Abstract
Endoscopic eradication therapy (EET) has become a standard of care for treatment of dysplastic Barrett’s esophagus (BE) and early Barrett’s neoplasia. EET mainly consists of removal of any visible lesions via endoscopic resection and eradication of all remaining Barrett’s mucosa using endoscopic ablation. Endoscopic mucosal resection and endoscopic submucosal dissection are the two available resection techniques. After complete resection of all visible lesions, it is crucial to perform endoscopic ablation to ensure complete eradication of the remaining Barrett’s segment. Endoscopic ablation can be done either with thermal techniques, including radiofrequency ablation and argon plasma coagulation, or cryotherapy techniques. The primary end point of EET is achieving complete remission of intestinal metaplasia (CRIM) to decrease the risk of dysplastic recurrence after successful EET. After CRIM is achieved, a standardized endoscopic surveillance protocol needs to be implemented for early detection of BE recurrence.
Keywords: Barrett’s esophagus, esophageal adenocarcinoma, endoscopic eradication therapy, endoscopic mucosal resection, endoscopic submucosal dissection
Introduction
Barrett’s esophagus (BE) is an acquired condition in which the esophageal squamous epithelium is replaced by specialized intestinal metaplasia (IM) as an adaptive response to long-standing reflux-induced injury. BE may progress to esophageal adenocarcinoma (EAC) through various dysplastic stages: from non-dysplastic BE (NDBE) to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and eventually to EAC. The estimated annual risk of progression to EAC in NDBE, LGD, and HGD is 0.3%, 1%, and 8%, respectively [1, 2]. Hence, the management of patients with BE is predominantly based on their grade of dysplasia, with the overall goal of preventing progression to HGD or EAC. Patients with NDBE are recommended to undergo periodic endoscopic surveillance (at 3- to 5-year intervals) to detect dysplasia or EAC, given their low rate of progression to EAC [3]. In contrast, patients with HGD are recommended to undergo endoscopic eradication therapy (EET) proactively, given their substantial risk of progressing to EAC. Treatment recommendations for LGD, which has an intermediate risk of progression to EAC, remain challenging given a substantial interobserver variation in the diagnosis of LGD amongst even expert pathologists.
In recent years, EET has become the standard of care for treatment of dysplastic BE. EET is now considered a treatment option for patients with confirmed LGD, as it has been shown to reduce the risk of progression to HGD/EAC in patients with LGD compared with endoscopic surveillance based on several studies, including a randomized–controlled trial [4–6]. EET has also replaced esophagectomy for the treatment of HGD and early-stage EAC given the comparable survival with significantly lower procedural morbidity and mortality [6–8]. In this article, we provide an in-depth review of the current data on the use of EET for the treatment of dysplastic BE and early BE-related neoplasia, including indications, techniques, efficacy, potential complications, and post-EET follow-up.
Endoscopic eradication therapy
The principle of EET consists of several components: (i) identification and removal of any visible focal lesions that may reflect advanced BE-related neoplastic lesions via endoscopic resection (ER); (ii) eradication of all remaining flat BE mucosa using endoscopic ablation; (iii) optimization of acid-suppression therapy to ensure replacement of BE mucosa by neo-squamous epithelium; and (iv) endoscopic surveillance for early detection of recurrence disease.
Early BE-related neoplastic lesions can be subtle and often hard to detect. Thus, a thorough endoscopic inspection of the BE mucosa using high-resolution endoscopes (using high-definition monitors) is critical. In addition, virtual or dye-based chromoendoscopy techniques such as acetic acid chromoendoscopy, narrow-band imaging (NBI), blue laser imaging (BLI), and I-scan should be used to improve the visualization of mucosal lesions [9, 10]. These techniques have been shown to improve dysplasia detection by 33% when compared with conventional white-light endoscopy [11]. This multimodal treatment approach has the potential to enable durable elimination of BE epithelium and replacement of metaplastic BE epithelium with neo-squamous epithelium (complete eradication of intestinal metaplasia, CE-IM), which ultimately reduces the risk of neoplastic progression to EAC.
ER
If a visible focal lesion is identified, its macroscopic appearance should be described using the Paris classification, which provides a standard terminology and has been shown to correlate with the depth of invasion in some studies [12]. Generally, flat lesions (such as Paris type 0–IIb) and some protruded lesions (such as Paris type 0–Ip) can be safely resected, given the low likelihood of deep submucosal invasion and lymph-node metastasis. ER is not recommended for the removal of deep excavated lesions (Paris type 0–III lesions), given the high likelihood of deep submucosal or muscularis propria invasion [13]. ER serves as both a diagnostic/staging and a therapeutic tool. It is performed not only to remove early neoplastic lesions for therapeutic purposes but also to obtain a more definitive histological diagnosis, often resulting in a change of the level of dysplasia and disease management [14]. ER provides larger specimens that significantly reduce the interobserver variability amongst gastrointestinal (GI) pathologists and lead to upstaging of histological diagnosis in 30%–40% of cases compared with surveillance biopsies [15]. In the case of carcinoma, an ER specimen also provides a more accurate T staging compared with conventional ER, allowing better assessment of margin involvement and other prognostic histologic features such as poor differentiation, deep submucosal invasion, and lymphovascular invasion [16].
ER techniques
If the lesion is deemed appropriate for ER, two main ER techniques can be considered: cap-assisted endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). EMR can be performed as a band-ligation technique or cap-and-snare technique. The size and macroscopic appearance of the lesions are the two most important factors for choosing the appropriate ER technique. If the lesion is suspected of deep submucosal invasion (such as Paris type 0–IIc) or >15–20 mm in size, ESD should likely be performed [17] to enable en bloc resection, particularly in lesions containing EAC. ESD might also be preferable in patients with visible lesions located within the scar of a previous treatment zone (with either EMR or ablative therapy). EMR is significantly more challenging in this case, given the poor submucosal lift in the setting of scar formation/fibrosis from previous therapy [18]. ESD involves dissection in the submucosal plane and allows en bloc resection of larger lesions, providing a more accurate assessment of resection status and lymphovascular invasion [19]. In the absence of expertise for ESD, piecemeal EMR may be acceptable, but with the limitation of the inability to assess lateral margins, given the fragmented nature of the specimen, which unavoidably compromises the accuracy of histopathologic evaluation. Piecemeal EMR has been associated with a higher risk of recurrence after curative ER [20]. Before performing ER, delineation of the lesion is done by placing coagulation markers a few millimeters beyond the lesion to help delineate the lateral margin and ensure complete resection of the lesion.
Cap-assisted EMR techniques
Band-ligation technique (band EMR)
Band EMR (or multiband mucosectomy) is the most frequently used ER technique. It is performed using a cap with pre-loaded rubber bands. The lesion is sucked into the cap directly and the rubber band is deployed to create a pseudopolyp. The pseudopolyp is then snared (just below the rubber band) using electrocautery (Figure 1). This technique may not require a submucosal fluid injection to separate the lesion from muscularis propria given that the rubber band is thought to be flexible enough to prevent entrapment of the muscularis propria.
