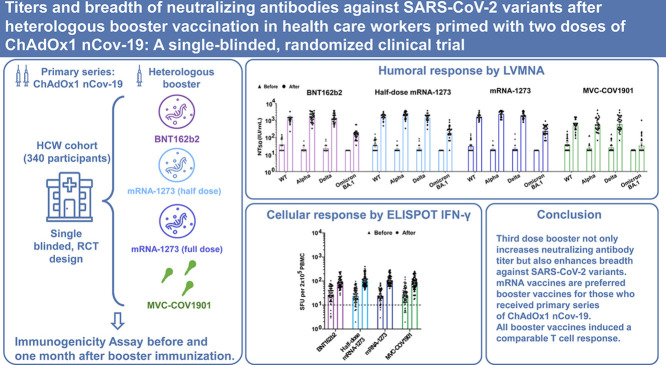
Abstract
Objectives
We conducted a single-blinded, randomized trial to evaluate the safety, reactogenicity, and immunogenicity of heterologous booster vaccination in health care workers (HCW) who had received two doses of ChAdOx1 nCov-19.
Methods
HCW who had at least 90 days after the second dose were enrolled to receive one of the four vaccines: BNT162b2 (30 μg), half-dose mRNA-1273 (50 μg), mRNA-1273 (100 μg), and MVC-COV1901 (15 μg). The primary outcomes were humoral and cellular immunogenicity and secondary outcomes assessed safety and reactogenicity at 28 days post-booster.
Results
MVC-COV1901 Three hundred and forty HCW were enrolled: 83 received BNT162b2 (2 excluded), 85 half-dose mRNA-1273, 85 mRNA-1273, and 85 MVC-COV1901. mRNA vaccines had more reactogenicity than protein vaccine. The fold-rise of anti-spike IgG geometric mean titer was 8.4 (95% CI 6.8–10.4) for MVC-COV1901, 32.2 (27.2–38.1) for BNT162b2, 47.6 (40.8–55.6) for half-dose mRNA-1273 and 63.2 (53.6–74.6) for mRNA-1273. The live virus microneutralization assays (LVMNA) against the wild type, alpha and delta variants were consistent with anti-spike IgG for all booster vaccines. The LVMNA in the four groups against omicron BA.1 variant were 6.4 to 13.5 times lower than those against the wild type. All booster vaccines induced a comparable T cell response.
Conclusions
Third dose booster not only increases neutralizing antibody titer but also enhances antibody breadth against SARS-CoV-2 variants. mRNA vaccines are preferred booster vaccines for those who received primary series of ChAdOx1 nCov-19.
Keywords: COVID-19, Vaccine, Heterologous booster, Third dose, Immunogenicity, Safety
Graphical abstract
1. Introduction
The pandemic of Coronavirus Disease 2019 (COVID-19) has been shaped by the successive emergence of different SARS-CoV-2 variants with increased transmissibility and/or immune escape, compared to the ancestral strain, since 2020 [1,2]. Delta variant (B.1.617.2) emerged in India in February 2021 and became dominant over the following months [2,3]. Omicron variant (B.1.1.529) that carries a large number of mutations in the spike protein gene was first reported from Gauteng province, South Africa in November 2021 [4]. Because these mutation sites are the major target of neutralization antibodies, omicron variant is able to avoid neutralization by antibodies in the serum of vaccinated or recovered individuals as well as by a large range of human monoclonal antibodies in use [1,[5], [6], [7]].
Taiwan experienced its first large wave of COVID-19 caused by alpha variant (B.1.1.7) from May to August 2021. The COVID-19 vaccination program in Taiwan started in March 2021. However, the first large batch of vaccines that arrived in Taiwan was ChAdOx1 nCov-19 (AstraZeneca). The majority of the health care workers (HCW) in Taiwan received two doses of ChAdOx1 nCov-19; first and second doses were given 8 weeks apart.
