ABSTRACT.
The bidirectional interaction between undernutrition and infection can be devastating to child health. Nutritional deficiencies impair immunity and increase susceptibility to infection. Simultaneously, infections compound undernutrition by increasing metabolic demand and impairing nutrient absorption. Treatment of acute malnutrition (wasting) can reverse some of its deleterious effects and reduce susceptibility to infectious diseases. Nutrition-specific approaches may be packaged with other interventions, including immunization, to support overall child health. To understand how mass nutritional supplementation, treatment of wasting, and vaccination affect the dynamics of a vaccine-preventable infection, we developed a population-level, compartmental model of measles transmission stratified by age and nutrition status. We simulated a range of scenarios to assess the potential reductions in measles infection and mortality associated with targeted therapeutic feeding for children who are wasted and with a mass supplementation intervention. Nutrition interventions were assumed to increase engagement with the health sector, leading to increased vaccination rates. We found that the combination of wasting treatment and mass supplementation coverage followed by an increase in vaccination coverage of non-wasted children from a baseline of 75% to 85%, leads to 34% to 57% and 65% to 77% reduction in measles infection and mortality and 56% to 60% reduction in overall mortality among wasted children, compared with the wasting treatment alone. Our work highlights the synergistic benefits that may be achieved by leveraging mass nutritional supplementation as a touch point with the health system to increase rates of vaccination and improve child survival beyond what would be expected from the additive benefits of each intervention.
INTRODUCTION
Undernutrition and infection interact in a bidirectional manner. Both micronutrient and macronutrient deficiencies stunt a child’s growth, impair immunity, increase susceptibility to infection, and worsen outcomes from common infectious diseases.1 The risk of adverse outcomes from infection is correlated with the degree of undernutrition; concurrently, infection contributes to undernutrition by reducing a child’s ability to consume food, contributing to nutrient malabsorption, and increasing metabolic demand.2–4 As an example, a higher cumulative burden of diarrhea during the first 2 years of life increases the risk of stunting at 24 months of age.5 Improving the baseline nutritional status of children and providing treatment of acute malnutrition (wasting) lowers the negative impacts of infections on growth by strengthening children’s immune systems, preventing poor appetite, compensating for malabsorption, and favoring the growth of beneficial gut microorganisms.2,6
Undernutrition can lead to long-lasting immune deficits, rendering undernourished children at a significantly increased risk of respiratory infections, diarrhea, malaria, and measles.3,7–9 Even seemingly mild manifestations of these common infectious diseases can have long-term effects on children’s physical and cognitive development.10 Identifying patterns of association between undernutrition and infection are important to the clinical and public health efforts in reducing childhood morbidity and mortality.1 The bidirectional relationship between the two events has been well documented, and its importance recognized for decades.4,11 Few studies, though, have explored the impact of undernutrition on the transmission dynamics of infectious diseases. Among these, there has been investigation into the effect of undernutrition, or specific nutritional therapies, on transmission of tuberculosis12 and cholera,13 emphasizing the need to address nutrition to reduce the burden of the infection. There are also animal models of infection and undernutrition that show infection can cause undernutrition; specifically, weanling undernutrition exacerbates infection and mucosal disruption,14–17 and increases the intensity of infection in neonatal mice, as assessed by stool shedding, by one to four orders of magnitude.15
Undernutrition is a primary contributor to death in 44.8% of childhood fatalities from measles.18 Malnourished children are more likely to develop complications of measles and have a higher case-fatality ratio,19 whereas measles infection can in turn worsen the nutritional status of children. Koster et al.20 found that measles had an adverse impact on both mortality and the nutritional status of surviving children. In their prospective study in Bangladesh, children 7 to 23 months of age experienced a persistent nutritional deficit of about 10% after measles infection, followed by prolonged diarrhea. Another study in an urban settlement in Guinea-Bissau also found a negative impact of measles on the nutritional status of children aged 9 to 35 months old.21 Several studies have shown that measles was a precipitating cause of kwashiorkor22–24 and that there have been reports of a reduction in the incidence of kwashiorkor after measles vaccine campaigns.23
Interventions focused on treating or preventing undernutrition often rely on increasing the engagement of mothers and children with the health sector. Intentionally packaging nutrition-specific care with routine immunization could lead to enhanced effectiveness in reducing undernutrition but also improve prevention efforts against infectious disease. In fact, nutritional commodities may be perceived as an incentive to draw patients to health centers where other services may be offered. Previous work has shown that an incentive as simple as a bag of lentils for each immunization visit, has large positive impacts on the uptake of immunization services in resource-poor areas.25 In addition, vaccination uptake can enhance long-term nutrition outcomes, and targeted vaccination of children with poor sociodemographic characteristics can improve their overall nutrition status.26
We developed a population-level, dynamic model of measles transmission stratified by nutrition status to understand how nutrition-based interventions and vaccination collectively affect measles infection and mortality. In particular, we modeled two scenarios. In the first, wasted children received treatment with ready-to-use therapeutic food (RUTF). The second included treatment of wasted children, as well as mass supplementation of all children aged 6 to 23 months with small quantity lipid-based nutritional supplements (SQ-LNS). LNS are a class of ready‐to‐use food supplements that are highly nutrient‐dense and fortified with vitamins and minerals at levels designed to treat and prevent acute malnutrition. They range in ration sizes based on their use to either treat or prevent acute malnutrition, with ready-to-use therapeutic or supplementary foods (RUTF, RUSF) coming in 92- or 100-g sachets, medium-quantity LNS in 50-g sachets, and 20-g sachets of SQ-LNS for home fortification of local diets to improve children’s complementary feeding. There is strong evidence in support of implementing mass SQ-LNS supplementation among at-risk children from 6 to 18 or 23 months, like those in Niger.27 Such supplementation has been shown to reduce stunting, wasting, anemia,28 and all-cause mortality,29 as well as improve developmental outcomes.30
We accounted for the nature, timing, and magnitude of interactions between measles and undernutrition under the different simulated scenarios. Model parameters reflected the situation in Niger, which was considered to be an appropriate case study due to its high rates of undernutrition and its persistent measles burden. Undernutrition is the leading cause of infant mortality and morbidity in Niger.31 The levels of undernutrition in Niger are among the highest in the world, with 43.5% and 12.5% of children under 5 years of age being stunted and wasted in 2021, respectively; in the rural Zinder region, stunting and wasting rates were even higher at 57.4% and 14.3%.32 These rates are much higher than the average for the African continent as a whole (30.7% and 6.0%, respectively).33 Outbreaks of vaccine-preventable diseases like measles are also common in Niger.34,35
Our modeling scenarios can help us understand the complex connections between undernutrition and measles that have not been disentangled by previous studies and may help inform approaches to intervention. There is strong evidence for the positive impact on preventing undernutrition and mortality through mass supplementation with child-adapted foods like SQ-LNS. If our model demonstrates an additive benefit of increased measles vaccination, it would justify further investment by governments and donors in scaling up such programs especially in areas with a simultaneous high burden of malnutrition and low coverage for measles vaccination.
