Abstract
Heavy menstrual bleeding (HMB) has an estimated prevalence of 18–32% but is known to be under-reported due to poor recognition and estimation of menstrual blood loss (MBL). HMB can negatively impact quality of life, affecting social interactions, work productivity and sexual life. Abnormal menstrual bleeding may have an underlying structural or systemic cause, such as endometrial and myometrial disorders; however, for some, there is no identified pathological cause. Several methods are available for assessing MBL, including the alkaline hematin (AH) method and the menstrual pictogram (MP). The AH method is considered to be the most accurate way to monitor MBL; however, it is associated with inconvenience and expense, therefore limiting its value outside of research. The MP requires the user to select an icon from a chart that reflects the appearance of a used sanitary product; the icon is associated with a blood volume that can be used to determine MBL. Validation studies have demonstrated that the results of the MP and AH method are well correlated, showing that the MP can measure MBL with sufficient accuracy. Additionally, the MP is more convenient for users, less expensive than the AH method, may be used in regions where the AH method is unavailable and may also be used as part of a digital application. Overall, the MP offers a convenient approach to monitor MBL both in research and clinical practice settings.
Keywords: heavy menstrual bleeding, menstrual blood loss, uterine fibroids, menstrual pictogram, alkaline hematin method, abnormal uterine bleeding
Introduction
Heavy menstrual bleeding (HMB) is defined as blood loss of >80 ml per menstrual cycle (Hallberg et al., 1966), or excessive menstrual blood loss (MBL) that interferes with a woman’s physical, emotional, social and material quality of life (QoL). HMB can occur alone or with other symptoms (National Institute for Health and Care Excellence, 2018).
HMB is under-recognized and under-reported
There are sparse data on HMB global prevalence in the general population, although a prevalence of 18–32% at any time during reproductive life has been suggested. This can vary across regions according to sample population characteristics (e.g. parity, age) and is dependent on HMB definition (Karlsson et al., 2014; Fraser et al., 2015, Hapangama and Bulmer, 2016; Ding et al., 2019).
HMB is known to be extensively under-reported, and potentially only 6% of women with HMB seek medical help (Fraser et al., 2015). This is largely due to inaccurate individual self-perception of HMB (Magnay et al., 2018) and normalization of symptoms (Fraser et al., 2015). For example, one population study demonstrated poor correlation between women’s perception of and actual MBL: 37% and 4% of women with blood loss >80 ml considered their MBL to be moderate or scanty, respectively; 14% of women with blood loss <20 ml considered their MBL to be heavy (Hallberg et al., 1966). This is supported by studies including self-reported data, which report a lower HMB prevalence than primary clinical studies (Fraser et al., 2015). HMB recognition is also influenced by cultural factors (Edelman et al., 2007; Bitzer et al., 2013): menstrual taboos can promote a culture of silence (Harlow and Campbell, 2004) and some cultures consider menstrual blood to be ‘medically cleansing’ or a sign of health (Bitzer et al., 2013).
The International Federation of Gynecology and Obstetrics Working Group on Menstrual Disorders developed the PALM-COEIN (polyp; adenomyosis; leiomyoma; malignancy and hyperplasia; coagulopathy; ovulatory dysfunction; endometrial; iatrogenic; and not yet classified) classification system for causes of abnormal uterine bleeding (AUB), defined as menstrual bleeding that is abnormal in duration, volume and/or frequency for ≥3 months. The term AUB also encompasses HMB (Munro et al., 2011). Although, for some women, there is no obvious pathological cause of their AUB, others may experience one or more entities that can cause or contribute to AUB, including structural causes (polyps, adenomyosis, leiomyoma [fibroids], malignancies) or non-structural causes (coagulation, ovulatory, endometrial iatrogenic disorders) (Munro et al., 2011; Hapangama and Bulmer, 2016; Cheong et al., 2017). Depending on severity, HMB may also lead to anemia (Borah et al., 2013; Stewart et al., 2013; Salehi et al., 2015; David et al., 2016; Soliman et al., 2017). It is therefore clear that under-reporting of HMB is of concern and improving identification methods may lead to timely diagnosis and treatment options.