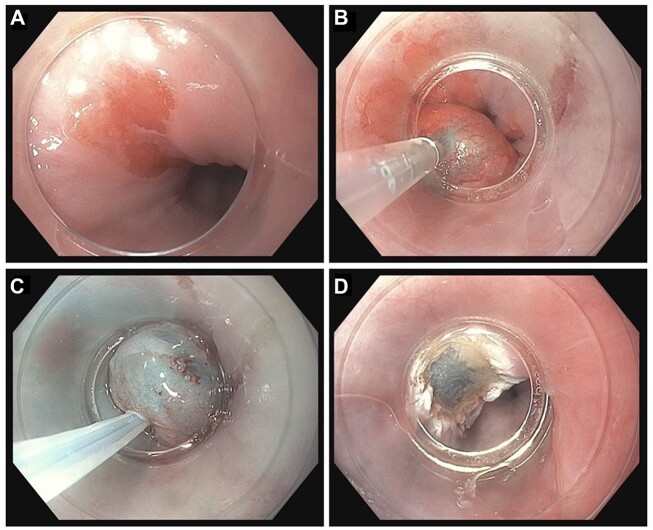
Figure 1.
Band-ligation EMR technique (band EMR). (A) Margins of the visible lesion are marked with electrocautery (optional). (B) Pseudopolyp is created after the deployment of pre-loaded rubber band. (C) The pseudopolyp is snared just below the rubber band. (D) Post-resection bed after complete removal of pseudopolyp.
Cap-and-snare technique (cap EMR)
With this technique, the mucosal lesion is first lifted with a submucosal saline injection to separate the lesion from muscularis propria. A flexible crescent-shaped snare is seated on a ledge on the inner aspect of the cap. The lesion is then sucked into the cap and resected with the snare using electrocautery (Figure 2).
Figure 2.
Cap-and-snare EMR technique (cap EMR). (A) Visible lesion is identified. (B) The lesion is lifted using a submucosal injection. (C) The lesion is sucked into the cap and resected with the pre-looped snare using electrocautery. (D) Post-resection bed after complete removal of the lesion.
A randomized–controlled trial by Pouw et al. [21] demonstrated that the efficacy of both EMR techniques is comparable, with similar side-effect profiles. However, band EMR may have a shorter learning curve and be less time-consuming, given the absence of the need for seating a snare for each resection, enabling multiple resections to be accomplished quickly. Thus, band EMR is more commonly used and may be a preferred EMR technique for early Barrett’s neoplasia [22].
ESD technique
ESD is an ER technique that dissects through the submucosal plane using a specialized endoscopic knife. Before performing ESD, the resection margin is marked 5–10 mm from the lateral edge of the lesion using electrocautery or the tip of the endoscopic knife. Next, submucosal injection is performed to lift the lesion from muscularis propria, granting adequate access to the submucosal space. Finally, the dissection starts with creating an incision circumferentially around the lesion and the submucosal layer is then carefully dissected using the endoscopic knife, removing the lesion entirely (Figure 3).
Figure 3.
ESD technique. (A) Margins of the visible lesion are marked with electrocautery. (B) Submucosal injection is performed to lift the lesion from muscularis propria. (C) The lesion is circumferentially dissected using endoscopic knife. (D) Post-resection bed after complete removal of the lesion.
Several submucosal injection solutions have been developed to achieve durable lesion elevation, given the time-consuming nature of the ESD procedure. Normal saline solution has been traditionally used but later replaced by alternative solutions to overcome its main limitation, which is the lack of durability due to rapid resorption. Hydroxypropyl methylcellulose is a widely used submucosal solution in the West and has been shown to be effective for long-lasting submucosal elevation, facilitating safe submucosal dissection [23]. In the East, sodium hyaluronate is more commonly used and has also been shown to provide a durable submucosal cushion in comparative studies [24]. Various types of ESD knives have been used, including tip-cutting knives (e.g. Dual knife, Olympus Co.), blunt-tip knives (e.g. IT knife 2, Olympus Co.), and scissors-type knives (e.g. Clutch Cutter, Fujifilm Co.). Several comparative trials have been conducted to determine the ideal ESD knives in various clinical scenarios [25]. However, the overall evidence of superiority is not conclusive, and ultimately the choice of ESD knives likely depends on individual expertise.
A growing body of literature supports the use of ESD as an alternative resection technique for nodular BE dysplasia/neoplasia. A multicenter retrospective study by Yang et al. [26] assessing histologic outcomes of patients who underwent ESD for HGD and EAC showed that ESD achieved a high rate of en bloc and curative resection rates (up to 96% and 70%, respectively). Additionally, all patients with curative resection achieved CE-IM at a mean follow-up period of 11 months. Another large multicenter study from Europe also demonstrated high rates of en bloc (90.8%) and R0 resection (79%) after ESD for treatment of BE neoplasia with a low overall complication rate of 3.5% [27]. The most common adverse event was stricture formation (2.1%) and no perforations occurred in this study.
EMR vs ESD
Cap-assisted EMR is currently the most frequently used ER technique for the treatment of BE-related neoplasia, given its lower technical complexity and a large body of evidence on its role in EET. However, the use of ESD is also rapidly expanding, with the potential of becoming the preferred treatment strategy in selected patients with Barrett’s neoplasia. ESD allows en bloc resection of larger lesions, providing an ideal specimen for precise histopathological assessment of both lateral and deep margins. On the other hand, ESD is also technically more challenging with a steep learning curve, higher rates of complications, and is more time-consuming when compared with EMR.
Several studies have compared clinical and histologic outcomes of EMR and ESD (Table 1). A systematic review and meta-analysis by Guo et al. [28], including 1,081 patients with superficial esophageal squamous cancer, showed that ESD resulted in a significantly higher en bloc resection rate than EMR (97.1% in ESD group vs 49.3% in EMR group) with longer procedural duration and higher perforation rates [odds ratio (OR), 2.19; 95% confidence interval (CI), 1.08–4.47; P = 0.03]. A small randomized clinical trial of 40 patients with HGD or early EAC from Germany also showed that ESD achieved a significantly higher rate of en bloc resection (100% with ESD vs 15% with EMR, P < 0.001) and R0 resection (58.8% with ESD vs 11.8% with EMR, P = 0.01); however, there was no difference in the remission rates at 3 months and ESD caused more complications including perforation [29]. A more recent retrospective cohort study compared the histologic outcomes of cap-assisted EMR and ESD, followed by endoscopic ablation in the treatment of dysplastic BE and intramucosal EAC, and showed that the ESD group achieved complete remission of dysplasia (CRD) at higher rates compared with the EMR group but with similar complete remission of intestinal metaplasia (CRIM) rates at 2 years [30].
Table 1.
Comparative studies on the outcomes of EMR vs ESD for early Barrett’s neoplasia
| Study reference, year | Study characteristics |
Outcome |
Adverse event |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Centers | No. of patients | En bloc resection (%) | R0 resection (%) | Complete remission of dysplasia (%) | Complete remission of intestinal metaplasia (%) | Recurrence (%) | Perforation (%) | Bleeding (%) | Stricture (%) | |
| Terheggen, 2017 [29] | Randomized–controlled trial | 1 | 40 | ||||||||
| Cap EMR | 20 | 15 | 11.8 | 94.1 (at 3 months) | 58.8 (at 3 months) | NA | 0 | 0 | 0 | ||
| ESD | 20 | 100 | 58.8 | 93.8 (at 3 months) | 37.5 (at 3 months) | NA | 10 | 0 | 0 | ||
| Codipilly, 2022 [30] | Retrospective | 1 | 537 | ||||||||
| Cap EMR | 456 | 41.9 | 20.2 | 75.8 (at 2 years) | 59.3 (at 2 years) | NA | 0 | 0.4 | 3.8 | ||
| ESD | 81 | 97.5 | 58 | 85.6 (at 2 years) | 50.6 (at 2 years) | NA | 0 | 2.5 | 5.9 | ||
| Mejia-Perez, 2022 [31] | Retrospective | 8 | |||||||||
| EMR | 150 | 43 | 56 | NA | NA | 31.4a | 0.7 | 4 | 10 | ||
| ESD | 93 | 89 | 73 | NA | NA | 3.5a | 0 | 3.1 | 16 | ||
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; NA, not available.