MATERIALS AND METHODS
Measles-undernutrition model with wasting treatment (Scenario 1: Treatment).
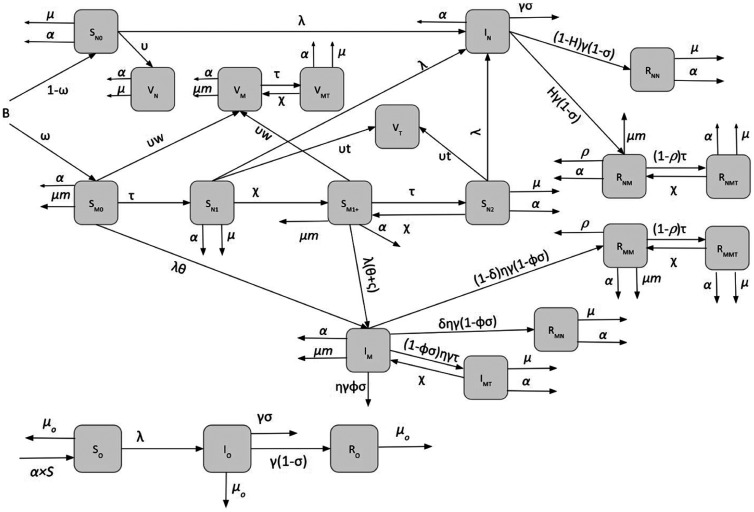
We developed a seasonally forced, deterministic, continuous-time susceptible-infectious-recovered (SIR) model of measles transmission that accounts for vaccination and for nutritional interventions (Figure 1). We stratified the population into children 6 to 23 months and individuals of all other ages, with children aging from the former into the latter compartment at a rate α. We assumed a fixed population of N0 = 100,000 and the demographic characteristics of Niger such as birth rate and death rates.36–38 The population size of infants up to 6 months is assumed to be = 5000. A full list of parameter values is given in Table 1, and model equations are also given in supplementary material.
Figure 1.
Schematic of measles-undernutrition model with no mass nutritional supplementation (Scenario 1).
Table 1.
Parameter values used in the dynamical measles-undernutrition model
| Parameters | Symbol | Value | Reference |
|---|---|---|---|
| Demographic and undernutrition parameters | |||
| Population size | N0 | 100,000 | – |
| Population of surviving infants under 6 months old | B | 5,000 | – |
| Aging rate of age group 6–23 months | α | 1/(6 + 23)/2 ∼1/15 month−1 | – |
| Under 2 mortality rate | µ | 2.05 deaths per 10,000 per day ∼0.075 per year | 36 |
| Proportion of infants under 6 months who are wasted | ω | 20% | 52 |
| Wasting mortality rate | µm | 0.016 + 0.075 | 36 , 53 |
| Wasting treatment coverage | τ | Variable (defined as a rate) | 54 |
| Relapse rate | χ | 2% at 1 month postdischarge | 39 |
| Measles parameters | |||
| Measles basic reproductive ratio | R0 | 15 | 55 |
| Seasonality amplitude of measles | b1 | 0.6 | 45 |
| Force of infection | See Supplemental Material | – | |
| Measles infectious period | 1/γ | 14 days | 45 |
| Immigration rate of measles | 10 infected per year | 56 | |
| Vaccination coverage of measles | υ | Defined as a rate to be equivalent to 75% | 40 |
| Vaccination coverage of wasted children | υw | Defined as a rate to be equivalent to 67.5% | 41 |
| Vaccination coverage of children treated for wasting | υt | Variable (defined as a rate) | – |
| Measles case fatality rate | σ | 2.8% | 57 |
| Increased measles mortality due to wasting | φ | (6 + 3.7)/2 ∼5 | 7 |
| Increased susceptibility to measles infection due to wasting | θ | 2 | Assumed |
| Proportion of children who become wasted after measles infection | H | 4% | 24,42 |
| Increased susceptibility to measles due to subsequent wasting event | ς | 0 | Assumed |
| Measles-associated wasting mortality | ρ | 44% | 42 |
| Infectious period extension for wasted children* | η | Assuming infectious period among wasted children is 20 days, η = 14/20 ∼0.7 | 58 |
| Proportion of wasted infected children who recover from wasting | 20% | Assumed | |
| Mass nutritional supplementation parameters | |||
| Mass supplementation coverage | MC | 60% | Assumed |
| Measles vaccine coverage after mass supplementation | υ′ | 75%, 80%, 85% | Assumed |
| Reduced all-cause mortality due to mass supplementation | µm′ | 0.075 (1 – 0.27) + 0.016 | 29 |
| Reduced probability of first wasting event due to mass supplementation | K | 29% | 43 |
Measles latent and infectious periods are approximately 6 and 8 days, respectively. Infectiousness starts ∼4 days before the onset of rash and lasts ∼4 days after the onset of rash.
