Abstract
Infected individuals often obtain or lose immunity after recovery in medical studies. To solve the problem, this paper proposes a stochastic SIRS epidemic model with a general incidence rate and partial immunity. Through an appropriate Lyapunov function, we obtain the existence and uniqueness of a unique globally positive solution. The disease will be extinct under the threshold criterion. We analyze the asymptotic behavior around the disease-free equilibrium of a deterministic SIRS model. By using the Khasminskii method, we prove the existence of a unique stationary distribution. Further, solutions of the stochastic model fluctuate around endemic equilibrium under certain conditions. Some numerical examples illustrate the theoretical results.
Keywords: SIRS epidemic model, General incidence, Stationary distribution, Partial immunity
Introduction
Infectious diseases have been a severe threat to human health. Many disasters in history have been due to the outbreak of contagious diseases, such as the plague pandemic, smallpox, the Black Death, AIDS, SARS [1], and recently COVID-19 [2, 3]. Mathematical models are effective ways to investigate the spread of epidemics. Kermark and McKendrick [4] first studied the dynamic behaviors of epidemics by ordinary differential equations. Since then, researchers have proposed and studied many deterministic epidemic models, see [5–12]. Suppose the total population is divided into three classes at time t: susceptible (S), infective (I), and recovered (R) individuals, respectively. Based on the structure of compartments, researchers have proposed and studied some infectious-disease models according to the transmission characteristics and pathogenicity of the disease itself, such as SIR, SIRS, SIRI, and SIRIS models. For the SIR model, the recovered individuals have permanent immunity. However, for some diseases, the recovered individuals may lose immunity after a certain period and become susceptible individuals or relapse with reactivation of latent infection and revert to the infective class [13, 14]. The former can be described by the SIRS model, while the SIRI model can describe the latter. In recent years, much work has been carried out to study the SIRS model from all aspects, see [15–20]. Let , , and be the number of susceptible, infectious, and recovered individuals at time t, respectively. Through ordinary differential equations, the deterministic SIRS model is usually expressed by
| 1 |
In the model (1), Λ denotes the recruitment rate of susceptible individuals, ρ and α are the natural and disease-related mortality rates, β is the contact transmission coefficient, η is the recovery rate, and θ is the immunity loss rate of recovered individuals.
In epidemic models, the incidence rate plays a vital role. It not only describes the characteristics of the disease but also measures the speed at which the disease spreads. There are two widely used incidence rates: the bilinear incidence rate [21–24] and the standard incidence rate [25–27]. The bilinear incidence is commonly used to model communicable diseases, for example, influenza [8, 28]. The standard incidence is more suitable for disease modeling when the total population is huge [1]. However, it is invalid to assume homogeneous mixing in a heterogeneous population. In this case, the transmission characteristics of the disease can be described through a suitable nonlinear incidence rate [8, 17, 29–31]. Capasso and Serio [29] introduced a saturated nonlinear-incidence rate into the epidemic model. The transmission rate between the infected and susceptible will be saturated if the number of infected individuals is larger in the population. Other nonlinear incidences are used one after another, including , , and . Lahrouz et al. [8] proposed an SIRS model with a general incidence rate , where φ is a positive function such that and . Most of the above incidence rates are the special cases of . For instance, if , then it is a bilinear incidence rate; if , and the incidence rate is . Another advantage of the general incidence rate can be used to describe the psychological effect: the infection force may decrease with the number of infective individuals.
In the real world, there are kinds of infectious disease such as bacterial meningitis. Some infective individuals obtain immunity after recovery and become recovered individuals, but others have no immunity after recovery and become susceptible. This kind of character is called partial immunity. In the above kinds of SIRS models, there are two main limitations: (i) the classical SIRS model (1) with bilinear or standard incidence rates does not always effectively analyze the dynamic properties of the disease in a heterogeneous population; and (ii) although the SIRS model with a general incidence rate, proposed by [8], is more practical than the classical model, the partial immunity of infectious individuals has not been considered in the model. Generally, the individuals can be divided into two sections: one section of them after recovery have immunity and go to the recovered class. Another section after recovery has no immunity and returns to the susceptible class. For this reason, in the work we propose an SIRS epidemic model with a general incidence rate and partial immunity as follows
| 2 |
where p is the immunity rate. In the model (2), corresponds to the infected individuals who lost immunity after recovery, while represents the infected individuals who gain immunity after recovery. In particular, if and , then the model (1) is a special case of the model (2). Define the basic reproduction number as
Suppose and for in the model (2). Similar to [8] and [32], it is easy to obtain the dynamic properties of equilibria in the model (2) by constructing the Lyapunov function as follows:
-
(i)
The model (2) has a unique disease-free equilibrium denoted by . If , the disease-free equilibrium is globally asymptotically stable.