Previous studies have shown that effectiveness of two doses of ChAdOx1 nCov-19 vaccine against symptomatic disease among persons infected with alpha or delta variant was less than that of two doses of BNT162b2 vaccine (Pfizer BioNTech) [3]. Moreover, given the occurrence of rare, but severe adverse events after vaccination with vector-based vaccines such as ChAdOx1 nCov-19, heterologous prime-boost regimens have been recommended in many countries [8,9]. On the other hand, significant waning of humoral responses within 6 months after receipt of the second dose of BNT162b2 vaccine or ChAdOx1 nCov-19 has been observed in recent studies [10,11]. Vaccine effectiveness in preventing COVID-19 declined because of such waning immunity. Furthermore, variant immune evasion also played a significant role [12]. It is therefore critical to give a third dose, also known as the first booster, to protect the vulnerable persons, and mitigate health care and economic impacts.
“Mixing and matching” COVID-19 vaccines may enhance the flexibility of vaccination and induce broader immune responses [13]. Immunological and safety assessments of the mRNA and Ad26.COV2.S (Johnson & Johnson-Janssen) boosters in persons who received different priming regimens had been reported previously [14]. However, these clinical trials were conducted with mRNA-1273 (100 μg) instead of current recommendation of half-dose mRNA-1273 (50 μg) [13,14]. Protein vaccines are based on established technology with a good safety record. Besides, protein vaccines are easier to be transported and stored than that of mRNA vaccines. MVC-COV1901 is a CpG1018 and aluminum hydroxide-adjuvanted SARS-COV-2 pre-fusion-stabilized spike protein S-2P vaccine developed by Medigen Vaccine Biologics Corporation, Taiwan. After a large phase II trial demonstrating good safety profile and promising immunogenicity, it was authorized for emergency use by Taiwan Food and Drug Administration (FDA) in July 2021. MVC-COV1901 is one of the two vaccines for inclusion in the WHO Solidarity Trial. To support decision making for people in Taiwan and other countries who received primary immunization with two doses of ChAdOx1 nCov-19, we performed this single-blinded, randomized study for a head-to-head comparison of safety and immunogenicity of different heterologous boosters administered to HCW.
2. Methods
2.1. Study design
This trial is to investigate the safety, reactogenicity and immunogenicity of heterologous booster of either BNT162b2 (30 μg), half-dose mRNA-1273 (50 μg) (Moderna), mRNA-1273 (100 μg) or MVC-COV1901 (15 μg) COVID-19 vaccine among HCW in a single institute at Chang Gung Memorial Hospital (CGMH), Taoyuan, Taiwan. All participants gave written informed consent before entering this trial. The study protocol was approved by institutional review board of CGMH (202101767A3).
2.2. Participants
Participants were between 20 and 65 years of age and had received 2 doses of ChAdOx1 nCoV-19 vaccine more than 90 days at enrollment. The main exclusion criteria were history of laboratory confirmed COVID-19 and anaphylaxis or severe allergic reaction to any components of study vaccines. The detailed inclusion and exclusion criteria were included in Appendix 1.
2.3. Randomization and masking
Participants were randomly assigned, in a 1:1:1:1 ratio, to receive a single dose of BNT162b2, half-dose mRNA-1273, mRNA-1273 or MVC-COV1901. Computer generated randomization list was conducted by a blinded statistical consultant. Participants were allocated by random block sizes of 4. Clinical research nurses who were not involved in vaccine administration and immunogenicity evaluation did the randomization process. Participants were blinded to the boost vaccine until 180 days postvaccination. Laboratory staff who processed immunological samples were blinded to vaccine allocation. Clinical research nurses and research staff accessing adverse events were unblinded.
2.4. Procedures
After an online screening procedure, participants were invited to join the study. Baseline demographic data were collected via electronic questionnaire. Blood samples were taken for baseline hematological and biochemical testing before booster at first visit. Blood samples were collected for immunogenicity analysis and checking for any previous infection before and 28 days after vaccination. Blood samples will be collected 180 days after booster vaccination for persistence of immunogenicity. We accessed adverse events by use of a modified US FDA toxicity grading scale [15]. Participants were asked to record electronic questionnaire on solicited local and systemic adverse events daily for 7 days and unsolicited adverse events weekly for 28 days post-booster. Serious adverse events and adverse events of special interest were reported by telephone or mobile message app for 180 days after booster vaccination.