Initially, children enter the two classes of susceptibility based on their nutritional status: nourished (non-wasted, SN0) or wasted (SM0). A proportion, ω, of children become wasted during the first 6 months of life. Some of the wasted children, regardless of their status in the model (susceptible to measles or vaccinated or recovered), receive therapeutic feeding and move to the non-wasted and treated compartments. The treatment rate of wasted children was varied to represent the coverage of 5% to 100%, with 75% effectiveness. Previous studies have shown that after recovery from wasting, some children relapse and become wasted postdischarge.39 We accounted for relapse by moving treated individuals to different compartments at rate . The susceptible children who received treatment (SN1), were moved to compartment (SM1+). After individuals in SM1+ are treated again for wasting, they move to (SN2) and can, in turn, move back to (SM1+) after experiencing relapse for the second time or more. We assume that wasted children have a higher mortality rate (µm) than in other states.
A proportion of individuals from all aforementioned compartments are vaccinated and enter the vaccinated compartment (V). The vaccination coverage is assumed to be 75%, close to Niger national immunization coverage in 2016.40 We model single-dose vaccination and assume 95% of vaccinated children are no longer susceptible to measles infection. We also assumed that vaccination coverage of wasted children are 10% lower than nourished children (υw = 67.5%), based on the Demographic and Health Surveys data.41 The vaccination coverages in the model were defined as rates, and the proportion vaccinated was calculated from the model outputs. On the basis of observations of changes in overall care-seeking as a result of engagement of mothers through nutrition intervention programs,25 we hypothesize that receiving therapeutic feeding for wasting among children who are susceptible to measles, increases the measles vaccine uptake by νt.
Upon contracting measles, non-wasted and wasted individuals enter the infectious compartments (IN and IM, respectively). Depending on the host age and condition, measles may be fatal. This is presented by the measles case fatality rate, here defined as probability (σ). We assume that wasting leads to increased susceptibility to measles (θ), longer infectious period (η), and increased measles mortality (φ). In this work, we assumed that the subsequent wasting event increases susceptibility to measles to the same degree as the first wasting event (ς = 0). Individuals who recover from measles move to the recovered class (R). A proportion, H, of children develop wasting after measles infection.23 Children who survive from measles but become wasted post–measles infection move from IN to RNM, and those who stay nourished move to RNN class. There is also a chance, ρ, of mortality due to measles-associated wasting.24,42 These parameter values were informed by literature on kwashiorkor specifically, due to a lack of studies on measles-associated marasmus. We assume that a proportion, , of wasted children who get infected with measles, eventually recover from wasting and move from IM to RMN upon infection. Those who stayed wasted move from IM to RMM. We assumed a proportion of individuals in RMM experience death due to measles-associated wasting.
We depict the findings as heatmaps displaying measles infections averted across different combinations of therapeutic feeding rate for children with wasting (τ) and vaccination rate among wasted children receiving therapeutic feeding (υt). We vary these two quantities while fixing values of the remaining model parameters. The percentage reduction in infections and mortality due to measles among wasted children aged 6 to 23 months old, total infections and mortality in the population to measles among wasted children, and overall mortality among wasted children are the model outcomes. To calculate the percentage reduction, the sum of number of each outcome over the past year of simulations were calculated versus the baseline sum (τ = 0.05 and υt = 67.5). We also calculated the rates of infected cases and measles deaths per 100,000 wasted children and per 100,000 population per year, as well as rate of overall mortality per 100,000 wasted children.
Measles-undernutrition model with wasting treatment (Scenario 2: Prevention).
Instead of wasting treatment, we model the effect of community-wide mass nutritional supplementation on dynamics of measles by assuming that 60% of the population aged 6 to 23 months (parameter MC) receive daily doses of SQ-LNS (20 g per sachet) at a health center, per coverage found in a recent study in Mali29 (Table 3, Supplemental Figure 1). We hypothesize that the mass supplementation results in an increase in the first dose of measles vaccine uptake in the region during the time the cohort receives supplementation. We represent this effect by conducting three scenarios and varying measles vaccination rate in a way that the estimated coverage changes from the baseline value of 75% to 80%, and 85% among susceptible individuals who are not wasted (SN0) after receiving the SQ-LNS. The increased measles vaccine uptake rate is shown as υ′ (Supplemental Figure 1). Recent studies have shown significant improvement in children’s nutritional status as a result of receiving SQ-LNS. A cluster-randomized controlled trial in Mali has shown a 29% reduction in the probability of a first wasting event.43 We account for this effect in the model by parameter k (Supplemental Figure 1). Also, a recent pooled analysis of randomized controlled trials of LNS showed a 27% reduced probability of all-cause mortality.29 This effect is shown as µm′ in the model.
The number of measles cases is calculated as the sum of (1) the number of measles cases where 40% of the population (1-MC) do not receive the mass supplementation, and (2) the number of measles case where 60% of population (MC) receive the mass supplementation, per unit time. The number of measles cases among wasted children, mortality due to measles among wasted children, and overall mortality among wasted children as well as their rates were calculated in a similar manner.
Measles-undernutrition model with mass nutritional supplementation and wasting treatment (Scenario 3: Treatment + Prevention).
We combine Scenarios 1 and 2 and we model the combined effects of wasting treatment and the community-wide mass nutritional supplementation on dynamics of measles (Supplemental Figure 2).
Sensitivity analysis.
