Abstract
Purpose
A systematic literature review was conducted to assess the use of home injections (self/partner/healthcare provider [HCP]-administered) of somatostatin analogs (SSAs) as an alternative to healthcare-setting injections in patients with acromegaly and neuroendocrine tumors (NETs).
Methods
MEDLINE/Embase/the Cochrane Library (2001–September 2021), key congresses (2019–2021), and bibliographies of relevant systematic reviews were searched. Eligible studies reported on efficacy/effectiveness, safety, adherence, patient-reported outcomes (PROs), and economic outcomes in populations receiving home injections of SSAs.
Results
Overall, 12 studies were included, all reporting on SSAs (lanreotide Autogel/Depot or octreotide long-acting release) in acromegaly or NETs. Across four studies, home injection was associated with similar disease control in patients with acromegaly/NETs compared with healthcare-setting administration. High rates of treatment adherence were shown in two studies of patients with acromegaly receiving lanreotide injections at home. Two studies reported non-serious adverse events; incidence of adverse reactions was similar in both the home and healthcare administration settings. Preference for injection setting varied between studies and indications; nonetheless, higher satisfaction/convenience (>75% patients) was reported for home injections. Self- or partner-injection was associated with economic savings compared with administration in the healthcare setting across five studies.
Conclusion
Efficacy/effectiveness, adherence, and safety outcomes of SSAs in the home injection setting were similar to those in the healthcare setting, with high reported satisfaction and convenience. Self/partner injection also resulted in cost savings. These findings provide a basis to understand outcomes related to home injection and encourage healthcare providers to discuss optimal treatment choices with their patients.
Keywords: Home injection, Injection modality, Acromegaly, Neuroendocrine tumors, Somatostatin analogs, Lanreotide
Plain language summary
Acromegaly and neuroendocrine tumors (NETs) are two diseases that affect the production of hormones, leading to a variety of symptoms in different parts of the body. Patients can be treated with medications called somatostatin analogs, which include lanreotide Autogel/Depot (LAN) and octreotide long-acting release (OCT). These treatments are given by injections, usually performed by a doctor or nurse in a healthcare setting such as a hospital or clinic. However, patients can sometimes receive LAN or OCT injections at home by a healthcare professional or—for LAN only—independently by a partner or the patient themself. Home injection may be less disruptive for patients and could free up healthcare resources, but there is limited evidence to support the choice. To address this, we reviewed all publications on home injection of somatostatin analogs in the last 20 years, finding 12 relevant studies. Results generally showed that home injections and injections in the healthcare setting had a similar effect on disease signs and symptoms, and were equally safe. Patients receiving home injections were also successfully able to follow the treatment plan prescribed by their doctor. Although some patients still preferred to receive injections in the healthcare setting, patients generally found injections at home more convenient. Home injections also resulted in lower costs as fewer appointments at the hospital or clinic were needed. The findings of this review indicate that injections of somatostatin analogs at home, instead of in the healthcare setting, could be a potential option for patients whose circumstances allow it.
Introduction
Acromegaly and neuroendocrine tumors (NETs) are two endocrine disorders with insidious onset [1, 2]. While the clinical features of acromegaly and NETs vary, both conditions negatively impact patients’ quality of life (QoL) and lead to an increased rate of mortality [3, 4].
Acromegaly is generally caused by a growth hormone (GH) secreting pituitary adenoma, resulting in GH excess and elevation in insulin-like growth factor-1 (IGF-1) [5]. Patients also experience changes in physical characteristics, such as enlarged hands, feet, and coarse facial features [6]. One of the most prevalent long-term complications of acromegaly is joint disease [6]. Pharmacological therapies are used for patients ineligible for surgical treatment or those with elevated levels of GH and IGF-1 after surgery [5, 6].
NETs represent a heterogeneous group of tumors, which in some cases may secrete hormones causing a variety of symptoms, including those associated with carcinoid syndrome [2, 7, 8]. The primary treatment goal is curative surgery, whereas pharmacological control of tumor size and the signs and symptoms of disease is recommended for patients with metastatic inoperable disease [9].
Treatment with somatostatin analogs (SSAs) is the mainstay medical treatment for acromegaly and grade 1–2 metastatic gastroenteropancreatic (GEP) NETs [10, 11]. Lanreotide Autogel (LAN, lanreotide Depot in the US) and octreotide long-acting release (OCT) are two first-generation SSAs with long-acting formulations, which are indicated for both acromegaly and NETs [12–15]. SSAs interact with targets throughout the body, inhibiting various endocrine, neuroendocrine, exocrine, and paracrine functions. Both OCT and LAN bind with high affinity to the somatostatin receptor subtypes SST2 and with lower affinity to SST5 [12, 16]. In acromegaly, SSAs suppress the production of GH and IGF-1, resulting in a reduction of clinical symptoms [17, 18]. In NETs, LAN and OCT have antitumor effects and also reduce symptoms associated with carcinoid syndrome and VIPoma syndrome such as diarrhea and flushing, through inhibition of hormone secretion [17, 18].
LAN is administered by deep subcutaneous (SC) injection every 4 weeks [12], while OCT is administered by deep intramuscular injection at the same treatment interval [14, 15]. The long-term, frequent administration of SSAs in the healthcare setting can lead to high treatment burden and accumulation of healthcare resource use and costs [1, 19]. Like other injected treatments for chronic diseases, life-long injections of SSAs may impact patients’ well-being and daily lives. Patients report a loss of independence and productivity, as well as inconvenience related to the time required to travel to and attend injection-related visits with their healthcare provider (HCP) [20].