2.5. Immunogenicity
All blood samples were measured for quantitative anti-spike IgG, SARS-CoV-2 nucleocapsid IgG, surrogate neutralizing antibody by an ELISA kit (Formosa Biomedical Technology Corporation), and T-cell ELISpot kit. Serum anti-spike IgG concentrations were evaluated by Abbott AdviseDx SARS-CoV-2 IgG II assay [16]. SARS-CoV-2 nucleocapsid IgG were measured for confirmation of previous infection by Roche Elecsys Anti-SARS-CoV-2 [17]. The MeDiPro SARS-CoV-2 Antibody ELISA kit, based on the binding affinity of S1 and RBD domains to antibodies, was designed to indirectly quantify SARS-CoV-2 neutralizing antibodies in the serum [18]. Values <12 IU/mL were considered negative results. Similar to other quantitative serological tests using different technologies, the MeDiPro SARS-CoV-2 Antibody ELISA assay was able to detect the trend in terms of humoral response magnitude and of variants of concern (VOCs) neutralizing ability levels [18,19]. The kit was approved by Taiwan FDA (No. 1,106,803,303). SARS-CoV-2 spike protein-specific T cell response was evaluated by ex vivo stimulating peripheral blood mononuclear cells using the Human IFN-γ ELISpot Kit (EL285, R&D) [20]. All these kits were used following manufacturers’ instructions.
A random subset of 120 participants (30 in each group) was tested for live virus neutralization with wild-type, alpha, delta and omicron BA.1 variants of SARS-CoV-2, as well as pseudovirus neutralization with the pseudoviruses either expressing SARS-CoV-2 D614G or omicron BA.1 spike protein. The live virus neutralizing antibody test followed the standard protocol of a live virus microneutralization assay (LVMNA) [21]. The lower limit of LVMNA was 34.45 IU/ml. SARS-CoV-2 pseudovirus expressing D614G or omicron BA.1 spike protein was prepared and titrated by the National RNAi Core Facility, Academia Sinica, Taiwan. SARS-CoV-2 pseudovirus neutralization assay (PNA) was performed as previously described [22]. The lower limit of PNA was 8 ID50. Values below the limit were substituted with half of the cutoff value. The detail methods for the analysis of immunogenicity were available in Appendix 2.
2.6. Outcomes
The primary outcomes were immunogenicity assessed 28 days after booster vaccination, including serum SARS-CoV-2 anti-spike IgG concentration, the 50% neutralizing antibody titers (NT50) against wild-type, alpha, delta and omicron BA.1 variants, and IFN-γ secreting T cells specific to whole spike protein of the wild type. Secondary outcomes were safety and reactogenicity including occurrence of solicited and unsolicited adverse events, adverse events of special interest and serious adverse events.
2.7. Statistical analysis
The sample size was calculated on the basis of a previous report with a minimum clinically important difference of 1.75-times difference between geometric mean titer, assuming a SD of 0.4 on log10 scale [13]. Since six comparisons were performed between 4 groups, a significance level of 0.05/6 = 0.0083 was adjusted by Bonferroni correction. Sixty-four participants in each group were required to achieve 90% power at a two-side 0.008% significance level with 20% dropout rate. We enrolled 85 participants in each group. Categorical data were expressed as number (percentage). Continuous variables were presented as median (interquartile range). Immunogenicity endpoints were reported as geometric means with 95% confidence intervals. The geometric mean of fold change was calculated as the antilogarithm of the mean difference between the log10 transformed titer of post-boost and that of pre-boost. Analysis of differences in immunogenicity between booster vaccine groups were performed by the Kruskal-Wallis test with Dunn's post hoc test. Correlations between different immunological tests were assessed using Spearman's correlation coefficient. Adverse events between groups were compared by Fisher's exact test. P < 0.008 was considered statistically significant. All analysis was performed with SPSS statistical software version 21.