To characterize the response of model outputs to parameter variations and to identify the influential parameters, we conducted a sensitivity analysis using Latin hypercube sampling-partial rank correlation coefficient (LHS-PRCC). LHS-PRCC is an efficient sampling-based sensitivity analysis that assumes the effect of a parameter on model outputs is monotonic after removing the linear effects of all parameters except the parameter of interest.44 Using LHS, we generated a matrix of 5,000 sample points in the nine-dimensional unit cube (i.e., all parameters), and we explored how these parameters affected the model outcomes by using different simulated parameter sets. Next, we determined the effect of each parameter on the model outcomes while averaging over the other variables by inspecting the PRCC between each of the parameters and the percent change in different outcomes.
RESULTS
Scenario 1.
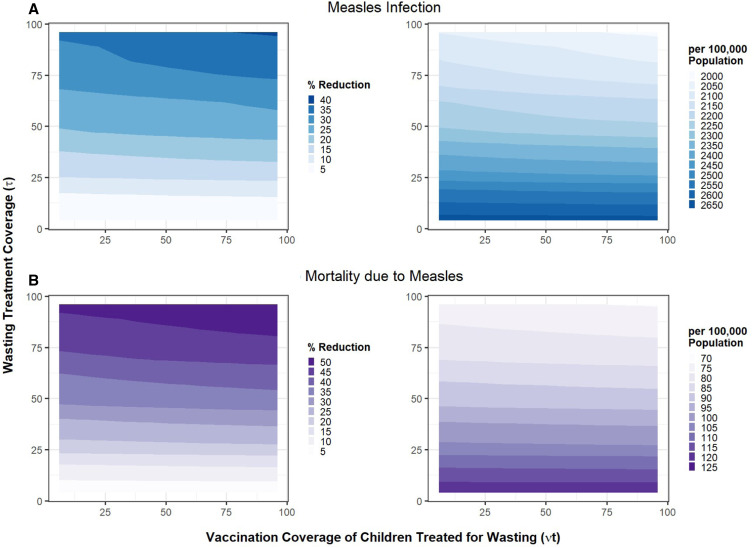
We modeled a 20% prevalence of wasting among children aged 6 to 23 months. Treating wasted children was assumed to increase vaccination coverage among wasted children who are susceptible to measles and seeking treatment. We first assumed a coverage of therapeutic treatment of wasted children to be 5% and explored the impact of incremental increases, along with constant or increased vaccination coverage among the treated children only, upon measles infection and measles mortality among wasted children and overall mortality. An underlying assumption is that unvaccinated wasted children who receive therapeutic feeding are given the measles vaccine during week 4 of treatment, as is indicated by most treatment protocols. Rates of treated and vaccinated individuals were converted to the proportions and shown in figures. Figure 2 shows how the combination of wasting treatment and measles vaccination coverage impacts measles outcomes. Increasing the treatment coverage of wasted children from 5% to 95%, followed by vaccination coverage of children who received treatment from 5% to 95%, leads to a reduction in measles cases of up to 36% (from 2,673 to 2,039 per 100,000 population), and 50% reduction in measles mortality (from 125 to 75 per 100,000 population) In addition to these benefits seen for the overall population of children, the combination of wasting treatment coverage from 5% to 95% followed by measles immunization coverage from 5% to 95% reduces the measles infection and mortality among wasted children specifically by 71%, and overall mortality of wasted children by 69% (Supplemental Figure 3).
Figure 2.
Combined impact of treatment of wasted children and their vaccination coverage (Scenario 1) on reduction in (A) measles infection and (B) mortality due to measles. The outcomes are shown as percentage reduction as well as incidence per 100,000 population.
Scenario 2.
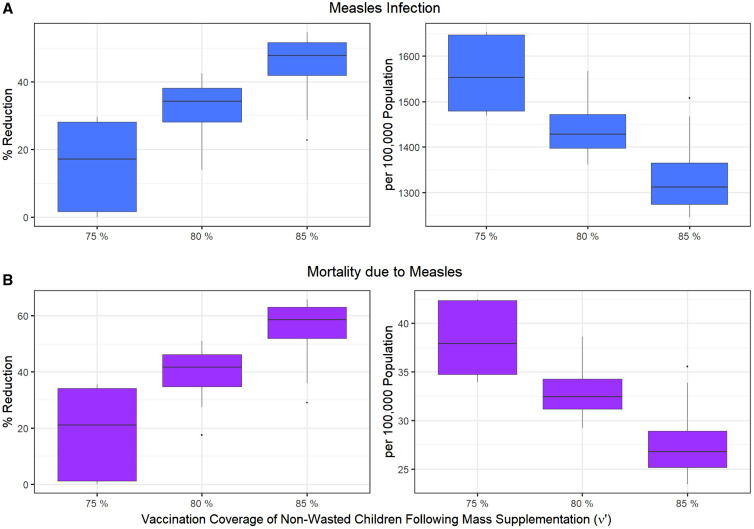
Instead of therapeutic feeding of wasted children, we included mass supplementation for nutritionally at-risk populations—a less targeted intervention with greater reach at the community level. We assumed that 60% of the children aged 6 to 23 months old received mass supplementation with SQ-LNS at health centers and hypothesized that vaccination coverage of non-wasted children (SN0) who received supplementation increased from 75% to 85%. This led to a 20% reduction in measles infection and mortality in the population and 15% reduction among wasted children. It also leads to a 10% reduction in overall mortality among wasted children.
Scenario 3.
We combined Scenarios 1 and 2 and assessed the effects of therapeutic feeding of wasted children and mass supplementation of 60% of children 6 to 23 months old, together on measles outcomes. We varied the treatment coverage of wasted children (τ) between 5% and 95% as well as the vaccination coverage of treated children for wasting (υt) between 67.5% and 95%. An increase in vaccination coverage of non-wasted children as a result of mass supplementation (υ′) from 75% to 85%, leads to a 55% reduction in measles infection (from 1,652 to 1,246 per 100,000 population) and 66% reduction in measles mortality (from 42 to 24 per 100,000 population) (Figure 3). The measles infection and mortality among wasted children aged 6 to 23 months were reduced by 71%, and overall mortality among wasted children was reduced by 67% (Supplemental Figure 4).
