ABSTRACT.
Early detection of SARS-CoV-2 infection is crucial to prevent its spread. This study aimed to document test sensitivity/specificity, correlation with cycle threshold value from polymerase chain reaction (PCR), fitness-for-use in different populations and settings, and user perspectives that could inform large-scale implementation. In this study, we evaluated the performance of a rapid antigen detection test, BD Veritor, and compared this (and another rapid test, Standard Q) against reverse transcription PCR (RT-PCR) in terms of sensitivity and specificity in 130 symptomatic and 130 asymptomatic adults. In addition, we evaluated the suitability and ease of use of the BD Veritor test in a subsample of study participants (n = 42) and implementers (n = 5). At 95% confidence interval, the sensitivity of the BD Veritor and Standard Q test were 70% and 63% in symptomatic and 87% and 73% in asymptomatic individuals, respectively, regarding positive SARS-CoV-2 RT-PCR results. Overall, the BD Veritor test was 78% sensitive and 99.5% specific compared with RT-PCR irrespective of the cycle threshold. This warrants large field evaluation as well as use of the rapid antigen test for quick assessment of SARS-CoV-2 for containment of epidemics in the country.
INTRODUCTION
Rapid antigen detection tests are point-of-care immunochromatographic assays that detect protein antigens specific to the SARS-CoV-2 (e.g., nucleocapsid).1 The ease of use and quick turnaround time of such tests can expand access to testing and decrease delays in diagnosis.2 Furthermore, modeling studies on SARS-CoV-2 have demonstrated that even if rapid antigen testing is associated with decreased sensitivity, the accessibility and short turnaround time in reporting results may be advantageous for decreasing transmission.3 Rapid antigen testing is particularly useful if deployed in the context of repeated testing over time.4,5
The performance of the rapid antigen tests has been determined by comparing their sensitivity and specificity with nucleic acid detection-based reference reaction.6 The current gold standard for identifying the presence of SARS-CoV-2 is reverse transcription polymerase chain reaction (RT-PCR) in samples collected by nasopharyngeal (NP) swab.7 Despite their high sensitivity, nucleic acid amplification tests are associated with the need for laboratory processing, high costs, and a longer turnaround from sampling to return of results.8,9 The NP swabs are also more challenging and uncomfortable (for patients) to collect than anterior nares swabs. For this reason, rapid antigen testing is a valuable tool for contact tracing and early detection of COVID-19 patients to triage for treatment options, especially in settings where RT-PCR is less available or where follow-up reporting of RT-PCR results is more difficult, and particularly when anterior nares samples can be used.
In this study among asymptomatic and symptomatic adults, we evaluated the performance (sensitivity/specificity) of two rapid antigen detection tests, the BD Veritor (Becton-Dickenson, Franklin Lakes, NJ) and the Standard Q (SD-Biosensor, Gyeonggi-do, Korea) rapid antigen test, in comparison to NP swab RT-PCR as the reference standard. The BD Veritor was performed according to the manufacturer’s recommendations using an anterior nares swab specimen, and the Standard Q and reference RT-PCR were performed on NP swab specimens. We also evaluated the performance of the rapid antigen tests across the spectrum of RT-PCR cycle threshold (Ct) values. Finally, we assessed the implementation characteristics of the BD Veritor rapid antigen test, including fitness-for-use in different populations and settings in Bangladesh.
METHODS
Study design and participants.
We enrolled study participants at a triage and sample collection booth at Kurmitola General Hospital (n = 49) as well as at the institute for developing Science and Health initiatives (ideSHi) COVID-19 testing facility (n = 211) in Dhaka, Bangladesh. Among these 211 subjects, 46 (21.8%) were defined as travelers who were Bangladeshi nationals planning to travel abroad and came for a COVID-19 positivity test at the ideSHi facility before traveling. Adults aged 18 and older were eligible for inclusion. For this analysis, we aimed to enroll 130 symptomatic patients with Covid-19 like symptoms including fever, cough, headache, sore throat, shortness of breath and fatigue10 who had their onset of first symptom within five days, including 100 individuals with negative RT-PCR results and 30 individuals with positive RT-PCR results. In addition, we aimed for a similar target for positive and negative asymptomatic individuals who presented for routine COVID-19 screening at the above sites (primarily occupational screening or for known contact with an individual who tested positive). Written informed consent was obtained from participants. The study was approved by the Research Review Committee (RRC) and Ethical Review Committee (ERC) of the International Center for Diarrheal Disease Research (icddr,b; Protocol no: PR-20042). All procedures were performed in accordance with relevant Good Clinical Practice guidelines.
