Background:
Pretreatment HIV drug resistance (PDR) undermines individual treatment success and threatens the achievement of UNAIDS 95-95-95 targets. In many African countries, limited data are available on PDR as detection of recent HIV infection is uncommon and access to resistance testing is limited. We describe the prevalence of PDR among South African women with recent HIV infection from the Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial.
Methods:
HIV-uninfected, sexually active women, aged 18–35 years, and seeking contraception were enrolled in the ECHO Trial at sites in South Africa, from 2015 to 2018. HIV testing was done at trial entry and repeated quarterly. We tested stored plasma samples collected at HIV diagnosis from women who seroconverted during follow-up and had a viral load >1000 copies/mL for antiretroviral resistant mutations using a validated laboratory-developed population genotyping assay, which sequences the full protease and reverse transcriptase regions. Mutation profiles were determined using the Stanford Drug Resistance Database.
Results:
We sequenced 275 samples. The median age was 23 years, and majority (98.9%, n = 272) were infected with HIV-1 subtype C. The prevalence of surveillance drug resistance mutations (SDRMs) was 13.5% (n = 37). Nonnucleoside reverse transcriptase inhibitor (NNRTI) mutations were found in 12.4% of women (n = 34). Few women had NRTI (1.8%, n = 5) and protease inhibitor (1.1%, n = 3) mutations. Five women had multiple NRTI and NNRTI SDRMs.
Conclusions:
The high levels of PDR, particularly to NNRTIs, strongly support the recent change to the South African national HIV treatment guidelines to transition to a first-line drug regimen that excludes NNRTIs.
Key Words: antiretroviral resistance, women, South Africa, pretreatment resistance, HIV-1
INTRODUCTION
According to UNAIDS (Joint United Nations Program on HIV/AIDS), in 2020, there were 7.8 million people living with HIV in South Africa (SA), with 230,000 new infections and approximately 60% of new infections occurring in women.1 An ambitious treatment target of 95-95-95 was set by UNAIDS in 2014 to try to end the AIDS epidemic by 2030.2 Critical to achieving this target is the prevention, monitoring, and timely response to population levels of HIV drug resistance.3 The last “95” reflects the goal of achieving 95% of individuals on treatment being virally suppressed. This goal is vital to eliminate the risk of onward transmission of HIV.4
Over the last decade, an increase in the use of antiretrovirals (ARVs) to treat HIV has been accompanied by the emergence of drug resistance.5 According to the World Health Organization (WHO) 2017 report on HIV drug resistance, there has been a steady increase in the prevalence of pretreatment HIV drug resistance (PDR) from 2001 in several regions, particularly in Eastern and Southern Africa, with levels of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance exceeding 10%.6 Similarly, a systematic review found that PDR was increasing at a substantial rate in several low-income and middle-income countries (LMICs), especially in sub-Saharan Africa.7 Trends in SA have also shown an increasing prevalence in PDR.8 The recent 2021 WHO HIV drug resistance report found that the level of PDR to nevirapine (NVP) or efavirenz (EFV) in populations initiating first-line antiretroviral therapy (ART) exceeded 10% in several countries including SA.9 HIV drug resistance, if unmitigated, can result in HIV-associated morbidity and mortality as well as onward transmission of resistant variants.5
The WHO Global Action Plan on drug resistance aims to minimize the emergence and transmission of HIV drug resistance, and one of the key strategic objectives of this plan is the monitoring and surveillance of HIV drug resistance that can be used to guide national ARV programmes.6 In many resource-limited settings, data on transmitted drug resistance is sparse because detection of recent HIV acquisition is uncommon and access to resistance testing is limited. This study describes the prevalence and patterns of PDR among South African women who were diagnosed with recent HIV infection during the Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial, which was a prospective trial. Given that the ECHO Trial enrolled young, HIV-uninfected, sexually active women who account for the majority of new HIV infections in SA,10 and that follow-up in the trial was quarterly which allowed for the measurement of prospective PDR, our study findings provide important data for the monitoring and surveillance of HIV drug resistance.
