Abstract
Introduction
Leber congenital amaurosis (LCA) type 2, due to disease‐causing variants in RPE65, is characterized by severe visual loss in early infancy. Current treatments include voretigene neparvovec‐rzyl (VN) for RPE65‐associated LCA. Herein, we present the long‐term follow‐up of a patient treated with VN using quantitative autofluorescence (488 nm excitation).
Case Report
A 9‐year‐old girl with a diagnosis of LCA with biallelic variants in RPE65 presented for evaluation. The patient underwent VN treatment at the age of 11. The patient returned to clinic at age of 19 at which time imaging revealed evidence of chorioretinal atrophy. Quantitative autofluorescence performed prior to gene therapy and at 6‐ and 8‐year follow‐up revealed a central area of fundus autofluorescence.
Discussion
This case report demonstrates acquisition of fundus autofluorescence at 6‐ and 8‐year follow‐up despite the development of chorioretinal atrophy.
Keywords: chorioretinal atrophy, gene therapy, inherited retinal disease, leber congenital amaurosis, quantitative autofluorescence, voretigene neparvovec‐rzyl
We present eight‐year follow‐up of a patient treated with voretigiene neparvovec‐rzyl (VN) who presented with chorioretinal atrophy at eight‐year follow‐up. Quantitative autofluorescence at six‐ and eight‐year follow‐up demonstrates presence of autofluorescence suggestive of the continued efficacy of VN despite the presence of chorioretinal atrophy.
1. INTRODUCTION
Leber congenital amaurosis (LCA) is a clinical diagnosis applied to a group of inherited retinal dystrophies presenting with severe visual loss from early infancy. Vision loss is progressive, often characterized by the onset of nystagmus, and commonly progresses to total blindness by the third or fourth decade (Kondkar & Abu‐Amero, 2019). Of the nearly 30 genes associated with LCA (Kondkar & Abu‐Amero, 2019), LCA2, secondary to biallelic variations in RPE65 (MIM 180069), is the only subtype of LCA with an FDA‐approved treatment: an adeno‐associated virus 2 vector, voretigene neparvovec‐rzyl (VN) (Maguire et al., 2019). The RPE65 gene encodes the isomerase responsible for the conversion of all‐trans‐retinyl ester to 11‐cis‐retinol in the visual cycle (Kondkar & Abu‐Amero, 2019). As clinical trial subjects and patients who received VN gene therapy following FDA approval continue to be monitored post‐surgery, recent findings have identified patients who have progressive perifoveal chorioretinal atrophy (Gange et al., 2021). Herein, we present findings, including quantitative autofluorescence (qAF), from an 8‐year follow‐up of a patient who received VN and presented with chorioretinal atrophy at the most recent visit.
2. REPORT OF A CASE
A patient diagnosed with LCA at 6 months of age presented to the Harkness Eye Institute at Columbia University, New York, NY, for evaluation at ages 9, 13, 18, and 20. Genetic evaluation revealed biallelic variants in the RPE65 gene leading to the protein changes p.(Lys295Ter) and p.(His68Tyr) (NM_000329.3). At initial presentation, best‐corrected visual acuities (BCVA) were 20/400 and 20/150 in the right (OD) and left (OS) eyes, respectively. Anterior segment examination was unremarkable. Dilated fundus examination revealed retinal vasculature attenuation (Figure 1a).
FIGURE 1.