3. Results
3.1. Baseline characteristics of the participants
From November 29 to December 14, 2021, 852 participants were contacted, among whom 340 participants were enrolled and randomly assigned to 4 groups (Fig. 1 ). No participants dropped out of the study. Two participants had detectable anti-nucleocapsid protein IgG antibody at baseline and during follow-up and were excluded for analysis. One participant had pregnancy after booster vaccine and was still under analysis. Among the 338 participants, the median age was 36 years old (range: 22–64) and 228 (67.5%) participants were female. The intervals between the second dose and booster ranged from 91 to 199 days. Generally, the baseline demographic characteristics and laboratory tests were balanced across the 4 vaccine groups (Table 1 ). There were no differences in immunological studies including anti-spike IgG levels, neutralizing antibodies and S-specific T cell response at baseline across the 4 vaccine groups (Table 2 ).
Fig. 1.
Study population and analysis. 340 participants were enrolled and randomly assigned to 4 groups. Two participants had detectable anti-nucleocapsid protein IgG antibody at baseline and during follow-up and were excluded for analysis.
Table 1.
. Baseline characteristics of the participants.
| Characteristic | BNT162b2 | Half-dose mRNA-1273 |
mRNA-1273 | MVC—COV1901 |
|---|---|---|---|---|
| No. of participants | 83 | 85 | 85 | 85 |
| Sex – no. (%) | ||||
| Female | 53 (64) | 58 (68) | 60 (71) | 57 (67) |
| Male | 30 (36) | 27 (32) | 25 (29) | 28 (33) |
| Age - years | ||||
| Median (IQR) | 35.0 (30.0–44.0) | 35.0 (30.0–45.5) | 37.0 (30.5–44.0) | 39.0 (32.5–44.5) |
| Intervals between first and second doses – days | ||||
| Median (IQR) | 63 (59–71) | 68 (60–71) | 63 (61–71) | 68 (61–71) |
| Intervals between second doses and booster – days | ||||
| Median (IQR) | 138 (126–177) | 140 (128–182) | 138 (127–149) | 138 (131–177) |
| Body-mass index* | 22.6 (20.3–24.8) | 23.5 (21.9–26.3) | 23.1 (21.5–25.8) | 22.2 (20.1–25.5) |
| Comorbidities | ||||
| Cardiovascular disease –no. (%) | 6 (7) | 2 (2) | 10 (12) | 6 (7) |
| Diabetes mellitus –no. (%) | 0 (0) | 1 (1) | 4 (5) | 2 (2) |
| Liver disease –no. (%) | 2 (2) | 3 (4) | 4 (5) | 2 (2) |
| Kidney disease –no. (%) | 0 (0) | 1 (1) | 0 (0) | 1 (1) |
| White blood cell count – per mm3 | ||||
| Median (IQR) | 5900 (5300–7200) | 6500 (5500–7850) | 6500 (5500–7750) | 6000 (5300–7700) |
| Hemoglobin – per g/dl | ||||
| Median (IQR) | 13.8 (13.0–15.1) | 13.7 (13.0–14.7) | 13.5 (12.7–14.7) | 13.7 (12.8–14.6) |
| Platelet count – 103 per mm3 | ||||
| Median (IQR) | 272 (232–311) | 282 (240–334) | 278 (247–310) | 263 (233–311) |
| AST – U/liter | ||||
| Median (IQR) | 18 (14–23) | 17 (13–20) | 17 (14–21) | 17 (14–21) |
| ALT – U/liter | ||||
| Median (IQR) | 15 (10–24) | 15 (11–22) | 16 (11–23) | 16 (10–21) |
IQR= interquartile range. AST=aspartate aminotransferase. ALT=alanine aminotransferase.
* Body-mass index is the weight in kilogram divided by the square of the height in meters.
Table 2.