The primary end point of this study was recurrence, which was defined as the presence of HGD or EAC on biopsies from a lesion at or near the post-ER scar (within 2 cm).
Another retrospective cohort study comparing recurrence rates and resection outcomes of EMR and ESD for early Barrett’s neoplasia reported that ESD resulted in lower recurrence rates compared to EMR (31.4% with ESD vs 3.5% with EMR, P < 0.001) with less need for repeat ER treatment [31]. In this study, recurrence was defined as the presence of any dysplastic or neoplastic lesion after initial ESD. Minimal data exist on the recurrence rates following CE-IM achieved with ESD followed by ablation. Overall, EMR and ESD are both highly effective treatment strategies for early Barrett’s neoplasia, but ESD may result in lower disease recurrence, though this is not well established, particularly after achieving CE-IM. ESD is also not limited by the diameter of the cap and is thus a preferred ER technique in patients with large visible lesions (size >15–20 mm) to allow en bloc resection, providing better histopathologic assessment. ESD should also be considered for visible lesions with worrisome macroscopic appearance concerning for submucosal invasion and in areas where scarring is evident.
Complications of ER
Both ESD and EMR have been shown to be safe ER techniques for the treatment of early Barrett’s neoplasia, but ESD may be associated with a higher rate of severe complications, specifically perforation. Other potential complications include bleeding and stricture formation. A systematic review and meta-analysis showed that the rate of bleeding and stricture were similar in both ESD and EMR groups for the treatment of esophageal squamous cell carcinoma. However, the perforation rate was higher in the ESD group (OR, 2.2; 95% CI, 1.08–4.47; P = 0.03) [28]. Another systematic review and meta-analysis showed that ESD and EMR had similar complication rates, including delayed bleeding, stricture, and perforation, for the treatment of early Barrett’s neoplasia [32].
Endoscopic ablation techniques
Following successful ER of macroscopic lesions, it is essential to perform endoscopic ablation to ensure complete eradication of the remaining BE segment. Without complete ablation of the residual Barrett’s mucosa, the recurrence rate of HGD and early EAC after ER can be strikingly high, up to 36% [33]. Endoscopic ablation can be done with either thermal techniques, including radiofrequency ablation (RFA) and argon plasma coagulation (APC), or cryotherapy techniques. RFA is currently the only ablative modality supported by Level 1 (randomized trial) evidence and thus remains the first-line ablative modality, based on all current GI society guidelines. However, head-to-head trials comparing the available endoscopic ablation techniques, including the data on combination ablative therapies, are lacking.
Thermal ablation techniques
RFA
RFA is currently the most commonly used ablative technique. It has been shown in multiple studies to be highly effective for eradication of IM, which ultimately reduces the risk of neoplastic progression [4, 34]. In the Ablation of Intestinal Metaplasia (AIM) Dysplasia trial, which was a multicenter, randomized, sham-controlled trial, patients who underwent RFA for dysplastic BE were shown to have a higher rate of CRIM (77.4%) when compared with patients who underwent endoscopic surveillance alone (2.3%) [5]. Recurrence following RFA can occur as shown in multiple studies. A large retrospective cohort from the UK, which followed the patients for 10 years, reported an EAC rate of 4.1% at 10 years after initiating the RFA treatment [35]. With this technique, a radiofrequency catheter is applied directly to esophageal mucosa to generate thermal energy within the targeted tissue, causing coagulation necrosis. BE mucosa can be ablated focally using a focal ablation device (Figure 4) or circumferentially using a balloon-based device (Figure 5). The balloon-based device (enabling a circumferential 4-cm treatment zone) is typically used to treat long circumferential BE segments with a length of >3 cm. If the BE mucosa is circumferential but short (< 3 cm) or non-circumferential in nature, the focal ablation catheter can be used. RFA ablation is typically done in multiple endoscopic sessions every 3 months until CE-IM is reached. The safety profile of RFA has also been shown to be acceptable in multiple clinical trials. The most common complication of RFA is esophageal stricture requiring dilations, which occurs in 6%–12% of the patients. Severe complications such as bleeding or perforation are rare [36, 37].
Figure 4.
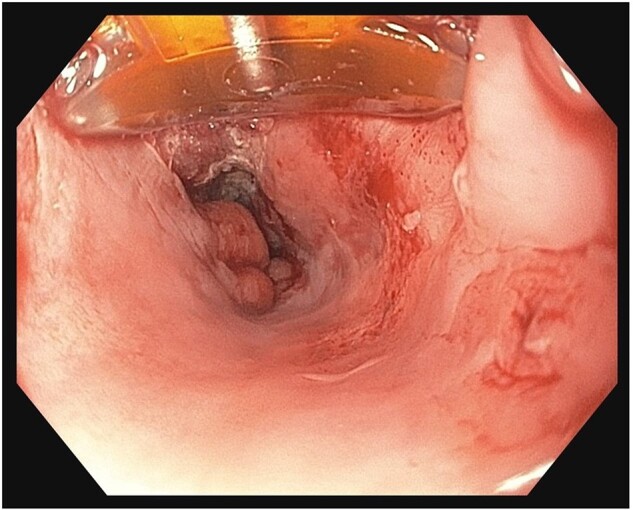
RFA technique using focal ablation device
Figure 5.
RFA technique using balloon-based device. (A) Long circumferential BE segment is noted. (B) Inflated RFA balloon catheter in the esophageal lumen. (C) Post-ablation mucosa.
APC
APC was one of the first endoscopic thermoablation techniques developed for the treatment of BE. The APC device generates electrical energy to ablate the targeted tissue through ionized plasma of argon gas, without direct contact. The APC device consists of an argon-gas source, a high-frequency electrosurgical unit that delivers the electric current, and a flexible Teflon tube that acts as a delivery system (APC probe). The APC probe is introduced through the therapeutic channel of the endoscope and applied to the esophageal mucosa, causing thermal coagulation of the targeted tissue surface. Conventional APC is used mostly for focal ablation of small islands and the most common adverse event is esophageal stricture [38]. To address this complication, hybrid APC has been developed to reduce the stricture rate using submucosal fluid injection prior to the application of APC to prevent unnecessary damage to deeper esophageal layers and allow the application of higher energy levels to treat larger BE segments [39]. A prospective multicenter study from Europe showed that the CRIM rate was 87% with the hybrid APC technique with a low stricture rate of 4% [40]. No randomized trial data or comparative data to RFA are currently available.