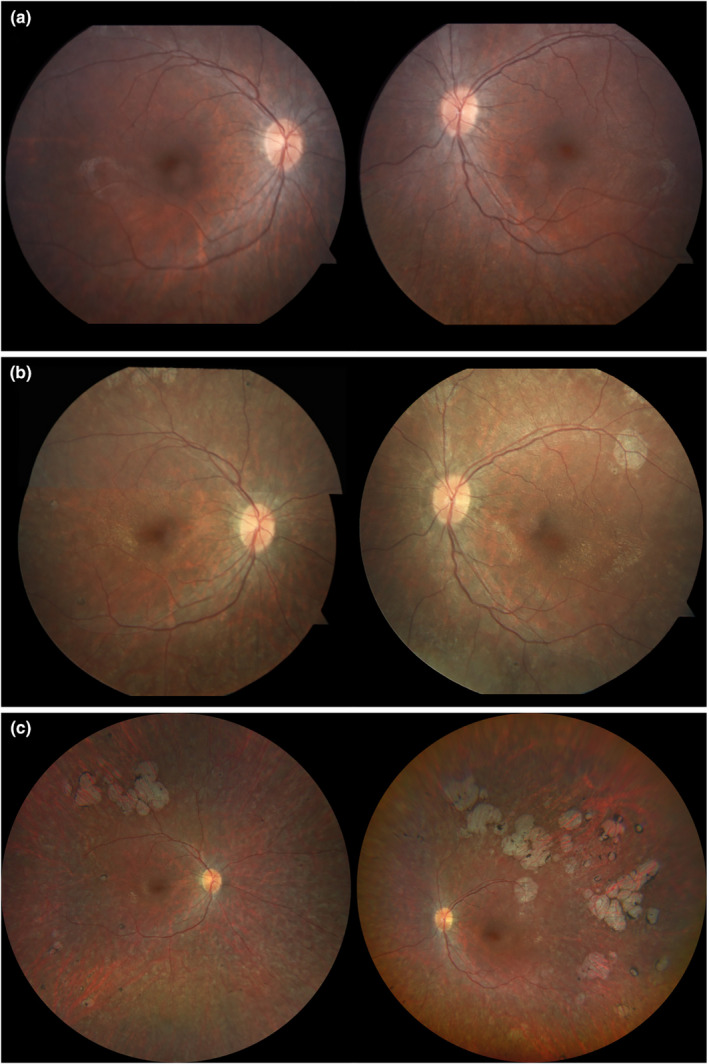
Color fundus photographs of a patient with biallelic variants in the RPE65 gene prior to treatment with voretigene neparvovec‐rzyl (VN) and 2 and 8 years after subretinal injection. (a) Baseline color fundus photograph at 9 years of age is largely unremarkable with retinal vasculature attenuation noted bilaterally. (b) Two years after VN intervention, color fundus imaging reveals salt‐and‐pepper mottling and lacunae superotemporally in both eyes. (c) Wide‐angle fundus imaging 8 years after treatment reveals extensive chorioretinal atrophy in both eyes, with more atrophy in the left eye compared with the right.
At age 11, the patient received bilateral subretinal injections of VN along the superotemporal arcade as part of a phase III clinical trial (NCT00999609). Two‐year follow‐up revealed the BCVA was stable in the left eye and improved to 20/150 in the right eye, with a subjective improvement in low‐light conditions. Eight‐year follow‐up revealed a BCVA of 20/400 OD and 20/150 OS and the patient noted decreased peripheral vision (Levi et al., 2020). Anterior segment examination remained unremarkable until 8‐year follow‐up when trace posterior subcapsular cataracts were noted bilaterally. Dilated fundus examination at 8‐year follow‐up disclosed bilateral superotemporal confluent atrophic lesions representative of chorioretinal atrophy (Figure 1c). No color fundus photographs were obtained at 6‐year follow‐up.
Quantitative fundus autofluorescence (qAF) was performed as previously described (Sparrow et al., 2020). Prior to intervention, short‐wavelength fundus autofluorescence (SW‐AF) imaging revealed a hypo‐autofluorescent fundus with absent contrast between the optic nerve and surrounding retina. The qAF color‐coded image revealed near absence of autofluorescence, compared with an age‐matched control (Figure 2a,b). At 6‐year follow‐up, qAF revealed newly detectable autofluorescence within a parafoveal ring that was also observed at 8‐year follow‐up (Figure 2c,e). Age‐matched controls are shown for comparison (Figure 2d,f).
FIGURE 2.