HMB negatively impacts QoL
HMB has been shown to adversely affect QoL, including impacting physical activities, social interactions, work productivity, well-being and sexual life (Lukes et al., 2012; Gokyildiz et al., 2013; Su et al., 2020). It is the impact on daily life that will often lead individuals to seek care from their healthcare providers (Lukes et al., 2012).
Post-hoc analysis of data from two randomized, placebo-controlled studies of an oral tranexamic acid formulation in women with HMB revealed that higher daily MBL was associated with worse ratings of health-related QoL (Menorrhagia Impact Questionnaire) (Lukes et al., 2012). Data from both a case–control study and a Swedish cross-sectional study found that scores on all eight domains of the Short Form-36 QoL scale (physical functioning, physical role, pain, general health, vitality, social role functioning, emotional role functioning and mental health) were significantly lower in women who reported HMB than in women in the control group (either relatives of the participants without any specific health problems or women with normal MBL) (Gokyildiz et al., 2013; Karlsson et al., 2014). To improve QoL in women with HMB, efforts to assess and reduce MBL should be a priority for healthcare providers (Lukes et al., 2012).
This report presents the advantages and limitations of the most commonly used methods for MBL assessment, with a focus on the potential use of the menstrual pictogram (MP), a tool with relevance for both research and routine clinical practice, compared with the alkaline hematin (AH) method.
Quantitative assessment of MBL
AH method
The AH method was established for the quantitative assessment of MBL and is considered to be the ‘gold standard’ in terms of accuracy (Wyatt et al., 2001; Magnay et al., 2018). Based on current United States Food and Drug Administration guidance, the AH method is typically used for the diagnosis and assessment of HMB in research settings (Magnay et al., 2018). The AH method was developed >50 years ago and involves chemical extraction of hemoglobin from used sanitary products. It was initially validated for use with cotton-based sanitary products and blood recovery was 96% after a 20 h incubation. Following protocol modifications to simplify and improve the speed (Wyatt et al., 2001), the efficiency of blood extraction from a selection of sanitary products ranged from 75 to 107% (Magnay et al., 2018).
More than a decade ago, most sanitary towels (also referred to as sanitary pads) contained cotton as the main component of the absorbent core, whereas today, the majority of products contain superabsorbent polymer (SAP) granules (Magnay et al., 2014; Woeller and Hochwalt, 2015; P&G (Proctor and Gamble), 2019). The AH method was subsequently adapted and revalidated for use with a selected brand of SAP-containing towels (Magnay et al., 2011); recovery of at least 90% (≥85% with automation) of simulated menstrual fluid volumes was observed (Magnay et al., 2018).
However, the AH method requires women to collect and send used sanitary products for laboratory analysis, which presents some challenges: it can be impractical and inconvenient, and it requires laboratory expertise and costs to interpret and report results (The Menorrhagia Research Group et al., 2004; Schumacher et al., 2012; Magnay et al., 2014, 2018). The AH method is subject to incomplete patient compliance and collection variability, including variation in sanitary products, with associated variability in recovered amount of AH and requirement for calibration curves for each product (El-Nashar et al., 2015). Underestimation of blood loss due to overflow from the sanitary product is exacerbated by non-blood components not being detected by the AH method (Fraser et al., 1985; Fraser et al., 2001; Wyatt et al., 2001; The Menorrhagia Research Group et al., 2004; Magnay et al., 2014, 2018).
These practical limitations prevent the AH method from being used beyond research settings. Furthermore, women may be deterred from participating in clinical trials and complying with the study requirements, due to the inconvenience of having to collect, store and send used sanitary wear (Magnay et al., 2018).
Pictorial methods
As the focus of treatment must be the improvement of women’s symptoms and QoL (Mohan et al., 2007; Cheong et al., 2017), it follows that the AH method of quantifying MBL has less relevance in clinical practice. Pictorial methods of measuring MBL, such as the original pictorial blood loss assessment chart (PBAC) and MP, are simple, quick to use, semi-quantitative, patient-reported outcome tools used to determine MBL volume utilizing icon-based visual scoring systems for commonly used sanitary products (Higham et al., 1990; Janssen et al., 1995; Wyatt et al., 2001; Magnay et al., 2013, 2014). These tools may be beneficial in both adult and adolescent patients and are particularly useful for monitoring treatment response (Mohan et al., 2007; Magnay et al., 2018).