Specimen collection.
NP swab specimens were collected by trained personnel and placed in a 3-mL tube of viral transport medium (Citoswab, Citotest Labware Manufacturing Co. Ltd., Jiangsu, China) to be used for both Standard Q antigen testing and RT-PCR testing. Anterior nares swab samples were also collected by trained personnel according to the manufacturers’ instructions for BD Veritor. Specifically, the swab provided with the kit was inserted into the anterior nasal cavity up to 2.5 cm and rolled five times along the mucosal surface in both nostrils. The NP and anterior nares swab specimens were collected simultaneously until the first 200 individuals with negative RT-PCR results were enrolled in each group (100 symptomatic and 100 asymptomatic). Thereafter, the anterior nares samples were collected within 24 hours of the NP specimen until 60 individuals with positive RT-PCR results were accrued (30 symptomatic and 30 asymptomatic).
RT-PCR on NP swab specimens.
Viral RNA was extracted from 200 μL of viral transport media using the magnetic bead based Nexor 32 Fully Automated Nucleic Acid Extractor (Nucleic Acid Extraction or Purification Kit, Beijing Lepu Medical Technology Co., Ltd., Beijing, China). RT-PCR was carried out using the China CDC primer and probes. In brief, this was performed in a 20 μL reaction volume and each reaction contained extracted RNA, 2x iTaq Universal Probes Reaction Master Mix (Bio-Rad, Hercules, CA), iScript Reverse Transcriptase, the CDC_ORF1ab and N forward and reverse primers, and probe.11,12 Specimens were determined to be positive for SARS-CoV-2 when the ORF1ab and N genes were detected with an exponential growth curve and a Ct value < 40, and negative when these genes could not be detected. The test was considered positive even if one gene was detected. The quality of the NP specimen extracted was determined by analyzing the curve generated with the RNase P housekeeping gene.
Rapid antigen testing.
The rapid tests were performed in accordance with the manufacturer’s instructions. For the BD Veritor assay, the anterior nares swab was inserted into the extraction reagent tube and mixed in the fluid for a minimum of 15 seconds before discarding. Three drops of the processed specimen were added to the sample well of the device and incubated for 15 minutes. The test device was then inserted into the Veritor Plus Analyzer (BD) for reading.
For the Standard Q kit, 350 μL of freshly obtained NP swab specimen in viral transport medium was reconstituted in the extraction buffer supplied by the manufacturer and incubated for 45 to 50 minutes. For testing, three drops (∼80 μL) of extracted NP specimen was applied to the sample well of device, and results were interpreted after 15 minutes, based on the manufacturer’s instructions.
Assessment of implementation characteristics.
We surveyed five test implementers and 42 participants about the BD Veritor test with a user acceptability and adoption assessment form for implementers and a feedback form for participants. Five-point Likert scale was used for documenting the level of satisfaction and level of difficulties in addition to the qualitative aspects in the questionnaire. We also assessed the BD Veritor test compared with NP swab RT-PCR regarding resources to collect and transport samples, and use of personal protective equipment and consumables. We evaluated turnaround times (from the sample collection time until the result was reported to the participant) for each sample type, assay, and platform from the time of collection to delivery of results.
Statistical analysis.
We calculated the sensitivity and specificity13 of each rapid test compared with the NP RT-PCR gold standard and reported these as a percentage with 95% confidence intervals (CIs). The sensitivity of both rapid tests (combined) was also analyzed in RT-PCR positive samples stratified by Ct value > 30, 20–30, and < 20. The sensitivity of the two rapid tests was compared and the P value was calculated using McNemar’s chi-square test. In addition, the comparison of Ct values between symptomatic and asymptomatic individuals was performed using the Mann-Whitney U test.