Home injection options, including administration by the patient, a partner, or HCP, provide an alternative to injections administered in the healthcare setting. LAN is approved for HCP, self- or partner-administration using a prefilled ready-to-use syringe in numerous countries worldwide, including in Europe, while OCT is approved for HCP administration only, in the healthcare setting or at home [13, 14]. LAN and OCT are not, however, approved for self- or partner-injection in the US [12, 15]. Home injection options allow for more autonomy in patients’ care, in particular for those with limited time or mobility, as observed in acromegaly patients with more severe joint disease [21–23], and have been increasingly used in times of high burden on the healthcare system, such as during the COVID-19 pandemic [24, 25].
Where therapies are expected to have similar efficacy and safety outcomes, individual patients’ preferences are important when considering the most suitable treatment for each patient. Shared treatment decision-making accommodating patients’ individual preferences has been associated with increased adherence and improved clinical outcomes [26]. However, despite the development of home injection treatment options for SSA injections, the advantages and disadvantages of home injections have not been widely examined [24]. A systematic literature review was therefore conducted with the primary objective of identifying and summarizing outcomes related to home administration of SSAs by self/partner injections (LAN) or injections by a HCP (OCT and LAN).
Methods
This systematic literature review was conducted in accordance with a pre-specified protocol and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27]. Details of the protocol for this systematic literature review were registered on PROSPERO 2021: CRD42021279886 [28].
Searches for this systematic literature review were separated into two streams of evidence: home injection of SSAs (primary objective) or other comparable treatments for chronic conditions (secondary objective). Comparable treatments were considered those administered every 4–8 weeks by subcutaneous injection, in both the healthcare and non-healthcare setting, to align with the route of administration of SSAs. Only the methodology and results pertaining to the primary objective (home injection of SSAs) are presented in this manuscript; results from the secondary objective have been previously reported [29].
Search strategy
Searches were conducted in MEDLINE (MEDLINE® Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations and Daily), Embase, and the Cochrane Library (including the Cochrane Database of Systematic Reviews [CDSR] and the Cochrane Central Register of Controlled Trials [CENTRAL]), on September 2, 2021. The searches were performed using terms for home injection (Table S1–Table S3) and limited to literature published since January 2001.
Bibliographies of relevant systematic literature reviews identified in the database searches were reviewed to identify additional relevant studies for inclusion. Proceedings from relevant congresses from the last 3 years (2019–2021) were also screened (Table S4).
Study selection, data extraction, and quality assessment
Eligible studies reported on efficacy/effectiveness, safety, adherence, patient-reported outcomes (PROs) or economic outcomes in populations receiving home injections of SSAs. Full eligibility criteria are presented in Table S5.
Records were screened according to the processes recommended by the Cochrane Collaboration [30]. Two independent reviewers screened the abstracts and full-text publications of the literature against pre-specified eligibility criteria. In case of disagreement, a third reviewer was consulted, and any conflicts were resolved by consensus. Publications reporting on the same study were considered as a single unit, with the primary article reporting the main results of the study and additional articles considered as secondary articles.
Data extractions were performed in line with guidelines from the University of York Centre for Reviews and Dissemination (CRD) [31], and performed by a single individual into pre-specified extraction tables. A second individual independently verified the extracted information. The quality of included studies was assessed using an adaptation of the Downs and Black checklist [32]; questions not relevant to the current review were removed.
Results
Characteristics of included studies
A total of 13 records comprising 12 unique studies were eligible for inclusion (Table 1, Table S6, Fig. S1). Seven studies were observational [33–39], alongside three interventional trials [40–42], and two budget impact models [43, 44]. Seven studies were conducted in Europe [33–35, 37, 39–41], two in the US, and one in Israel [36, 38, 42]. Both budget impact models used a UK perspective [43, 44]. Eight of the studies reported industry funding, all from Ipsen Biopharmaceuticals Inc. [34, 36, 37, 40, 41, 43, 44] or its subsidiary Tercica Inc. [42].
Table 1.
