Abstract
Background
Understanding protection conferred by natural SARS-CoV-2 infection versus COVID-19 vaccination is important for informing vaccine mandate decisions. We compared protection conferred by natural infection versus that from the BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) vaccines in Qatar.
Methods
We conducted two matched retrospective cohort studies that emulated target trials. Data were obtained from the national federated databases for COVID-19 vaccination, SARS-CoV-2 testing, and COVID-19-related hospitalisation and death between Feb 28, 2020 (pandemic onset in Qatar) and May 12, 2022. We matched individuals with a documented primary infection and no vaccination record (natural infection cohort) with individuals who had received two doses (primary series) of the same vaccine (BNT162b2-vaccinated or mRNA-1273-vaccinated cohorts) at the start of follow-up (90 days after the primary infection). Individuals were exact matched (1:1) by sex, 10-year age group, nationality, comorbidity count, and timing of primary infection or first-dose vaccination. Incidence of SARS-CoV-2 infection and COVID-19-related hospitalisation and death in the natural infection cohorts was compared with incidence in the vaccinated cohorts, using Cox proportional hazards regression models with adjustment for matching factors.
Findings
Between Jan 5, 2021 (date of second-dose vaccine roll-out) and May 12, 2022, 104 500 individuals vaccinated with BNT162b2 and 61 955 individuals vaccinated with mRNA-1273 were matched to unvaccinated individuals with a documented primary infection. During follow-up, 7123 SARS-CoV-2 infections were recorded in the BNT162b2-vaccinated cohort and 3583 reinfections were recorded in the matched natural infection cohort. 4282 SARS-CoV-2 infections were recorded in the mRNA-1273-vaccinated cohort and 2301 reinfections were recorded in the matched natural infection cohort. The overall adjusted hazard ratio (HR) for SARS-CoV-2 infection was 0·47 (95% CI 0·45–0·48) after previous natural infection versus BNT162b2 vaccination, and 0·51 (0·49–0·54) after previous natural infection versus mRNA-1273 vaccination. The overall adjusted HR for severe (acute care hospitalisations), critical (intensive care unit hospitalisations), or fatal COVID-19 cases was 0·24 (0·08–0·72) after previous natural infection versus BNT162b2 vaccination, and 0·24 (0·05–1·19) after previous natural infection versus mRNA-1273 vaccination. Severe, critical, or fatal COVID-19 was rare in both the natural infection and vaccinated cohorts.
Interpretation
Previous natural infection was associated with lower incidence of SARS-CoV-2 infection, regardless of the variant, than mRNA primary-series vaccination. Vaccination remains the safest and most optimal tool for protecting against infection and COVID-19-related hospitalisation and death, irrespective of previous infection status.
Funding
The Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, Weill Cornell Medicine-Qatar; Qatar Ministry of Public Health; Hamad Medical Corporation; Sidra Medicine; Qatar Genome Programme; and Qatar University Biomedical Research Center.
Introduction
COVID-19 vaccines induce protection against SARS-CoV-2 infection and COVID-19-related hospitalisation and death.1, 2, 3, 4 Natural infection with SARS-CoV-2 also induces protection against subsequent SARS-CoV-2 infection and COVID-19-related hospitalisation and death.5, 6 An increasing number of studies suggest that differences exist in the level and durability of protection conferred by natural infection versus vaccination.7, 8, 9, 10, 11 This variation might arise from differences in several factors, including the mechanism of action,12, 13 mucosal immunity,14, 15 the volume and nature of neutralising antibody titres,12, 16, 17 and circulating variants.18, 19, 20, 21, 22
Understanding protection conferred by natural SARS-CoV-2 infection versus COVID-19 vaccination is important for informing vaccine mandate decisions. Qatar experienced SARS-CoV-2 epidemic waves with the index virus, the alpha (B.1.1.7) variant, the beta (B.1.351) variant, a low-incidence phase with the delta (B.1.617.2) variant, and a large wave with the omicron (BA.1 and BA.2) subvariants.20, 21, 22, 23, 24 In this study, we compared protection conferred by natural infection to that conferred by the BNT162b2 (Pfizer–BioNTech)1 and mRNA-1273 (Moderna)2 vaccines in Qatar, using a matched target-trial cohort study design.25, 26 This design fundamentally emulates a retrospective, unmasked, controlled trial.25, 26 In the present study, we used this approach for a head-to-head comparison of protection conferred by natural infection versus vaccination against SARS-CoV-2 infection, and against severe, critical, or fatal COVID-19.
Research in context.
Evidence before this study
COVID-19 vaccines induce protection against SARS-CoV-2 infection and COVID-19-related hospitalisation and death. Natural infection with SARS-CoV-2 also induces protection against subsequent SARS-CoV-2 infection and COVID-19-related hospitalisation and death. On May 17, 2022, we searched PubMed and Google Scholar with the keywords “natural infection”, “vaccination”, “immunity”, “protection”, “infection”, “SARS-CoV-2”, and “COVID-19”, for studies published since Feb 1, 2020. We found no studies with head-to-head comparison of protection conferred by natural infection without vaccination, and protection conferred by the primary-series vaccination with the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines in those with no previous infection.
Added value of this study
This study analysed the national federated databases for SARS-CoV-2 infection, COVID-19 vaccination, and COVID-19-related hospitalisation and death in Qatar according to a matched target-trial cohort study design that essentially emulates a retrospective, unmasked controlled trial. Cohorts were followed up for a duration of 10 months starting from 3 months after natural infection or first-dose vaccination. We found that natural infection was associated with lower incidence of SARS-CoV-2 infection, regardless of the variant, than mRNA primary-series vaccination. Additionally, natural infection was associated with lower incidence of COVID-19-related hospitalisation and death. Differences between protection from natural infection and protection from vaccination were smaller soon after the second vaccine dose, with divergence between the two types of protection occurring in subsequent months, consistent with waning of vaccine immunity and slow waning of natural infection immunity.
Implications of all the available evidence
Although natural infection was associated with stronger protection against SARS-CoV-2 infection and COVID-19 disease than primary-series mRNA vaccination, vaccination remains the safest and most optimal tool for protection against infection and COVID-19-related hospitalisation and death. Natural infection can lead to COVID-19-related hospitalisation and death at the time of primary infection, and long COVID-19 after the infection, which are risks not present with vaccination. The rapid waning of protection of primary-series vaccination supports the need for scaling up of booster vaccination and development of more potent vaccines to mitigate the effect of emerging variants.
Methods
Study setting and data sources
This retrospective cohort study used data on the resident population of Qatar. We analysed the national, federated databases for COVID-19 vaccination, SARS-CoV-2 testing, COVID-19-related hospitalisation, and COVID-19-related death, retrieved from the integrated nationwide digital health information platform of the Qatar Ministry of Public Health. The databases include all SARS-CoV-2-related data and associated demographic information, with no missing information, since pandemic onset on Feb 28, 2020, including all PCR test results and, more recently, rapid antigen test results conducted at health-care facilities (from Jan 5, 2022 onward).