RESULTS
We enrolled 262 individuals in this study. Two study participants were subsequently excluded from the analysis because of incomplete information in the case record forms, resulting in a study set of 260 individuals. Forty-nine of the symptomatic individuals were enrolled at the Kurmitola General Hospital, and the remaining 211 participants included in the analysis were enrolled at the ideSHi testing facility. Demographic characteristics are shown in Table 1. The median age of the symptomatic individuals was 35 years (range, 18–81 years), and 53% were male. The most common symptom was fever (N = 88, 68%), and the median duration (and range) of symptoms were 3 days (1–5 days) in the symptomatic patients. The clinical team at the study site and at home visits recorded the vital signs such as pulse, blood pressure, temperature, and respiratory rate during enrollment. The symptoms (duration and onset) were reported by the patients themselves. The underarm temperature cutoff was set as 99°F. Oral temperature was not measured considering the COVID situation. The median age of the asymptomatic individuals was 33 years (range, 18–74), and 81% were male. The history of contact was denoted as positive in case of contact with a PCR positive patient. Participants were asked about their mask use when going out, during social gatherings, and during any close contact with other persons. From Table 1, it can be observed that the PCR-positive asymptomatic individuals reported lower use of masks than the other groups.
Table 1.
Demographic characteristics in different groups of participants
| Characteristic | Symptomatic | Asymptomatic | ||
|---|---|---|---|---|
| PCR+ (N = 30) | PCR– (N = 100) | PCR+(N = 30) | PCR– (N = 100) | |
| Median age, years | 46.5 | 32 | 33.5 | 33 |
| Sex | ||||
| Male | 15 (50%) | 54 (54%) | 29 (96%) | 76 (76%) |
| Female | 15 (50%) | 46 (46%) | 1 (4%) | 24 (24%) |
| Population category | ||||
| Traveler | 0 (0%) | 0 (0%) | 19 (63.3%) | 27 (27%) |
| Student | 4 (13.3%) | 19 (19%) | 2 (6.7%) | 1 (1%) |
| Healthcare worker | 3 (10%) | 6 (6%) | 0 (0%) | 2 (2%) |
| General population | 23 (76.6%) | 75 (75%) | 9 (30%) | 70 (70%) |
| Symptom spectrum† | – | – | ||
| Fever alone | 1 (3.3%) | 13 (13%) | ||
| Fever+cough | 4 (13.3%) | 7 (7%) | ||
| Fever+headache | 0 (0%) | 5 (5%) | ||
| Fever+cough+headache | 5 (16.7%) | 10 (10%) | ||
| Symptoms without fever | 7 (23.3%) | 35 (35%) | ||
| History of contact | 15 (50%) | 43 (43%) | 7 (23.3%) | 3 (3%) |
| History of regular use of mask | 27 (90%) | 89 (89%) | 24 (80%) | 97 (97%) |
Other symptoms not mentioned include sore throat, shortness of breath, diarrhea, loss of taste and/or smell, generalized weakness
Distribution of other demographic characteristics such as age, sex, body mass index, and comorbidities among the groups defined by the rapid tests and RT-PCR result interpretation was analyzed (Table 2). Parameters were similar in all groups. However, the presence of comorbid conditions such as diabetes mellitus and asthma was higher among the false-negative groups by both rapid kits.
Table 2.