Summary table of results, by indication
| Reference | Study design | Efficacy/effectiveness | Safety | Adherence | PROs | Economic outcomes |
|---|---|---|---|---|---|---|
| Acromegaly population | ||||||
| Akirov 2021 [38] | Prospective cohort study; Israel (n = 88, LAN) | • HCP at home: 86% achieved IGF-1 normalization | NR | • HCP at home: 74%/92% had excellent/good adherence (>90%/>80% of expected number of injections) | NR | NR |
| Bevan 2008 [40] | Phase IV, open-label non-randomized controlled trial; UK (n = 30, LAN) |
• Self/partner: 93%/100% achieved GH/IGF-1 control at Week 40 • HC setting: 100%/93% achieved GH/IGF-1 control at Week 40 |
• Majority of patients experienced no or mild pain, redness or swelling in either self/partner or HC setting injection (data NR) • No clinically relevant differences in tests, vital signs or examinations (data NR) |
• Self/partner: 93% successfully injected (based on assessment by study investigators and maintained disease control) • HC setting: 100% successfully injected |
NR | NR |
| Follin 2016 [34] | Cross-sectional study; Sweden (n = 23, SSA NR) | NR | NR | NR | • 13%/4.4% preferred self/partner injection; 82.6% preferred injection by nurse in HC setting | NR |
| Salvatori 2010 [42] | Open-label single-arm trial; US (n = 52, LAN) |
• Self/partner (switching from HC setting): 76.9%/93.7% achieved GH/IGF-1 normalization at Week 24 • Self/partner (other patients): 39.1%/46.2% achieved GH/IGF-1 normalization at Week 24 |
NR | NR |
• Self/partner: 75.0%/15.6% rated treatment very/somewhat convenient • HC setting: 18.8%/18.8% rated treatment very/somewhat convenient • 81.3% preferred self/partner injection; 12.5% HC setting; 6.2% no preference |
NR |
| Salvatori 2014 [36] | Prospective cohort study; US (n = 166, LAN) |
• Self/partner: 88% achieved IGF-1 normalization • HC setting/other: 67% achieved IGF-1 normalization (p = 0.01) |
• Self: 19%/58% had injection site reaction/≥1 AE • Partner: 2%/56% had injection site reaction/≥1 AE • HC setting: 7%/52% had injection site reaction/≥1 AE |
NR |
Proportion rating treatment very/somewhat convenient at Month 12: • Self: 86.7% • Partner: 81.8% • HC setting: 45.7% • Combination/other: 86.6% |
NR |
| NETs population | ||||||
| Cortez 2021 [33] | Cross-sectional study; UK (n = 18, LAN) | NR | NR | NR | • 100% preferred self-injection; 77.78% found the process ‘effortless’ |
• Self/partner: saved 4 h/£10 every 4 weeks on average • 36 clinic appointments expected to be released every 36 weeks due to the program |
| Gertner 2020 [35] | Cross-sectional study; UK (n = 34, LAN) | NR | NR | NR |
• 94% were (very) satisfied with home injection (any) • 6%/50% of those receiving self/partner injection found it ‘very easy’ |
• Home injection (any): 91% reported travel to hospital would take at least half a day |
| Harrow 2021 [44] | Budget impact model; UK (n = NR, LAN or OCT | NR | NR | NR | NR | • Switching to self/partner injection would save 14.5 nurse contacts/4 hospital visits per patient per year (£2458 [16.4%] saving) |
| Johanson 2012 [41] | Phase IV, open-label randomized crossover trial; Sweden, Norway, Denmark (n = 25, LAN) |
• Self/partner: 16%/12% reported worsened/improved diarrhea; n = 1/n = 2 reported worsened/improved flushing • HC setting: 0%/4% reported worsened/improved diarrhea; n = 1/n = 4 reported worsened/improved flushing |
• Self/partner: n = 6/5/3 had moderate AE/mild AE/injection site reaction • HC setting: n = 9/7/7 had moderate AE/mild AE/injection site reaction |
NR | • 88% preferred self/partner injection; 12% preferred injection in HC setting |
• Self/partner: 23 days sick leave (22 days by n = 1 patient); 17 HCP visits; 205 telephone contacts • HC setting: 0 days sick leave; 25 HCP visits; 312 telephone contacts. Estimated cost for patient: €7.95 per injection |
| Ström 2019 [37] | Cross-sectional study; Sweden (n = 119, LAN or OCT) | NR | NR | NR | • Of N = 43 patients who did not know about availability of self-injection, 16% said they would consider starting | • HC setting: treatment visit lasted <2 h in 93% of patients |
| Opalinska 2021 [39] | Retrospective cohort study; Poland (n = 184, LAN or OCT) |
• Self/partner: 28.1% experienced tumor progression; TTP: 56.0 (6–152) months • HC setting: 18.2% experienced tumor progression (p = 0.079); TTP: 56.8 (8–144) months |
NR | NR | NR | NR |
| Mixed acromegaly and NETs population | ||||||
| Harrow 2020 [43] | Budget impact model; UK (n = 3921 acromegaly; n = 2073 NETs, LAN or OCT) | NR | NR | NR | NR | • Switching to self/partner injection in patients with acromegaly/GEP-NETs would save 13.3/14.5 nurse contacts per patient per year (£1262 [9.1%]/£2458 [16.4%] savings) |
AE adverse event, GEP gastroenteropancreatic, GH growth hormone, h hour, HC healthcare, HCP healthcare professional, IGF-1 insulin-like growth factor 1, LAN lanreotide Autogel/Depot, NET Neuroendocrine tumor, NR not reported, OCT octreotide long-acting release, PRO patient-reported outcome, TTP time to progression, UK United Kingdom, US United States
Five and six studies evaluated patients with acromegaly [34, 36, 38, 40, 42] and NETs, respectively [33, 35, 37, 39, 41, 44]. One of the budget impact models analyzed SSA usage in both acromegaly and NETs patient populations [43]. Most studies included fewer than 200 patients (18–184 patients), while one budget impact model estimated a population of 3921 patients with acromegaly and 2073 patients with NETs [43] (the other budget impact model did not report the size of the modelled population [44]). All patient characteristics are presented in Table S7.
Four studies assessed home injection only and did not include a comparison to injections administered in the healthcare setting [33, 35, 38, 42]. The majority of studies evaluated home injection of LAN only (7/12 studies) [33, 35, 36, 38, 40–42]. A further four studies investigated patients treated with LAN or OCT [37, 39, 43, 44]. One study did not specify which SSA treatment patients were administered [34].
Efficacy and effectiveness outcomes
Across the two indications, three clinical trials assessed treatment efficacy [40–42], and an additional three real-world evidence studies examined treatment effectiveness (Table 1 and Table S8) [36, 38, 39].
In patients with acromegaly, efficacy/effectiveness assessed by change in IGF-1 and GH values was similar in patients receiving home injections compared with injections in the healthcare setting, across two studies [36, 40]. Overall, >85% of participants receiving home injections were reported to achieve normalization or control of IGF-1 and GH in three studies (defined on the basis of study-specific thresholds; Table S8) [36, 38, 40].