Cryoablation techniques
Cryoablation causes cellular damage leading to necrosis of targeted tissue via direct and indirect mechanisms. It causes cold-induced cellular injury via rapid freezing, inducing intracellular/extracellular ice-crystal formation leading to cell-membrane disruption [41]. It also indirectly causes an unfavorable microenvironment that negatively affects tissue viability through several mechanisms, including cellular ischemia and reperfusion injury in the post-thaw period. There are currently two main cryoablation techniques: liquid-nitrogen cryospray and nitrous-oxide-based cryoballoon therapies. The histologic outcomes of this ablative modality appear to be comparable with those of RFA but with a slightly higher rate of esophageal stricture [using cryoballoon ablation (CBA)]. Cryoablation also might have a role in patients with persistent dysplasia or IM after RFA. A systematic review and meta-analysis including 148 patients with RFA-refractory BE treated with liquid-nitrogen cryoablation showed that CE-IM was achieved in 45.9% [42].
Liquid-nitrogen cryospray
The cryospray technique delivers liquid nitrogen at −196°C via a flexible spray catheter in a non-contact fashion. After delivery, the liquid nitrogen rapidly expands into gas and freezes the tissue. Typically, each application is cautiously performed in a 20-s treatment cycle to avoid deep submucosal injury, followed by a 60-s thaw. This is typically repeated three times. Simultaneously, the nitrogen gas is continuously suctioned via a multiport orogastric decompression catheter. Spray cryotherapy has been shown to be a safe and reasonably effective treatment option for dysplastic BE, particularly in patients with short-segment BE. A prospective multicenter trial including 96 patients with dysplastic BE showed that spray cryotherapy achieved a CE-IM rate of 61% in patients with LGD, 66% in patients with HGD, and 77% in patients with short-segment BE with any grade of dysplasia [43].
Nitric-oxide CBA
CBA is a newer generation of cryoablation therapy developed to address the risk of barotrauma requiring decompression in cryospray therapy. With this technique, the nitrous-oxide cryogen is delivered via a rotatable catheter, remaining contained in the balloon, eliminating the need for a decompression catheter. The inflatable cryoballoon is applied directly to the targeted tissue and freezes a 1.5- to 2.0-cm area of the esophageal mucosa in contact with the balloon (Figure 6). A systematic review and meta-analysis including 272 patients showed that cryoballoon is safe and effective for the treatment of BE neoplasia; the study reported a pooled CRIM rate of ≤86% with an esophageal stricture rate of 5% [44]. Another prospective multicenter trial, including 120 patients with dysplastic BE and intramucosal carcinoma, showed that a cryoballoon had a CRIM rate of 72% in the intention-to-treat analysis and a stricture rate of 12% [45]. The efficacy and safety of CBA also appear to be comparable with those of RFA. A recent non-randomized study by Agarwal et al. [46] showed comparative histologic outcomes of CBA and RFA using propensity score-matched analysis. The histologic outcomes of both ablative modalities were comparable (CBA vs RFA: HR, 1.24; 95% CI, 0.79–1.96; P = 0.35); however, the rate of esophageal stricture requiring dilation was higher in the cryoballoon group than in the RFA group (10.6% vs 4.4%, P = 0.04) [46].
Figure 6.
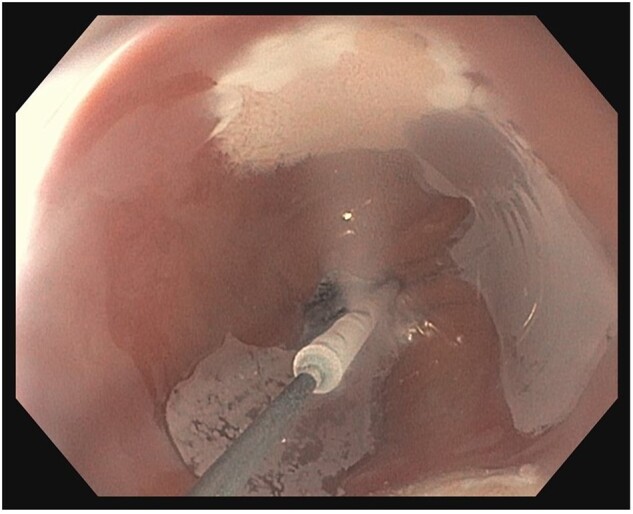
Cryoballoon ablation. Nitrous-oxide cryogen is delivered via a rotatable catheter and contained within the balloon, freezing the BE mucosa by surface contact.
Role of endoscopic ultrasound
Endoscopic ultrasound (EUS) has revolutionized the diagnostic and staging process of upper-GI-tract tumors. Different layers of the GI tract can be endosonographically visualized using EUS, which allows assessment of the depth of tumor invasion (T category) and lymph-node involvement (N category). The role of EUS in patients with advanced EAC (>T1b) and evaluation of nodal disease are well established, but the utility of EUS in staging patients with dysplastic BE and early Barrett’s neoplasia remains unclear. EUS is mainly incorporated into this setting to help differentiate superficial (T1) EAC from deeper disease invading the muscularis propria (T2 or deeper), excluding lymph-node metastasis, thus determining which patients are suitable for EET and avoiding the need for esophagectomy.
A systematic review and meta-analysis from Japan, including 1,019 patients from 19 studies, has reported fairly high sensitivity and specificity of EUS for both T1a and T1b EAC staging [47]. However, several studies from the West have shown that the accuracy of tumor staging with conventional EUS varied significantly between studies and could be suboptimal. A retrospective cohort study by Bartel et al. [48], including 335 patients with dysplastic BE and early EAC, showed that the sensitivity and specificity of patient selection for endoscopic vs surgical resection (≥T1b) were 50% and 93%, respectively. Additionally, 11% of the patients in this study were staged inaccurately, with overstaging more common (7%), which would have resulted in unnecessary esophagectomy [48]. The overstaging is thought to be related to peritumoral inflammation causing esophageal wall thickening, which mimics deeper esophageal layer involvement. A systematic review and meta-analysis by Qumseya et al. [49], including 895 patients of 11 studies, showed that EUS is associated with high false-positive (9.1%) and false-negative rates (9.2%) based on random-effects models. Another study by Bulsiewicz et al. [50], including 135 patients with confirmed HGD and intramucosal EAC who underwent EUS prior to EET, showed that EUS rarely changed the course of management in patients with HGD or early EAC, particularly in patients with non-nodular BE in which submucosal invasion and lymph-node metastasis are extremely rare. Given these controversies, the clinical utility of EUS in the evaluation of dysplastic BE and early EAC is still unclear, with the role of EUS being primarily to exclude metastatic lymphadenopathy and exclude deep muscularis propria invasion [6, 51].
Indications for EET
EET is indicated in patients with BE-related LGD, HGD, and intramucosal (T1a) EAC. EET may also be an alternative to esophagectomy in patients with submucosal (T1b) EAC with low-risk histologic features (<500 µm of submucosal invasion, good to moderate differentiation grade, and no lymphovascular invasion). EET is preferred in these settings given the significantly lower procedure-related morbidity and mortality with comparable survival rates to esophagectomy. Given the low risk of neoplastic progression in non-dysplastic BE (0.2%–0.5% per year), EET is not recommended, with endoscopic surveillance every 3–5 years to detect incident dysplasia or neoplasia. Before proceeding with each treatment strategy, the grade of dysplasia should be confirmed by gastrointestinal pathologists with expertise in BE pathology given the poor interobserver agreement among pathologists on identifying the grade of dysplasia, particularly in LGD [52, 53].