METHODS
ECHO Trial Overview and Procedures
The ECHO Trial, conducted between December 2015 and October 2018, enrolled HIV-negative women, aged 16–35 years, from 12 sites in 4 African countries (Eswatini, Kenya, SA, and Zambia).11 This analysis is limited to ECHO Trial participants, aged 18–35 years, who were enrolled at 8 of the 9 SA trial sites (Brits, Durban, East London, Edendale, Johannesburg, Klerksdorp, Ladysmith, and Soshanguve).
The primary ECHO Trial outcome was HIV incidence. HIV-negative women, desiring contraception, and willing to be randomized to 1 of 3 highly effective contraceptives were enrolled and followed for 12–18 months. At study entry, 2 different rapid HIV tests were conducted in parallel, and both tests had to be negative to be eligible for the trial. Parallel rapid HIV testing was repeated quarterly. If one or both rapid tests were reactive during study follow-up, additional serologic and HIV RNA testing was done, and all HIV seroconversions were confirmed by an endpoints committee. Detailed ECHO Trial procedures have been previously described.11 Women who reported testing for HIV at off-site facilities before their study visits were tested for HIV upon returning to the research sites as per the study protocol for HIV testing.
Women who seroconverted were counseled by trial staff and referred to local health facilities for HIV care. All women were asked at every follow-up study visit whether they had used HIV pre-exposure prophylaxis (PrEP) since the previous visit, and if PrEP was used, dates of PrEP use were recorded on case report forms. Oral PrEP was provided on-site by trial staff at the SA trial sites during the latter 8 months of the trial.12 Women with confirmed seroconversion were asked if they had taken any ARVs since the previous visit, and ART regimens and dates of use were recorded.
Among women who seroconverted, additional blood samples were collected at the seroconversion visit. Women who consented to sample storage and future testing had these samples stored beyond the duration of the ECHO Trial. For the 8 South African trial sites, samples were stored in freezers at Bio Analytical Research Corporation (BARC) laboratory in Johannesburg, SA at −80°C.
Sexually transmitted infection (STI) testing for Chlamydia trachomatis and Neisseria gonorrhoeae was done via nucleic acid amplification testing of a provider-collected genital swab. Herpes simplex virus type 2 infection (HSV-2) status was determined using serology.
HIV Resistance Testing Procedures
HIV drug resistance testing was performed on plasma samples from women with confirmed HIV infection during study follow-up, who had a HIV RNA PCR >1000 copies/mL at the HIV detection visit, and who consented to sample storage and future testing. Women who tested positive for HIV at screening were excluded from the main ECHO Trial. Population-based HIV drug resistance testing, using a laboratory developed assay,13 of the complete HIV-1 protease and reverse transcriptase was performed using a laboratory-developed assay that was certified by Division of AIDS (DAIDs), National Institute of Health virology quality assessment (VQA) at BARC-SA/Lancet Laboratories, SA. Known HIV drug resistance mutations and scores were determined using the Stanford Drug Resistance Database (v8.9),14 and HIV subtype was determined using REGA subtyping tool version 3.15 Surveillance drug resistance mutations (SDRMs) were defined according to the Stanford University HIV drug resistance database.16
Study participants were contacted to receive their HIV resistance testing results (where HIV resistance was detected) in-person at study sites. In addition, participants who were unable or unwilling to come to the research sites were given the option of having their HIV resistance results sent to their healthcare providers.
Statistical Methods
We present overall participant characteristics including counts and percentages for categorical variables. Demographic characteristics, including age, education, marital status, cohabitation with partner, and STIs, were collected at enrolment. Sexual behaviours, condom use, partner HIV status, CD4 counts, and viral load counts were collected at the HIV detection visit.
The prevalence of HIV drug-resistant mutations is presented overall and by location of trial sites. We evaluated factors associated with SDRM including sociodemographic and health factors. We constructed univariate Poisson regression models to evaluate prevalence ratios of correlates of SDRM vs. no SDRM using a 2-tailed test to evaluate significance, with a significance threshold of P < 0.05. Missing data were excluded from the analysis. All statistical analyses were conducted with STATA v.15.17
Ethics Statement
This study was approved by the University of the Witwatersrand Human Research Ethics Committee (Reference 141112). Women provided written informed consent to participate in the ECHO Trial and additional consent for long-term sample storage and future testing.