Unfortunately, there are important drawbacks to the currently used pictorial methods, including variable sensitivity and specificity versus the AH method, due to only three icons being used, contributing to reduced accuracy. The MP also requires a paper diary record for every used sanitary towel or tampon, a limitation associated with decreased accuracy (Magnay et al., 2013, 2018; El-Nashar et al., 2015). There is, therefore, an unmet need for an accurate, semi-quantitative method of MBL assessment that is acceptable for use in clinical trials and clinical practice.
The original PBAC and MP were validated for use with the cotton-containing sanitary products that were available more than a decade ago (Janssen et al., 1995; Wyatt et al., 2001; Magnay et al., 2018), and have subsequently been revalidated with SAP-containing products that are now commonly used (although validation has only been conducted for a limited number of current products) (Magnay et al., 2014, 2018). The revalidated PBAC and MP still both have the disadvantage of women having to recall/record results (Magnay et al., 2018); however, although the MP (which has the advantage of estimating MBL in milliliters and being directly comparable with the AH method) (Magnay et al., 2018) can differentiate between sanitary product absorbency ratings, the PBAC (which uses a scoring system that is proportional, but not equivalent to MBL) (Magnay et al., 2018) cannot. Furthermore, the PBAC has been shown to overestimate MBL in some women, thereby limiting its value in clinical practice (Magnay et al., 2014., 2018). An overview of some of the recent original and revalidated PBAC and MP data, highlighting sensitivity and specificity, and correlation with the AH method is provided in Table I. A full review of the currently available data for these, and other methods used to measure MBL, has been published (Magnay et al., 2018).
Table I.
Overview of pictorial blood-loss assessment chart and menstrual pictogram data for assessing menstrual blood loss in clinical trials.a
| PBAC | MP | |
|---|---|---|
| Sensitivity and specificity | Diagnosis of MBL >80 ml (n = 950) (Higham et al., 1990; Deeny and Davis, 1994; Janssen et al., 1995; Barr et al., 1999; Reid et al., 2000; Zakherah et al., 2011; Hald and Lieng, 2014):
|
Diagnosis of MBL >80 ml (Wyatt et al., 2001; Magnay et al., 2014) or identifying ≥50% decrease in MBL (n = 314) (Larsen et al., 2013):
|
| Predictive value | Predictive value of diagnosing HMB with a PBAC score cut-off of 100 (n = 103) (Reid et al., 2000):
|
Predictive value of diagnosing HMB (n = 170) (Larsen et al., 2013):
|
| Correlation with MBL assessed by AH method |
|
|
Data shown are for different versions of the MP and PBAC, which may have been adapted for use for study purposes. A full review of these data has been published (Magnay et al., 2018).
PBAC, pictorial blood-loss assessment chart; MP, menstrual pictogram; MBL, menstrual blood loss; HMB, heavy menstrual bleeding; AH, alkaline hematin; PPV, positive predictive value; NPV, negative predictive value.
Development and validation of the MP
The revalidated MP (for use with SAP-containing sanitary products; hereafter referred to as the MP) is a later modification of the PBAC developed to assess MBL in clinical trials (Magnay et al., 2014). The MP allows women to assess the visual appearance of used sanitary products (Magnay et al., 2013, 2014), and the pictograms are used to provide an estimation of MBL (Magnay et al., 2013).
The MP comprises diagrams, with five icons that depict a graded series of stained towels or tampons (Fig. 1); each icon is associated with a blood volume derived from measurements taken by the AH method (Magnay et al., 2013, 2014). Women are asked to complete the MP whenever a sanitary product is changed, by choosing a pictogram icon that corresponds with the degree of staining on the underside of the sanitary product (Magnay et al., 2014).
Figure 1.