Every PCR test (but not rapid antigen test) conducted in Qatar is classified on the basis of symptoms and the reason for testing (eg, clinical symptoms, contact tracing, surveys or random testing campaigns, individual requests, routine health-care testing, pre-travel, or at port of entry). Qatar has a young, diverse demographic profile,23 in that only 9% of its residents are aged 50 years or older, and 89% are expatriates from over 150 countries (as of 2022).27 Qatar launched its COVID-19 vaccination programme at the end of December, 2020, using both the BNT162b2 and mRNA-1273 mRNA vaccines.28 Most residents were vaccinated in Qatar, but if vaccinated elsewhere, those vaccinations were still recorded in the health system at the port of entry upon arrival in Qatar. Further descriptions of the study population and the national databases have been reported previously.3, 18, 19, 20, 21, 22, 23, 29
Study design and participants
Using two matched retrospective cohort studies that emulated target trials,25, 26 we compared the durability of protection conferred by natural infection with that conferred by a primary series of two-dose vaccination, for both the BNT162b2 and mRNA-1273 vaccines. Incidence of reinfection in the cohort of individuals with a documented SARS-CoV-2 primary (first) infection (designated the natural infection cohort) was compared with incidence of infection in the cohort of individuals who had received two vaccine doses (two doses of BNT162b2 or two doses of mRNA-1273), and had not yet received their third (booster) dose (designated the vaccinated cohorts). Documentation of infection was based on positive PCR or rapid antigen tests. Methods for real-time, quantitative reverse-transcriptase PCR testing and rapid antigen testing at health-care facilities approved by the Qatar Ministry of Public Health are provided in the appendix (pp 2–3).
Any individual with a documented primary infection from pandemic onset in Qatar to the end of the study (Feb 28, 2020 to May 12, 2022) was considered for inclusion in the natural infection cohort, provided that the individual had no vaccination record before the start of follow-up. Any individual with at least two doses of the same vaccine (BNT162b2 or mRNA-1273 primary series) between Jan 5, 2021 (date of roll-out of second-dose vaccination in Qatar) and May 12, 2022 was eligible for inclusion in either the BNT162b2-vaccinated or mRNA-1273-vaccinated cohorts, provided that the individual had no record of a previous documented infection and had not received a booster vaccination before the start of follow-up. Individuals who died before the start of follow-up and those who died during follow-up but had no ascertained death date were excluded.
Individuals in the natural infection cohort were exact matched (1:1) by the potential confounders of sex, 10-year age group, nationality, and comorbidity count with individuals in each of the vaccinated cohorts, to control for known differences in the risk of exposure to SARS-CoV-2 infection in Qatar.23, 30, 31, 32, 33 The characteristics for matching were retrieved from electronic health records in the Cerner-system national database that includes all citizens and residents registered in the national and universal public health-care system. Comorbidity count was based on International Classification of Diseases (10th revision) codes for chronic conditions. Matching by the selected factors was previously shown to provide adequate control of differences in the risk of exposure to infection in Qatar in studies of different epidemiological designs, including target-trial cohort studies.3, 4, 19, 28, 34 Matching was performed by an iterative process in Stata/SE (version 17.0) to ensure that each individual in the vaccinated cohorts was alive, infection-free, and had not received a booster dose at the start of follow-up. In this process, after individuals were matched and the start of follow-up established for the vaccinated individual, we set a condition that the vaccinated individual would be included if this person did not receive the booster vaccine before the start of follow-up, or the person would be replaced by an individual who fulfils the matching criteria.
The institutional review boards of Hamad Medical Corporation and Weill Cornell Medicine-Qatar approved this retrospective study with waiver of informed consent. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The STROBE checklist is provided in the appendix (pp 5–6).
Procedures
Onset of symptoms for SARS-CoV-2 infection occurs several days after acquisition of the virus.35 Accordingly, to control for time since inducement of SARS-CoV-2 immunity, and to control for epidemic phase and variant exposure throughout follow-up, each individual in the vaccinated cohorts was matched to an individual in the natural infection cohort who had a documented primary infection within a week after the vaccinated match received their first dose.
SARS-CoV-2 reinfection is conventionally defined as a documented infection at least 90 days after an earlier infection, to avoid misclassification of prolonged PCR positivity as reinfection if a shorter interval is used.6, 8, 9 Therefore, each matched pair was followed up from when the individual in the natural infection cohort completed 90 days since the documented primary infection. As a consequence of the inclusion and matching criteria and timing of the start of follow-up, and considering the interval between first and second doses was less than 30 days across Qatar (per the national vaccination regimen), no individual in the vaccinated cohorts had received only one vaccine dose. The start of follow-up was also more than 60 days after each individual in the vaccinated cohorts received the second dose.
If the individual in the natural infection cohort received a first vaccine dose, or the individual in the vaccinated cohort received a booster dose, both members of each matched pair were censored to ensure exchangeability.26, 36 Accordingly, individuals were followed up until the first of any of the following events: a documented SARS-CoV-2 infection (defined as the first PCR-positive or rapid antigen-positive test after the start of follow-up, regardless of symptoms or reason for testing), first-dose vaccination of the individual in the natural infection cohort (with matched pair censoring), booster-dose vaccination of the individual in the vaccinated cohort (with matched pair censoring), death, or end of study censoring (May 12, 2022).
Outcomes
The primary outcome was occurrence of a documented SARS-CoV-2 infection during follow-up according to a positive PCR or rapid antigen test, regardless of symptoms or reason for testing. We also investigated, as a secondary outcome, occurrence of severe, critical, or fatal COVID-19 cases. We followed WHO guidelines for classification of severe COVID-19 (acute care hospitalisation),37 critical COVID-19 (intensive care unit hospitalisation),37 and fatal COVID-19.38 Assessments were made by trained medical personnel independent of study investigators and using individual chart reviews, as part of a national protocol applied to every patient hospitalised with COVID-19. The severe, critical, and fatal COVID-19 classifications are detailed in the appendix (p 4).
Every hospitalised patient with COVID-19 underwent an infection severity assessment every 3 days until discharge or death. We classified individuals who progressed to severe, critical, or fatal COVID-19 between the time of the documented infection and the end of the study based on their worst outcome, starting with death, followed by critical disease, and then severe disease.
Statistical analysis
Eligible and matched cohorts were described with frequency distributions and measures of central tendency, and compared with standardised mean differences (SMDs). An SMD of less than 0·1 indicated adequate matching.39 We estimated cumulative incidence of infection (defined as the proportion of individuals at risk, whose primary endpoint during follow-up was a reinfection for the natural infection cohort, or an infection for the vaccinated cohort) in each cohort using the Kaplan-Meier estimator method.40 Additionally, we estimated incidence rate of infection in each cohort, defined as the number of identified infections divided by the number of person-weeks contributed by all individuals in the cohort, with 95% CI, using a Poisson log-likelihood regression model with the stptime command in Stata/SE (version 17.0). We present incidence up to month 10 of follow-up, after which sample size became too small for meaningful analysis.
Hazard ratios (HRs) and corresponding 95% CIs comparing incidence of infection, and incidence of severe, critical, and fatal COVID-19, in the natural infection versus vaccinated cohorts were calculated with Cox regression adjusted for the matching factors with the stcox command in Stata. Schoenfeld residuals and log–log plots for survival curves were used to test the proportional hazards assumption and to confirm its adequacy. 95% CIs were not adjusted for multiplicity; thus, they should not be used to infer definitive differences between cohorts. Interactions were not considered.
Subgroup analyses were conducted to estimate adjusted HRs stratified by month of follow-up. Sensitivity analyses were conducted by restricting the analysis to those aged 50 years or older to investigate whether effects could be different for older individuals, and by stratifying the analysis by sex to investigate differences between males and females. Sensitivity analyses were further conducted to adjust the estimates for differences in testing frequency between the cohorts, by dividing HRs by the ratio of testing frequency. Testing frequency for each cohort was measured by dividing the number of tests during follow-up by the number of people in the cohort.