-
(ii)If , the model (2) has a unique endemic equilibrium denoted by , satisfying
Further, the endemic equilibrium is globally asymptotically stable. Detailed proofs of these are provided in the Appendix.
For the deterministic SIRS model (2), an important assumption is that the disease is not affected by stochastic perturbations. In practice, some parameters of the SIRS model (2) always fluctuate due to stochastic perturbations in the environment. Thus, the stochastic epidemic model can provide more realism than the corresponding deterministic models. In the past few years, many researchers have considered stochastic epidemic models and have obtained significant results [27, 30, 32–42]. For example, Jiang et al. [42] proved a global positive solution of a stochastic SIR model and investigated the asymptotic behaviors. Lahrouz and Omari [30] studied a stochastic SIRS model with a nonlinear incidence rate in a population of varying sizes. However, they did not investigate the asymptotic behavior of the solution. Zhang et al. [27] analyzed an SIRS model with a standard incidence rate under stochastic perturbations. Recently, Fatini et al. [33] analyzed a stochastic model with a nonlinear incidence and obtained the asymptotic behavior of the disease. Koufi et al. [34] considered a stochastic SIRS system with switching among different environments. Ding and Zhang [35] proposed a stochastic SIRS epidemic model with bilinear incidence. However, to the best of our knowledge, there are few reports of research about the stochastic SIRS model with general incidence and partial immunity. As an extension of the above results, we introduce various stochastic perturbations into the system (2). Then, we obtain a stochastic SIRS epidemic model with a general incidence rate and partial immunity as follows
| 3 |
where () are independent standard Brownian motions defined on the complete probability space with a filtration satisfying the usual conditions, and () are the nonnegative intensities of the standard Gaussian white noises.
The rest of the paper is organized as follows. We first review some basic concepts and useful lemmas in Sect. 2. The existence and uniqueness of the globally positive solution are proved in Sect. 3. In Sect. 4, we obtain sufficient conditions for the extinction of the disease under a stochastic system. Asymptotic behaviors of the solution are discussed around the disease-free equilibrium of the deterministic model in Sect. 5. In Sect. 6, we prove that the model (3) has a unique stationary distribution under certain conditions and discuss the asymptotic behaviors of the solution around the endemic equilibrium. A brief conclusion is provided in Sect. 7.
Preliminaries
Let be a three-dimensional time-homogeneous Markov process described by the following stochastic differential equation (SDE)
where , are locally Lipschitz functions in and is a four-dimensional standard Brownian motion. Denote . The operator of is defined as
Denote as the family of all nonnegative functions defined on such that they are continuously once in t and twice in z. The following formula can be obtained by acting on a function
where
By Itô’s formula, we have .
Lemma 1
([43])
Let be the quadratic variation of a continuous local martingale with initial value . Then, for almost all , there exists a random integer such that
for all , where is a number and , are two sequences.
Lemma 2
([44])
There exists a bounded open domain with regular boundary Γ, having the following properties:
In the domain U and some neighborhood thereof, the smallest eigenvalue of the diffusion matrix is bounded away from zero.
If , the mean time τ at which a path issuing from z reaches the set U is finite, and for every compact subset .
If (A.1) and (A.2) hold, then the Markov process has a stationary distribution . Further,
for all , where be a function integrable concerning measure π.
In Lemma 2, Assumption (A.1) can be verified by the existence of a positive number M such that , , . To verify Assumption (A.2), it is sufficient to prove that there is a nonnegative -function ψ such that for some , , (see [45]).
Existence and uniqueness of a positive solution
In this section, we first prove that the solution of the stochastic model (3) satisfies the following properties.