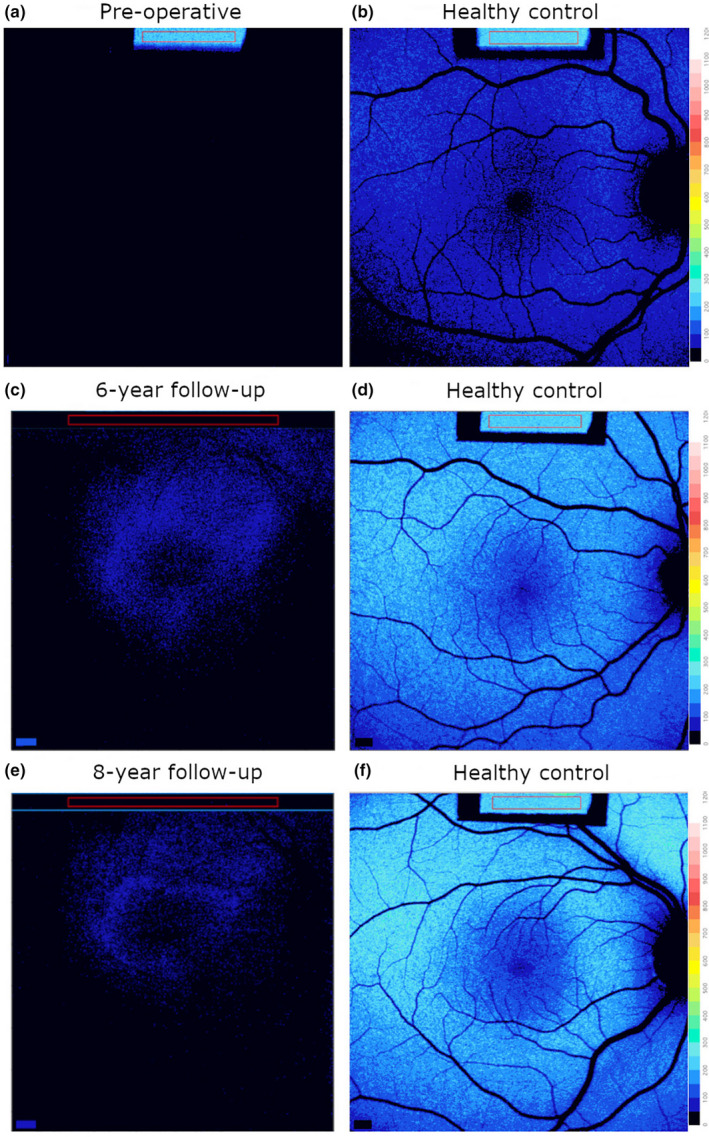
Quantitative fundus autofluorescence (qAF) color‐coded images in the patient prior to treatment and at 6‐ and 8‐year follow‐up. Right eyes. (a,b) qAF prior to intervention; near absence of autofluorescence (a) relative to a healthy control (b). (c,d) qAF image revealed parafoveal autofluorescence at 6‐year follow‐up (c) compared with healthy age‐matched control (d). (e,f) 8‐year (e) follow‐up compared with control (f).
3. DISCUSSION
Phase I and III clinical trials of VN demonstrated only mild adverse effects up to 4 years post‐treatment (Maguire et al., 2019). New data from 2021, following 10 patients (18 eyes) over a period of 18 months, revealed a previously unpublished adverse effect following treatment: chorioretinal atrophy (Gange et al., 2021). Gange et al. defined this feature as atrophy if (1) it was not directly related to the touch‐down site of the subretinal cannula and (2) if it enlarged overtime. We found no association between the atrophy and subjective or objective measurements of vision. The etiology of the atrophy is not known but could be a result of direct toxicity of AAV2 vector, inflammation, or surgical delivery through fast subretinal injection (OMIM®, 2021).
In this case report, we present the 8‐year follow‐up of a patient who developed chorioretinal atrophy, as defined by Gange et al., following treatment with VN. Despite the development of extramacular atrophy, the presence of central autofluorescence at six‐ and 8‐year follow‐up, even if slightly diminished at latest follow‐up, is indicative of a functioning visual cycle and thus continued efficacy of VN. At 8‐year follow‐up, the patient's BCVA had returned to baseline in both eyes suggesting that the intervention had stabilized the patient's vision such that it did not deteriorate below baseline.
CONFLICT OF INTEREST
Stephen H. Tsang receives financial support from Abeona Therapeutics, Inc. and Emendo. He is also the founder of Rejuvitas and is on the scientific and clinical advisory board for Nanoscope Therapeutics and Medical Excellence Capital.