In patients with NETs, comparable efficacy/effectiveness in the home and healthcare administration settings was also observed when assessing the proportion of patients with tumor progression, time to progression, and the proportion of participants with symptomatic control of diarrhea and flushing (n = 2 studies) [39, 41].
Safety/tolerability outcomes and treatment adherence
Only three studies investigated the safety and tolerability of home injection, with a similar proportion of patients experienced adverse events (AEs) in both the home and healthcare administration settings (n = 2 studies in acromegaly, n = 1 study in NETs, Table 1 and Table S9) [36, 40, 41]. Of the two studies reporting injection site reactions, the first study showed no differences between self- or partner-injection and the healthcare administration setting, though statistical significance was not reported [40]. In the second study, a greater number of patients with acromegaly experienced injection site reactions following self-injection (19%) as compared with partner injections (2%; p < 0.05) or injections in the healthcare setting (7%; statistical significance not reported) [36].
Adherence and successful administration rates were high in two studies reporting on patients with acromegaly [38, 40]. Across both studies, >90% of participants achieved either good adherence (>80% of expected injections) or successful administration of treatment as assessed by the study investigators [38, 40].
Patient-reported outcomes
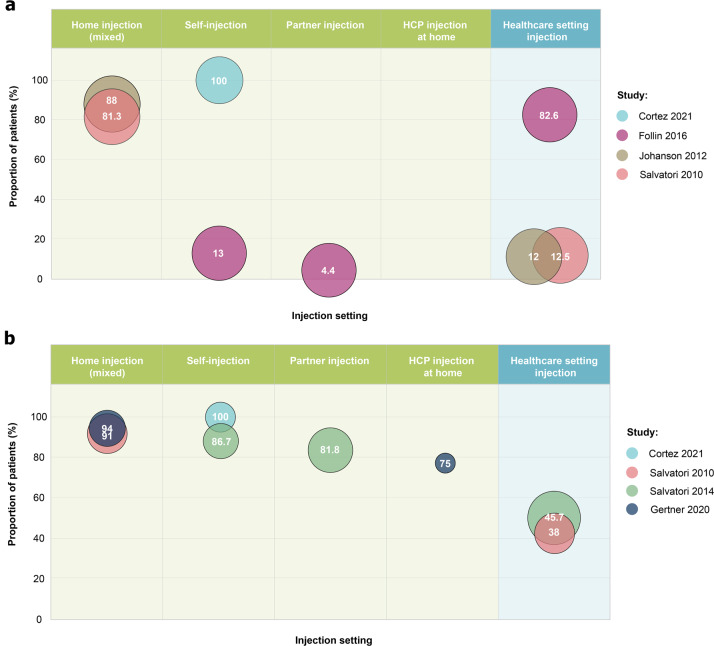
Of the 12 included studies, 4 reported on patient preferences for setting of administration (Fig. 1a, Table 1 and Table S10) [33, 34, 41, 42]. In patients with acromegaly, one study found that 82.6% of patients favored healthcare-setting administration, while another study reported that the majority (81.3%) of patients preferred self- or partner-administration to healthcare-setting administration [34, 42]. For studies assessing NETs, 88–100% of patients preferred self- or partner-injection (n = 2 studies) [33, 41].
Fig. 1.
Summary of patient-reported outcomes. a Patient preference for administration setting. b Treatment convenience and satisfaction associated with administration setting. Size of each bubble is proportional to the sample size contributing data for the relevant injection setting, while the labelled number indicates the proportion of patients (%). Injection setting was categorized as “home injection (mixed)” for studies reporting on a combined group of patients receiving home injections from different types of injectors (partner, HCP or by self-injection). HCP healthcare professional
A total of 75–100% of patients with receiving home injection were satisfied with their treatment or found it to be very/somewhat convenient at study end, compared to 38%–46% of patients for healthcare-setting administration (n = 4 studies; Fig. 1b, Table 1, and Table S10) [33, 35, 36, 42]. Fewer patients with NETs receiving self- or partner-injections (8%) reported that their treatment interfered with daily activities compared with those receiving injections in the healthcare setting (24%) [41].
Economic outcomes
Home injection was associated with economic savings compared with healthcare-setting administration across six studies in patients with NETs (Table 1 and Table S11) [33, 35, 37, 41, 43, 44]. Direct cost savings were attributed to reduced healthcare resource use (n = 4 studies) [37, 41, 43, 44]. Time saved by patients (including travel and attendance at appointments) was estimated to range from 1.4 h to at least half a day per visit, with implications for indirect and out-of-pocket costs (n = 3 studies) [33, 35, 41].
Two budget impact models estimated that the overall expenses would be cut by 16.4% or 9.1% per year in patients with GEP-NETs and acromegaly, respectively, if a patient treated in the healthcare setting with OCT switched to self- or partner-injections of LAN. In-hospital nurse contact and hospital visits would also be reduced in patients with GEP-NETs or acromegaly [43, 44].
Quality assessment
A detailed summary of the quality assessment is provided in Table S12. Risk of bias varied between studies, with the reviewers finding six studies did not meet or provided insufficient information to determine ≥4/8 items of the modified Downs and Blacks checklist [36, 39, 40, 42–44]. Potential sources of bias identified related to both external and internal validity of included studies, including limitations in reporting such as details of the statistical analyses performed, recruitment of participants, and whether the participants were representative of the larger population.