. Binding antibody, neutralizing antibody, and T cell response.
| Characteristic | BNT162b2 | Half-dose mRNA-1273 |
mRNA-1273 | MVC—COV1901 | P value* |
|---|---|---|---|---|---|
| SARS-CoV-2 anti-spike IgG, BAU/mL |
N = 83 | N = 85 | N = 85 | N = 85 | |
| GMC before boost | 35 (30–42) | 36 (31–43) | 38 (32–46) | 42 (35–50) | P = 0.52 |
| GMC after boost | 1133 (971–1323) | 1723 (1521–1952) | 2400 (2067–2788) | 352 (301–411) | P <0.001 |
| Fold change | 32.18 (27.18–38.11) | 47.60 (40.78–55.56) | 63.20 (53.56–74.56) | 8.40 (6.82–10.35) | |
| Surrogate neutralizing antibody by ELISA, IU/mL | N = 83 | N = 85 | N = 85 | N = 85 | |
| GMT before boost | 16 (14–19) | 17 (14–20) | 15 (13–17) | 17 (15–20) | P = 0.17 |
| GMT after boost | 524 (481–573) | 657 (609–708) | 709 (658–764) | 219 (187–256) | P < 0.001 |
| Fold change | 32.27 (27.38–38.04) | 39.75 (33.18–47.61) | 47.71 (41.36–55.03) | 12.69 (10.20–15.79) | |
| Live virus neutralizing antibody (wild type), NT50, IU/mL |
N = 30 | N = 30 | N = 30 | N = 30 | |
| Before boost | 39 (29–51) | 37 (28–47) | 32 (25–42) | 40 (30–54) | P = 0.64 |
| After boost | 1154 (981–1357) | 1673 (1459–1918) | 1607 (1423–1815) | 501 (384–654) | P <0.001 |
| Fold change | 29.81 (24.06–36.93) | 45.53 (36.03–57.53) | 49.97 (38.23–65.31) | 12.34 (8.41–18.10) | |
| Pseudovirus neutralizing antibody (D614G), ID50 | N = 30 | N = 30 | N = 30 | N = 30 | |
| Before boost | 123 (90–167) | 139 (99–194) | 110 (78–156) | 108 (78–149) | P = 0.74 |
| After boost | 3298 (2786–3905) | 4140 (3558–4817) | 5375 (4576–6314) | 1428 (1050–1941) | P < 0.001 |
| Fold change | 26.77 (19.50–36.77) | 29.72 (22.34–39.52) | 48.94 (33.64–71.22) | 13.22 (8.54–20.47) | |
| Live virus neutralizing antibody (alpha), NT50, IU/mL |
N = 30 | N = 30 | N = 30 | N = 30 | |
| Before boost | 20 (18–23) | 20 (18–23) | 20 (18–22) | 23 (19–29) | P = 0.88 |
| After boost | 1566 (1256–1951) | 2022 (1669–2450) | 2351 (2017–2739) | 535 (386–740) | P < 0.001 |
| Fold change | 76.86 (61.57–95.95) | 99.31 (82.74–119.20) | 118.11 (101.52–137.42) | 23.01 (15.97–33.17) | |
| Live virus neutralizing antibody (delta), NT50, IU/mL |
N = 30 | N = 30 | N = 30 | N = 30 | |
| Before boost | 23 (19–27) | 21 (18–25) | 21 (17–25) | 26 (21–33) | P = 0.29 |
| After boost | 1325 (1069–1641) | 1427 (1177–1730) | 1879 (1606–2200) | 624 (441–883) | P < 0.001 |
| Fold change | 57.77 (47.34–70.50) | 73.16 (55.24–96.90) | 97.79 (70.22–136.19) | 23.81 (15.47–36.63) | |
| Live virus neutralizing antibody (omicron), NT50, IU/mL |
N = 30 | N = 30 | N = 30 | N = 30 | |
| Before boost | 17 (17–17) | 17 (17–17) | 17 (17–17) | 17 (17–17) | P = 1.0 |
| After boost | 146 (119–179) | 204 (152–275) | 250 (195–322) | 37 (25–53) | P < 0.001 |
| Fold change | 8.38 (6.82–10.29) | 11.75 (8.73–15.82) | 14.41 (11.22–18.50) | 2.10 (1.44–3.07) | |
| Pseudovirus neutralizing antibody (omicron), ID50 |
N = 30 | N = 30 | N = 30 | N = 30 | |