AUTHOR CONTRIBUTIONS
Conception and design: JRS, SHT; Provision of patients: SHT, VBM; Collection and assembly of data: MK, JRLC, RP, AHK; Analysis and interpretation of data: JRLC, RP, JRS; Manuscript writing and revision: all authors.
ETHIC STATEMENT
Written informed consent was obtained from the patient as regulated by the Columbia University Institutional Review Board, protocol #AAAB6560.
ACKNOWLEDGMENTS
The authors would like to thank the Jonas Children's Vision Care (JCVC) team for their support and comradery. The Jonas Children's Vision Center is supported by the NIH R01EY09076, 5P30CA013696, U01 EY030580, U54OD020351, R24EY028758, R24EY027285, 5P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437, the Schneeweiss Stem Cell Fund, New York State [SDHDOH01‐C32590GG‐3450000], the Foundation Fighting Blindness New York Regional Research Center Grant [TA‐NMT‐0116‐0692‐COLU], Nancy & Kobi Karp, the Crowley Family Funds, The Rosenbaum Family Foundation, Alcon Research Institute, the Gebroe Family Foundation, the Research to Prevent Blindness (RPB) Physician‐Scientist Award, unrestricted funds from RPB, New York, NY, USA. Vinit B. Mahajan is supported by NIH grants (R01 EY030151, R01 EY031952, and P30EY026877), Stanford ChEM‐H IMA, the Stanford Center for Optic Disc Drusen, and Research to Prevent Blindness, New York, New York. Additionally, this work was supported by NIH grant EY024091 (JRS) and by Foundation Fighting Blindness (SHT and JRS).
Kolesnikova, M. , Lima de Carvalho, J. R. , Parmann, R. , Kim, A. H. , Mahajan, V. B. , Tsang, S. H. , & Sparrow, J. R. (2022). Chorioretinal atrophy following voretigene neparvovec despite the presence of fundus autofluorescence. Molecular Genetics & Genomic Medicine, 10, e2038. 10.1002/mgg3.2038
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Gange, W. S. , Sisk, R. A. , Besirli, C. G. , Lee, T. C. , Havunjian, M. , Schwartz, H. , Borchert, M. , Sengillo, J. D. , Mendoza, C. , Berrocal, A. M. , & Nagiel, A. (2021). Perifoveal chorioretinal atrophy after subretinal voretigene neparvovec‐rzyl for RPE65‐mediated leber congenital amaurosis. Ophthalmology Retina, 6, 58–64. 10.1016/j.oret.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondkar, A. A. , & Abu‐Amero, K. K. (2019). Leber congenital amaurosis: Current genetic basis, scope for genetic testing and personalized medicine. Experimental Eye Research, 189, 107834. 10.1016/j.exer.2019.107834 [DOI] [PubMed] [Google Scholar]
- Levi, S. R. , Oh, J. K. , de Carvalho, J. R. L., Jr. , Mahajan, V. B. , Tsang, S. H. , & Sparrow, J. R. (2020). Quantitative autofluorescence following gene therapy with voretigene neparvovec. JAMA Ophthalmology, 138(8), 919–921. 10.1001/jamaophthalmol.2020.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, A. M. , Russell, S. , Wellman, J. A. , Chung, D. C. , Yu, Z. F. , Tillman, A. , Wittes, J. , Pappas, J. , Elci, O. , Marshall, K. A. , McCague, S. , Reichert, H. , Davis, M. , Simonelli, F. , Leroy, B. P. , Wright, J. F. , High, K. A. , & Bennett, J. (2019). Efficacy, safety, and durability of voretigene neparvovec‐rzyl in RPE65 mutation‐associated inherited retinal dystrophy: Results of phase 1 and 3 trials. Ophthalmology, 126(9), 1273–1285. 10.1016/j.ophtha.2019.06.017 [DOI] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man, OMIM® . (2021). Johns Hopkins University. MIM Number: 180069. https://omim.org/ [Google Scholar]
- Sparrow, J. R. , Duncker, T. , Schuerch, K. , Paavo, M. , & de Carvalho, J. R. L., Jr. (2020). Lessons learned from quantitative fundus autofluorescence. Progress in Retinal and Eye Research, 74, 100774. 10.1016/j.preteyeres.2019.100774 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