The revalidated menstrual pictogram. The menstrual pictogram requires women to assess their sanitary product upon changing, by selecting the image that looks the most like the underside of their sanitary product. In Magnay et al. (2014), blood loss (ml) was assigned to each pictogram: 0.5, 1.5, 4, 6.5 and 12.5 for icons 1–5 of the ‘normal’ sanitary products, 0.5, 1.5, 3.5, 6.5 and 12.5 for icons 1–5 for the ‘long’ sanitary products, and 0.5, 2, 4.5, 8 and 14 for icons 1–5 of the ‘night’ sanitary products. Reprinted with permissions from Magnay et al. (2014).
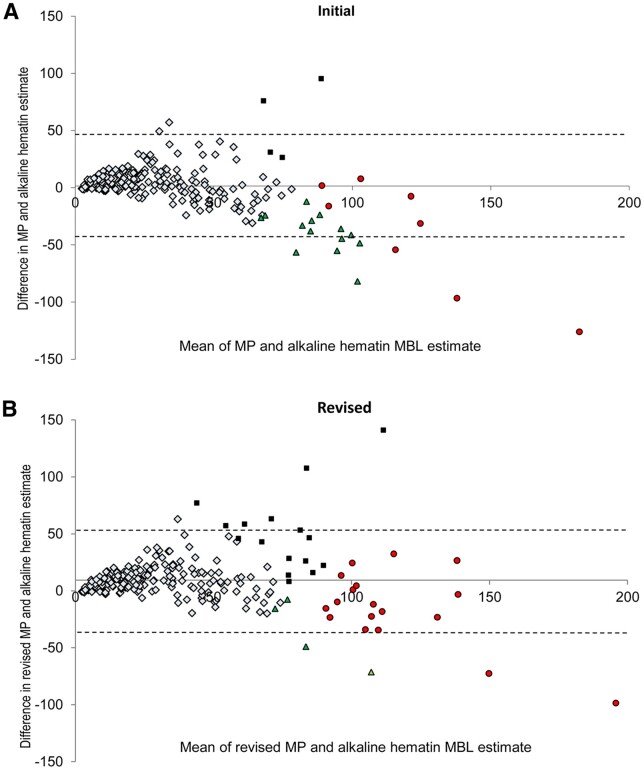
In the validation study of the MP for measuring MBL (with the AH method as the reference standard) (Magnay et al., 2014), the median blood loss for the 22 HMB cycles was 111 ml (range, 80.1–245 ml). In contrast, the median blood loss for the 213 normal cycles was 17 ml (range: 1–80 ml). Information on patient compliance/adherence was not reported. Overall, of 3325 sanitary towels collected, only 10 were excluded from analysis due to missing participant icon data (Magnay et al., 2014). Following correction for the incremental rise in blood fraction with volume, the MP demonstrated high sensitivity (82% [participant assessments identified 18/22 HMB cycles as >80 ml]) and specificity (92% [197/213 normal cycles were identified as ≤80 ml]) in diagnosing HMB. Figure 2 shows the Bland–Altman analysis of participant MP estimate of MBL versus AH estimate of MBL, after revision of icon blood volume. The expert ratings revealed a sensitivity of 95% (21/22 HMB cycles) and a specificity of 89% (190/213 normal cycles). Furthermore, AH and MP scores were significantly correlated (r = 0.81, P < 0.0001) (Magnay et al., 2014).
Figure 2.
Bland–Altman analysis of menstrual blood loss based on participant estimates with the menstrual pictogram versus alkaline hematin method. Bland–Altman analysis of participant revalidated menstrual pictogram (MP) estimate of menstrual blood loss (MBL) versus alkaline hematin estimate of menstrual blood loss (A) before and (B) after revision of icon blood volume, in Magnay et al. (2014). Symbol interpretation: light blue diamond = true negative; red circle = true positive; green triangle = false negative; black square = false positive. Dotted lines indicate 95% limits of agreement. Reprinted with permissions from Magnay et al. (2014).