RESULTS
Of the 345 women who seroconverted from the South Africa trial sites, 314 were from the 8 study sites included in this analysis. In total, samples from 284 women (284 of 314, 90.4%) met the criteria for HIV drug resistance testing, and 275 samples (275 of 284, 96.8%) were sequenced. Nine samples were not sequenced for the following reasons: 4 had insufficient plasma available for testing, 4 failed amplification, and 1 failed sequencing. Among the 275 women included in this analysis, the median age was 23 years (range: 18–35 years), and over two-thirds were ≤24 years (n = 192, 69.8%) (Table 1).
TABLE 1.
Participant Characteristics at Enrolment Into the Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial and at the HIV Seroconversion Visit (N = 275)
| Characteristics (N = 275) | n (%) |
| Baseline characteristics | |
| Age, yrs | |
| 18–20 | 80 (29.1) |
| 21–24 | 112 (40.7) |
| 25–30 | 73 (26.6) |
| 31–35 | 10 (3.6) |
| Marital status | |
| Never married | 273 (99.3) |
| Married | 1 (0.4) |
| Previously married | 1 (0.4) |
| Lives with partner | 24 (8.7) |
| Education | |
| Primary school (any) | 1 (0.4) |
| Secondary school (any) | 222 (80.7) |
| Postsecondary school (any) | 52 (18.9) |
| Sexually transmitted infections* | |
| Chlamydia trachomatis | 75 (27.4) |
| Neisseria gonorrhoea | 33 (12.0) |
| HSV-2 | |
| Negative | 117 (42.5) |
| Indeterminate | 36 (13.1) |
| Positive | 121 (44.0) |
| Unavailable | 1 (0.4) |
| Participant characteristics at seroconversion visit† | |
| Sexual behaviours (in the last 3 mo) | |
| Number of sex partners in the last 3 mo | |
| 0 | 3 (1.1) |
| 1 | 246 (89.8) |
| ≥2 | 25 (9.1) |
| New sex partner | 20 (7.3) |
| Condom use in the last 3 mo | |
| Never | 46 (16.8) |
| Rarely | 12 (4.4) |
| Sometimes | 123 (44.9) |
| Often | 18 (6.6) |
| Always | 61 (22.3) |
| N/A (did not have vaginal sex in the last 3 mo/no sex partner) | 14 (5.1) |
| Sex in exchange for money or gifts (yes) | 2 (0.7) |
| HIV status of primary sex partner‡ | |
| Negative | 159 (58.7) |
| Unknown | 106 (39.1) |
| Positive | 6 (2.2) |
| Primary sex partner on ARVs (yes)‡ | 3 (1.1) |
| Sexually transmitted infections§ | |
| Chlamydia trachomatis | 59 (23.0) |
| Neisseria gonorrhoea | 39 (15.2) |
| CD4 (cells/mm3) | |
| <200 | 4 (1.5) |
| 200–500 | 116 (42.3) |
| >500 | 154 (56.2) |
| Viral load (copies/mL) | |
| 1000–10,000 | 77 (28.0) |
| 10,001–100,000 | 99 (36.0) |
| >100,000 | 99 (36.0) |
One participant had missing laboratory results (n = 274).
One participant had missing data for all variables at possible seroconversion visit except viral load (n = 274).
Only refers to women who had a primary sex partner (n = 271).
Seventeen with missing lab results (n = 257).