Discussion
The results of this systematic literature review evaluating home injection versus healthcare-setting administration of SSAs showed that efficacy/effectiveness, safety, and adherence outcomes were similar in both settings for patients with acromegaly or NETs. Combined with high patient satisfaction, convenience, and cost savings for home injection, these results support the use of SSAs administered in the home setting (Fig. 2). Although patients predominantly preferred home injection options [33, 41, 42], conflicting results were reported in one study [34], highlighting the importance of patient choice in the decision to inject at home or in the healthcare setting.
Fig. 2.
Graphic summary of balance of evidence. Empty categories indicate an absence of relevant evidence. CRU costs and resource use, GH growth hormone, IGF-1 insulin-like growth factor 1
Home injection of SSAs was found to have comparable efficacy/effectiveness to healthcare-setting administration, which may be expected given that the treatment regimens themselves do not differ between settings. Similar findings have been found in other conditions; for example in patients requiring injection of golimumab or methotrexate for rheumatoid arthritis [45, 46]. These results, in addition to competent self- and partner-injection techniques demonstrated by patients [47–50], may alleviate potential concerns relating to effectiveness of home injections.
Few patients experienced adverse events related to home injection, with the most notable finding being a higher rate of injection-site reactions in patients self-injecting compared to those receiving partner injections, in one study of patients with acromegaly [36]. However, the results of this study may be impacted by physical limitations associated with the disease, including abnormal enlargement of the hands in patients with acromegaly, which may impact self-injection techniques [51]. A more ergonomic, newly designed delivery system was developed for LAN in 2019; this system was not available at the time of that study’s publication [52]. Understanding how device design impacts patient outcomes will be important to optimize patients’ experience of home injection therapies, particularly given the low number of comparative studies regarding safety identified in this review.
Home injection was associated with high adherence rates and successful injection administration, though these outcomes were only reported in studies of patients with acromegaly [38, 40]. Differences in treatment adherence rates may be impacted by various factors, including disease indication and the process of injection [23, 53, 54]. Additional barriers to treatment adherence, including treatment side effects and convenience, financial issues, and patient-related factors such as age, may impact adherence to home injection therapies and should be further investigated.
The evidence relating to patient experience of home injections identified in this systematic review is supported by the findings from the HomeLAN survey of patients receiving home injections of SSAs, first presented after the completion of the literature review searches. In this survey, over 95% of participants reported being satisfied with home injection, citing independence, flexibility, and time and cost savings as reasons underlying their choice to receive injections at home [20]. Individual patient preference for injection setting may be affected by a variety of factors. For example, research has suggested higher satisfaction in patients with acromegaly and GEP-NETs receiving LAN via a prefilled syringe compared with the octreotide LAR syringe [55], reflecting variations between therapies. Evidence from patients with rheumatoid arthritis similarly supports the contribution of individual-level patient factors to the preference for particular administration settings, including frequency of hospital visits, anxiety, flexible administration schedules, and age (e.g., due to cognitive impairment [56]). This evidence, along with the findings of this systematic literature review, highlight the importance of patient choice in the decision to inject in the healthcare setting or at home with support from patient education and training programs [57].
Unsurprisingly, the use of self- or partner-injection was associated with economic savings compared with healthcare-setting administration [33, 35, 37, 41, 43, 44]. Therefore, increased uptake of home injections may substantially reduce the high costs and resource burden associated with acromegaly and NETs. The results of this systematic literature review also support home treatment options to offset the high burden placed on healthcare services in times of particular strain. As evidenced by the COVID-19 pandemic, stringent public health measures have disrupted routine clinical care for patients [58]. Home treatment options, therefore, allow for effective patient management in times of limited availability of HCPs and healthcare-setting resources [24, 25]. Treatments for home injection are increasingly being developed for a wide range of chronic illnesses, including plaque psoriasis, ankylosing spondylitis, and psoriatic arthritis, and their adoption is expected to rise. The impact of provider reimbursement fees and financial incentives on HCP uptake of home injection, which is currently unclear, therefore deserves further study to ensure relevant programs can be made available to all patients who wish to receive treatment at home.
Strengths of this review include adherence to best-practice systematic review methods for publication searches, data extraction and analysis as recommended by the Cochrane Collaboration [59]. A variety of outcomes were also assessed, with the inclusion of data from real-world studies representing administration settings in the community setting. However, few studies provided statistical analyses directly comparing home injection with healthcare-setting administration, limiting the conclusions that could be drawn. Differences in study design, patient populations and reported outcomes may have introduced heterogeneity in the interpretation and inferences made, potentially inflated by small sample sizes. Given this heterogeneity between studies, a meta-analysis could not be conducted; thus, further research is required to extend the findings of this literature review.
Overall, the findings of this systematic literature review highlight that home injection of SSAs is associated with disease control, high treatment adherence, and an acceptable safety profile. Home injection of SSAs may allow for optimal use of healthcare resources, while allowing for a greater choice in the management of patients who wish for more independence and are suitable for home injection. Nevertheless, patients’ preference for setting of injection varied across studies, emphasizing the need to provide tailored options and support to each patient, including programs delivering HCP-led home injections and/or providing training on self- or partner-injection for those wishing to receive SSA injections at home.
Supplementary information
Acknowledgements
The authors acknowledge Jill Crich, BSc, MSL for medical writing and editorial assistance based on the authors’ input and direction.
Author contributions
All authors made substantial contributions to study conception and design, and the analysis and interpretation of the data. All authors also contributed to drafting the paper or revising it critically for important intellectual content, and approved the final approval of the version of the paper to be published.