Figure 3.
Impacts of mass nutritional supplementation and wasting treatment (Scenario 3), on reducing (A) measles infection (blue) and (B) mortality due to measles (purple), assuming the vaccination coverage of non-wasted children (υ′) after receiving the SQ-LNS changes from its baseline value of 75% to 80%, and 85%. The variation in boxplots is produced by changing the treatment coverage of wasted children (τ) between 5% and 95%, and the vaccination coverage of wasted children between 67.5% and 95%.
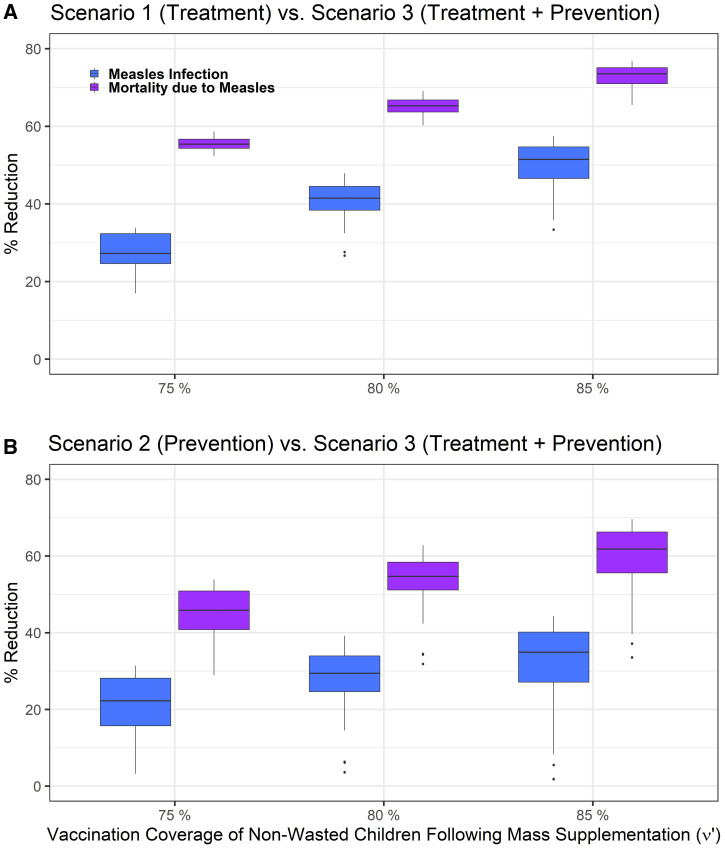
Comparing Scenario 1 with Scenario 3 reveals that the absolute numbers of measles cases and measles mortality are lower in Scenario 3 than Scenario 1. Eighty-five percent vaccination coverage of non-wasted children after mass supplementation, in addition to varying wasting treatment and vaccination coverage of children treated for wasting, leads to 36% to 57% and 67% to 77% reductions in measles infection and mortality, respectively, compared with wasting treatment only (Figure 4 and Supplemental Figure 5). Among wasted children specifically, the infection and mortality due to measles also reduced by 74% to 78%, and overall mortality by 56% to 60%, for the 85% vaccination coverage (Supplemental Figures 6 and 7).
Figure 4.
Difference between (A) wasting treatment (Scenario 1) and mass nutritional supplementation and wasting treatment (Scenario 3) and (B) mass nutritional supplementation (Scenario 2) and scenario 3, in reducing measles infection (blue) and mortality due to measles (purple), assuming the vaccination coverage of non-wasted children (υ′) after receiving SQ-LNS varies to 75%, 80%, and 85%. The variation in boxplots is produced by changing the treatment coverage of wasted children (τ) between 5% and 95%, and the vaccination coverage of wasted children between 67.5% and 95%.
If mass supplementation leads to 85% vaccination coverage of non-wasted children in both Scenarios 2 and 3, comparing Scenario 3 outcomes with Scenario 2 shows 5% to 44% reduction in measles infection and 37% to 70% reduction in measles mortality (Figure 4 and Supplemental Figure 5). The variation in boxplots is produced by changing the treatment coverage of wasted children as well as vaccination coverage of children who received treatment of wasting. Increasing the treatment coverage of wasted children increases the effect size of Scenario 3 compared with Scenario 2 and leads to larger reduction in measles infection, measles mortality, and overall mortality (Supplemental Figures 6 and 7).
Overall, comparing the three scenarios shows that prevention could be a more effective approach than treatment. Assuming that in the prevention scenario 75% of non-wasted children who received nutrition supplementation received vaccination, and in the treatment scenario, 75% of wasted children receive treatment, and 75% vaccination coverage of treated children, number of infected cases per 100,000 wasted children reduced from 13,574 (Scenario 1, treatment scenario) to 6,260 (Scenario 2, prevention scenario) and to 4,929 (Scenario 3, treatment + prevention scenario). Number of measles deaths per 100,000 wasted children was also reduced from 1,900 (treatment scenario) to 876 (prevention scenario) and to 690 (treatment + prevention scenario) (Supplemental Figure 9). Similar results were obtained for the measles infection as well as measles mortality per 100,000 population (Supplemental Figure 8). Provision of mass nutritional supplementation reduced the number of wasted children and therefore the number of wasted infected children. This effect was much larger than the treatment effect. Scenarios 2 and 3 have similar impact sizes on measles burden, emphasizing that prevention is a more effective approach than treatment (Supplemental Figure 9).