Theorem 1
For any given initial value in the model (3), there exists a unique positive solution for . The solution remains in with probability one, that is to say, for all almost certainly.
Proof
Since the coefficients of model (3) are locally Lipschitz continuous, there exists a unique local solution on for any given initial value , where is the explosion time. The solution is global if we can prove that a.s. To do this, define the stopping time as
Set as usual, where ∅ represents the empty set. Obviously, . We now only need to prove a.s. If , there must exist a positive constant C satisfying . Define a function as
By Itô’s formula, we have
where
| 4 |
The inequality (4) holds since . Then,
Integrate both sides of from 0 to t, to yield
| 5 |
From the definition of , we have . Hence, it follows that (5) satisfies
| 6 |
for . On the other hand, since . Then,
| 7 |
Combining (6) with (7), we have
Obviously, this is a contradiction. Therefore, . The proof is complete. □
Extinction of a disease
The extinction of a disease has always been a concern. In the deterministic model (2), the disease will be extinct if . That is to say, the disease-free equilibrium is globally asymptotically stable. However, the condition of disease extinction in model (3) is different from that of the model (2). Define a parameter
Theorem 2
For any initial value in model (3), if , then the disease will die out exponentially with probability one; that is,
Proof
Through Itô’s formula, we have
Integrating both sides of the above equation from 0 to t leads to
Denote . Obviously, is a continuous local martingale, and the quadratic variation satisfies
Based on Lemma 1, take , and . Hence, it follows that
| 8 |
Then,
Through calculation, we have
Hence,
Dividing the inequality by t (), we obtain
From the strong law of large numbers, when , i.e., , this yields
The desired inequality holds by letting . The proof is complete. □
Example 1
Let in the models (2) and (3).
-
(i)
Take , , and . After direct calculation, and . Figure 1 shows that the disease will become extinct in both a random and deterministic environment.
-
(ii)
Let ; other parameters and initial values are the same as those in (i). After direct calculation, and . Figure 2 shows that the disease will become extinct in a random environment but persist in a deterministic environment.
Figure 1.
Dynamical curves of compartments: (a) S, (b) I, and (c) R under and
Figure 2.
Dynamical curves of compartments: (a) S, (b) I, and (c) R under and
Asymptotic behavior around the disease-free equilibrium
In the model (2), the disease-free equilibrium is globally asymptotically stable when . However, is no longer the equilibrium of model (3) due to the stochastic perturbations. Thus, it is interesting to study the asymptotic behavior of the solution of model (3) around .
Theorem 3
If and , , , then the solution of model (3) satisfies
for any given initial value , where
Proof
Let , and . Substituting these variables into the model (3), one can obtain the following equations
Denote , and . Define a function . Then,
where
Through calculation, we have
| 9 |
| 10 |
| 11 |
The inequalities (9) and (11) follow from the fact that and for any . The inequality (10) holds due to . Combining these equations, we have
| 12 |
Hence,
Integrating both sides of the inequality from 0 to t, and taking the expectation yields
which implies
Therefore,
Then,
i.e.,
The result follows. □
Remark 1
Theorem 3 shows that if and () satisfy certain conditions, the solution of model (3) oscillates around , and the intensity of the oscillation is determined by (). Further, when () decrease, the solution of model (3) is close to the disease-free equilibrium . If , (12) is simplified as
which is negative-definite when , . Therefore, the disease-free equilibrium of model (3) is stochastically asymptotically stable.
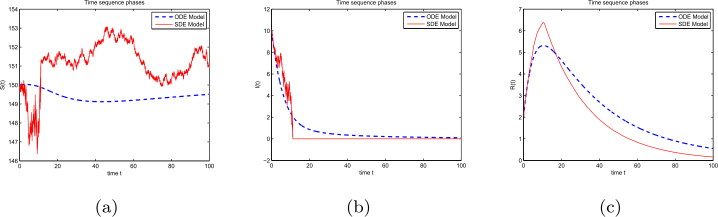
Example 2
Assume that , , and . After calculation, one can see that and , , , satisfying the conditions of Theorem 3. Figure 3 shows the trajectories of models (2) and (3). The disease-free equilibrium is global asymptotically stable. The solution of model (3) is around the solution of model (2).