Distribution of demographic characteristics and comorbidities among the groups categorized by the different rapid test and RT-PCR result interpretation
| Parameters | BD+ (N = 47) | BD– (N = 199) | BD FP (N = 1) | BD FN (N = 13) | Standard Q+ (N = 41) | Standard Q– (N = 200) | Standard Q FP (N = 0) | Standard Q FN (N = 19) |
|---|---|---|---|---|---|---|---|---|
| Age, years | ||||||||
| Mean | 39 | 37 | 30 | 41 | 40 | 37 | – | 39 |
| Range | 18–67 | 18–81 | – | 22–74 | 18–61 | 18–81 | 22–74 | |
| Sex | ||||||||
| Male | 37 (78.72) | 129 (64.82) | 1 (100) | 7 (53.85) | 32 (78.05) | 130 (65.0) | – | 12 (63.16) |
| Female | 10 (21.28) | 70 (35.18) | – | 6 (46.15) | 9 (21.95) | 70 (35.0) | – | 7 (36.84) |
| BMI | – | – | ||||||
| Normal | 21 (44.68) | 114 (57.29) | – | 5 (38.46) | 19 (46.34) | 114 (57.0) | – | 7 (36.84) |
| Overweight | 23 (48.94) | 66 (33.17) | 1 (100) | 7 (53.45) | 20 (48.78) | 67 (33.50) | – | 10 (52.63) |
| Obese | 3 (6.38) | 18 (9.05) | – | 1 (7.69) | 2 (4.88) | 18 (9.0) | – | 2 (10.53) |
| Underweight | – | 1 (0.50) | – | – | – | 1 (0.50) | – | – |
| Comorbidities | – | |||||||
| DM | 8 (17.02) | 23 (11.56) | – | 3 (23.08) | 7 (17.07) | 23 (11.50) | – | 4 (21.05) |
| HTN | 7 (14.89) | 27 (13.57) | – | 2 (15.38) | 7 (17.07) | 27 (13.50) | – | 2 (10.53) |
| Asthma | 3 (6.38) | 9 (4.52) | – | 2 (15.38) | 2 (4.87) | 9 (4.50) | – | 3 (15.79) |
| CKD | – | 1 (0.50) | – | – | – | 1 (0.50) | – | – |
| CHD | 3 (6.38) | 5 (2.51) | – | – | 3 (7.32) | 5 (2.50) | – | – |
RT-PCR = reverse transcriptase polymerase chain reaction. BD+ = specimens positive by both BD Veritor kit and RT-PCR; BD– = specimens negative by both BD Veritor kit and RT-PCR; BD FP = false-positive result by BD Veritor kit; BD FN = false-negative result by BD Veritor kit; Standard Q+ = specimens positive by both Standard Q kit and RT-PCR; Standard Q– = specimens negative by both Standard Q kit and RT-PCR; Standard Q FP = false-positive result by Standard Q kit; Standard Q FN = false-negative result by Standard Q kit.
The sensitivity and specificity of both rapid tests are reported in Table 3. The sensitivity of the BD Veritor test (78%) was higher than that of the Standard Q test (68%; P = 0.041). The sensitivity of both tests was higher in asymptomatic individuals than in symptomatic individuals. Only one false-positive BD Veritor test was identified in a screened symptomatic individual who reported a 4-day history of cough with no fever, myalgia, or loss of smell. No false-positive Standard Q tests were observed.
Table 3.
Performance of two rapid antigen detection tests for detection of SARS-CoV-2 compared with nasopharyngeal RT-PCR
| Category | BD+ (n)* | BD– (n)* | BD performance (95% CI) | Standard* Q+ (n) | Standard* Q– (n) | Standard Q performance (95% CI) | |
|---|---|---|---|---|---|---|---|
| Symptomatic | PCR+ (n = 30) | 21 | 9 | Sensitivity 70% (54–88%) | 19 | 11 | Sensitivity 63% (47–83%) |
| PCR– (n = 100) | 1 | 99 | Specificity 99% (95–100%) | 0 | 100 | Specificity 100% (69–100%) | |
| Asymptomatic | PCR+ (n = 30) | 26 | 4 | Sensitivity 87% (69–96%) | 22 | 8 | Sensitivity 73% (61–92%) |
| PCR– (n = 100) | 0 | 100 | Specificity 100% (69–100%) | 0 | 100 | Specificity 100% (69–100%) | |
| Overall sensitivity and specificity (both symptomatic and asymptomatic participants) | |||||||
| Positive PCR | n = 60 | 47 | 13 | Sensitivity 78% (66–88%) | 41 | 19 | Sensitivity 68% (55–80%) |
| Negative PCR | n= (200) | 1 | 199 | Specificity 99.5% (97–100%) | 0 | 200 | Specificity 100% (98–100%) |
| Combined symptomatic and asymptomatic categorized by RT-PCR Ct value | |||||||
| Ct > 30 | n = 13 | 1 | 12 | Sensitivity 8% (0–36%) | 0 | 13 | Sensitivity 0% (0–25%) |
| Ct 20–30 | n = 32 | 31 | 1 | Sensitivity 97% (84–100%) | 26 | 6 | Sensitivity 81% (64–93%) |
| Ct < 20 | n = 15 | 15 | 0 | Sensitivity 100% (78–100%) | 15 | 0 | Sensitivity 100% (78–100%) |
CI = confidence interval; Ct = cycle threshold; RT-PCR = reverse transcriptase polymerase chain reaction; BD+ = specimens positive by BD Veritor kit, BD– = specimens negative by BD Veritor kit; Standard Q+ = specimens positive by Standard Q kit; Standard Q– = specimens negative by Standard Q kit.