LGD
After the histopathological diagnosis of LGD is confirmed by expert pathologist review, endoscopic ablative therapy may be considered, if no visible lesion requiring ER is identified on endoscopy. An evaluation by expert endoscopists includes careful inspection and resection of visible abnormalities. Endoscopic ablation is recommended given the substantial neoplastic progression rate of LGD to HGD/EAC if the presence of LGD is confirmed by the review of an experienced GI pathologist. A retrospective cohort from the Netherlands, including 293 patients with LGD, has shown that the annual incidence rate of HGD/EAC was 9.1% in patients with an expert review diagnosis of LGD [54]. Additionally, a multicenter randomized control trial from Europe included 136 patients with BE and confirmed LGD showed that patients who underwent RFA had a significantly lower rate of progression to HGD or adenocarcinoma than patients who underwent only endoscopic surveillance (1.5% with RFA vs 26.5% with surveillance; P < 0.001) over a 3-year follow-up period [4]. The CRD rate was 92.6% in the RFA group compared with 27.9% in the surveillance group. Another systematic review and meta-analysis, including 2,746 patients from 19 studies, showed that there was an 86% reduction in the risk of neoplastic progression in the RFA group compared with the surveillance group [relative risk (RR), 0.14; P < 0.001] [55]. Endoscopic surveillance every 6–12 months is an acceptable treatment strategy, particularly if the patients have significant life-limited co-morbidities or are risk-averse [6].
HGD
The risk of neoplastic progression to EAC in HGD is significantly higher than LGD (7%–8% per year). Thus, careful endoscopic examination of the entire BE segment with high-definition white-light endoscopy and advanced imaging techniques such as chromoendoscopy, NBI, or BLI is essential to detect subtle visible lesions, which are more common in patients with HGD. If the visible lesion is localized, ER should be performed either with the EMR or ESD technique depending on the size (>15–20 mm) and macroscopic appearance of the lesion. EET is the first-line treatment option in patients with HGD and has been shown to be a safe and effective treatment strategy for patients with HGD across multiple studies [20, 36]. In the AIM Dysplasia trial, the rate of neoplastic progression in patients without RFA was significantly higher than that of the RFA group (19% in control group vs 2.4% in RFA group, P = 0.04) [5]. EET is now the preferred treatment over esophagectomy in patients with HGD given the lower perioperative morbidity and mortality. A study by Prasad et al. [56] assessed the long-term clinical outcomes following the treatment of HGD with endoscopic therapy (photodynamic therapy with or without EMR) and esophagectomy. The study showed that the overall mortality and survival were comparable in these two groups over the mean follow-up period of 5 years [56]. A systematic review and meta-analysis by Wu et al. [57], including 870 patients with HGD and intramucosal EAC, showed that the 5-year overall survival rate (RR, 1; 95% CI, 0.93–1.06; P = 0.9) and neoplasia-related mortality were similar between the EET and esophagectomy groups but with fewer major post-procedural complications included bleeding, perforation, stenosis, anastomotic leakage, and death (RR, 0.38; 95% CI, 0.2–0.73; P = 0.004).
Intramucosal (T1a) EAC
EET is currently recommended for patients with intramucosal EAC (IMC) given the relatively low risk (0%–2%) of lymph-node metastasis, particularly if high-risk histologic features (such as poor differentiation or lymphovascular invasion) are not present [58]. To date, there is no randomized–controlled trial providing head-to-head data comparing EET and esophagectomy. However, several observational studies have shown that EET is highly effective and safe for patients with intramucosal EAC with a similar overall survival rate and superior cost-effectiveness compared with patients who underwent esophagectomy [57, 59, 60]. A large case series including 1,000 patients with IMC who underwent EET showed that EET achieved complete remission of HGD and EAC in ≤96% of the patients with a mean follow-up period of 5 years [61]. A population-based study of 2,016 patients who underwent EET and esophagectomy for patients with T1a EAC showed no difference in 5-year EAC-related mortality (36.7% with EET vs 42.8% with esophagectomy, P = 0.16) between the two groups [62]. There may be a few circumstances under which surgical resection could be considered a primary treatment modality over EET in IMC. The annual risk of lymph-node metastasis in patients with IMC can be higher (6.9%) if high-risk histologic features (poor differentiation grade, lymphovascular invasion) are present [63]. In these cases, esophagectomy could be discussed as a treatment option with patients.
Submucosal (T1b) EAC
Esophagectomy has been the gold standard for the treatment of submucosal EAC given the higher rate of metastatic lymphadenopathy (20%–25%) [64]. Esophagectomy allows lymph-node dissection and removal in suitable surgical candidates. However, there has been growing evidence in recent years that EET may be an effective alternative treatment option for patients with submucosal EAC without high-risk histologic features, given a relatively low risk for lymph-node metastasis in this setting (0.7%–2%) [63, 65]. Therefore, EET seems to be a reasonable approach in this patient group when being compared with the relatively high mortality rate of esophagectomy at 3%. However, every patient with submucosal EAC should be managed in a multidisciplinary fashion with surgical and oncology team consultation before proceeding with EET. High-risk histologic features include poor differentiation grade, lymphovascular invasion, and deep submucosal invasion (>500 µm). The presence of high-risk histologic features was strongly associated with increased overall mortality [66] and thus esophagectomy should still be the mainstay treatment in these patients with high-risk characteristics unless the patients are poor surgical candidates. A retrospective cohort study by Manner et al. [67], including 66 patients who had submucosal EAC with low-risk histologic features after ER, found that complete endoluminal remission (negative for HGD/EAC in both deep and lateral margins) was achieved in 87% of the patients with a 5-year survival rate of 84%.
End point of EET
The primary end point of EET is achieving CRIM. The definition of CRIM varies slightly among the current literature but is typically defined as one or two surveillance endoscopies negative for IM from surveillance biopsies taken from both tubular esophagus and gastroesophageal junction (GEJ). The main rationale for achieving CRIM is to lower the risk of dysplastic/EAC recurrence after successful EET. By achieving only CRD, the risk of neoplastic progression of the remaining histologically non-dysplastic BE segment to a higher grade of dysplasia or advanced BE neoplasias remains elevated. A systematic review and meta-analysis by Sawas et al. [68], including 4,410 patients from 40 studies, showed that the incidence of dysplastic recurrence was twice as high in patients who only achieved CRD (12%) compared with patients who achieved CRIM (5%). The incidence rate of HGD/EAC recurrence was also significantly higher in the CRD-only group (6% with CRD only vs 3% with CRIM) [68]. In most cases, CRIM is achieved after two to four endoscopic ablative therapy sessions unless the patients have risks factor associated with a lower probability of achieving CRIM, such as long BE segment length (>3 cm), advanced age, and high body mass index (>30) [30, 69].