All statistical analyses were conducted with Stata/SE (version 17.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
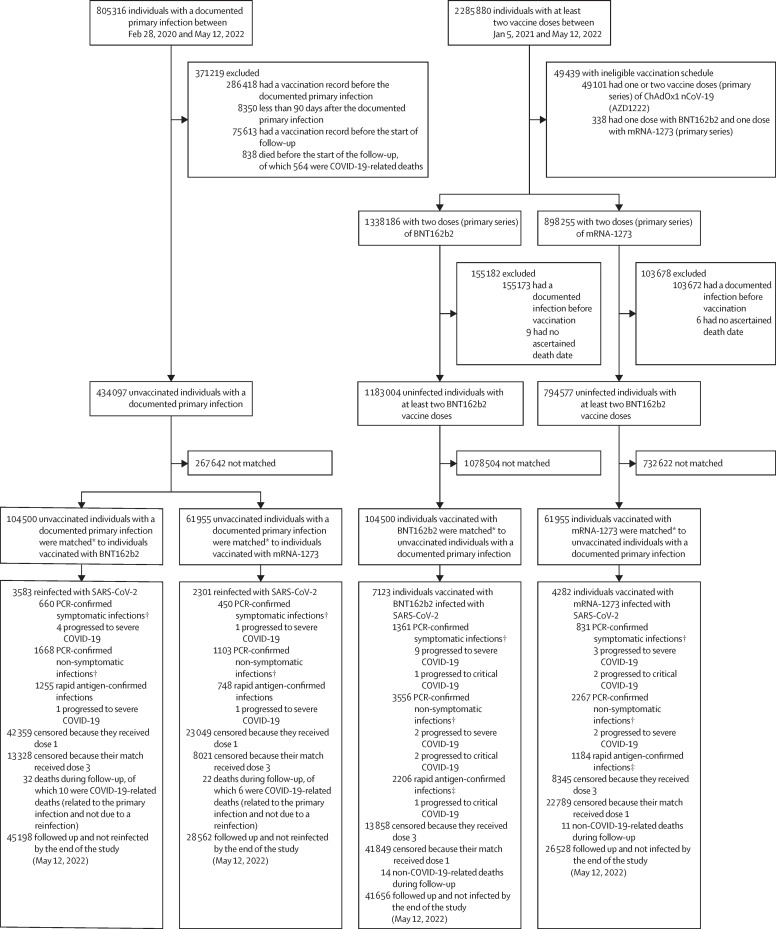
Between Feb 28, 2020 and May 12, 2022, 805 316 individuals had a primary SARS-CoV-2 infection confirmed by PCR or rapid antigen test in Qatar. The median date of infection was Sept 6, 2021. Of the total infected population, 518 898 (64·4%) individuals were unvaccinated at the time of diagnosis. The median date of infection in the total infected population (n=805 316) was Sept 6, 2021. After exclusions, 434 097 unvaccinated individuals with a documented primary infection were matched with the vaccinated cohorts (figure 1 ).
Figure 1.
Selection of cohorts with protection from natural SARS-CoV-2 infection versus BNT162b2 or mRNA-1273 vaccination
*Individuals with a documented SARS-CoV-2 primary infection were exact matched (1:1) to the first eligible individual with two-dose vaccination by sex, 10-year age group, nationality, comorbidity count, and timing of primary infection or first-dose vaccination; matched vaccinated individuals had to be alive, infection free, and to have received dose 2 but not dose 3 vaccination as of the start of follow-up (90 days after the documented primary infection of their match). †A PCR-confirmed symptomatic infection was defined as a PCR-positive test conducted because of clinical suspicion due to presence of symptoms compatible with a respiratory tract infection; a PCR-confirmed non-symptomatic infection was defined as an infection diagnosed after PCR testing for a reason other than clinical suspicion; some individuals with PCR-confirmed non-symptomatic infections developed symptoms after diagnosis and some progressed to severe forms of COVID-19. ‡There was no record of reason for testing or presence of symptoms for the rapid antigen testing.
Between Jan 5, 2021 and May 12, 2022, 1 338 186 individuals received two BNT162b2 doses (primary series), and 422 347 (31·6%) of these individuals received a booster dose. The median date was May 5, 2021 for the first dose, May 25, 2021 for the second dose, and Jan 4, 2022 for the booster dose. The median time elapsed between the first and second doses was 21 days (IQR 21–22 days), and between the second and booster doses was 254 days (235–283 days). 104 500 individuals vaccinated with BNT162b2 who were alive, infection-free, and had received vaccine dose 2 but not dose 3 at the start of follow-up were matched to unvaccinated individuals with a documented primary infection (figure 1).
Jan 24, 2021 was the roll-out date of second-dose mRNA-1273 in Qatar. Between this date and May 12, 2022, 898 255 individuals received two mRNA-1273 doses (primary series), and 199 947 (22·3%) of these individuals received a booster dose. The median date was May 28, 2021 for the first dose, June 27, 2021 for the second dose, and Jan 31, 2022 for the booster dose. The median time elapsed between the first and second doses was 28 days (IQR 28–30 days), and between the second and booster doses was 240 days (214–270 days). 61 955 individuals vaccinated with mRNA-1273 were matched to unvaccinated individuals with a documented primary infection (figure 1).
Characteristics of the study populations are shown in table 1 . The appendix (p 7) shows the distribution of documented primary infections and of first-dose vaccinations by calendar month in the matched cohorts. More than 50% of first-dose mRNA-1273 vaccinations occurred in March–April, 2021, when large shipments of this vaccine were received in Qatar, whereas first-dose BNT162b2 vaccinations tended to be more broadly distributed over time. The appendix (p 8) shows the distribution of the durations of follow-up in the cohorts. The two matched cohort studies were based on the total population of Qatar; thus, study populations were broadly representative of the internationally diverse population with a skew towards young age groups and male sex (table 1).
Table 1.
