NC, Englot DJ, Cage TA, Sughrue ME, Barbaro NM, Chang EF. A meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg. 2012;116(5):1035-1041. [DOI] [PubMed] [Google Scholar]
- 41.Jin B, Wang J, Zhou J, Wang S, Guan Y, Chen S. A longitudinal study of surgical outcome of pharmacoresistant epilepsy caused by focal cortical dysplasia. J Neurol. 2016;263(12):2403-2410. [DOI] [PubMed] [Google Scholar]
- 42.Kim DW, Lee SK, Chu K, et al. Predictors of surgical outcome and pathologic considerations in focal cortical dysplasia. Neurology. 2009;72(3):211-216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.