Management of dysplastic BE in special populations
Patients with esophageal varices
Dysplastic BE is often an actionable diagnosis even in high-risk patients with esophageal varices who often have co-existing portal hypertension, particularly in patients with HGD. Patients with compensated cirrhosis or those who are liver-transplantation candidates should have these dysplastic lesions treated, given the high risk of neoplastic progression to EAC, which ultimately precludes liver transplantation. Surveillance endoscopy may be considered in selective situations, such as in patients with decompensated cirrhosis who are not transplantation candidates, with poor short-term survival. Bleeding complications are the primary concern in patients with esophageal varices who undergo endoscopic treatment for dysplastic BE, especially when ER is performed. Esophagectomy is also not an ideal option for high-risk patients with the exceedingly high rate of post-surgical complications in the setting of portal hypertension. Several case series have shown that EMR appears to be relatively safe in selected patients with cirrhosis, particularly if the varices are completely eradicated prior to ER confirmed using EUS examination [70]. Endoscopic band ligation (EBL) without mucosal resection has also emerged as a safe alternative to EET for treating dysplastic BE but with relatively limited efficacy. Palmer et al. [71] compared histologic outcomes between patients with BE-HGD and concomitant esophageal varices in the setting of cirrhosis who underwent EBL and patients without esophageal varices who underwent EMR/RFA and found that the CRD and CRIM rates achieved in only 12.5% and 37.5%, respectively, in the EBL group. Another approach is to perform ER with modified techniques. A small case series by Hugo et al. [72] showed that modified EMR in patients with concomitant esophageal varices is feasible by performing traditional EBL distally to the targeted dysplastic lesion to block variceal flow prior to EMR.
Patients with dysplastic BE refractory to RFA
The CRIM rate with RFA for dysplastic BE has been reported to be 78% in a systematic review and meta-analysis by Orman et al. [73], indicating that failure to achieve CRIM happens in ∼20% of patients. Before proceeding with other ablative therapies for this patient group, acid-suppression therapy needs to be optimized. Chronic ongoing acid reflux injury has been demonstrated to reduce the efficacy of RFA in achieving CRIM. Optimal medical antireflux therapy, with twice-a-day proton-pump inhibitor dosage (taken 30–45 min before breakfast and dinner), is critical. In those with persistent symptoms or esophagitis on full-dose proton-pump inhibitor therapy, addition of a histamine 2 receptor blocker at bedtime can be considered. A study by Komanduri et al. [74], including 221 patients who underwent EET under a standardized reflux management protocol, reported a CRIM rate of 93%. Forty-five out of 48 patients (93.7%) who did not achieve CRIM after three RFA sessions later achieved CRIM after optimization of their reflux management either medically or with surgical fundoplication. Surgical fundoplication can also be considered in patients with ongoing reflux confirmed by physiological testing who fail RFA despite maximal medical therapy. After acid-suppression therapy is optimized, other ablative modalities can be used as a salvage therapy if the patients still have an inadequate response after RFA. Cryotherapy allows a deeper treatment effect and it is the most-used ablative modality for BE refractory to RFA. A systematic review and meta-analysis by Visrodia et al. [42], including nine studies of 148 patients with persistent dysplasia after RFA, reported a pooled CRIM rate of 45.9% after liquid-nitrogen spray cryotherapy. Apart from cryotherapy, hybrid APC has also been used in patients with BE refractory to RFA. A small case series by Trindade et al. [75] showed that dysplastic BE refractory to RFA could be successfully treated using hybrid APC even without prior cryotherapy.
Post-EET surveillance
The incidence of recurrence, defined by the presence of IM (either non-dysplastic or dysplastic) after achieving CRIM, is not negligible. A systematic review and meta-analysis by Krishnamoorthi et al. [76], including 4,443 patients from 41 studies, showed that the annual recurrence rate of IM (with and without dysplasia), dysplastic BE, and HGD/EAC were 9.5%, 2%, and 1.2% respectively. Thus, a standardized endoscopic surveillance protocol after successful EET is crucial for early detection of BE recurrence. The most typical location for BE recurrence is at or near the GEJ (75%), followed by the distal esophagus within 2–5 cm above the GEJ [77]. Before surveillance biopsies are performed, thorough inspection using white-light endoscopy and chromoendoscopy in both antegrade and retroflexed fashion of the GEJ and tubular esophagus, particularly distal esophagus and in the area of prior BE segment, is mandatory. Any visible nodular lesions should be removed via ER with the same consideration as the initial EET. The threshold for biopsies in suspicious areas should also be very low. If a visible lesion is not present, four-quadrant surveillance biopsies should be obtained from the GEJ in a separate bottle and from the distal 2–5 cm above the GEJ from the neo-squamous epithelium replacing the prior BE segment. The recognized predictors of recurrence are older age, male sex, long BE segment, and pretreatment histology of HGD/early-stage EAC (compared with LGD) [76, 78].
Based on updated American College of Gastroenterology clinical guidelines, the recommended endoscopic surveillance interval depends on the worst pre-ablation histology. Patients with LGD should undergo surveillance endoscopy with biopsies at 1 year post-CRIM and then every 2 years thereafter. In cases of HGD/EAC, the suggested surveillance interval is at 3, 6, and 12 months post-CRIM, and then yearly thereafter [6]. These intervals were suggested given the lower rates of recurrent disease, which justifies the need for less intensive surveillance than previously recommended [79]. If the recurrent disease is present, it should be managed similarly to initial therapy based on histology.
Conclusions
Dysplastic BE should ideally be managed by expert endoscopists in centers with a dedicated high-volume BE endoscopic therapy unit. EET has revolutionized the field of BE treatment and is currently a well-established first-line therapy for dysplastic BE and intramucosal (T1a) adenocarcinoma with the aim of achieving CE-IM. EET may also be a viable treatment option for submucosal (T1b) with low-risk histologic features for lymph-node metastasis, but further prospective studies are needed. In addition, protocolized endoscopic surveillance should be implemented after successful EET to monitor neoplastic progression and BE recurrence.
There are still several gaps in the literature in this field requiring further studies, including identifying the predictors and approaches to treatment failure and disease recurrence. Newer ER and ablative techniques, such as ESD and balloon cryotherapy, have demonstrated promising clinical and histologic outcomes and could become first-line treatment options in selected patients as evidence continues to grow. However, comparative data among these endoscopic treatment techniques are still needed.
Authors’ Contributions
K.V. drafted the manuscript and prepared the figures and table. P.G.I. provided a significant intellectual contribution and revised the manuscript. Both authors reviewed, edited, and approved the final version of the manuscript.
Funding
Research funding: Exact Sciences, Pentax Medical, CDx Medical, Castle Biosciences.
Consultant: Exact Sciences, Pentax Medical, CDx Medical, Castle Biosciences, Ambu, Symple Surgical.
Supported in part by the Freeman Foundation.
Conflict of Interest
None declared.