Baseline characteristics of the study cohorts
|
Natural infection versus BNT162b2 vaccination study |
Natural infection versus mRNA-1273 vaccination study |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unmatched cohorts |
Matched cohorts* |
Unmatched cohorts |
Matched cohorts* |
||||||||||
| Natural infection, n=434 097 | BNT162b2 vaccination, n=1 183 004 | SMD† | Natural infection, n=104 500 | BNT162b2 vaccination, n=104 500 | SMD† | Natural infection, n=434 097 | mRNA-1273 vaccination, n=794 577 | SMD† | Natural infection, n=61 955 | mRNA-1273 vaccination, n=61 955 | SMD† | ||
| Median age, years | 30 (17–39) | 36 (28–44) | 0·55‡ | 33 (26–40) | 33 (27–40) | 0·06‡ | 30 (17–39) | 35 (30–42) | 0·63‡ | 33 (28–40) | 33 (28–40) | 0·05‡ | |
| Age group, years | |||||||||||||
| 0–9 | 73 770 (17·0%) | 10 927 (0·9%) | 0·65 | 537 (0·5%) | 537 (0·5%) | 0·00 | 73 770 (17·0%) | 0 | 0·85 | .. | .. | 0·00 | |
| 10–19 | 42 101 (9·7%) | 121 921 (10·3%) | .. | 11 736 (11·2%) | 11 736 (11·2%) | .. | 42 101 (9·7%) | 4544 (0·6%) | .. | 2238 (3·6%) | 2238 (3·6%) | .. | |
| 20–29 | 96 373 (22·2%) | 209 629 (17·7%) | .. | 27 397 (26·2%) | 27 397 (26·2%) | .. | 96 373 (22·2%) | 180 542 (22·7%) | .. | 19 071 (30·8%) | 19 071 (30·8%) | .. | |
| 30–39 | 121 961 (28·1%) | 397 339 (33·6%) | .. | 37 511 (35·9%) | 37 511 (35·9%) | .. | 121 961 (28·1%) | 340 759 (42·9%) | .. | 24 364 (39·3%) | 24 364 (39·3%) | .. | |
| 40–49 | 64 466 (14·9%) | 262 398 (22·2%) | .. | 19 481 (18·6%) | 19 481 (18·6%) | .. | 64 466 (14·9%) | 189 276 (23·8%) | .. | 11 965 (19·3%) | 11 965 (19·3%) | .. | |
| 50–59 | 25 190 (5·8%) | 120 948 (10·2%) | .. | 6010 (5·8%) | 6010 (5·8%) | .. | 25 190 (5·8%) | 64 702 (8·1%) | .. | 3430 (5·5%) | 3430 (5·5%) | .. | |
| 60–69 | 7754 (1·8%) | 45 539 (3·9%) | .. | 1442 (1·4%) | 1442 (1·4%) | .. | 7754 (1·8%) | 12 501 (1·6%) | .. | 707 (1·1%) | 707 (1·1%) | .. | |
| ≥70 | 2482 (0·6%) | 14 303 (1·2%) | .. | 386 (0·4%) | 386 (0·4%) | .. | 2482 (0·6%) | 2253 (0·3%) | .. | 180 (0·3%) | 180 (0·3%) | .. | |
| Sex | |||||||||||||
| Male | 294 819 (67·9%) | 814 527 (68·9%) | 0·02 | 69 581 (66·6%) | 69 581 (66·6%) | 0·00 | 294 819 (67·9%) | 641 980 (80·8%) | 0·30 | 43 048 (69·5%) | 43 048 (69·5%) | 0·00 | |
| Female | 139 278 (32·1%) | 368 477 (31·1%) | .. | 34 919 (33·4%) | 34 919 (33·4%) | .. | 139 278 (32·1%) | 152 597 (19·2%) | .. | 18 907 (30·5%) | 18 907 (30·5%) | .. | |
| Nationality§ | |||||||||||||
| Bangladeshi | 29 444 (6·8%) | 129 476 (10·9%) | 0·19 | 7154 (6·9%) | 7154 (6·9%) | 0·00 | 29 444 (6·8%) | 153 988 (19·4%) | 0·67 | 4518 (7·3%) | 4518 (7·3%) | 0·00 | |
| Egyptian | 22 657 (5·2%) | 66 179 (5·6%) | .. | 4796 (4·6%) | 4796 (4·6%) | .. | 22 657 (5·2%) | 31 263 (3·9%) | .. | 2097 (3·4%) | 2097 (3·4%) | .. | |
| Filipino | 32 521 (7·5%) | 110 866 (9·4%) | .. | 13 807 (13·2%) | 13 807 (13·2%) | .. | 32 521 (7·5%) | 71 258 (9·0%) | .. | 8499 (13·7%) | 8499 (13·7%) | .. | |
| Indian | 107 399 (24·7%) | 268 264 (22·7%) | .. | 31 804 (30·4%) | 31 804 (30·4%) | .. | 107 399 (24·7%) | 218 861 (27·5%) | .. | 21 084 (34·0%) | 21 084 (34·0%) | .. | |
| Nepalese | 42 771 (9·9%) | 97 241 (8·2%) | .. | 8521 (8·2%) | 8521 (8·2%) | .. | 42 771 (9·9%) | 110 004 (13·8%) | .. | 5571 (9·0%) | 5571 (9·0%) | .. | |
| Pakistani | 22 310 (5·1%) | 51 098 (4·3%) | .. | 5567 (5·3%) | 5567 (5·3%) | .. | 22 310 (5·1%) | 42 777 (5·4%) | .. | 3660 (5·9%) | 3660 (5·9%) | .. | |
| Qatari | 70 354 (16·2%) | 161 214 (13·6%) | .. | 10 527 (10·1%) | 10 527 (10·1%) | .. | 70 354 (16·2%) | 14 603 (1·8%) | .. | 4244 (6·9%) | 4244 (6·9%) | .. | |
| Sri Lankan | 11 849 (2·7%) | 35 753 (3·0%) | .. | 3198 (3·1%) | 3198 (3·1%) | .. | 11 849 (2·7%) | 32 615 (4·1%) | .. | 2107 (3·4%) | 2107 (3·4%) | .. | |
| Sudanese | 11 588 (2·7%) | 26 047 (2·2%) | .. | 2650 (2·5%) | 2650 (2·5%) | .. | 11 588 (2·7%) | 13 614 (1·7%) | .. | 1273 (2·1%) | 1273 (2·1%) | .. | |
| Other¶ | 83 204 (19·2%) | 236 866 (20·0%) | .. | 16 476 (15·8%) | 16 476 (15·8%) | .. | 83 204 (19·2%) | 105 594 (13·3%) | .. | 8902 (14·4%) | 8902 (14·4%) | .. | |
| Comorbidity count | |||||||||||||
| None | 348 751 (80·3%) | 942 329 (79·7%) | 0·13 | 89 265 (85·4%) | 89 265 (85·4%) | 0·00 | 348 751 (80·3%) | 713 941 (89·9%) | 0·27 | 54 720 (88·3%) | 54 720 (88·3%) | 0·00 | |
| 1 | 52 944 (12·2%) | 119 339 (10·1%) | .. | 8882 (8·5%) | 8882 (8·5%) | .. | 52 944 (12·2%) | 44 981 (5·7%) | .. | 4171 (6·7%) | 4171 (6·7%) | .. | |
| 2 | 18 481 (4·3%) | 56 644 (4·8%) | .. | 3554 (3·4%) | 3554 (3·4%) | .. | 18 481 (4·3%) | 20 522 (2·6%) | .. | 1737 (2·8%) | 1737 (2·8%) | .. | |
| 3 | 6560 (1·5%) | 27 795 (2·3%) | .. | 1346 (1·3%) | 1346 (1·3%) | .. | 6560 (1·5%) | 8129 (1·0%) | .. | 619 (1·0%) | 619 (1·0%) | .. | |
| ≥4 | 7361 (1·7%) | 36 897 (3·1%) | .. | 1453 (1·4%) | 1453 (1·4%) | .. | 7361 (1·7%) | 7004 (0·9%) | .. | 708 (1·1%) | 708 (1·1%) | .. | |
Data are n (%) or median (IQR). SMD=standardised mean difference.
Individuals with a documented SARS-CoV-2 primary infection were exact matched (1:1) to the first eligible individual with two-dose vaccination by sex, 10-year age group, nationality, comorbidity count, and timing of primary infection or first-dose vaccination.
SMD is the difference in the mean of a covariate between groups divided by the pooled SD.
SMD is for the mean difference between groups divided by the pooled SD.
Nationalities were chosen to represent the most populous groups in Qatar.23
In the natural infection versus BNT162b2 study, these comprise 184 other nationalities in individuals with natural infection and 189 other nationalities in individuals with BNT162b2 vaccination in the unmatched cohorts, and 111 other nationalities in individuals with natural infection and 111 other nationalities in individuals with BNT162b2 vaccination in the matched cohorts; in the natural infection versus mRNA-1273 study, these comprise 184 other nationalities in individuals with natural infection and 172 other nationalities in individuals with mRNA-1273 vaccination in the unmatched cohorts, and 105 other nationalities in individuals with natural infection and 105 other nationalities in individuals with mRNA-1273 vaccination in the matched cohorts.