Funding
This work was sponsored by Ipsen Biopharmaceuticals, Inc. Support for third-party writing assistance for this article was funded by Ipsen in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Compliance with ethical standards
Conflict of interest
C.L.B.: Speaker’s bureau from Novartis; principal investigator for Novartis and Crinetics clinical trials; M.K.: Speaker’s bureau from Ipsen, NovoNordisk, Pfizer; grant support from Crinetics and ONO; A.A.: Employee of Costello Medical; A.M.G.: Member of the Spanish Association of Acromegaly; A.H.: Employee of Ipsen; holds stock options in Ipsen; A.R.O.J.: Employee of Ipsen; holds stock options in Ipsen; W.W.d.H.: Speaker’s bureau from Ipsen and Novartis; research support from Novartis; advisory board member for Camurus and Crinetics.
Footnotes
Patient Author: Almudena Martín García
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1007/s12020-022-03227-0.
References
- 1.Fleseriu M, Molitch M, Dreval A, Biermasz NR, Gordon MB, Crosby RD, Ludlam WH, Haviv A, Gilgun-Sherki Y, Mathias SD. Disease and treatment-related burden in patients with acromegaly who are biochemically controlled on injectable somatostatin receptor ligands. Front. Endocrinol. 2021;12:627711. doi: 10.3389/fendo.2021.627711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofland J, Kaltsas G, de Herder WW. Advances in the diagnosis and management of well-differentiated neuroendocrine neoplasms. Endocr. Rev. 2020;41(2):371–403. doi: 10.1210/endrev/bnz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adelman DT, Liebert KJ, Nachtigall LB, Lamerson M, Bakker B. Acromegaly: The disease, its impact on patients, and managing the burden of long-term treatment. Int J. Gen. Med. 2013;6:31–38. doi: 10.2147/ijgm.S38594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raphael MJ, Chan DL, Law C, Singh S. Principles of diagnosis and management of neuroendocrine tumours. CMAJ. 2017;189(10):e398–e404. doi: 10.1503/cmaj.160771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adigun OO, Nguyen M, Fox TJ, Anastasopoulou C. Acromegaly. Florida: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 6.National Health Service (NHS): Acromegaly. https://www.nhs.uk/conditions/acromegaly/. Accessed 16 August 2022
- 7.Wolin EM, Leyden J, Goldstein G, Kolarova T, Hollander R, Warner RRP. Patient-reported experience of diagnosis, management, and burden of neuroendocrine tumors: results from a large patient survey in the United States. Pancreas. 2017;46(5):639–647. doi: 10.1097/mpa.0000000000000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basuroy R, Bouvier C, Ramage JK, Sissons M, Srirajaskanthan R. Delays and routes to diagnosis of neuroendocrine tumours. BMC Cancer. 2018;18(1):1122. doi: 10.1186/s12885-018-5057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandostatin LAR: The goal of neuroendocrine tumor therapy. https://www.sandostatin.com/en/neuroendocrine-tumors/treating-net/treatment-goal/. Accessed 16 August 2022
- 10.Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin analogs in clinical practice: a review. Int J. Mol. Sci. 2020;21(5):1–27. doi: 10.3390/ijms21051682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paragliola RM, Prete A, Papi G, Torino F, Corsello A, Pontecorvi A, Corsello SM. Clinical utility of lanreotide Autogel(®) in gastroenteropancreatic neuroendocrine tumors. Drug Des. Devel Ther. 2016;10:3459–3470. doi: 10.2147/dddt.S76732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration (FDA): Somatuline depot prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022074s024lbl.pdf. Accessed 16 August 2022
- 13.Electronic Medicines Compendium (EMC): Somatuline autogel 60mg, somatuline autogel 90mg, somatuline autogel 120mg. https://www.medicines.org.uk/emc/medicine/25104#gref. Accessed 16 August 2022
- 14.European Medicines Agency (EMA): Sandostatin LAR summary of product characteristics. https://www.ema.europa.eu/en/documents/referral/sandostatin-lar-article-30-referral-annex-iii_en.pdf. Accessed 16 August 2022
- 15.Food and Drug Administration (FDA): Sandostatin LAR prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021008s041lbl.pdf. Accessed 16 August 2022
- 16.Günther T, Tulipano G, Dournaud P, Bousquet C, Csaba Z, Kreienkamp H-J, Lupp A, Korbonits M, Castaño JP, Wester H-J. International union of basic and clinical pharmacology. CV. Somatostatin receptors: structure, function, ligands, and new nomenclature. Pharm. Rev. 2018;70(4):763–835. doi: 10.1124/pr.117.015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ipsen Biopharmaceuticals: Somatuline autogel product monograph. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/61/2021/04/23163136/PM-Somatuline-Autogel-EN-25Mar2021.pdf (2021). Accessed 16 August 2022
- 18.Novartis Pharmaceuticals Canada Inc.: Sandostatin LAR Product Monograph. https://www.ask.novartispharma.ca/download.htm?res=sandostatin_scrip_e.pdf&resTitleId=789 (2021). Accessed 16 August 2022
- 19.Ortendahl JD, Pulgar SJ, Mirakhur B, Cox D, Bentley TG, Phan AT. Budget impact of somatostatin analogs as treatment for metastatic gastroenteropancreatic neuroendocrine tumors in US hospitals. Clinicoecon Outcomes Res. 2017;9:495–503. doi: 10.2147/ceor.S140866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernando J, Kolarova T, Verslype C, Kaltsas G, Houchard A, Gueguen D, de Herder W. HomeLAN: An international online survey to assess satisfaction with injection experience of patients with neuroendocrine tumors (NETs) enrolled in lanreotide autogel (LAN) Patient Support Programmes (PSPs) J. Neuroendocrinol. 2022;34:176. doi: 10.1111/jne.13281. [DOI] [PubMed] [Google Scholar]