Nearly all women were infected with HIV-1 subtype C (272 of 275, 98.9%), 2 had subtype A, and 1 had subtype D. Of the 275 samples sequenced, SDRMs were detected in 37 women (13.5%), and the majority of SDRMs were NNRTI mutations (34 of 37, 91.9%). NRTI and protease inhibitor (PI) SDRMs were detected in 5 (1.8%) and in 3 (1.1%) women, respectively. The most frequently occurring NNRTI mutation was K103N (n = 21, 7.6%), which was found as a mixture with wild-type (K103KN), in isolation, or in combination with other NRTI and/or NNRTI mutations (Fig. 1). The E138A mutation, which is polymorphic in subtype C HIV-1 and not classified as a SDRM, was found in 17 women (6.2%). Among women with NRTI resistance mutations (n = 5, 1.8%), 4 (1.5%) had the M184V mutation and 2 (0.7%) had the K65R mutation (Table 2). Thymidine analogue mutations (TAMs) included K219Q or K219E (n = 3) and D67N or D67G (n = 2). Five women (1.8%) had dual NNRTI and NRTI resistance mutations. Major PI mutations were found in 3 women (1.1%), and included L90M, V82L, and M46L, with no other mutations detected among these women. There were 8 (2.9%) minor PI mutations detected, namely, Q58E (n = 5), L33F (n = 2), and K43T (n = 1). One woman with Q58E also had the K103N mutation, and one woman with Q58E had the E138A mutation. Among the 8 trial sites, the prevalence of SDRMs ranged from 6.1% (95% CI = 1.7%, 19.6%) in Klerksdorp (Northwest Province) to 23.1% (95% CI = 8.2%, 50.3%) in Brits (Northwest Province) (Fig. 2).
FIGURE 1.
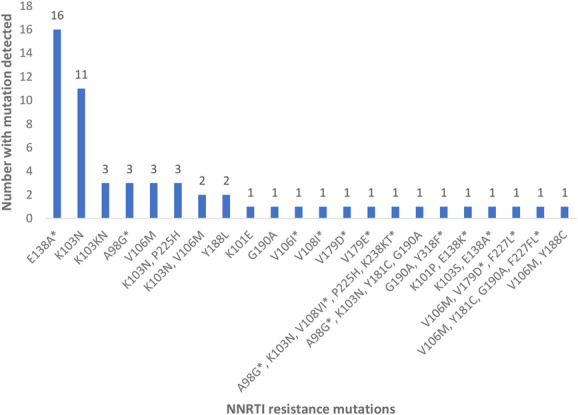
Detailed NNRTI resistance mutations including polymorphisms. *Designated mutations are not on the Stanford list of HIV-1 mutations for drug-resistance surveillance and may represent polymorphisms of “wild-type” virus.
TABLE 2.
Combined NNRTI and NRTI Resistance Mutations
| Participant Number | Mutations |
| 1 | K103N, K219Q |
| 2 | A98G*, K103N, V108VI*, P225H, K238KT*, K70N, L74I, M184V |
| 3 | V106M, V179D*, F227L*, D67G, M184V, K219E |
| 4 | V106M, Y181C, G190A, F227FL*, A62V, K65R, M184V |
| 5 | V106M, Y188C, K65R, D67N, M184V, K219E |
Designated mutations are not on the Stanford list of HIV-1 mutations for Drug-Resistance Surveillance and may represent polymorphisms of “wild-type” virus.
FIGURE 2.
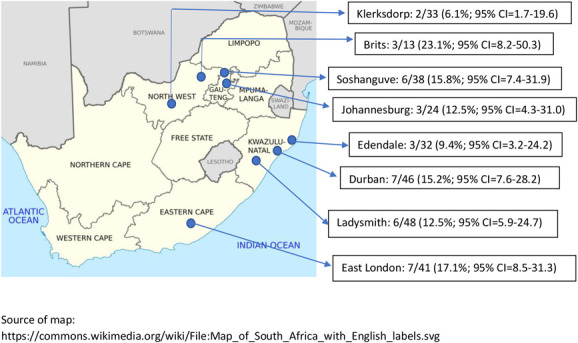
Prevalence of SDRMs with 95% confidence intervals per trial site.
Six women had reported the use of ARVs before HIV detection in the study. Of these, 4 reported using ART and 2 women reported using oral PrEP (tenofovir/emtricitabine). Three of the 4 women on ART had no resistance mutations detected, and had used ART for 5, 12, and 35 days, respectively, before HIV detection in the study. The remaining participant was on ART for more than 6 months before HIV detection in the study and had the following mutations: K70N, L74I, M184V, A98G, K103N, V108VI, P225H, and K238KT. This participant tested negative for HIV on rapid antibody and retrospective PCR testing at enrolment into the trial; thereafter, she missed quarterly follow-up study visits for 15 months, and at her subsequent follow-up visit, she was confirmed to be HIV infected. Among the 2 women using oral PrEP, one had initiated oral PrEP 81 days before seroconversion and reported continuous use of oral PrEP until the day before HIV diagnosis in the study and had the E138A subtype C polymorphism with no NRTI mutations. The second woman on oral PrEP had used oral PrEP for 27 days and then was off oral PrEP for approximately 2 months, and thereafter was confirmed to have HIV infection at the study site. This participant had HIV-1 with the NNRTI mutations K103N and P225H but no NRTI mutations.