These differences in model outcomes were estimated by fixing the input parameter values to best estimates informed by the literature and to represent the dynamics of measles in the Zinder region of Niger45 (Table 1). However, precise quantities to inform some of the model parameters are unknown or unavailable—specifically, the increase in infectious period and increase in susceptibility to measles due to wasting (η, θ). Simulation over a range of values for one parameter with others fixed allows us to examine the effect of Scenario 1 on multiple model outcomes. As the magnitude of wasting effects, such as susceptibility effect and duration of infectious period, grow larger, they result in an increase in the number of infected children and number of deaths per 100,000 wasted children aged 6 to 23 months old, assuming the vaccination coverage of children who received treatment varied between 67.5% and 100% and the wasting treatment coverages varies between 5–100% (Supplemental Figures 10 and 11). For instance, an increase in susceptibility effect from θ = 2 to θ = 4, leads to, on average, a 50% increase in measles infection among wasted children (from 13,988 to 22,085 per 100,000 wasted children aged 6 to 23 months old per year) (Supplemental Figure 10). Varying other parameter values such as increased measles mortality due to wasting (φ) or proportion who recover from wasting after measles infection (δ) does not lead to a noticeable difference in the simulation results for the number of infected cases. Further sensitivity analyses using the LHS-partial rank correlation coefficient (LHS-PRCC) index and generating a matrix of 5,000 sample points in the nine-dimensional unit cube (all parameters) indicate that σ (measles-induced mortality probability) θ (susceptibility impact of measles infection) have the strongest influence on both measles infection and measles mortality outcomes (Supplemental Figure 12). In addition, mortality due to measles among wasted children aged 6 to 23 months old is sensitive to φ (increased measles mortality due to wasting).
DISCUSSION
Substantive barriers to improving child health in resource-limited settings include persistently high rates of undernutrition and low rates of vaccine coverage for common infections such as measles. Given the close bidirectional relationship between childhood undernutrition and infectious diseases among vulnerable populations, there may be opportunities for synergy wherein the treatment and/or prevention of one condition leads to decreased rates of the other. Our work aims to capture the dynamic interactions between undernutrition and measles, so as to estimate the impact of treatment of wasting, mass nutritional supplementation for nutritionally at-risk populations, and measles vaccine coverage on measles infection and mortality.
No other nutrition intervention has shown such significant, simultaneous impacts on childhood malnutrition as mass supplementation with SQ-LNS from 6 to 18 or to 23 months, including reductions in wasting, stunting, anemia, and mortality as well as improved developmental outcomes.27–30 Our models demonstrate a strong effect of mass nutritional supplementation in reducing measles infection and death, as well as overall mortality, if such an intervention could be leveraged to incentivize measles vaccination uptake. Importantly, modestly increasing measles vaccination coverage by providing vaccination at the same time and location as blanket nutritional supplementation leads to sizeable reductions in measles cases and mortality well above treatment of wasting or increased vaccine uptake alone. In addition to the synergic effect, our dynamical model demonstrates the nonlinear impacts of these interventions on measles burden.
Our results show that mass supplementation alone and mass supplementation combined with wasting treatment have a similar effect size on reducing measles infection and mortality. Mass supplementation with SQ-LNS coupled with vaccination would likely be more cost-effective than the individual treatment of wasting alone, which, in high-burden countries, puts financial pressures on vulnerable and poorly resourced health systems. Future work should explore the cost-effectiveness of integrated interventions.
We used model parameters based on data from Niger, a nation plagued by a high burden of undernutrition, where interventions focused on the early childhood nutrition have the potential for a large impact on measles infection rates. Niger has made little progress toward achieving international targets for decreasing stunting and wasting.46 Our results suggest that mass nutritional supplementation among vulnerable populations using SQ-LNS has over twice the effect on reducing measles infection and mortality as well as overall mortality among wasted children, compared with treatment of wasted children alone, assuming both methods lead to increased vaccination coverage. Therefore, expanding the reach of mass nutritional supplementation, including providing nutrition supplementation during routine immunization visits, could help Niger make progress toward lowering the incidence of wasting, as well as indirectly improve vaccination coverage. The models could be applied to other countries like Chad, Nigeria, or the Democratic Republic of Congo that have a similar burden of undernutrition as Niger but much lower measles vaccination coverage.
As with all models, there are some limitations to our work. Our work is theoretical; however, our sensitivity analyses show that our model is robust to choice of parameter values and thus is a call for more empirical studies. Although we account for multiple interactions between measles and undernutrition, there are several features of the complex system of measles epidemiology that were not included in the model. For instance, we did not account for any potential impact of undernutrition on measles vaccine leading to a potential delay in response to vaccine or waning of vaccine because it is underexplored in the literature.47 In addition, one of the complications that malnourished children experience after measles infection is post-measles diarrhea, which exacerbates nutrient loss.1 Accounting for this could help us better identify the impacts of infection on children’s nutritional status. Also, after measles infection a certain percentage of children experience blindness, a certain percentage have some residual pneumonia, and, quite significantly, a certain percentage have residual post-measles immunodeficiency. The reported deaths due to measles do not account for mortality from these various comorbidities.48,49 In addition, assessing the potential increased pathogenicity of measles as a result of undernutrition would be useful in understanding the cyclic relationship between the two events to develop more effective interventions. More field data to constrain model parameters around the relationship between measles infection and undernutrition would allow for increasingly rigorous quantification of direct and indirect impacts of nutrition interventions. Also, nutrition interventions that led to rises in vaccination coverage were assumed to be delivered at health centers and at regular intervals. It will also be important to investigate different distribution models for supplementation to retain contact with health personnel without placing undue burden on already overworked caretakers.