A second validation study for a further adapted version of the MP (MPv3) has been published (Haberland et al., 2020). The MPv3 (on an electronic device given to patients) was included alongside the AH method in a Phase 2 study of a novel medical treatment for uterine fibroids. Comparison and quantitative assessment of the MPv3 was performed based on participant use of sanitary pads or tampons (Haberland et al., 2020). Full details of comparisons and statistical analyses have been described (Haberland et al., 2020). The results demonstrated that bleeding outcomes measured by the MPv3 strongly correlated with those from the AH method. Outcomes were determined by intraclass correlation coefficients (ICCs) for reliability of the MPv3 to provide reproducible scores over time (test–retest), correlation coefficients for the extent to which MBL measured by MPv3 is related to observed MBL (criterion validity) and responsiveness (Haberland et al., 2020). Correlation coefficients showed a strong association between the MPv3 and the AH method with regard to test–retest reliability (ICC estimate [95% CI] of 0.93 [0.88–0.96] during screening and randomization periods, and 0.96 [0.94–0.97] during treatment in AH-defined stable women); criterion validity (rs = 0.72 at randomization and rs = 0.97 at end of treatment); and responsiveness (rs = 0.86 for change in monthly sum scores) (Haberland et al., 2020). There was also a lower frequency of missing data for the MPv3, versus the AH method, indicating improved compliance with the MPv3—a key benefit (Haberland et al., 2020).
Overall, currently available evidence suggests that the MP and MPv3 meet the unmet need for more accurate and patient-friendly methods for quantitative MBL evaluation, potentially supporting improved clinical care and more informed decision-making. Although all methods for the assessment of MBL have limitations, pictorial methods (especially the MP/MPv3) offer a good balance between ease of use and validated accuracy (Magnay et al., 2018).
Clinician opinion of the MPv3 and opportunities for future use
The MPv3 is simple, user-friendly and inexpensive, and has demonstrated responsiveness and reliability during validation for use in clinical trials evaluating MBL (Magnay et al., 2014; Haberland et al., 2020). MP data are available from a number of studies (Wyatt et al., 2001; Wyatt et al., 2002; Larsen et al., 2013; Magnay et al., 2013, 2014) and, similar to the AH method, the MPv3 can assess change in MBL over time to determine treatment response, as demonstrated in women with uterine fibroids (Haberland et al., 2020). Advantages of the MPv3 over the AH method include:
it is easy to use (Magnay et al., 2014; Haberland et al., 2020),
it does not require women to mark, label, store and send used sanitary products for analysis (The Menorrhagia Research Group et al., 2004; Magnay et al., 2018),
it does not require a clinic to receive, store and analyze used sanitary products (The Menorrhagia Research Group et al., 2004),
it is used as part of a digital application, making recording data straightforward, and allowing data to be easily entered alongside electronic medical records without a paper diary record for every used sanitary towel or tampon (Haberland et al., 2020),
it can be used in areas where the AH method is not available and
it is of lower cost (Schumacher et al., 2012; Magnay et al., 2014).
Positive results support an opportunity for using the MPv3 to monitor treatment response for HMB-associated conditions in clinical practice. Based on the convenience and ease of use versus the AH method, the MPv3 may support patient recruitment and retention in clinical trials, and potentially improve compliance and increase the accuracy of reported results, ultimately facilitating research (Haberland et al., 2020). One drawback of paper pictorial methods is that patients must record details of used sanitary products in a paper record; when used as a digital application (ideally available on any mobile device), physician and patient access to the MPv3, along with data storage and sharing, would be key advantages. Although it should be noted that not all patients may have access to a phone, be able to download the digital application or readily have access to an internet connection.
Beyond clinical trials, the MPv3 may be a valuable diagnostic tool for HMB. Reports indicate that HMB is under-reported and under-recognized, underlining the importance of de-normalizing this pathological condition (Fraser et al., 2015). The MPv3 may also educate women on whether their MBL volume is abnormal and if it may indicate an underlying condition. It would allow physicians and women to gauge the severity of HMB and facilitate personalized bleeding management, as well as evaluate the efficacy of treatment of any underlying condition. Increasing awareness of HMB will, in turn, improve knowledge around its impact on reproductive health, enabling the identification and management of any adverse effects on QoL and fertility.