Figure 3.
(a) Time-series phases of solutions (, , ) for model (2); (b) Time-series phases of solutions (, , ) for model (3). The parameters are taken from Example 2
Existence of the stationary distribution
In this section, we mainly study two properties: (i) the asymptotic behavior of the solution of the model (3) around the endemic equilibrium of the model (2), and (ii) the existence and uniqueness of the stationary distribution of the solution for the model (3). Denote
Theorem 4
If and , then
| 13 |
Further, there is a unique stationary distribution π for the solution of model (3).
Proof
We first prove the inequality (13). Since , model (2) has a unique endemic equilibrium satisfying
Let , and . Define a function
Then,
By Itô’s formula, we have
where
| 14 |
| 15 |
| 16 |
| 17 |
| 18 |
The inequalities (15) and (18) hold because , (14) and (17) hold because , while (16) holds because of the fact that . Combined with the above inequalities, one can obtain
| 19 |
Note that
Integrating both sides of dΨ from 0 to t, and taking the expectations, from (19), this yields
Dividing both sides by t and letting , we have
Then, (13) has been proved.
On the other hand, we only prove Assumptions (A.1) and (A.2) for the existence and uniqueness of the stationary distribution. Consider the ellipsoid , i.e.,
If , and , i.e., , then the ellipsoid is fully contained in . Let U be the neighborhood of the ellipsoid such that . Thus,
for any , i.e. for any . Assumption (A.2) is then satisfied. We rewrite the model (3) as
The diffusion matrix of model (3) is
Suppose . Then, for any and , we have
which meets Assumption (A.1). Therefore, model (3) has a unique stationary distribution π. □
Remark 2
Theorem 4 shows that the solution of model (3) oscillates around if when () and some parameters satisfy certain conditions.
Example 3
Let , , , and , one can obtain that , , , and . Then, Theorem 4 shows that there exists a unique stationary distribution π of model (3). Figures 4(a), (b), and (c) reflect the dynamical population of the susceptible, infective, and recovered individuals in model (2) and model (3), respectively. In Figs. 4(d), (e), and (f), we provide the frequency histogram and corresponding marginal density function curves of compartments S, I, and R, respectively. These two kinds of figures indicate that there exists a unique stationary distribution for model (3).
Figure 4.
Dynamical curves of compartments: (a) S, (b) I, (c) R. The frequency histograms and marginal density functions of compartments: (d) S, (e) I, (f) R
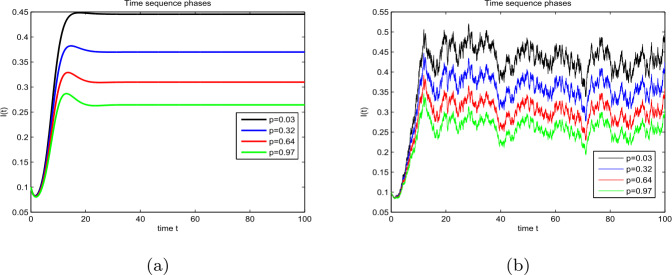
The following numerical example focuses on the effect of the partial immunization rate p on the dynamics of disease transmission.
Example 4
Let , , and . We analyze the effect of partial immunity p on the dynamic behavior of model (3). Take , and 0.97. The corresponding curves of are shown in Figs. 5(a) and (b), respectively. As the value of p increases, the growth rate and stability level of will decrease. This indicates that a large partial immunity rate can better control the epidemic than a small one. Thus, it is effective to increase the immunity rate and control the outbreak of the disease.
Figure 5.