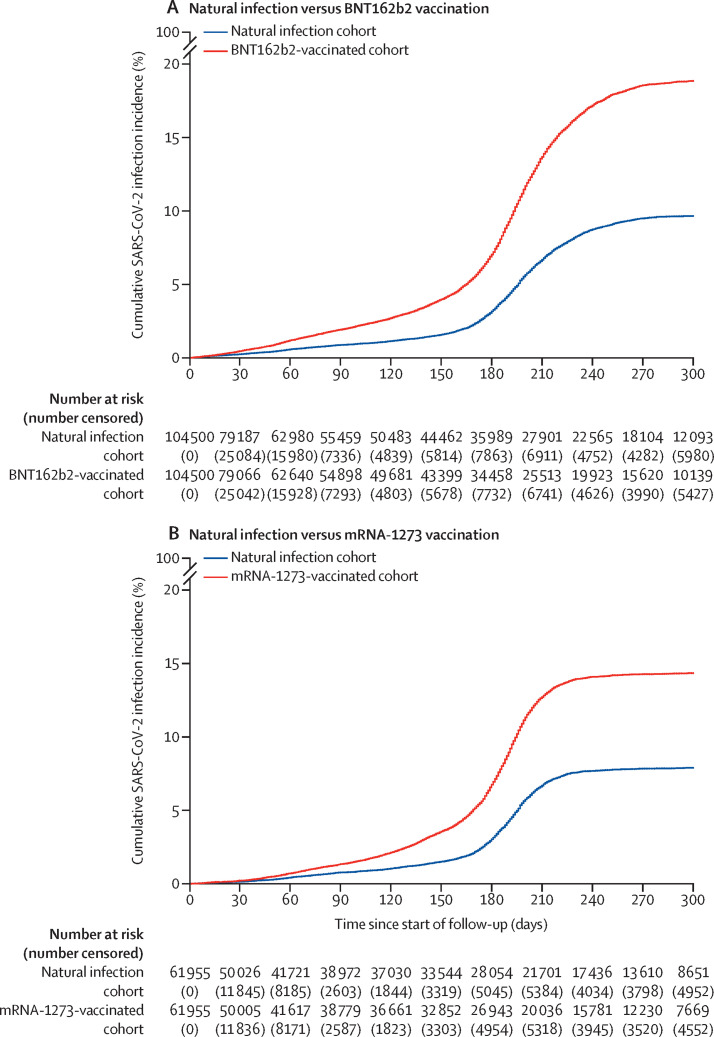
In the natural infection versus BNT162b2 vaccination study, median follow-up was 104 days (IQR 30–207 days) for the natural infection cohort and 109 days (31–218 days) for the BNT162b2-vaccinated cohort (figure 2A ). 3583 reinfections with SARS-CoV-2 were recorded in the matched natural infection cohort during follow-up (figure 1). Five (0·1%) of these reinfections progressed to severe COVID-19, but none to critical or fatal COVID-19. 7123 infections were recorded in the BNT162b2-vaccinated cohort. Of these, 11 (0·2%) progressed to severe COVID-19 and four to critical COVID-19; none progressed to fatal COVID-19.
Figure 2.
Cumulative incidence of infection in the natural infection cohort and in the BNT162b2-vaccinated cohort (A) and the mRNA-1273-vaccinated cohort (B)
Numbers censored due to reasons other than infection are shown in parentheses.
Cumulative incidence of infection was estimated to be 9·7% (95% CI 9·3–10·0) for the natural infection cohort and 18·9% (18·4–19·3) for the BNT162b2-vaccinated cohort, after 300 days (10 months) of follow-up (figure 2A). Incidence was low in the natural infection cohort until day 160 (month 6) of follow-up, then increased rapidly. Based on indirect, qualitative comparisons of our analysis with the timing of COVID-19 waves in Qatar, this increase appeared to coincide with onset of the omicron (B.1.1.529) variant wave on Dec 19, 2021, which peaked in mid-January of 2022.6, 26, 41 Before around day 160, incidence would have been dominated by the alpha, beta, and delta variants.3, 20, 42, 43 Incidence was higher in the BNT162b2-vaccinated cohort versus the natural infection cohort throughout follow-up, before and after the omicron wave, with the difference becoming pronounced around the time of onset of this wave.
The overall HR for infection after previous natural infection versus BNT162b2 vaccination, adjusted for sex, 10-year age group, ten nationality groups, comorbidity count, and time since primary infection or first-dose vaccination, was estimated at 0·47 (95% CI 0·45–0·48; table 2 ). However, the adjusted HR varied by month of follow-up (table 3 ). The adjusted HR was 0·55 (0·46–0·65) in the first month of follow-up (the fourth month after primary infection or first-dose vaccination), but decreased gradually to 0·33 (0·28–0·38) in the fifth month of follow-up (the eighth month after primary infection or first-dose vaccination). The adjusted HR appeared to increase after onset of the omicron wave with HR values of around 0·50 from month 6 of follow-up, which coincided with this wave.
Table 2.
HRs for the incidence of SARS-CoV-2 infection and severe, critical, and fatal COVID-19
|
Natural infection versus BNT162b2 vaccination study |
Natural infection versus mRNA-1273 vaccination study |
|||
|---|---|---|---|---|
| Natural infection cohort | BNT162b2-vaccinated cohort | Natural infection cohort | mRNA-1273-vaccinated cohort | |
| Total follow-up, person-weeks | 1 985 243 | 1 921 539 | 1 374 220 | 1 338 649 |
| Incidence rate of infection, per 10 000 person-weeks | 18·1 (17·5–18·7) | 37·1 (36·2–37·9) | 16·7 (16·1–17·4) | 32·0 (31·0–33·0) |
| Unadjusted HR for SARS-CoV-2 infection | 0·48 (0·46–0·50) | 1 (ref) | 0·51 (0·49–0·54) | 1 (ref) |
| Adjusted HR for SARS-CoV-2 infection* | 0·47 (0·45–0·48) | 1 (ref) | 0·51 (0·49–0·54) | 1 (ref) |
| Unadjusted HR for severe, critical, or fatal COVID-19† | 0·25 (0·08–0·77) | 1 (ref) | 0·27 (0·06–1·32) | 1 (ref) |
| Adjusted HR for severe, critical, or fatal COVID-19*† | 0·24 (0·08–0·72) | 1 (ref) | 0·24 (0·05–1·19) | 1 (ref) |
95% CIs for HRs and incidence rate are shown in parentheses. HR=hazard ratio.
Cox regression analysis adjusted for sex, 10-year age group, ten nationality groups, comorbidity count (table 1), and timing of primary infection or first-dose vaccination.
Table 3.