For BD Veritor testing kit, anterior nares specimens and for Standard Q, nasopharyngeal specimen were tested based on manufacturer’s instruction.
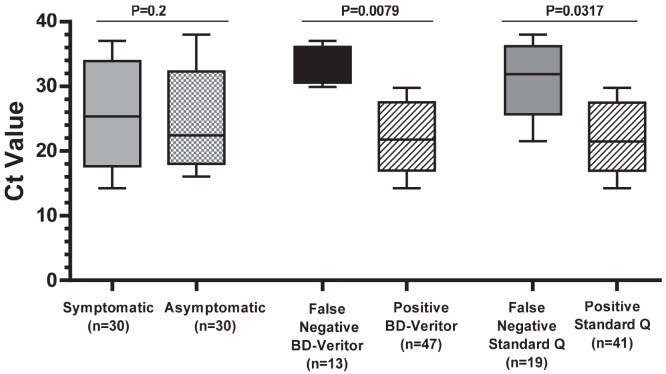
We compared the performance of both rapid antigen tests stratified by the observed Ct value of the NP RT-PCR. There was no difference in the median Ct value between the symptomatic and asymptomatic individuals (Figure 1). We also observed no significant difference (P = 0.2 by Mann-Whitney U) in the range of Ct values between the two groups and hence considered such individuals together in the analysis stratified by Ct value (Table 3). For RT-PCR samples with a Ct < 20 (i.e., those with a high amount of viral genetic material), the sensitivity of both rapid antigen tests was 100%. For RT-PCR samples with a Ct > 30 (i.e., those with little viral genetic material), neither rapid antigen test performed well (sensitivity < 10%). For RT-PCR samples with Ct values in the 20 to 30 range, the sensitivity of the BD Veritor test was 97% (95% CI: 84–100%), compared with 81% (95% CI: 64–93%) for the Standard Q.
Figure 1.
Comparison of cycle threshold (Ct) values among symptomatic and asymptomatic study participants and across rapid antigen tests. There was no significant difference in the median Ct value in polymerase chain reaction–positive symptomatic and asymptomatic participants; however, the median Ct values were significantly higher in individuals with false-negative rapid antigen tests.
We also interviewed a subgroup of participants (n = 42) within 24 hours after having obtained the anterior nares swab for the BD Veritor rapid test and asked them about the suitability of the test, the ease of the sample collection process, the ease of testing, the accuracy of the test, and the turnaround time. Four participants were dissatisfied with the accuracy of the result by the BD Veritor, and the remainder of the participants (n = 38) were satisfied. All 42 interviewees expressed satisfaction with the sample collection process, ease of testing, and the 15- to 20-minute turnaround time. The five implementers surveyed, each of whom performed more than 50 tests, were satisfied with the kit components, design of the device, kit storage conditions, quality controls, time taken for the sequence of steps, readout of the results, and the suitability for batch testing along with sequential testing. Based on their experience, testers estimated > 100 patients could be tested and results given with the BD Veritor kit in one 8-hour working day.