The effect of partial immunity on compartment I: (a) model (2) and (b) model (3)
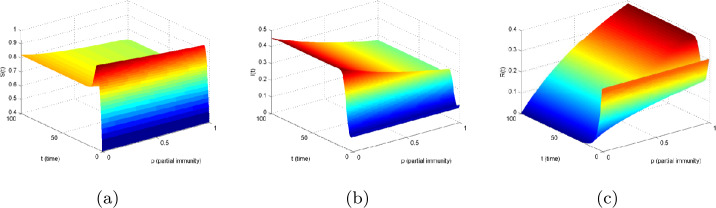
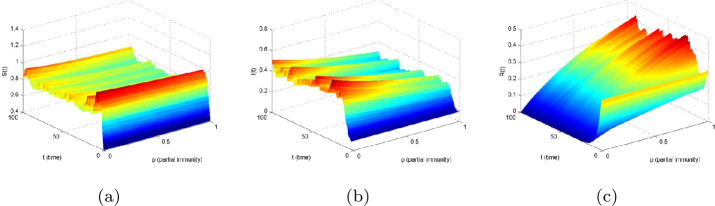
Based on the models (2) and (3), Figs. 6 and 7 show the sensitivity of partial immunity rate on , , and in three-dimensional changes in time, respectively. As the increases of p in , the value of becomes smaller, and reduces faster than others. However, increases with the increase of p.
Figure 6.
The effect of partial immunity p with respect to time t for compartments: (a) S, (b) I, and (c) R in model (2)
Figure 7.
The effect of partial immunity p with respect to time t for compartments: (a) S, (b) I, and (c) R in model (3)
Conclusion
In this paper, we propose a stochastic SIRS model with partial immunity and noninear incidence. Through a theoretical derivation, the following results are obtained for the kind of models: (i) By constructing a suitable function, the SIRS model has a unique global positive solution starting from the positive initial value (see Theorem 1). (ii) If , the disease will become extinct under the stochastic system (see Theorem 2). The result reveals that the large stochastic perturbations may lead to disease extinction. (iii) If and some parameter limits are satisfied, the solution of model (3) oscillates around . Significantly, the disease-free equilibrium of model (3) is stochastically asymptotically stable when (see Theorem 3 and Remark 1). (iv) A sufficient condition is given for the existence of the stationary distribution by using the Khasminskii method. Under this sufficient condition, the solution of model (3) will oscillate around (see Theorem 4).
The numerical simulations are provided to illustrate the theoretical analysis. Take . Four examples are given according to the following aspects: (i) Effects of the stochastic perturbations on the extinction of the infectious disease. (ii) Asymptotic behavior around the disease-free equilibrium. (iii) The existence of the stationary distribution. (iv) Effect of the partial immunization rate p on the disease-transmission dynamics. Through these numerical simulations, we observe that: (i) The sufficient condition for the extinction of the disease in model (3) is . Also, large perturbations may lead to the extinction of the disease even though it will be persistent in model (2). (ii) A large partial immunity rate can better control the epidemic than a small one.
In this work, we focus on using white noise to describe the randomness of the SIRS model. Other interesting topics for further work should be considered, such as a stochastic SIRS model with regime switching or Lévy Jumps. In recent years, the application of fractional differential equations in biological and epidemic models has increased significantly. Combined with the theory of fractional differential equations [46], the model can be extended to a fractional SIRS epidemic model. In addition, the partial-immune mechanism studied may also exist in some cells or viruses, which can be described by branching process [47]. We leave these for further investigation.
Acknowledgements
The authors are grateful to the editor and referees for their helpful comments.
Appendix
The proofs of the results (i) and (ii) of model (2) are provided as follows.
Proof of (i)
The disease-free equilibrium can be directly obtained by setting in model (2). Next, we prove that if , the disease-free equilibrium is globally asymptotically stable.
For convenience, we replace with . Through model (2), this yields
Obviously, model (2) is equivalent to the following model
| 20 |
Thus, the global stability of equilibria can be discussed by the model (20). Construct a function as
Clearly, is a positive-definite function. The first-order derivative of with respect to t is
The fact that and implies . Under , one can easily obtain that is negative-definite. By the Lyapunov asymptotic theorem, is globally asymptotically stable. □
Proof of (ii)
To obtain the endemic equilibrium , let the right sides of (2) be equal to zero, i.e.,
Since and , these equations can be simplified as
Substitute the first two equations into the third equation, i.e.,
Define
Thus, , that is, is the solution of the equation . Differentiating with respect to I, we have
Note that , we have . Thus, decreases for . Based on the existence theorem of zero, Moreover, , which leads to . In addition,
According to the existence theorem of the zero point, has a unique positive solution if and only if , i.e., . In other words, a unique endemic equilibrium exists in model (2).