- 21.Chilton F, Collett RA. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskelet. Care. 2008;6(1):1–14. doi: 10.1002/msc.110. [DOI] [PubMed] [Google Scholar]
- 22.Arthur AB, Klinkhoff AV, Teufel A. Safety of self-injection of gold and methotrexate. J. Rheumatol. 1999;26(2):302–305. [PubMed] [Google Scholar]
- 23.Schiff M, Saunderson S, Mountian I, Hartley P. Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol. Ther. 2017;4(2):445–463. doi: 10.1007/s40744-017-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cella D, Evans J, Feuilly M, Neggers S, Van Genechten D, Herman J, Khan MS. Patient and healthcare provider perspectives of first-generation somatostatin analogs in the management of neuroendocrine tumors and acromegaly: a systematic literature review. Adv. Ther. 2021;38(2):969–993. doi: 10.1007/s12325-020-01600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.S. Khawaja, M. Marks, M. Gurnell, ACROCOVID II: An international survey on acromegaly management more than 1 year into the COVID-19 pandemic era. Presented at NORD Rare Summit (2021), Virtual
- 26.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, Vollmer WM. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am. J. Respir. Crit. Care Med. 2010;181(6):566–577. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health Research (NIHR): PROSPERO. Evaluating patient outcomes and preferences for independent injection of somatostatin analogues: a systematic literature review. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=279886. Accessed 16 August 2022
- 29.Boguszewski CL, Korbonits M, Artignan A, Martin GA, Houchard A, Ribeiro-Oliveira A. Independent injection vs healthcare-setting administration of somatostatin analogues: A systematic literature review. Endocr. Abstr. 2022;81:P411. [Google Scholar]
- 30.Zhang C, Cao X, Li D. Home treatment of bortezomib: a preliminary exploration of a new treatment mode in China. Blood. 2019;134:2127. doi: 10.1182/blood-2019-125505. [DOI] [Google Scholar]
- 31.Centre for Reviews and Dissemination (CRD). Systematic Reviews: CRD’s guidance for undertaking reviews in health care. (York Publ. Services, York, 2009).
- 32.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Commun. Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortez L. An evaluation of the impact of self or partner administration of lanreotide within a pharmacist-led homecare service for patients with neuroendocrine tumours (NET) J. Oncol. Pharm. Pr. 2021;27(2):60–61. [Google Scholar]
- 34.Follin C, Karlsson S. Attitudes and preferences in patients with acromegaly on long-term treatment with somatostatin analogues. Endocr. Connect. 2016;5(4):167–173. doi: 10.1530/ec-16-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gertner J, McSweeney A, O'Mahony FL, Quaglia E, John A, Mandair D, Caplin M, Toumpanakis C. Homecare Service for Administration of Lanreotide Autogel Injections: Assessment of Patients’ Experience. Neuroendocrinol. 2020;110:192. [Google Scholar]
- 36.Salvatori R, Woodmansee WW, Molitch M, Gordon MB, Lomax KG. Lanreotide extended-release aqueous-gel formulation, injected by patient, partner or healthcare provider in patients with acromegaly in the United States: 1-year data from the SODA registry. Pituitary. 2014;17(1):13–21. doi: 10.1007/s11102-012-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ström T, Kozlovacki G, Myrenfors P, Almquist M. Patient and nurse experience of using somatostatin analogues to treat gastroenteropancreatic neuroendocrine tumors: results of the somatostatin treatment experience trial (STREET) Patient Prefer Adherence. 2019;13:1799–1807. doi: 10.2147/ppa.S213472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akirov A, Masri-Iraqi H, Gorshtein A, Duskin-Bitan H, Kaminer K, Shimon I. Benefits of a nurse-led home injection service for acromegaly patients treated with somatuline autogel. Endocrine. 2021;71(2):453–458. doi: 10.1007/s12020-020-02529-5. [DOI] [PubMed] [Google Scholar]
- 39.Sowa-Staszczak A, Opalińska M, Kurzyńska A, Morawiec-Sławek K, Gilis-Januszewska A, Palen-Tytko J, Olearska H, Hubalewska-Dydejczyk A. Self-Administration of Long-Acting Somatostatin Analogues in NET Patients—Does It Affect the Clinical Outcome? Medicina. 2021;57(12):1287. doi: 10.3390/medicina57121287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bevan JS, Newell-Price J, Wass JA, Atkin SL, Bouloux PM, Chapman J, Davis JR, Howlett TA, Randeva HS, Stewart PM, Viswanath A. Home administration of lanreotide Autogel by patients with acromegaly, or their partners, is safe and effective. Clin. Endocrinol. 2008;68(3):343–349. doi: 10.1111/j.1365-2265.2007.03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johanson V, Wilson B, Abrahamsson A, Jianu C, Calissendorff J, Wall N, Grønbæk H, Florholmen J, Ohberg A, Granberg D. Randomized crossover study in patients with neuroendocrine tumors to assess patient preference for lanreotide Autogel(®) given by either self/partner or a health care professional. Patient Prefer Adherence. 2012;6:703–710. doi: 10.2147/ppa.S34337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvatori R, Nachtigall LB, Cook DM, Bonert V, Molitch ME, Blethen S, Chang S. Effectiveness of self- or partner-administration of an extended-release aqueous-gel formulation of lanreotide in lanreotide-naïve patients with acromegaly. Pituitary. 2010;13(2):115–122. doi: 10.1007/s11102-009-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrow B, Cristeau O, Clay E, Francois C, Frankcom I, Marteau F. PDG12 In the current COVID reality, can independent injection of somatostatin analogues lead to greater health savings? A budget impact analysis in the UK. Value Health. 2020;23:S522–S523. doi: 10.1016/j.jval.2020.08.695. [DOI] [Google Scholar]