Contributor Information
Kornpong Vantanasiri, Barrett’s Esophagus Unit, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Prasad G Iyer, Barrett’s Esophagus Unit, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
References
- 1. Singh S, Manickam P, Amin AV. et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:897–909.e4. quiz 83.e1–e3. [DOI] [PubMed] [Google Scholar]
- 2. Rastogi A, Puli S, El-Serag HB. et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 3. American Gastroenterological A, Spechler SJ, Sharma P. et al. ; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:1084–91. [DOI] [PubMed] [Google Scholar]
- 4. Phoa KN, van Vilsteren FG, Weusten BL. et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- 5. Shaheen NJ, Sharma P, Overholt BF. et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277–88. [DOI] [PubMed] [Google Scholar]
- 6. Shaheen NJ, Falk GW, Iyer PG. et al. Diagnosis and management of Barrett's esophagus: an updated ACG guideline. Am J Gastroenterol 2022;117:559–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu Y, Puri V, Shami VM. et al. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg 2016;263:719–26. [DOI] [PubMed] [Google Scholar]
- 8. Zehetner J, DeMeester SR, Hagen JA. et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011;141:39–47. [DOI] [PubMed] [Google Scholar]
- 9. Canto MI. Chromoendoscopy and magnifying endoscopy for Barrett's esophagus. Clin Gastroenterol Hepatol 2005;3:S12– 5. [DOI] [PubMed] [Google Scholar]
- 10. Coletta M, Sami SS, Nachiappan A. et al. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc 2016;83:57–67 e1. [DOI] [PubMed] [Google Scholar]
- 11. Qumseya BJ, Wang H, Badie N. et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett's esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562–70 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. [DOI] [PubMed] [Google Scholar]
- 13. Pech O, Gossner L, Manner H. et al. Prospective evaluation of the macroscopic types and location of early Barrett's neoplasia in 380 lesions. Endoscopy 2007;39:588–93. [DOI] [PubMed] [Google Scholar]
- 14. Wani S, Abrams J, Edmundowicz SA, Gaddam S. et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett's esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci 2013;58:1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wani S, Mathur SC, Curvers WL. et al. Greater interobserver agreement by endoscopic mucosal resection than biopsy samples in Barrett's dysplasia. Clin Gastroenterol Hepatol 2010;8:783–8. [DOI] [PubMed] [Google Scholar]
- 16. Prasad GA, Buttar NS, Wongkeesong LM. et al. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett's esophagus. Am J Gastroenterol 2007;102:2380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pimentel-Nunes P, Libanio D, Bastiaansen BAJ. et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2022. Endoscopy 2022;54:591–622. [DOI] [PubMed] [Google Scholar]
- 18. Tomizawa Y, Friedland S, Hwang JH.. Endoscopic submucosal dissection (ESD) for Barrett's esophagus (BE)-related early neoplasia after standard endoscopic management is feasible and safe. Endosc Int Open 2020;8:E498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martelli MG, Duckworth LV, Draganov PV.. Endoscopic submucosal dissection is superior to endoscopic mucosal resection for histologic evaluation of Barrett's esophagus and Barrett's-related neoplasia. Am J Gastroenterol 2016;111:902–3. [DOI] [PubMed] [Google Scholar]
- 20. Pech O, Behrens A, May A. et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200–6. [DOI] [PubMed] [Google Scholar]
- 21. Pouw RE, van Vilsteren FG, Peters FP. et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc 2011;74:35–43. [DOI] [PubMed] [Google Scholar]
- 22. Sharma P, Shaheen NJ, Katzka D. et al. AGA clinical practice update on endoscopic treatment of Barrett's esophagus with dysplasia and/or early cancer: expert review. Gastroenterology 2020;158:760–9. [DOI] [PubMed] [Google Scholar]
- 23. Hyun JJ, Chun HR, Chun HJ, Jeen YT. et al. Comparison of the characteristics of submucosal injection solutions used in endoscopic mucosal resection. Scand J Gastroenterol 2006;41:488–92. [DOI] [PubMed] [Google Scholar]
- 24. Fujishiro M, Yahagi N, Kashimura K. et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 2004;36:579–83. [DOI] [PubMed] [Google Scholar]
- 25. Harlow C, Sivananthan A, Ayaru L. et al. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv Gastrointest Endosc 2020;13:2631774520957220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang D, Coman RM, Kahaleh M. et al. Endoscopic submucosal dissection for Barrett's early neoplasia: a multicenter study in the United States. Gastrointest Endosc 2017;86:600–7. [DOI] [PubMed] [Google Scholar]
- 27. Subramaniam S, Chedgy F, Longcroft-Wheaton G. et al. Complex early Barrett's neoplasia at 3 Western centers: European Barrett's Endoscopic Submucosal Dissection Trial (E-BEST). Gastrointest Endosc 2017;86:608–18. [DOI] [PubMed] [Google Scholar]
- 28. Guo HM, Zhang XQ, Chen M. et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terheggen G, Horn EM, Vieth M. et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Codipilly DC, Dhaliwal L, Oberoi M. et al. Comparative outcomes of cap assisted endoscopic resection and endoscopic submucosal dissection in dysplastic Barrett's esophagus. Clin Gastroenterol Hepatol 2022;20:65–73.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mejia Perez LK, Yang D, Draganov PV. et al. Endoscopic submucosal dissection vs. endoscopic mucosal resection for early Barrett's neoplasia in the West: a retrospective study. Endoscopy 2022;54:439–46. [DOI] [PubMed] [Google Scholar]
- 32. Komeda Y, Bruno M, Koch A.. EMR is not inferior to ESD for early Barrett's and EGJ neoplasia: an extensive review on outcome, recurrence and complication rates. Endosc Int Open 2014;2:E58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manner H, Rabenstein T, Pech O. et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6–12. [DOI] [PubMed] [Google Scholar]
- 34. Fleischer DE, Overholt BF, Sharma VK. et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy 2010;42:781–9. [DOI] [PubMed] [Google Scholar]
- 35. Wolfson P, Ho KMA, Wilson A, UK RFA Study Group et al. Endoscopic eradication therapy for Barrett's esophagus-related neoplasia: a final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc 2022;96:223–33. [DOI] [PubMed] [Google Scholar]
- 36. Desai M, Saligram S, Gupta N. et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482–95 e4. [DOI] [PubMed] [Google Scholar]
- 37. Qumseya BJ, Wani S, Desai M. et al. Adverse events after radiofrequency ablation in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:1086–95 e6. [DOI] [PubMed] [Google Scholar]
- 38. Manner H, May A, Miehlke S. et al. Ablation of nonneoplastic Barrett's mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006;101:1762–9. [DOI] [PubMed] [Google Scholar]
- 39. Manner H, Neugebauer A, Scharpf M. et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (Hybrid-APC) versus standard APC: a randomized ex-vivo study. United European Gastroenterol J 2014;2:383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knabe M, Beyna T, Rosch T. et al. Hybrid APC in combination with resection for the endoscopic treatment of neoplastic Barrett's esophagus: a prospective, multicenter study. Am J Gastroenterol 2022;117:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whittaker DK. Mechanisms of tissue destruction following cryosurgery. Ann R Coll Surg Engl 1984;66:313–8. [PMC free article] [PubMed] [Google Scholar]