Let , , and . Substituting these variables into model (20), one can obtain the following equations
| 21 |
Next, we only need to prove that the trivial solution of model (21) is globally asymptotically stable. Define
Obviously, is a positive-definite function and
Recall that , one has . Evidently, is negative-definite. Thus, is a Lyapunov function for model (21). By the Lyapunov asymptotic stability theorem, the trivial solution of model (21) is globally asymptotically stable. That is to say, the endemic equilibrium state is globally asymptotically. □
Author contributions
Chen conducted the theoretical results, simulation, analyzed the examples and drafted the manuscript. Li supervised the work and revised the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (No: 12061070) and the Natural Science Foundation of Xinjiang Uygur Autonomous Region of China (Nos: 2021D01E13).
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
References
- 1.Ma Z., Li J. Dynamical Modeling and Analysis of Epidemics. Singapore: World Scientific; 2009. [Google Scholar]
- 2.Lü X., Hui H., Liu F., Bai Y. Stability and optimal control strategies for a novel epidemic model of COVID-19. Nonlinear Dyn. 2021;106:1491–1507. doi: 10.1007/s11071-021-06524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Tang B., Bragazzi N.L., Xiao Y., Wu J. Modeling the impact of mass influenza vaccination and public health interventions on COVID-19 epidemics with limited detection capability. Math. Biosci. 2020;325:108378. doi: 10.1016/j.mbs.2020.108378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kermack W.O., McKendrick A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A. 1927;115:700–721. doi: 10.1098/rspa.1927.0118. [DOI] [Google Scholar]
- 5.Anderson R.M., May R.M. Population biology of infectious diseases: part I. Nature. 1979;280(5721):361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 6.Mena-Lorcat J., Hethcote H.W. Dynamic models of infectious diseases as regulators of population sizes. J. Math. Biol. 1992;30(7):693–716. doi: 10.1007/BF00173264. [DOI] [PubMed] [Google Scholar]
- 7.Ma Z., Zhou Y., Wu J. Modeling and Dynamics of Infectious Diseases. Singapore: World Scientific; 2009. [Google Scholar]
- 8.Lahrouz A., Omari L., Kiouach D., Belmaâti A. Complete global stability for an sirs epidemic model with generalized non-linear incidence and vaccination. Appl. Math. Comput. 2012;218(11):6519–6525. [Google Scholar]
- 9.Gao S., Ouyang H., Nieto J.J. Mixed vaccination strategy in SIRS epidemic model with seasonal variability on infection. Int. J. Biomath. 2011;4(04):473–491. doi: 10.1142/S1793524511001337. [DOI] [Google Scholar]
- 10.Feng T., Qiu Z. Global analysis of a stochastic TB model with vaccination and treatment. Discrete Contin. Dyn. Syst. 2019;24(6):2923–2939. [Google Scholar]
- 11.Li J., Ma Z. Global analysis of SIS epidemic models with variable total population size. Math. Comput. Model. 2004;39(11–12):1231–1242. [Google Scholar]
- 12.Xiao D., Ruan S. Global analysis of an epidemic model with nonmonotone incidence rate. Math. Biosci. 2007;208(2):419–429. doi: 10.1016/j.mbs.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatini M.E., Lahrouz A., Pettersson R., Settati A. Stochastic stability and instability of an epidemic model with relapse. Appl. Math. Comput. 2018;316:326–341. [Google Scholar]
- 14.Lahrouz A., Settati A., Fatini M.E., Pettersson R., Taki R. Probability analysis of a perturbed epidemic system with relapse and cure. Int. J. Comput. Methods. 2020;17(03):1850140. doi: 10.1142/S0219876218501402. [DOI] [Google Scholar]