Given the complex interactions between undernutrition and infectious diseases, it is still challenging to recognize where and at what level specific interventions can be most effective. The large contribution of undernutrition and infection to the global burden of disease increases the importance of studying these two processes. Our study offers a more nuanced understanding of the complex interactions between measles transmission dynamics and undernutrition and helps motivate advocacy for nutrition-focused interventions based on their multidimensional impacts. This work also helps understand how the addition of nutritional status, preventive nutrition interventions, and therapeutic treatments into mathematical models of vaccine-preventable diseases can better explain increased susceptibility to infection despite achieving certain vaccine coverage levels. This model also has implications for understanding other indirect benefits of increased care-seeking due to engagement with the health system for nutrition-based interventions. When considering how health systems with limited budgets should spend their money to achieve maximal benefit, we have shown here that leveraging mass nutritional supplementation as a contact point with the health system to increase, even modestly, measles vaccination coverage has a synergistic benefit beyond either intervention alone. Such a double impact could be even more important in areas where both malnutrition and measles are rising because of conflict, climate shocks, and disruptions from the COVID-19 pandemic.50,51
Supplemental files
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1. Humphries DL, Scott ME, Vermund SH, 2021. Nutrition and Infectious Diseases: Shifting the Clinical Paradigm. Towata, NJ: Humana Press. [Google Scholar]
- 2. Scrimshaw NS, 2003. Historical concepts of interactions, synergism and antagonism between nutrition and infection. J Nutr 133: 316–321. [DOI] [PubMed] [Google Scholar]
- 3. Schaible UE, Kaufmann SH, 2007. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4: 0806–0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walson JL, Berkley JA, 2018. The impact of malnutrition on childhood infections. Curr Opin Infect Dis 31: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Mølbak K, Valentiner-Branth P, Lanata CF, Black RE, 2008. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 37: 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dewey KG, Mayers DR, 2011. Early child growth: how do nutrition and infection interact? Matern Child Nutr 7: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J, 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371: 243–260. [DOI] [PubMed] [Google Scholar]
- 8. Katona P, Katona-Apte J, 2008. The interaction between nutrition and infection. Clin Infect Dis 46: 1582–1588. [DOI] [PubMed] [Google Scholar]
- 9. Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A, 2017. Severe childhood malnutrition. Nat Rev Dis Primers 3: 17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Black RE. et al. , 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 11. Bourke CD, Berkley JA, Prendergast AJ, 2016. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 37: 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oxlade O, Huang CHC, Murray M, 2015. Estimating the impact of reducing under-nutrition on the tuberculosis epidemic in the central eastern states of India: a dynamic modeling study. PLoS One 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hove-Musekwa SD, Nyabadza F, Chiyaka ch, Das P, Tripathi A, Mukandavire Z, 2011. Modelling and analysis of the effects of malnutrition in the spread of cholera. Math Comput Model 53: 1583–1595. [Google Scholar]
- 14. Coutinho BP, Oriá RB, Vieira CM, Sevilleja JE, Warren CA, Maciel JG, Thompson MR, Pinkerton RC, Lima AA, Guerrant RL, 2008. Cryptosporidium infection causes undernutrition and, conversely, weanling undernutrition intensifies infection. J Parasitol 94: 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL, 2010. Enteroaggregative Escherichia coli (EAEC) impairs growth while malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J Infect Dis 202: 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costa LB, Noronha FJ, Roche JK, Sevilleja JE, Warren CA, Oriá R, Lima A, Guerrant RL, 2012. Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of cryptosporidium infection and malnutrition. J Infect Dis 205: 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartelt LA, Roche J, Kolling G, Bolick D, Noronha F, Naylor C, Hoffman P, Warren C, Singer S, Guerrant R, 2013. Persistent G. lamblia impairs growth in a murine malnutrition model. J Clin Invest 123: 2672–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ibrahim MK, Zambruni M, Melby CL, Melby PC, 2017. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev 30: 919–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fenn B, 2014. Malnutrition in humanitarian emergencies. Midwifery 4. [Google Scholar]
- 20. Koster FT, Curlin GC, Aziz KN, Haque A, 1981. Synergistic impact of measles and diarrhoea on nutrition and mortality in Bangladesh. Bull World Health Organ 59: 901–908. [PMC free article] [PubMed] [Google Scholar]
- 21. Smedman L, Lindeberg A, Jeppsson O, Zetterstrom R, 1983. Nutritional status and measles: a community study in Guinea-Bissau. Ann Trop Paediatr 3: 169–176. [DOI] [PubMed] [Google Scholar]
- 22. Morley D, 1969. Severe measles in the tropics. BMJ 1: 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morley DC, 1974. Measles in the developing world. Proc R Soc Med 67: 1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scrimshaw NS, Salomon JB, Bruch HA, Gordon JE, 1966. Studies of diarrheal disease in Central America VIII. measles, diarrhea, and nutritional deficiency in rural Guatemala. Am J Trop Med Hyg 15: 625–631. [DOI] [PubMed] [Google Scholar]
- 25. Banerjee AV, Duflo E, Glennerster R, Kothari D, 2010. Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. BMJ 340: 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sato R, 2021. Association between uptake of selected vaccines and undernutrition among Nigerian children. Hum Vaccin Immunother 17: 2630–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keats EC, Das JK, Salam RA, Lassi ZS, Imdad A, Black RE, Bhutta ZA, 2021. Effective interventions to address maternal and child malnutrition: an update of the evidence. Lancet Child Adolesc Health 5: 367–384. [DOI] [PubMed] [Google Scholar]
- 28. Dewey KG, Stewart CP, Wessells KR, Prado EL, Arnold CD, 2021. Small-quantity lipid-based nutrient supplements for the prevention of child malnutrition and promotion of healthy development: overview of individual participant data meta-analysis and programmatic implications. Am J Clin Nutr 114 (Suppl 1): 3S–14S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stewart CP, Wessells KR, Arnold ChD, Huybregts L, Ashorn P, Beccquey E, Humphrey JH, Dewey KG, 2020. Lipid-based nutrient supplements and all-cause mortality in children 6–24 months of age: a meta-analysis of randomized controlled trials. Am J Clin Nutr 111: 207–218. [DOI] [PubMed] [Google Scholar]
- 30. Prado EL. et al. , 2021. Small-quantity lipid-based nutrient supplements for children age 6–24 months: a systematic review and individual participant data meta-analysis of effects on developmental outcomes and effect modifiers. Am J Clin Nutr 114 (Suppl 1): 43S–67S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. International Federation of Red Cross and Red Crescent Societies , 2020. Case Study: Tackling Malnutrition in Niger. Available at: https://preparecenter.org/wp-content/sites/default/files/tackling_malnutrition_in_niger-en.pdf.