HRs for the incidence of SARS-CoV-2 infection stratified by month* since the start of follow-up
|
Natural infection cohort† |
mRNA-vaccinated cohort† |
Natural infection versus mRNA vaccination (ref) |
||||
|---|---|---|---|---|---|---|
| n | Cumulative incidence, % | n | Cumulative incidence, % | Unadjusted HR | Adjusted HR‡ | |
| Natural infection versus BNT162b2 vaccination study§ | ||||||
| Month 1 of follow-up (month 4 after primary infection or first-dose vaccination) | 79 187 | 0·2% (0·2–0·3) | 79 066 | 0·5% (0·4–0·5) | 0·55 (0·47–0·65) | 0·55 (0·46–0·65) |
| Month 2 of follow-up (month 5 after primary infection or first-dose vaccination) | 62 980 | 0·6% (0·5–0·6) | 62 640 | 1·2% (1·1–1·3) | 0·45 (0·39–0·53) | 0·44 (0·38–0·51) |
| Month 3 of follow-up (month 6 after primary infection or first-dose vaccination) | 55 459 | 0·9% (0·8–0·9) | 54 898 | 1·9% (1·8–2·0) | 0·41 (0·34–0·49) | 0·39 (0·33–0·46) |
| Month 4 of follow-up (month 7 after primary infection or first-dose vaccination) | 50 483 | 1·1% (1·1–1·2) | 49 681 | 2·7% (2·6–2·8) | 0·33 (0·27–0·40) | 0·32 (0·26–0·39) |
| Month 5 of follow-up (month 8 after primary infection or first-dose vaccination) | 44 462 | 1·6% (1·5–1·7) | 43 399 | 4·0% (3·8–4·1) | 0·34 (0·29–0·39) | 0·33 (0·28–0·38) |
| Month 6 of follow-up (month 9 after primary infection or first-dose vaccination) | 35 989 | 3·2% (3·0–3·3) | 34 458 | 7·0% (6·8–7·3) | 0·49 (0·44–0·54) | 0·48 (0·43–0·53) |
| Month 7 of follow-up (month 10 after primary infection or first-dose vaccination) | 27 901 | 6·7% (6·4–6·9) | 25 513 | 13·7% (13·3–14·0) | 0·50 (0·47–0·54) | 0·49 (0·46–0·53) |
| Month 8 of follow-up (month 11 after primary infection or first-dose vaccination) | 22 565 | 8·7% (8·4–9·0) | 19 923 | 17·2% (16·8–17·6) | 0·55 (0·49–0·61) | 0·54 (0·49–0·60) |
| Month 9 of follow-up (month 12 after primary infection or first-dose vaccination) | 18 104 | 9·5% (9·2–9·8) | 15 620 | 18·6% (18·1–19·0) | 0·50 (0·42–0·60) | 0·49 (0·41–0·59) |
| Month 10 of follow-up (month 13 after primary infection or first-dose vaccination) | 12 093 | 9·7% (9·3–10·0) | 10 139 | 18·9% (18·4–19·3) | 0·49 (0·32–0·76) | 0·47 (0·30–0·73) |
| Natural infection versus mRNA-1273 vaccination study§ | ||||||
| Month 1 of follow-up (month 4 after primary infection or first-dose vaccination) | 50 026 | 0·1% (0·1–0·2) | 50 005 | 0·2% (0·2–0·3) | 0·68 (0·51–0·91) | 0·68 (0·51–0·90) |
| Month 2 of follow-up (month 5 after primary infection or first-dose vaccination) | 41 721 | 0·4% (0·4–0·5) | 41 617 | 0·7% (0·6–0·8) | 0·55 (0·44–0·69) | 0·54 (0·43–0·68) |
| Month 3 of follow-up (month 6 after primary infection or first-dose vaccination) | 38 972 | 0·8% (0·7–0·9) | 38 779 | 1·3% (1·2–1·4) | 0·58 (0·47–0·71) | 0·57 (0·46–0·70) |
| Month 4 of follow-up (month 7 after primary infection or first-dose vaccination) | 37 030 | 1·0% (0·9–1·1) | 36 661 | 2·1% (2·0–2·3) | 0·33 (0·26–0·41) | 0·33 (0·26–0·41) |
| Month 5 of follow-up (month 8 after primary infection or first-dose vaccination) | 33 544 | 1·5% (1·4–1·6) | 32 852 | 3·6% (3·4–3·7) | 0·33 (0·27–0·39) | 0·32 (0·27–0·38) |
| Month 6 of follow-up (month 9 after primary infection or first-dose vaccination) | 28 054 | 3·0% (2·9–3·2) | 26 943 | 6·7% (6·5–7·0) | 0·45 (0·40–0·51) | 0·44 (0·40–0·50) |
| Month 7 of follow-up (month 10 after primary infection or first-dose vaccination) | 21 701 | 6·7% (6·4–7·0) | 20 036 | 12·7% (12·3–13·1) | 0·58 (0·53–0·62) | 0·57 (0·53–0·62) |
| Month 8 of follow-up (month 11 after primary infection or first-dose vaccination) | 17 436 | 7·7% (7·4–8·0) | 15 781 | 14·1% (13·7–14·5) | 0·68 (0·58–0·81) | 0·68 (0·58–0·81) |
| Month 9 of follow-up (month 12 after primary infection or first-dose vaccination) | 13 610 | 7·8% (7·5–8·2) | 12 230 | 14·3% (13·9–14·7) | 0·81 (0·49–1·36) | 0·82 (0·49–1·37) |
| Month 10 of follow-up (month 13 after primary infection or first-dose vaccination) | 8651 | 7·9% (7·6–8·2) | 7669 | 14·3% (13·9–14·8) | 0·70 (0·26–1·87) | 0·66 (0·25–1·78) |
95% CIs for HRs and cumulative incidence rates are shown in parentheses. HR=hazard ratio.
A month was defined as 30 days; documented primary infection (natural infection cohort) could have occurred up to a week after first-dose vaccination (vaccinated cohorts) within a matched pair, with follow-up starting 90 days after the primary infection.
Cohorts were exact matched (1:1) by sex, 10-year age group, nationality, comorbidity count, and timing of primary infection or first-dose vaccination.
Cox regression analysis adjusted for sex, 10-year age group, ten nationality groups, comorbidity count (table 1), and timing of primary infection or first-dose vaccination.
No results were generated for months 1–3 after primary infection or first-dose vaccination. This is because a SARS-CoV-2 reinfection is conventionally defined as a documented infection occurring at least 90 days after an earlier infection, to avoid misclassification of prolonged PCR positivity as reinfections;6, 8, 9 therefore, cohorts were followed up starting from 90 days (3 months) after the documented primary infection.
The overall adjusted HR for severe, critical, or fatal COVID-19 after previous natural infection versus BNT162b2 vaccination was estimated at 0·24 (95% CI 0·08–0·72; table 2). The wide 95% CI reflected the rarity of severe or critical COVID-19 in both the natural infection and BNT162b2-vaccinated cohorts (figure 1).
In the sensitivity analysis, the overall adjusted HR for infection after previous natural infection versus BNT162b2 vaccination was 0·51 (95% CI 0·44–0·60) for those aged 50 years or older, 0·54 (0·51–0·57) for women, and 0·39 (0·37–0·42) for men (appendix p 9). The overall adjusted HR for severe, critical, or fatal COVID-19 was 0·28 (0·09–0·89) for those aged 50 years or older, 0·77 (0·15–3·83) for women, and 0·08 (0·01–0·63) for men.
The number of individuals with at least one SARS-CoV-2 test during follow-up was 26 412 (25·3%) of 104 500 in the natural infection cohort and 40 914 (39·2%) of 104 500 in the BNT162b2-vaccinated cohort. The testing frequency was 0·53 tests per person in the natural infection cohort and 0·86 tests per person in the BNT162b2-vaccinated cohort. In the sensitivity analysis adjusting the HR by the ratio of testing frequencies, the adjusted HR for infection after previous natural infection versus BNT162b2 vaccination was 0·76 (95% CI 0·73–0·78).
In the natural infection versus mRNA-1273 vaccination study, median follow-up was 166 days (IQR 39–256 days) for the natural infection cohort and 162 days (39–242 days) for the mRNA-1273-vaccinated cohort (figure 2B). 2301 SARS-CoV-2 reinfections were recorded in the natural infection cohort during follow-up (figure 1). Two of these reinfections progressed to severe COVID-19, but none to critical or fatal COVID-19. 4282 infections were recorded in the mRNA-1273-vaccinated cohort. Of these infections, five progressed to severe COVID-19 and two to critical COVID-19; none progressed to fatal COVID-19.