Categorical participant age was lower among women with seroconversions with SDRMs (81.1% <25 years vs. 68.1% in those with no SDRMs), although this was not statistically significant (relative risk [RR] = 0.54, 95% CI = 0.24, 1.23, P = 0.142) (Table 3). Having a primary partner on ARVs represented an increased risk of SDRMs, with 2 women reporting a partner with HIV (5.4%) compared with 1 woman in the non-SDRM group (0.4%) (RR = 5.41, 95% CI = 1.30, 22.56, P = 0.020). In addition, having an STI at the seroconversion visit was associated with an increased risk of SDRMs compared with not having an STI, and this association was significant (RR = 2.10, 95% CI = 1.03, 4.30, P = 0.042). No other demographic or behavioral factors were associated with SDRMs.
TABLE 3.
Factors Associated With the Detection of SDRMs
| Characteristics | Total (N = 275) | SDRM Detected (n = 37) | No SDRM (n = 238) | Relative Risk (95% Confidence Interval); P* |
| Baseline characteristics | ||||
| Age, yrs | ||||
| 18–24 | 192 (69.8) | 30 (81.1) | 162 (68.1) | Ref |
| 25–35 | 83 (30.2) | 7 (18.9) | 76 (31.9) | 0.54 (0.24 to 1.23); P = 0.142 |
| Marital status | ||||
| Married/previously married | 2 (0.7) | 0 (0) | 2 (0.8) | |
| Unmarried | 273 (99.3) | 37 (100) | 236 (99.2) | — |
| Does not live with partner | 251 (91.3) | 34 (91.9) | 217 (91.2) | Ref |
| Lives with partner | 24 (8.7) | 3 (8.1) | 21 (8.8) | 0.92 (0.28 to 3.00); P = 0.894 |
| Education | ||||
| Primary/secondary | 223 (81.1) | 29 (78.4) | 194 (81.5) | Ref |
| Postsecondary school | 52 (18.9) | 8 (21.6) | 44 (18.5) | 1.18 (0.54 to 2.59); P = 0.674 |
| Sexually transmitted infections† | ||||
| No STIs | 184 (67.2) | 26 (70.3) | 158 (66.7) | Ref |
| Chlamydia trachomatis and/or Neisseria gonorrhoea | 90 (32.9) | 11 (29.7) | 79 (33.3) | 0.85 (0.40 to 1.80); P = 0.664 |
| HSV-2† | ||||
| Negative/indeterminate | 153 (55.8) | 23 (62.2) | 130 (54.9) | Ref |
| Positive | 121 (44.2) | 14 (37.8) | 107 (45.1) | 0.77 (0.40 to 1.50); P = 0.440 |
| Study site | ||||
| Urban | 182 (66.2) | 25 (67.6) | 157 (66.0) | Ref |
| Rural | 93 (33.8) | 12 (32.4) | 81 (34.0) | 0.94 (0.47 to 1.87); P = 0.859 |
| Province | ||||
| KwaZulu-Natal | 126 (45.8) | 16 (43.2) | 110 (46.2) | Ref |
| Gauteng | 62 (22.5) | 9 (24.3) | 53 (22.3) | |
| Eastern Cape | 41 (14.9) | 7 (18.9) | 34 (14.3) | 1.01 (0.99 to 1.03); P = 0.268 |
| North-West | 46 (16.7) | 5 (13.5) | 41 (17.2) | |
| Participant characteristics at seroconversion visit | ||||
| Sexual behaviours (in the last 3 mo)‡ | ||||