There is also a need to shift regional and cultural views around HMB (Harlow and Campbell, 2004; Edelman et al., 2007; Bitzer et al., 2013), making this a topic that women feel comfortable and confident discussing. Tools such as the MPv3 may help to empower women to openly discuss HMB with their physician. Self-reporting of symptoms and outcomes is strongly encouraged by many physicians, and helps women to play a more active role in their diagnosis and treatment.
Conclusion
The MPv3 menstrual loss evaluation tool has been validated for the assessment of HMB and offers several opportunities for use both in research and clinical practice to evaluate treatment response and disease progression/patient follow-up. Based on these benefits, and its advantages over the AH method, the MPv3 has the potential to broaden the perception and awareness of HMB and its associated pathologies in women and clinicians, resulting in improved outcomes for these women.
Acknowledgements
Medical writing support was provided by Afsaneh Khetrapal at Huntsworth Health Ltd, with funding from Bayer AG. All authors attended a meeting to discuss the topics and develop the expert opinions included in this manuscript. This meeting was financially supported by Bayer AG. The Menstrual Pictogram was originally developed at Keele University, UK, and refined in cooperation between Keele University and Bayer AG, Germany. Intellectual property in the instrument is jointly owned by Keele University and Bayer.
Authors’ roles
The manuscript was formulated after extensive discussion over several months between all authors (J.C.A., W.C., J-.P.E., K.K., A.L.S-.F., S.S.S, S.V. and X.Y.). All authors provided equal substantial intellectual contribution during the writing and revising of the manuscript, and all authors approved the final version.
Funding
Funding was provided by Bayer AG.
Conflict of interest
J.C.A. is a member of the HELP group, which is financially supported by Bayer, and has received honoraria from Gedeon Richter for developing educational materials. W.C. is a member of the HELP group, which is financially supported by Bayer. He has also acted as a consultant at AbbVie, Allergan (now owned by AbbVie), Bayer, Myovant and ObsEva. J.-P.E. is a member of the HELP group, which is financially supported by Bayer. K.K. is a member of the HELP group, which is financially supported by Bayer, and has received honorarium and support for travel to meetings for the study by Bayer. A.L.S.-F. is a member of the HELP group, which is financially supported by Bayer, and has received honoraria for lecturing and acting in an advisory capacity for Bayer. S.S.S. is a member of the HELP group, which is financially supported by Bayer, has participated in advisory boards, and provided consulting services and educational presentations for AbbVie, Allergan, Bayer, Myovant and Hologic. He has also been an investigator for trials related to endometriosis and uterine fibroids for AbbVie, EndoDiag and Bayer, for which his institution received funding. S.V. is a member of the HELP group, which is financially supported by Bayer, and has received honoraria and reimbursement for travel and accommodation. She is also part of the Bayer IUS Insertion Training steering committee. X.Y. is a member of the HELP group, which is financially supported by Bayer, and has received honoraria for lecturing and acting in an advisory capacity for Bayer and Abbott.
Contributor Information
Sukhbir S Singh, University of Ottawa & Ottawa Hospital Research Institute, Ottawa, Ontario, Canada.
Joaquin Calaf Alsina, Hospital de la Santa Creu i Sant Pau, Universitat Autónoma, Barcelona, Spain.
Silvia Vannuccini, Careggi University Hospital, Florence, Italy.
Kaori Koga, University of Tokyo, Tokyo, Japan.
Agnaldo Lopes Silva-Filho, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
Xin Yang, Peking University People’s Hospital, Beijing, China.
Jean-Philippe Estrade, Clinique Bouchard-Elsan, Marseille, France.
William Catherino, Uniformed Services University of the Health Sciences, Bethesda, MD, USA.
Data Availability
No new data have been generated or analyzed in support of this publication.