- 15.Kusmawati I., Chandra T. Stability analysis of SIRS epidemic model on measles disease spreading with vaccination and migration. J. Phys. Conf. Ser. 2021;1872:012033. doi: 10.1088/1742-6596/1872/1/012033. [DOI] [Google Scholar]
- 16.Chen J. An SIRS epidemic model. Appl. Math. J. Chin. Univ. Ser. A. 2004;19(1):101–108. doi: 10.1007/s11766-004-0027-8. [DOI] [Google Scholar]
- 17.Hu Z., Bi P., Ma W., Ruan S. Bifurcations of an SIRS epidemic model with nonlinear incidence rate. Discrete Contin. Dyn. Syst., Ser. B. 2011;15(1):93. [Google Scholar]
- 18.Jana S., Haldar P., Kar T.K. Complex dynamics of an epidemic model with vaccination and treatment controls. Int. J. Dyn. Control. 2016;4(3):318–329. doi: 10.1007/s40435-015-0189-7. [DOI] [Google Scholar]
- 19.Li T., Zhang F., Liu H., Chen Y. Threshold dynamics of an SIRS model with nonlinear incidence rate and transfer from infectious to susceptible. Appl. Math. Lett. 2017;70:52–57. doi: 10.1016/j.aml.2017.03.005. [DOI] [Google Scholar]
- 20.Nguyen D.H., Yin G., Zhu C. Long-term analysis of a stochastic SIRS model with general incidence rates. SIAM J. Appl. Math. 2020;80(2):814–838. doi: 10.1137/19M1246973. [DOI] [Google Scholar]
- 21.Zhou Y., Zhang W., Yuan S. Survival and stationary distribution of a SIR epidemic model with stochastic perturbations. Appl. Math. Comput. 2014;244:118–131. [Google Scholar]
- 22.Ji C., Jiang D. Threshold behaviour of a stochastic sir model. Appl. Math. Model. 2014;38(21–22):5067–5079. doi: 10.1016/j.apm.2014.03.037. [DOI] [Google Scholar]
- 23.Wang J.J., Zhang J.Z., Jin Z. Analysis of an SIR model with bilinear incidence rate. Nonlinear Anal., Real World Appl. 2010;11(4):2390–2402. doi: 10.1016/j.nonrwa.2009.07.012. [DOI] [Google Scholar]
- 24.Liu S., Zhang L., Zhang X.B., Li A. Dynamics of a stochastic heroin epidemic model with bilinear incidence and varying population size. Int. J. Biomath. 2019;12(01):1950005. doi: 10.1142/S1793524519500050. [DOI] [Google Scholar]
- 25.Xu J., Wang Y., Cao Z. Dynamics of a stochastic SIRS epidemic model with standard incidence under regime switching. Int. J. Biomath. 2022;15(02):2150074. doi: 10.1142/S1793524521500741. [DOI] [Google Scholar]
- 26.Arino J., McCluskey C.C., van den Driessche P. Global results for an epidemic model with vaccination that exhibits backward bifurcation. SIAM J. Appl. Math. 2003;64(1):260–276. doi: 10.1137/S0036139902413829. [DOI] [Google Scholar]
- 27.Zhang X.B., Wang X.D., Huo H.F. Extinction and stationary distribution of a stochastic SIRS epidemic model with standard incidence rate and partial immunity. Phys. A, Stat. Mech. Appl. 2019;531:121548. doi: 10.1016/j.physa.2019.121548. [DOI] [Google Scholar]
- 28.Korobeinikov A. Lyapunov functions and global stability for sir and SIRS epidemiological models with non-linear transmission. Bull. Math. Biol. 2006;68(3):615–626. doi: 10.1007/s11538-005-9037-9. [DOI] [PubMed] [Google Scholar]
- 29.Capasso V., Serio G. A generalization of the Kermack–McKendrick deterministic epidemic model. Math. Biosci. 1978;42(1–2):43–61. doi: 10.1016/0025-5564(78)90006-8. [DOI] [Google Scholar]
- 30.Lahrouz A., Omari L. Extinction and stationary distribution of a stochastic SIRS epidemic model with non-linear incidence. Stat. Probab. Lett. 2013;83(4):960–968. doi: 10.1016/j.spl.2012.12.021. [DOI] [Google Scholar]