| ≤ 1 sex partner | 249 (90.9) | 31 (83.8) | 218 (92.0) | Ref |
| >1 sex partner | 25 (9.1) | 6 (16.2) | 19 (8.0) | 2.04 (0.85 to 4.90); P = 0.113 |
| No new sex partner(s) | 251 (92.6) | 32 (91.4) | 219 (92.8) | Ref |
| New sex partner | 20 (7.4) | 3 (8.6) | 17 (7.2) | 1.13 (0.35 to 3.66); P = 0.845 |
| No unprotected sex | 60 (21.9) | 8 (21.6) | 52 (21.9) | Ref |
| Any unprotected sex | 214 (78.1) | 29 (78.4) | 185 (78.1) | 0.97 (0.44 to 2.12); P = 0.935 |
| 236 (99.2) | ||||
| No sex in exchange for money or gifts | 272 (99.3) | 37 (100.0) | 236 (99.2) | |
| Sex in exchange for money or gifts | 2 (0.7) | 0 (0) | 2 (0.8) | — |
| HIV status of primary sex partner | ||||
| Negative | 159 (57.8) | 18 (48.6) | 141 (59.2) | Ref |
| Unknown | 106 (38.5) | 15 (40.5) | 91 (38.2) | |
| Positive | 6 (2.2) | 2 (5.4) | 4 (1.7) | 1.03 (0.75 to 1.43); P = 0.20 |
| No primary partner | 4 (1.5) | 2 (5.4) | 2 (0.8) | |
| Primary sex partner on ARVs (yes)‡ | 3 (1.1) | 2 (5.4) | 1 (0.4) | 5.41 (1.30 to 22.56); P = 0.020 |
| Sexually transmitted infections§ | ||||
| No STIs | 174 (67.7) | 19 (52.8) | 155 (70.1) | Ref |
| Chlamydia trachomatis and/or Neisseria gonorrhoea | 83 (32.3) | 17 (47.2) | 66 (29.9) | 2.10 (1.03 to 4.30); P = 0.042 |
| CD4 (cells/mm3)‖ | ||||
| <500 | 116 (42.3) | 14 (37.8) | 102 (43.0) | Ref |
| ≥500 | 158 (57.7) | 23 (62.2) | 135 (57.0) | 1.24 (0.61 to 2.53); P = 0.552 |
| Viral load (copies/mL) | ||||
| 1000–10,000 | 77 (28.0) | 10 (27.0) | 67 (28.2) | Ref |
| 10,001–100,000 | 99 (36.0) | 10 (27.0) | 89 (37.4) | |
| >100,000 | 99 (36.0) | 17 (45.9) | 82 (34.5) | 1.19 (0.79 to 1.80); P = 0.405 |
Bold: P < 0.05.
RR not calculated as one category had 0.
One participant had missing laboratory results (n = 274).
One participant had missing data and 3 participants did not have a primary sex partner in the last 3 months (n = 271).
Missing 18 laboratory results (n = 257).
One participant had a missing CD4 result.
DISCUSSION
In this analysis among South African women with recent seroconversion, who were enrolled in the ECHO Trial, SDRMs were detected in 13.5% of women, with NNRTI-resistant mutations being the most frequent. Of concern, several of the NNRTI SDRMs detected confer high-level resistance to EFV and NVP. Dual NRTI and NNRTI mutations were observed in about 2% of women, and approximately 1% had major PI mutations. Our study findings add robust data to the body of evidence on the prevalence and patterns of PDR among young South African women.