- 32. Niger Ministry of Public Health , Department of Nutrition, 2021. National Nutrition and Retrospective Mortality Survey, SMART Report. December 2021 (in French).
- 33. UNICEF/WHO/World Bank , 2021. Joint Child Malnutrition Estimates Expanded Database, Stunting, Wasting and Overweight. Available at: https://www.who.int/data/gho/data/themes/topics/joint-child-malnutrition-estimates-unicef-who-wb.
- 34. World Health Organization Regional Office for Africa , 2022. Weekly Bulletin on Outbreaks and Other Emergencies (Technical Report April; Week 17: 18–24. Geneva, Switzerland: World Health Organization.
- 35. Blake A, Djibo A, Guindo O, Bharti N, 2020. Investigating persistent measles dynamics in Niger and associations with rainfall. J R Soc Interface 17: 2020048020200480. Available at: https://royalsocietypublishing.org/doi/10.1098/rsif.2020.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oumarou M, Kanta MI, Le Duc G, 2011. The New Role of Nigerian Medical NGOs in Treating SAM. Field Exchange 41. Available at: https://www.ennonline.net/fex/41/role.
- 37. World Bank World Development Indicators 2016. Birth Rate, Crude (per 1,000 people)—Niger Data. Available at: https://data.worldbank.org/indicator/SP.DYN.CBRT.IN?locations=NE.
- 38. World Bank World Development Indicators , 2016. Mortality Rate, Infant (per 1,000 live births)—Niger Data. Available at: https://data.worldbank.org/indicator/SP.DYN.IMRT.IN?locations=NE.
- 39. Stobaugh HC, Mayberry A, McGrath M, Bahwere P, Zagre NM, Manary MJ, Black R, Lelijveld N, 2019. Relapse after severe acute malnutrition: a systematic literature review and secondary data analysis. Matern Child Nutr 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. WHO , 2020. WHO Vaccine-Preventable Diseases: Monitoring System. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 41. The DHS Program , 2018. Quality Information to Plan, Monitor and Improve Population, Health, and Nutrition Programs. Rockville, MD: IFC. [Google Scholar]
- 42. Morley D, Woodland M, Martin WJ, 1963. Measles in Nigerian children. J Hyg (Lond) 61: 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huybregts L, Le Port A, Becquey E, Zongrone A, Barba FM, Rawat R, Leroy JL, Ruel MT, 2019. Impact on child acute malnutrition of integrating small-quantity lipid-based nutrient supplements into community-level screening for acute malnutrition: a cluster-randomized controlled trial in Mali. PLoS Med 16: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu J, Dhingra R, Gambhir M, Remais JV, 2013. Sensitivity analysis of infectious disease models: methods, advances and their application. J R Soc Interface 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferrari MJ, Grais RF, Bharti N, Conlan AJK, Bjørnstad ON, Wolfson LJ, Guerin PJ, Djibo A, Grenfell BT, 2008. The dynamics of measles in sub-Saharan Africa. Nature 451: 679–684. [DOI] [PubMed] [Google Scholar]
- 46. Osgood-Zimmerman A. et al. , 2018. Mapping child growth failure in Africa between 2000 and 2015. Nature 555: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prendergast AJ, 2015. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laksono BM. et al. , 2018. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun 9: 4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mina MJ, Metcalf CJE, de Swart RL, Osterhaus ADME, Grenfell BT, 2015. Long-term measles-induced immunomodulation increase overall childhood infectious disease mortality. Science 348: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. UNICEF , 2022. Severe Wasting: An Overlooked Child Emergency. UNICEF Child Alert New York. Available at: https://www.unicef.org/child-alert/severe-wasting.
- 51. UNICEF and World Health Organization , 2022. UNICEF and WHO Warn of Perfect Storm of Conditions for Measles Outbreaks, Affecting Children (Joint News Release April 27, 2022). Available at: https://www.who.int/news/item/27-04-2022-unicef-and-who-warn-of–perfect-storm–of-conditions-for-measles-outbreaks–affecting-children.
- 52. Kerac M, Frison S, Connell N, Page B, McGrath KM, 2019. Informing the management of acute malnutrition in infants aged under 6 months (MAMI): risk factor analysis using nationally-representative demographic health survey secondary data. PeerJ 6: e5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Isanaka S, Nombela N, Djibo A, Poupard M, Van Beckhoven D, Gaboulaud V, Guerin PJ, Grais RF, 2009. Effect of preventive supplementation with ready-to-use- therapeutic food on the nutritional status, mortality and morbidity of children 6 to 60 months in Niger: a cluster randomized trial. JAMA 301: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Besada D. et al. , 2016. Niger’s child survival success, contributing factors and challenges to sustainability: a retrospective analysis. PLoS One 11: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS, 2017. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis 17: e420–e428. [DOI] [PubMed] [Google Scholar]
- 56. Ferrari MJ, Djibo A, Grais RF, Bharti N, Grenfell BT, Bjørnstad ON, 2010. Rural-urban gradient in seasonal forcing of measles transmission in Niger. Proc Biol Sci 277: 2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Portnoy A, Jit M, Ferrari MJ, Hanson M, Brenzel L, Verguet S, 2019. Estimates of case-fatality ratios of measles in low-income and middle-income countries: a systematic review and modelling analysis. Lancet Glob Health 7: e472–e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dossetor J, Whittle HC, Greenwood BM, 1977. Persistent measles infection in malnourished children. Br Med J 1: 1633–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.