- 31.Yang Y., Zou L., Zhang T., Xu Y. Dynamical analysis of a diffusive SIRS model with general incidence rate. Discrete Contin. Dyn. Syst., Ser. B. 2020;25(7):2433–2451. [Google Scholar]
- 32.Liu Q., Chen Q. Analysis of the deterministic and stochastic SIRS epidemic models with nonlinear incidence. Phys. A, Stat. Mech. Appl. 2015;428:140–153. doi: 10.1016/j.physa.2015.01.075. [DOI] [Google Scholar]
- 33.Fatini M.E., Sekkak I., Taki R., Guendouz T.E. A control treatment for a stochastic epidemic model with relapse and Crowly–Martin incidence. J. Anal. 2021;29:713–729. doi: 10.1007/s41478-020-00276-4. [DOI] [Google Scholar]
- 34.Koufi A.E., Bennar A., Yousfi N., Pitchaimani M. Threshold dynamics for a class of stochastic SIRS epidemic models with nonlinear incidence and Markovian switching. Math. Model. Nat. Phenom. 2021;16:55. doi: 10.1051/mmnp/2021047. [DOI] [Google Scholar]
- 35.Ding T., Zhang T. Asymptotic behavior of the solutions for a stochastic SIRS model with information intervention. Math. Biosci. Eng. 2022;19(7):6940–6961. doi: 10.3934/mbe.2022327. [DOI] [PubMed] [Google Scholar]
- 36.Ma J., Yi Q. Asymptotic behavior and stationary distribution of a nonlinear stochastic epidemic model with relapse and cure. J. Math. 2020;2020:1–12. doi: 10.1155/2020/4307083. [DOI] [Google Scholar]
- 37.Koufi A.E., Adnani J., Bennar A., Yousfi N. Dynamics of a stochastic sir epidemic model driven by Lévy jumps with saturated incidence rate and saturated treatment function. Stoch. Anal. Appl. 2021;40:1048–1066. doi: 10.1080/07362994.2021.1981382. [DOI] [Google Scholar]
- 38.Khan T., Zaman G., El-Khatib Y. Modeling the dynamics of novel coronavirus (COVID-19) via stochastic epidemic model. Results Phys. 2021;24:104004. doi: 10.1016/j.rinp.2021.104004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y., Jiao J., Gui Z., Liu Y., Wang W. Environmental variability in a stochastic epidemic model. Appl. Math. Comput. 2018;329:210–226. [Google Scholar]
- 40.Yang B., Cai Y., Wang K., Wang W. Optimal harvesting policy of logistic population model in a randomly fluctuating environment. Phys. A, Stat. Mech. Appl. 2019;526:120817. doi: 10.1016/j.physa.2019.04.053. [DOI] [Google Scholar]
- 41.Liu Q., Jiang D., Hayat T., Alsaedi A., Ahmad B. Dynamics of a multigroup SIQS epidemic model under regime switching. Stoch. Anal. Appl. 2020;38(5):769–796. doi: 10.1080/07362994.2020.1722167. [DOI] [Google Scholar]
- 42.Jiang D., Yu J., Ji C., Shi N. Asymptotic behavior of global positive solution to a stochastic SIR model. Math. Comput. Model. 2011;54(1–2):221–232. doi: 10.1016/j.mcm.2011.02.004. [DOI] [Google Scholar]
- 43.Mao X. Almost sure asymptotic bounds for a class of stochastic differential equations. Stoch. Stoch. Rep. 1992;41:57–69. doi: 10.1080/17442509208833794. [DOI] [Google Scholar]
- 44.Khasminskii R. Stochastic Stability of Differential Equations. Alphen aan den Rijn: Sijthoff & Noordhoff; 1980. [Google Scholar]
- 45.Zhu C., Yin G. Asymptotic properties of hybrid diffusion systems. SIAM J. Control Optim. 2007;46(4):1155–1179. doi: 10.1137/060649343. [DOI] [Google Scholar]
- 46.He J.W., Zhou Y., Peng L., Ahmad B. On well-posedness of semilinear Rayleigh–Stokes problem with fractional derivative on . Adv. Nonlinear Anal. 2021;11:580–597. doi: 10.1515/anona-2021-0211. [DOI] [Google Scholar]
- 47.Beznea L., Vrabie C. Continuous flows driving branching processes and their nonlinear evolution equations. Adv. Nonlinear Anal. 2022;11:921–936. doi: 10.1515/anona-2021-0229. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.