- 44.Harrow B, Cristeau O, Clay E, Francois C, Frankcom I, Cortez L. Independent administration of long-acting somatostatin analogues (SSAs) for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NET): Potential savings of increased uptake in the UK National Health Service (NHS) J. Neuroendocrinol. 2021;33:135. [Google Scholar]
- 45.Schulze-Koops H, Giacomelli R, Samborski W, Rednic S, Herold M, Yao R, Govoni M, Vastesaeger N, Weng HH. Factors influencing the patient evaluation of injection experience with the SmartJect autoinjector in rheumatoid arthritis. Clin. Exp. Rheumatol. 2015;33(2):201–208. [PubMed] [Google Scholar]
- 46.Amano K, Matsubara T, Tanaka T, Inoue H, Iwahashi M, Kanamono T, Nakano T, Uchimura S, Izumihara T, Yamazaki A, Karyekar CS, Takeuchi T. Long-term safety and efficacy of treatment with subcutaneous abatacept in Japanese patients with rheumatoid arthritis who are methotrexate inadequate responders. Mod. Rheumatol. 2015;25(5):665–671. doi: 10.3109/14397595.2015.1012786. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan I, Ross D, Hilton F, Morgenstern D, Wolter K. The role of training in effective simulated self-injection of subcutaneous depot medroxyprogesterone acetate: observations from a usability study. Contraception. 2016;94(4):314–320. doi: 10.1016/j.contraception.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Mohr DC, Cox D, Epstein L, Boudewyn A. Teaching patients to self-inject: pilot study of a treatment for injection anxiety and phobia in multiple sclerosis patients prescribed injectable medications. J. Behav. Ther. Exp. Psychiatry. 2002;33(1):39–47. doi: 10.1016/s0005-7916(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 49.Beauvais C, Fayet F, Rousseau A, Sordet C, Pouplin S, Maugars Y, Poilverd RM, Savel C, Ségard V, Godon B. Efficacy of a nurse-led patient education intervention in promoting safety skills of patients with inflammatory arthritis treated with biologics: a multicentre randomised clinical trial. RMD Open. 2022;8(1):e001828. doi: 10.1136/rmdopen-2021-001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afilal S, Rkain H, Berchane B, Moulay Berkchi J, Fellous S, Fatima Zahrae T, Ilham A, Alami N, Latifa T, Hajjaj-Hassouni N, Allali F. Evolution of the perceptions of RA patients after education patient session teaching methotrexate self-injection a prospective pilot study. Ann. Rheum. Dis. 2020;79(Suppl 1):566. doi: 10.1136/annrheumdis-2020-eular.5505. [DOI] [Google Scholar]
- 51.Chanson P, Salenave S. Acromegaly. Orphanet J. Rare Dis. 2008;3(1):1–17. doi: 10.1186/1750-1172-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ipsen Biopharmaceuticals: Ipsen announces U.S. FDA approval for newly designed pre-filled syringe for somatuline® depot (lanreotide). https://www.ipsen.com/us/blog/press-releases/ipsen-announces-u-s-fda-approval-for-newly-designed-pre-filled-syringe-for-somatuline-depot-lanreotide/. Accessed 16 August 2022
- 53.Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008;4(1):269–286. doi: 10.2147/tcrm.s1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tavares NU, Bertoldi AD, Thumé E, Facchini LA, França GV, Mengue SS. Factors associated with low adherence to medication in older adults. Rev. Saude Publica. 2013;47(6):1092–1101. doi: 10.1590/s0034-8910.2013047004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Toole D, Prebtani A, Webb S, Houchard A, Gueguen D, Boiziau S, Kunz P. PRESTO 2: An international patient survey to evaluate impact of injection and delivery system on local pain when administering somatostatin analogue (SSA) therapy. J. Neuroendocrinol. 2022;34:183. [Google Scholar]
- 56.Tomlin A, Sinclair A. The influence of cognition on self-management of type 2 diabetes in older people. Psychol. Res. Behav. Manag. 2016;9:7–20. doi: 10.2147/prbm.S36238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kishimoto M, Yamairi F, Sato N, Kobayashi J, Yamauchi S, Iwasaki T. Patient preference for treatment mode of biologics in rheumatoid arthritis: A 2020 web-based survey in Japan. Rheumatol. Ther. 2021;8(3):1095–1111. doi: 10.1007/s40744-021-00325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danhieux K, Buffel V, Pairon A, Benkheil A, Remmen R, Wouters E, van Olmen J. The impact of COVID-19 on chronic care according to providers: a qualitative study among primary care practices in Belgium. BMC Fam. Pr. 2020;21(1):255. doi: 10.1186/s12875-020-01326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The Joanna Briggs Institute: Critical appraisal tools for use in JBI systematic seviews: checklist for text and opinion. https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Text_and_Opinion2017_0.pdf (2017). Accessed 16 August 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.