The prevalence of PDR found in our study is consistent with other studies conducted in the region. A systematic review and meta-analysis found that the estimated prevalence of NNRTI PDR in Southern Africa was 11% in 2016.7 In SA, a systematic review that included several datasets found that the pooled annual prevalence of PDR was 12% in 2015.8 Similarly, a study conducted in a province (KwaZulu-Natal) with the highest HIV prevalence in SA found that PDR prevalence was 11%, with NNRTI PDR levels exceeding 10%.18 Of concern, several studies found that PDR was increasing rapidly, raising concerns about effective first-line ART regimens.7,8 In a nationally representative household survey conducted in SA in 2017, among those recently infected with HIV, drug resistance was detected in 22% of samples, all of which had NNRTI-resistant mutations.19 The WHO recommends using a first-line regimen that does not contain EFV or NVP in countries in which resistance to these drugs exceeds 10% and further recommends that the rapid adoption of dolutegravir (DTG)-based regimens as first-line treatment would help avert the negative effects of NNRTI resistance.3 DTG offers a high genetic barrier to resistance, minimal side effects, and provides rapid viral suppression. SA revised its national HIV guidelines in 2019 to include a DTG-based first-line ART regimen.20
One of the strengths of this study is that women who were enrolled in the ECHO Trial were HIV negative at enrolment into the trial, and HIV testing was conducted quarterly allowing for the prospective measure of PDR among women with recent HIV infection. Therefore, our data reflect transmitted drug resistance. This is of importance as PDR surveys have found that up to 26% of people initiating ART have had previous ARV drug exposure,3 and data on transmitted drug resistance is lacking because many surveys do not measure recent HIV infection, hence the timing between HIV seroconversion and PDR testing may be delayed or unknown. Furthermore, in the ECHO Trial, women were asked about the use of PrEP at every quarterly follow-up visit and the use of ART at every follow-up visit subsequent to HIV diagnosis in the study. Of the 2 women with SDRMs who had reported the use of ARVs, one was found to have dual NRTI and NNRTI resistance mutations and had missed several follow-up visits after enrolment and had started ART before HIV diagnosis in the study. The other woman who had used daily oral PrEP containing tenofovir and emtricitabine was found to have NNRTI-resistant mutations (K103N and P225H) that were unrelated to oral PrEP use.
We found that most mutations detected in our study were NNRTI mutations, with the K103N mutation occurring most frequently. Similarly, in the 2017 South African national household survey, among persons reporting not using ART and among those with recent HIV infection, the prevalence of drug resistance was 15% and 22%, respectively, and all had NNRTI-only resistance, with the K103N mutation occurring in almost all those with recent infection.19 The K103N mutation confers high level resistance to EFV and NVP compromising first-line ART regimens containing these drugs. Worryingly, WHO reported that in several LMICs, levels of PDR to NVP and/or EFV exceeded 10%, and overall, levels of NNRTI PDR are nearly twice as high among women as among men.3
We found that women with PDR in our study tended to be younger and were more likely to report having a partner living with HIV on ART. Although the South African national household survey and a study conducted in KwaZulu-Natal, SA did not show significant associations between age and PDR,18,19 a study in Kenya found a significant association between age and PDR prevalence, where lower age was associated with higher PDR prevalence.21 The mechanism that ties the risk of PDR and age is unknown but may be related to sexual behaviors in young women, for example, having multiple partners and condomless sex, compared with older women in more stable relationships.
In our study, ARV use was ascertained via participant self-report and no testing for ARVs was conducted; however, the frequency of HIV testing in the ECHO Trial and the prospective design of the study allowed for the assessment of recent HIV infection. Our analysis is limited to study sites located in 4 provinces in SA; therefore, our results may not be generalizable to the entire country, and it is possible that resistance might differ between different geographical areas and provinces. Furthermore, our analysis did not include the ECHO Trial sites located out of SA.
In conclusion, we found high levels of PDR, predominantly to NNRTIs, among young South African women with recent seroconversion who were enrolled in the ECHO Trial. Our study findings support the recent change to a DTG-based first-line ART regimen for HIV treatment in SA. Finally, ongoing drug resistance surveillance remains critical to monitor and evaluate trends in PDR, particularly transmitted drug resistance, in SA.
ACKNOWLEDGMENTS
The authors thank all the women who participated in the ECHO Trial.
Footnotes
This work and the Evidence for Contraceptive Options and HIV Outcomes (ECHO) Study were made possible by the combined generous support of the Bill & Melinda Gates Foundation (Grant OPP1032115), the American people through the United States Agency for International Development (Grants AID-OAA-A-15–00045; AID-OAA-A-15-0031), the Swedish International Development Cooperation Agency (Grant 2017/762965–0), the South Africa Medical Research Council, and the United Nations Population Fund. Contraceptive supplies were donated by the Government of South Africa and US Agency for International Development. The contents of this article are solely the responsibility of the authors and do not necessarily reflect the views, decisions or policies of the institutions with which they are affiliated, the ECHO Trial funders, or the supporting governments.
The authors have no conflicts of interest to disclose.