Cumulative incidence of infection was estimated to be 7·9% (95% CI 7·6–8·2) for the natural infection cohort and 14·3% (13·9–14·8) for the mRNA-1273-vaccinated cohort, after 300 days (10 months) of follow-up (figure 2B). Incidence was low in the natural infection cohort until day 170 (month 6) of follow-up, then increased rapidly, which coincided with onset of the omicron wave.6, 26, 41 Before day 170, incidence was dominated by the alpha, beta, and delta variants.3, 20, 42, 43 Incidence in the mRNA-1273-vaccinated cohort was higher than in the natural infection cohort throughout follow-up, before and after the omicron wave, with notably higher incidence around the time that coincided with this wave.
The overall adjusted HR for SARS-CoV-2 infection after previous natural infection versus mRNA-1273 vaccination was estimated at 0·51 (95% CI 0·49–0·54; table 2). However, the adjusted HR varied by month of follow-up (table 3). The adjusted HR was 0·68 (0·51–0·90) in the first month of follow-up (the fourth month after primary infection or first-dose vaccination), but decreased gradually to 0·32 (0·27–0·38) in the fifth month of follow-up (the eighth month after primary infection or first-dose vaccination). The adjusted HR appeared to increase with onset of the omicron wave with HR values of around 0·60–0·70 from month 7 of follow-up, which coincided with this wave.
The overall adjusted HR for severe, critical, or fatal COVID-19 after previous natural infection versus mRNA-1273 vaccination was estimated at 0·24 (95% CI 0·05–1·19; table 2). The wide 95% CI reflected the rarity of severe or critical COVID-19 in both the natural infection and mRNA-1273-vaccinated cohorts (figure 1).
In the sensitivity analysis, the overall adjusted HR for SARS-CoV-2 infection after previous natural infection versus mRNA-1273 vaccination was 0·65 (95% CI 0·54–0·78) for those aged 50 years or older, 0·61 (0·57–0·66) for women, and 0·41 (0·38–0·44) for men (appendix p 9). The overall adjusted HR for severe, critical, or fatal COVID-19 after previous natural infection versus mRNA-1273 vaccination was 0·43 (0·08–2·43) for those aged 50 years or older, 0·40 (0·07–2·27) for women, and 0·00 (0·00–2·37) for men.
The number of individuals with at least one SARS-CoV-2 test during follow-up was 15 705 (25·3%) of 61 955 in the natural infection cohort and 25 738 (41·5%) of 61 955 in the mRNA-1273-vaccinated cohort. The testing frequency was 0·52 tests per person in the natural infection cohort and 0·83 tests per person in the mRNA-1273-vaccinated cohort. After adjusting the HR by the ratio of testing frequencies, the adjusted HR for infection after previous natural infection versus mRNA-1273 vaccination was 0·81 (95% CI 0·78–0·86).
Discussion
Our results showed that previous natural infection was associated with an overall lower incidence of SARS-CoV-2 infection than mRNA primary-series vaccination, including among individuals aged 50 years or older. Natural infection was also associated with lower incidence of severe COVID-19 than mRNA vaccination; however, severe COVID-19 was rare for both the natural infection and vaccinated cohorts. Vaccine protection against infection waned with time after the second dose, whereas natural immunity showed little waning in protection for at least 8 months after the primary infection. However, onset of the omicron wave led to large increases in the incidence of reinfections in the natural infection cohort and the incidence of infections in the vaccinated cohorts. Nonetheless, even during the omicron wave, natural infection was associated with lower incidence than BNT162b2 and mRNA-1273 vaccination.
Although natural infection was more protective than vaccination, the differences in protection were smaller soon after the second dose, and increased with time after the second dose. These findings might be explained by different roles of mucosal immunity.14, 15 Vaccination induces systemic immunity that might not be retained in the upper respiratory tracts, unlike natural infection, which induces longer term and strong mucosal immunity at the site of virus entry and replication.14, 15
Although natural infection was associated with stronger protection, vaccination remains the safest and most optimal tool for protection against infection and COVID-19-related hospitalisation and death. Natural infection can lead to COVID-19-related hospitalisation and death at the time of primary infection, and long COVID-19 after the infection, which are risks not present with vaccination. Additionally, protection from natural infection was compared with only primary-series vaccination, and the differences in protection between natural infection and booster vaccination might be smaller.
The results of this study confirm findings that we reported in March, 2022, whereby protection from previous infection (with pre-omicron variants) against reinfection with omicron was estimated at 56·0% (95% CI 50·6–60·9).6 Given BNT162b2 vaccine protection is negligible 6 or more months after the second dose,44, 45 the adjusted HR in this study of around 0·50 during the omicron wave implies a level of protection of around 50% from natural infection against reinfection with omicron. The present study results confirm the waning of protection from mRNA vaccines against pre-omicron variants,3, 4 and the lower vaccine protection against omicron. 24, 26, 44, 45 The results also support stronger protection and slower waning with mRNA-1273 vaccination than with BNT162b2 vaccination.28, 29
This study has limitations. We investigated incidence of documented infections, but other infections might have occurred and been undocumented, perhaps because of minimal, mild, or no symptoms. Although the vaccinated cohorts excluded individuals with a previous documented infection, some of those who were vaccinated might have had an undocumented previous infection. Therefore, the higher protection from natural infection compared with vaccination might have been underestimated. SARS-CoV-2 testing frequency was higher in the vaccinated cohorts than in the natural infection cohorts. Based on reason for testing (data not shown), this higher testing frequency in the vaccinated cohorts was in part because of higher rates of routine testing, particularly for travel. At the time of follow-up, many countries required vaccination and testing to allow travel. The lower testing frequency in the natural infection cohort might also reflect less need for testing because of lower infection incidence. Therefore, the increased protection from natural infection against subsequent infection, regardless of variant, might have been overestimated. However, after adjustment for the differences in testing frequency between the cohorts in sensitivity analyses, natural infection was still associated with lower incidence of SARS-CoV-2 infection than mRNA vaccination. Differences in testing frequency, such as for travel, might also explain differences in HRs between women and men. The average woman in Qatar has high socioeconomic status and travels frequently.23, 30, 31, 32, 33, 46 The average man in Qatar is a young craft or manual worker with low socioeconomic status, and travels infrequently.23, 30, 31, 32, 33, 46
COVID-19-related mortality in the natural infection cohort at the time of primary infection might have biased this cohort towards healthier individuals with stronger immune responses. However, as reported in the national federated databases, COVID-19 mortality has been low in Qatar's predominantly young, working-age population,23, 47 totalling 677 COVID-19-related deaths (approximately 0·1% of primary infections) from the onset of the pandemic in early 2020 until May 12, 2022, which is a small proportion compared with the size of the cohorts in this study. Among all the COVID-19-related deaths in Qatar, 587 (86·7%) occurred before receiving any dose of vaccination. A survival effect seems unlikely to explain or appreciably affect the study findings, apart from the estimated protection against severe COVID-19.
We found no evidence for a reduction in reporting of cases over time to affect the study. Testing in Qatar is done at a mass scale, mostly for routine reasons, and is tracked centrally for the whole country.3, 24 About 75% of those diagnosed are diagnosed not because of appearance of symptoms, but because of routine testing.3, 24 Given the high and durable effectiveness of natural infection5, 6 and mRNA primary-series vaccination3, 4 against COVID-19-related hospitalisation and death, and the young population of Qatar,23 case numbers were insufficient for precise estimation of differences in protection between natural infection and vaccination against COVID-19 hospitalisation and death.