DISCUSSION
We evaluated the performance of the BD Veritor rapid antigen test and compared it with the Standard antigen test for detecting SARS-CoV-2 in asymptomatic and symptomatic adults in a real-world, community-based study in Bangladesh between January and April 2021. We compared both tests to the gold standard RT-PCR performed on a NP swab. We found the BD Veritor test to be more sensitive (78%) than the Standard Q (68%) in our study population.
The sensitivity of both rapid antigen tests was highly dependent on the Ct value of the specimen evaluated. Both tests were 100% sensitive among individuals with Ct < 20—those with high viral loads. These findings are consistent with a study in China that reported 68% sensitivity and 100% specificity when a Ct value ≤ 40 was used as a cutoff, compared with 98% sensitivity and 100% specificity when Ct value ≤ 30.14 This is expected because the higher Ct value represents lower viral copies present in the specimen.
Interestingly, we found that the sensitivity of both the rapid antigen tests was also high among asymptomatic individuals than the symptomatic individuals. Apart from the high viral loads indicated by Ct values among the asymptomatic individuals, no significant difference was found in demographic characteristics, clinical features, and comorbid conditions between the two groups. In Bangladesh, the national COVID-19 prevalence rate was 24% in April 2021; however, the prevalence rate varied throughout the study period (range 2–24%) from January 2021 to April 2021. Of note, most of the asymptomatic RT-PCR positive participants were enrolled during the period in which infections surged, whereas most of the symptomatic individuals were enrolled before the surge. Therefore, the performance of rapid antigen might vary among population groups due to different epidemiologic and geographic conditions and impact of the variants of concern circulating in that period, although no difference has been found to date.5,15 In Bangladesh, the B.1.1.7 variant, followed by the B.1.351 variant, predominated in Bangladesh at the time (https://www.epicov.org/epi3/frontend#45859a).
The use of anterior nares specimens for the BD Veritor rapid antigen test was an added advantage, and study participants found this test to be more acceptable than the NP swab. An additional advantage of the BD Veritor testing was that it was carried out directly at the study site, and results were available immediately. In several instances, study participants were informed to isolate themselves until RT-PCR results confirmed infection. In addition, the analyzer provided with the kit was able to detect very faint bands on the device that were barely visible by the naked eye, which reduces the chance of human bias. Inclusion of the positive and negative controls with other kit components was helpful for quality assessment of the analyzer before use.
A limitation of our study is that we compared rapid antigen tests that required different types of clinical specimens: For the BD Veritor, we used anterior nares specimens for rapid test and a NP sample for PCR; the Standard Q uses only one NP specimen, which is diluted in viral transport media and used for both PCR and rapid test. If the concentration of the virus differs between anterior nares and NP swabs—that is, in the two sites of the nostril, it can also affect results. However, we did not see significant discrepancies between the two tests, except when specimens had high RT-PCR Ct values, indicating low viral loads. In that case, both BD Veritor and the Standard Q failed to detect SARS-CoV-2 virus. Notably, we also found the BD Veritor rapid antigen test to be more sensitive even though the anterior nares specimen for it were collected later for the RT-PCR–positive individuals.
Our study on rapid antigen tests is timely for Bangladesh, which at the time of this writing is experiencing a second wave and high rates of COVID-19.16 Point-of-care tests are urgently needed for health facilities, travelers, workplaces, and the general population, and our findings can help guide the implementation of these tests in Bangladesh. The BD Veritor test is sensitive enough to detect cases with high viral load in presymptomatic and early symptomatic cases as well as asymptomatic persons—groups that likely contribute to a significant proportion of transmission and spread of the disease.17 The patients who test positive by rapid antigen tests can readily be diagnosed with a minimum turnaround time, which offers the opportunity for early interruption of transmission through targeted isolation and contact tracing, as infectivity may be high with a Ct of < 24.18 Persons with negative rapid test who are suspected of having COVID can be tested by RT-PCR, depending on the epidemiologic context. Our information can provide implementation guidance when deciding testing strategies in different settings.
Many countries are now planning the expanded use of rapid antigen tests, and our results will provide guidance on their implementation in real-world settings such as that performed our study site in Bangladesh. Rapid antigen tests will be a critical component of COVID-19 control for the foreseeable future.