Access to data from the ECHO Study may be requested through submission of a research concept to icrc@uw.edu. The concept must include the research question, data requested, analytic methods, and steps taken to ensure ethical use of the data. Access will be granted if the concept is evaluated to have scientific merit and if sufficient data protections are in place. As of the time of publication, data access applications are in process with the governing institutional review boards of the ECHO Study to make de-identified data publicly available.
I.B., J.M.B., and C.W. conceived of the study design. C.W., D.L.D., and I.B. analyzed the data. I.B., J.M.B., C.W., and D.L.D. drafted the initial manuscript. I.B., U.M.P., J.W.M., D.L.D., R.H., T.P., S.L.B., M.B., J.S., K.A., H.M., P.S., C.L., P.K., G.J.H., M.S., H.R., J.M.B., and C.W. contributed to data interpretation and critical review of the article. All authors have approved the final manuscript.
REFERENCES
- 1.Joint United Nations Program on HIV/AIDS (UNAIDS). Country Fact Sheets: South Africa; 2020. Available at: https://www.unaids.org/en/regionscountries/countries/southafrica. Accessed December 1, 2021. [Google Scholar]
- 2.Joint United Nations Program on HIV/AIDS (UNAIDS). Fast-Track—Ending the AIDS Epidemic by 2030. UNAIDS/JC2686; 2014. Available at: https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed December 1, 2021. [Google Scholar]
- 3.HIV Drug Resistance Report 2019. Geneva, Switzerland: World Health Organization; 2019. (WHO/CDS/HIV/19.21). Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 4.Beyrer C, Pozniak A. HIV drug resistance—an emerging threat to epidemic control. N Engl J Med. 2017;377:1605–1607. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Fact Sheet: HIV Drug Resistance; 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/hiv-drug-resistance. [Google Scholar]
- 6.Global Action Plan on HIV Drug Resistance 2017–2021. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 7.Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimukangara B, Lessells RJ, Rhee SY, et al. Trends in pretreatment HIV-1 drug resistance in antiretroviral therapy-naive adults in South Africa, 2000-2016: a pooled sequence analysis. EClinicalMedicine. 2019;9:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HIV Drug Resistance Report 2021. Geneva, Switzerland: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 10.South African National AIDS Council. South Africa's National Strategic Plan for HIV, TB and STIs 2017-2022; 2017. Available at: https://sanac.org.za/wp-content/uploads/2017/06/NSP_FullDocument_FINAL-1.pdf. [Google Scholar]
- 11.Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet. 2019;394:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beesham I, Welch JD, Heffron R, et al. Integrating oral PrEP delivery among African women in a large HIV endpoint-driven clinical trial. J Int AIDS Soc. 2020;23:e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis CL, Papathanasopoulos MA, Lakhi S, et al. Affordable in-house antiretroviral drug resistance assay with good performance in non-subtype B HIV-1. J Virol Methods. 2010;163:505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee SY, Gonzales MJ, Kantor R, et al. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pineda-Peña AC, Faria NR, Imbrechts S, et al. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: performance evaluation of the new REGA version 3 and seven other tools. Infect Genet Evol. 2013;19:337–348. [DOI] [PubMed] [Google Scholar]
- 16.Stanford HIV Drug Resistance Database. Major HIV-1 Drug Resistance Mutations. 2019. Available at: hivdb.stanford.edu. [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 18.Chimukangara B, Kharsany ABM, Lessells RJ, et al. Moderate-to-High levels of pretreatment HIV drug resistance in KwaZulu-Natal province, South Africa. AIDS Res Hum Retroviruses. 2019;35:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyo S, Hunt G, Zuma K, et al. HIV drug resistance profile in South Africa: findings and implications from the 2017 national HIV household survey. PLoS One. 2020;15:e0241071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South African National Department of Health. 2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy, Adolescents, Children, Infants and Neonates; 2019. Updated March 2020. Availabe at: https://sahivsoc.org/Files/2019%20ART%20Guideline%2028042020%20pdf.pdf. [Google Scholar]
- 21.Silverman RA, Beck IA, Kiptinness C, et al. Prevalence of pre-antiretroviral-treatment drug resistance by gender, age, and other factors in HIV-infected individuals initiating therapy in Kenya, 2013–2014. J Infect Dis. 2017;216:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]