As an observational study, investigated cohorts were not masked or randomised, and thus unmeasured or uncontrolled confounding cannot be excluded. Although we matched cohorts for sex, age, nationality, comorbidities, and timing of previous infection or first-dose vaccination, this was not possible for other factors such as occupation or geography, as these data were unavailable. However, Qatar is essentially a city state where around 89% of the population (as of 2020) are expatriates from more than 150 countries, immigrating because of employment.23 About 60% of the population are men and young craft and manual workers working in development projects.23, 46 Nationality, age, and sex provide a strong proxy for socioeconomic status in this country.23, 30, 31, 32, 33, 46 Nationality alone is strongly associated with occupation.23, 30, 31, 32, 33, 46 Based on our work in the national COVID-19 response, we know that infection incidence and vaccination were broadly distributed across neighbourhoods and areas. Therefore, geography is not likely to have been a confounding factor.
Matching was done to control for factors known to affect infection exposure in Qatar.23, 30, 31, 32, 33 The matching criteria have been investigated in previous studies that used different epidemiological designs, and with control groups to test for null effects.3, 4, 19, 28, 34 These control groups included unvaccinated cohorts versus vaccinated cohorts within 2 weeks of the first dose,3, 4, 19, 34 when vaccine protection is negligible,1, 2 and mRNA-1273-vaccinated versus BNT162b2-vaccinated cohorts, also in the first 2 weeks after the first dose.28 These studies indicated that the selected matching factors provide adequate control over differences in risk of exposure to the infection.3, 4, 19, 28, 34 The present results confirm initial results without matching for comorbidity in a preliminary, preprint analysis of this study.48 The present analysis was implemented on national population data from Qatar with large, representative sample sizes. The study was not implemented on a select segment of the population, but on the total population of the country, which possibly minimised the likelihood of bias.
In conclusion, previous natural infection with SARS-CoV-2 was associated with lower incidence of SARS-CoV-2 infection, regardless of the variant, than mRNA primary-series vaccination. Natural infection was also associated with slower waning in protection than mRNA vaccination. These findings might help to optimise policy and guidelines related to differential application of restrictions by previous infection status, and protocols for isolation and quarantine. Although natural infection was associated with stronger protection, vaccination remains the safest and most optimal tool for protection against SARS-CoV-2 infection and COVID-19-related hospitalisation and death.
Data sharing
The dataset in this study is the property of the Qatar Ministry of Public Health and was provided to the authors through a restricted access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Qatar's Minister of Public Health at https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-Health-Communication-Center.aspx. The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the manuscript and its appendix.
Declaration of interests
AAB has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge the many dedicated individuals at Hamad Medical Corporation, the Qatar Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine-Qatar for their diligent efforts and contributions which made this study possible. We are grateful for institutional salary support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar, and for institutional salary support provided by the Qatar Ministry of Public Health, Hamad Medical Corporation, and Sidra Medicine. We are also grateful for the provision of reagents required for viral genome sequencing from the Qatar Genome Programme and Qatar University Biomedical Research Center. Statements made herein are solely the responsibility of the authors.
Contributors
HC co-designed the study, performed the statistical analyses, and co-wrote the first draft of the Article. LJA-R conceived and co-designed the study, led the statistical analyses, and co-wrote the first draft of the Article. HHA provided statistical and mathematical modelling advice related to analyses, and conducted modeling on protection from natural infection that informed the study design. HMY, HAA-K, and MKS conducted viral genome sequencing. PT and MRH conducted the multiplex, quantitative reverse-transcriptase PCR variant screening and viral genome sequencing. HC and LJA-R accessed and verified the data in this study. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and the writing of the manuscript. All authors had full access to all the data in the study, have read and approved the final manuscript, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;386:1091–1093. doi: 10.1056/NEJMc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med. 2021;385:2487–2489. doi: 10.1056/NEJMc2108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386:1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima N, Shrestha NK, Klausner JD. A systematic review of the protective effect of prior SARS-CoV-2 infection on repeat infection. Eval Health Prof. 2021;44:327–332. doi: 10.1177/01632787211047932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilz S, Theiler-Schwetz V, Trummer C, Krause R, Ioannidis JPA. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209 doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaitelly H, Abu-Raddad LJ. Waning effectiveness of COVID-19 vaccines. Lancet. 2022;399:771–773. doi: 10.1016/S0140-6736(22)00277-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sette A, Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457–458. doi: 10.1038/s41577-020-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzi L, Dalla Gasperina D, Veronesi G, et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano K, Bhavsar D, Singh G, et al. SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals. Nat Commun. 2022;13 doi: 10.1038/s41467-022-32389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Raddad LJ, Chemaitelly H, Butt AA, for the National Study Group for COVID-19 Vaccination Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 20.Tang P, Hasan MR, Chemaitelly H, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 21.Chemaitelly H, Bertollini R, Abu-Raddad LJ. Efficacy of natural immunity against SARS-CoV-2 reinfection with the beta variant. N Engl J Med. 2021;385:2585–2586. doi: 10.1056/NEJMc2110300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Introduction and expansion of the SARS-CoV-2 B.1.1.7 variant and reinfections in Qatar: a nationally representative cohort study. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep. 2021;11 doi: 10.1038/s41598-021-85428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planning and Statistics Authority. State of Qatar Qatar monthly statistics. Statistics of July 2022. 2022. https://www.psa.gov.qa/en/statistics/Statistical%20Releases/General/QMS/QMS_PSA_103_Aug_2022.pdf
- 28.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med. 2022;386:799–800. doi: 10.1056/NEJMc2117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoub HH, Chemaitelly H, Seedat S, et al. Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J Glob Health. 2021;11 doi: 10.7189/jogh.11.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyle PV, Chemaitelly H, Ben Hadj Kacem MA, et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112 941 individuals. iScience. 2021;24 doi: 10.1016/j.isci.2021.102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Thani MH, Farag E, Bertollini R, et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeremijenko A, Chemaitelly H, Ayoub HH, et al. Herd immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities, Qatar. Emerg Infect Dis. 2021;27:1343–1352. doi: 10.3201/eid2705.204365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu-Raddad LJ, Chemaitelly H, Yassine HM, et al. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J Travel Med. 2021;28 doi: 10.1093/jtm/taab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 36.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO COVID-19 clinical management: living guidance. Nov 23, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2
- 38.WHO International guidelines for certification and classification (coding) of COVID-19 as cause of death. April 20, 2020. https://www.who.int/publications/m/item/international-guidelines-for-certification-and-classification-%28coding%29-of-covid-19-as-cause-of-death
- 39.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 40.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 41.Chemaitelly H, Ayoub HH, Coyle P, et al. Protection of omicron sub-lineage infection against reinfection with another omicron sub-lineage. Nat Commun. 2022;13 doi: 10.1038/s41467-022-32363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan MR, Kalikiri MKR, Mirza F, et al. Real-time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int J Infect Dis. 2021;112:52–54. doi: 10.1016/j.ijid.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Benslimane FM, Al Khatib HA, Al-Jamal O, et al. One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.768883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chemaitelly H, Ayoub H, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13 doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Planning and Statistics Authority. State of Qatar The simplified census of population, housing & establishments. 2019. https://www.psa.gov.qa/en/statistics/Statistical%20Releases/Population/Population/2018/Population_social_1_2018_AE.pdf
- 47.Seedat S, Chemaitelly H, Ayoub HH, et al. SARS-CoV-2 infection hospitalization, severity, criticality, and fatality rates in Qatar. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Protection of prior natural infection compared to mRNA vaccination against SARS-CoV-2 infection and severe COVID-19 in Qatar. medRxiv. 2022 doi: 10.1101/2022.03.17.22272529. published online March 18. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset in this study is the property of the Qatar Ministry of Public Health and was provided to the authors through a restricted access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Qatar's Minister of Public Health at https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-Health-Communication-Center.aspx. The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the manuscript and its appendix.