References
- Barr F, Brabin L, Agbaje O.. A pictorial chart for managing common menstrual disorders in Nigerian adolescents. Int J Gynaecol Obstet 1999;66:51–53. [DOI] [PubMed] [Google Scholar]
- Bitzer J, Serrani M, Lahav A.. Women’s attitudes towards heavy menstrual bleeding, and their impact on quality of life. Open Access J Contracept 2013;4:21–28. [Google Scholar]
- Borah BJ, Nicholson WK, Bradley L, Stewart EA.. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol 2013;209:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong Y, Cameron IT, Critchley HOD.. Abnormal uterine bleeding. Br Med Bull 2017;123:103–114. [DOI] [PubMed] [Google Scholar]
- David M, Pitz CM, Mihaylova A, Siedentopf F.. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol 2016;199:137–140. [DOI] [PubMed] [Google Scholar]
- Deeny M, Davis JA.. Assessment of menstrual blood loss in women referred for endometrial ablation. Eur J Obstet Gynecol Reprod Biol 1994;57:179–180. [DOI] [PubMed] [Google Scholar]
- Ding C, Wang J, Cao Y, Pan Y, Lu X, Wang W, Zhuo L, Tian Q, Zhan S.. Heavy menstrual bleeding among women aged 18-50 years living in Beijing, China: prevalence, risk factors, and impact on daily life. BMC Women’s Health 2019;19:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J.. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007;75:450–453. [DOI] [PubMed] [Google Scholar]
- El-Nashar SA, Shazly SAM, Famuyide AO.. Pictorial blood loss assessment chart for quantification of menstrual blood loss: a systematic review. Gynecol Surg 2015;12:157–163. [Google Scholar]
- Fraser IS, Mansour D, Breymann C, Hoffman C, Mezzacasa A, Petraglia F.. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Int J Gynaecol Obstet 2015;128:196–200. [DOI] [PubMed] [Google Scholar]
- Fraser IS, McCarron G, Markham R, Resta T.. Blood and total fluid content of menstrual discharge. Obstet Gynecol 1985;65:194–198. [PubMed] [Google Scholar]
- Fraser IS, Warner P, Marantos PA.. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstet Gynecol 2001;98:806–814. [DOI] [PubMed] [Google Scholar]
- Gokyildiz S, Aslan E, Beji NK, Mecdi M.. The effects of menorrhagia on women’s quality of life: a case-control study. ISRN Obstet Gynecol 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland C, Filonenko A, Seitz C, Börner M, Gerlinger C, Doll H, Wessiepe D.. Validation of a menstrual pictogram and a daily bleeding diary for assessment of uterine fibroid treatment efficacy in clinical studies. J Patient Rep Outcomes 2020;4:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald K, Lieng M.. Assessment of periodic blood loss: interindividual and intraindividual variations of pictorial blood loss assessment chart registrations. J Minim Invasive Gynecol 2014;21:662–668. [DOI] [PubMed] [Google Scholar]
- Hallberg L, Hogdahl AM, Nilsson L, Rybo G.. Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand 1966;45:320–351. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Bulmer JN.. Pathophysiology of heavy menstrual bleeding. Womens Health (Lond) 2016;12:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Campbell OM.. Epidemiology of menstrual disorders in developing countries: a systematic review. BJOG 2004;111:6–16. [DOI] [PubMed] [Google Scholar]
- Higham JM, O’Brien PM, Shaw RW.. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990;97:734–739. [DOI] [PubMed] [Google Scholar]
- Janssen CA, Scholten PC, Heintz AP.. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol 1995;85:977–982. [DOI] [PubMed] [Google Scholar]
- Karlsson TS, Marions LB, Edlund MG.. Heavy menstrual bleeding significantly affects quality of life. Acta Obstet Gynecol Scand 2014;93:52–57. [DOI] [PubMed] [Google Scholar]
- Larsen L, Coyne K, Chwalisz K.. Validation of the menstrual pictogram in women with leiomyomata associated with heavy menstrual bleeding. Reprod Sci 2013;20:680–687. [DOI] [PubMed] [Google Scholar]