- 42. Visrodia K, Zakko L, Singh S. et al. Cryotherapy for persistent Barrett's esophagus after radiofrequency ablation: a systematic review and meta-analysis. Gastrointest Endosc 2018;87:1396–404 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghorbani S, Tsai FC, Greenwald BD. et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's dysplasia: results of the National Cryospray Registry. Dis Esophagus 2016;29:241–7. [DOI] [PubMed] [Google Scholar]
- 44. Westerveld DR, Nguyen K, Banerjee D. et al. Safety and effectiveness of balloon cryoablation for treatment of Barrett's associated neoplasia: systematic review and meta-analysis. Endosc Int Open 2020;8:E172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canto MI, Trindade AJ, Abrams J. et al. Multifocal cryoballoon ablation for eradication of Barrett's esophagus-related neoplasia: a prospective multicenter clinical trial. Am J Gastroenterol 2020;115:1879–90. [DOI] [PubMed] [Google Scholar]
- 46. Agarwal S, Alshelleh M, Scott J. et al. Comparative outcomes of radiofrequency ablation and cryoballoon ablation in dysplastic Barrett's esophagus: a propensity score-matched cohort study. Gastrointest Endosc 2022;95:422–31 e2. [DOI] [PubMed] [Google Scholar]
- 47. Thosani N, Singh H, Kapadia A. et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:242–53. [DOI] [PubMed] [Google Scholar]
- 48. Bartel MJ, Wallace TM, Gomez-Esquivel RD. et al. Role of EUS in patients with suspected Barrett's esophagus with high-grade dysplasia or early esophageal adenocarcinoma: impact on endoscopic therapy. Gastrointest Endosc 2017;86:292–8. [DOI] [PubMed] [Google Scholar]
- 49. Qumseya BJ, Bartel MJ, Gendy S. et al. High rate of over-staging of Barrett's neoplasia with endoscopic ultrasound: systemic review and meta-analysis. Dig Liver Dis 2018;50:438–45. [DOI] [PubMed] [Google Scholar]
- 50. Bulsiewicz WJ, Dellon ES, Rogers AJ. et al. The impact of endoscopic ultrasound findings on clinical decision making in Barrett's esophagus with high-grade dysplasia or early esophageal adenocarcinoma. Dis Esophagus 2014;27:409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qumseya B, Sultan S, Bain P. et al. ; ASGE Standards of Practice Committee Chair. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 2019;90:335–59 e2. [DOI] [PubMed] [Google Scholar]
- 52. Downs-Kelly E, Mendelin JE, Bennett AE. et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett's esophagus biopsies. Am J Gastroenterol 2008;103:2333–40; quiz 2341. [DOI] [PubMed] [Google Scholar]
- 53. Montgomery E, Bronner MP, Goldblum JR. et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368–78. [DOI] [PubMed] [Google Scholar]
- 54. Duits LC, Phoa KN, Curvers WL. et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015;64:700–6. [DOI] [PubMed] [Google Scholar]
- 55. Qumseya BJ, Wani S, Gendy S. et al. Disease progression in Barrett's low-grade dysplasia with radiofrequency ablation compared with surveillance: systematic review and meta-analysis. Am J Gastroenterol 2017;112:849–65. [DOI] [PubMed] [Google Scholar]
- 56. Prasad GA, Wang KK, Buttar NS. et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology 2007;132:1226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu J, Pan YM, Wang TT. et al. Endotherapy versus surgery for early neoplasia in Barrett's esophagus: a meta-analysis. Gastrointest Endosc 2014;79:233–41 e2. [DOI] [PubMed] [Google Scholar]
- 58. Leers JM, DeMeester SR, Oezcelik A. et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271–8. [DOI] [PubMed] [Google Scholar]
- 59. Chu JN, Choi J, Tramontano A. et al. Surgical vs endoscopic management of T1 esophageal adenocarcinoma: a modeling decision analysis. Clin Gastroenterol Hepatol 2018;16:392–400 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prasad GA, Wu TT, Wigle DA. et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 2009;137:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pech O, May A, Manner H. et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652–60.e1. [DOI] [PubMed] [Google Scholar]
- 62. Wani S, Drahos J, Cook MB. et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc 2014;79:224–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nieuwenhuis EA, van Munster SN, Meijer SL. et al. ; Dutch Barrett Expert Centers. Analysis of metastases rates during follow-up after endoscopic resection of early “high-risk” esophageal adenocarcinoma. Gastrointest Endosc 2022;96:237–47 e3. [DOI] [PubMed] [Google Scholar]
- 64. Badreddine RJ, Prasad GA, Lewis JT. et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol 2010;8:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Manner H, Pech O, Heldmann Y. et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc 2015;29:1888–96. [DOI] [PubMed] [Google Scholar]
- 66. Otaki F, Ma GK, Krigel A. et al. Outcomes of patients with submucosal (T1b) esophageal adenocarcinoma: a multicenter cohort study. Gastrointest Endosc 2020;92:31–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Manner H, Pech O, Heldmann Y. et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol 2013;11:630–5; quiz e45. [DOI] [PubMed] [Google Scholar]
- 68. Sawas T, Alsawas M, Bazerbachi F. et al. Persistent intestinal metaplasia after endoscopic eradication therapy of neoplastic Barrett's esophagus increases the risk of dysplasia recurrence: meta-analysis. Gastrointest Endosc 2019;89:913–25.e6. [DOI] [PubMed] [Google Scholar]
- 69. Shimamura Y, Iwaya Y, Kobayashi R. et al. Clinical and pathological predictors of failure of endoscopic therapy for Barrett's related high-grade dysplasia and early esophageal adenocarcinoma. Surg Endosc 2021;35:5468–79. [DOI] [PubMed] [Google Scholar]
- 70. Prasad GA, Wang KK, Joyce AM. et al. Endoscopic therapy in patients with Barrett's esophagus and portal hypertension. Gastrointest Endosc 2007;65:527–31. [DOI] [PubMed] [Google Scholar]
- 71. Palmer WC, Di Leo M, Jovani M. et al. Management of high grade dysplasia in Barrett's oesophagus with underlying oesophageal varices: a retrospective study. Dig Liver Dis 2015;47:763–8. [DOI] [PubMed] [Google Scholar]
- 72. Uchima H, Ble M, Busquets D. et al. Eradication of neoplastic Barrett's esophagus in patients with esophageal varices with a modified endoscopic mucosal resection technique and radiofrequency ablation. Endoscopy 2022;54:E261–3. [DOI] [PubMed] [Google Scholar]
- 73. Orman ES, Li N, Shaheen NJ.. Efficacy and durability of radiofrequency ablation for Barrett's Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Komanduri S, Kahrilas PJ, Krishnan K. et al. Recurrence of Barrett's esophagus is rare following endoscopic eradication therapy coupled with effective reflux control. Am J Gastroenterol 2017;112:556–66. [DOI] [PubMed] [Google Scholar]
- 75. Trindade AJ, Wee D, Wander P. et al. Successful treatment of refractory Barrett's neoplasia with hybrid argon plasma coagulation: a case series. Endoscopy 2020;52:812–3. [DOI] [PubMed] [Google Scholar]
- 76. Krishnamoorthi R, Singh S, Ragunathan K. et al. Risk of recurrence of Barrett's esophagus after successful endoscopic therapy. Gastrointest Endosc 2016;83:1090–106.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sami SS, Ravindran A, Kahn A. et al. Timeline and location of recurrence following successful ablation in Barrett's oesophagus: an international multicentre study. Gut 2019;68:1379–85. [DOI] [PubMed] [Google Scholar]
- 78. Fujii-Lau LL, Cinnor B, Shaheen N. et al. Recurrence of intestinal metaplasia and early neoplasia after endoscopic eradication therapy for Barrett's esophagus: a systematic review and meta-analysis. Endosc Int Open 2017;5:E430–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kahn A, Crook J, Heckman MG. et al. Optimized surveillance intervals following endoscopic eradication of dysplastic Barrett's esophagus: an international cohort study. Clin Gastroenterol Hepatol 2022. 10.1016/j.cgh.2022.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]