ACKNOWLEDGMENTS
We acknowledge the support of Mass General Brigham Center for COVID Innovation for providing the BD Veritor plus analyzer antigen detection kits and the Massachusetts General Hospital for facilitation of the work. We thank the Bill and Melinda Gates Foundation for support to icddr,b investigators. ideSHi and icddr,b are grateful to the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support. We also acknowledge the overall support of Foundation Merieux to ideSHi. We express our gratitude to the participants for taking part and the field site staff of ideSHi for their effort to accomplish the work. The American Society of Tropical Medicine and Hygiene has waived the open-access fee for this article due to the ongoing COVID-19 pandemic.
REFERENCES
- 1. Chaimayo C. et al. , 2020. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J 17: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eshghifar N, Busheri A, Shrestha R, Beqaj S, 2021. Evaluation of analytical performance of seven rapid antigen detection kits for detection of SARS-CoV-2 virus. Int J Gen Med 14: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy-Shaffer L, Baym M, Hanage WP, 2021. Perfect as the enemy of good: tracing transmissions with low-sensitivity tests to mitigate SARS-CoV-2 outbreaks. Lancet Microbe 2: e219–e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johansson MA. et al. , 2020. Reducing travel-related SARS-CoV-2 transmission with layered mitigation measures: Symptom monitoring, quarantine, and testing. medRxiv. Available at: https://www.medrxiv.org/content/10.1101/2020.11.23.20237412v1. Accessed June 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holmdahl I, Kahn R, Hay JA, Buckee CO, Mina MJ, 2021. Estimation of transmission of COVID-19 in simulated nursing homes with frequent testing and immunity-based staffing. JAMA Netw Open 4: e2110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO , 2020. Antigen-detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays Interim Guidance. Geneva, Switzerland: World Health Organization. Available at: https://apps.who.int/iris/handle/10665/334253.
- 7. Olalekan A, Iwalokun B, Akinloye OM, Popoola O, Samuel TA, Akinloye O, 2020. COVID-19 rapid diagnostic test could contain transmission in low- and middle-income countries. Afr J Lab Med 9: 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambert-Niclot S, Cuffel A, Pape L, Vauloup-Fellous C, Morand-Joubert L. Evaluation of a rapid diagnostic assay for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. J Clin Microbiol 15635;58(May): e00977-20. [DOI] [PMC free article] [PubMed]
- 9. Udugama B. et al. , 2020. Diagnosing COVID-19: the disease and tools for detection. ACS Nano 14: 3822–3835. [DOI] [PubMed] [Google Scholar]
- 10. Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R, 2021. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed] [Google Scholar]
- 11. Jung Y. et al. , 2020. Comparative analysis of primer-probe sets for RT-qPCR of COVID-19 causative virus (SARS-CoV-2). ACS Infect Dis 6: 2513–2523. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention , 2019. Novel Coronavirus (2019-nCoV) Real-time rRT-PCR Panel Primers and Probes Note: Oligonucleotide Sequences are Subject to Future Changes as the 2019-Novel Coronavirus, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf.
- 13. Smith CJ, 2012. Diagnostic tests (1)—sensitivity and specificity. Phlebology 27: 250–251. [DOI] [PubMed] [Google Scholar]
- 14. Diao B. et al. , 2020. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv. doi: 10.1101/2020.03.07.20032524. [DOI] [Google Scholar]
- 15. Saeed U. et al. , 2021. Evaluation of SARS-CoV-2 antigen-based rapid diagnostic kits in Pakistan: formulation of COVID-19 national testing strategy. Virol J 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Experts: Bangladesh’s second covid wave has peaked, situation likely to improve in May Published online 2021. Available at: https://www.dhakatribune.com/bangladesh/2021/04/17/experts-bangladesh-s-second-covid-wave-has-peaked-situation-likely-to-improve-in-may.
- 17. European Centre for Disease Prevention and Control , 2020. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. Available at: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-first-update.
- 18. Street L, 2016. Predicting infectious SARS-CoV-2 from diagnostic samples. 306: 0–19.