- Lukes AS, Baker J, Eder S, Adomako TL.. Daily menstrual blood loss and quality of life in women with heavy menstrual bleeding. Women’s Health (Lond) 2012;8:503–511. [DOI] [PubMed] [Google Scholar]
- Magnay JL, Nevatte TM, O’Brien S, Gerlinger C, Seitz C.. Validation of a new menstrual pictogram (superabsorbent polymer-c version) for use with ultraslim towels that contain superabsorbent polymers. Fertil Steril 2014;101:515–522. [DOI] [PubMed] [Google Scholar]
- Magnay JL, Nevatte TM, Seitz C, O’Brien S.. A new menstrual pictogram for use with feminine products that contain superabsorbent polymers. Fertil Steril 2013;100:1715–1721. [DOI] [PubMed] [Google Scholar]
- Magnay JL, O’Brien S, Gerlinger C, Seitz C.. A systematic review of methods to measure menstrual blood loss. BMC Womens Health 2018;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnay JL, Schonicke G, Nevatte TM, O’Brien S, Junge W.. Validation of a rapid alkaline hematin technique to measure menstrual blood loss on feminine towels containing superabsorbent polymers. Fertil Steril 2011;96:394–398. [DOI] [PubMed] [Google Scholar]
- Mohan S, Page LM, Higham JM.. Diagnosis of abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol 2007;21:891–903. [DOI] [PubMed] [Google Scholar]
- Munro MG, Critchley HO, Broder MS, Fraser IS;. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113:3–13. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. National Evidence Based Clinical Guidelines. Heavy Menstrual Bleeding. 2018. https://www.nice.org.uk/guidance/ng88 (November 2019, date last accessed).
- P&G (Proctor and Gamble). What Ingredients are in Always Pads? 2019. https://alwayscom/en-us/about-us/what-ingredients-are-in-always-pads (November 2019, date last accessed).
- Reid PC, Coker A, Coltart R.. Assessment of menstrual blood loss using a pictorial chart: a validation study. BJOG 2000;107:320–322. [DOI] [PubMed] [Google Scholar]
- Salehi M, Jalilian N, Salehi A, Ayazi M.. Clinical efficacy and complications of uterine artery embolization in symptomatic uterine fibroids. Glob J Health Sci 2015;8:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher U, Schumacher J, Mellinger U, Gerlinger C, Wienke A, Endrikat J.. Estimation of menstrual blood loss volume based on menstrual diary and laboratory data. BMC Womens Health 2012;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AM, Margolis MK, Castelli-Haley J, Fuldeore MJ, Owens CD, Coyne KS.. Impact of uterine fibroid symptoms on health-related quality of life of US women: evidence from a cross-sectional survey. Curr Med Res Opin 2017;33:1971–1978. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Nicholson WK, Bradley L, Borah BJ.. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health 2013;22:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Yang X, Su Q, Zhao Y.. Prevalence and knowledge of heavy menstrual bleeding among gynecology outpatients by scanning a WeChat QR Code. PLoS One 2020;15:e0229123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Menorrhagia Research Group, Warrilow G, Kirkham C, Ismail KM, Wyatt K, Dimmock P, O’Brien S.. Quantification of menstrual blood loss. Obstet Gynaecol 2004;6:88–92. [Google Scholar]
- Woeller KE, Hochwalt AE.. Safety assessment of sanitary pads with a polymeric foam absorbent core. Regul Toxicol Pharmacol 2015;73:419–424. [DOI] [PubMed] [Google Scholar]
- Wyatt KM, Dimmock PW, Hayes-Gill B, Crowe J, O’Brien PM.. Menstrual symptometrics: a simple computer-aided method to quantify menstrual cycle disorders. Fertil Steril 2002;78:96–101. [DOI] [PubMed] [Google Scholar]
- Wyatt KM, Dimmock PW, Walker TJ, O’Brien PM.. Determination of total menstrual blood loss. Fertil Steril 2001;76:125–131. [DOI] [PubMed] [Google Scholar]
- Zakherah MS, Sayed GH, El-Nashar SA, Shaaban MM.. Pictorial blood loss assessment chart in the evaluation of heavy menstrual bleeding: diagnostic accuracy compared to alkaline hematin. Gynecol Obstet Invest 2011;71:281–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data have been generated or analyzed in support of this publication.