| Before boost | 10 (7–13) | 10 (8–14) | 13 (10–19) | 8 (6–11) | P = 0.21 |
| After boost | 682 (516–902) | 851 (681–1064) | 1165 (931–1457) | 273 (189–395) | P < 0.001 |
| Fold change | 71.12 (49.97–101.22) | 81.47 (62.39–106.38) | 86.56 (59.86–125.15) | 32.89 (20.11–53.79) | |
| Cellular response, SFU per 2 × 105 PBMCs |
N = 83 | N = 85 | N = 85 | N = 85 | |
| Before boost | 29 (25–34) | 27 (23–31) | 26 (23–30) | 26 (22–30) | P = 0.45 |
| After boost | 89 (80–99) | 105 (94–117) | 109 (99–120) | 84 (74–95) | P = 0.01 |
| Fold change | 3.05 (2.60–3.59) | 3.92 (3.30–4.65) | 4.21 (3.59–4.92) | 3.26 (2.77–3.85) |
Data are GM (95% CI). BAU=binding antibody unit. GMC=geometric mean concentration. GMT=geometric mean titer. ELISA=enzyme-linked immunosorbent assay. NT50= 50% neutralization titer. ID50=50% inhibitory dose. SFU=Spot-forming unit. PBMCs=peripheral blood mononuclear cells.
*P values were reported using Kruskal-Wallis.
3.2. Safety and reactogenicity
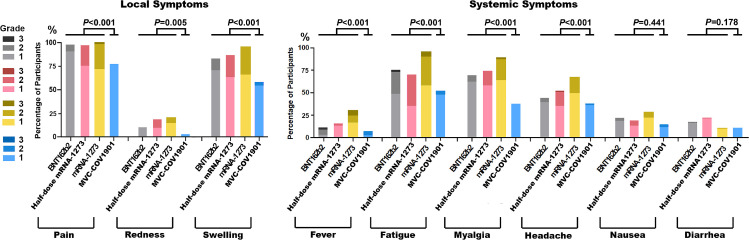
Neither potentially life-threatening, serious solicited or unsolicited adverse events nor adverse events of special interest were reported after any booster vaccine in the study. The most common local solicited adverse event was injection site pain (92%), followed by swelling (81%) and redness (13%). Fatigue (73%) was the most common systemic adverse event, followed by myalgia (67%), headache/dizziness (50%), nausea (21%), fever (15%), and diarrhea (15%) (Table S1). The proportion of severe adverse events were injection site pain, 0 to 2.4%; fever, 0 to 5.9%; fatigue, 0 to 4.7%; myalgia, 0 to 1.2% and headache, 0 to 1.2% (Fig. 2 ; Table S1). Local adverse events and some systemic adverse events such as fatigue, myalgia and headache could last for 1 week after booster vaccination (Fig. S1). Only 8 (2.4%) participants had fever for more than 48 h. Protein vaccine (MVC-COV1901) showed less local adverse events (pain, P < 0.001; redness, P = 0.005; swelling, P < 0.001) and systemic adverse events (fever, P < 0.001; fatigue, P <0.001; myalgia, P < 0.001; Headache, P < 0.001) than mRNA vaccines (Table S1). Full-dose mRNA-1273 had more local and systemic adverse events than half-dose mRNA1273. Half-dose mRNA-1273 had more local and systemic adverse events than BNT162b2 (Fig. 2; Table S1).
Fig. 2.
Severity of systemic and local reactions after booster vaccination. The percentage of participants with local symptoms (pain, redness, or swelling at the injection site) and the percentage of participants with systemic symptoms (fever, fatigue, myalgia, headache, nausea, or diarrhea) after booster vaccination are demonstrated. These reactions were monitored in the 7 days after the administration of the booster. The toxicity grading scale represents the highest grade of severity during the seven days. Grade 1 means mild reaction, grade 2 moderate reaction and grade 3 severe reaction. gray: BNT162b2; red: half-dose mRNA-1273; yellow: mRNA-1273; blue: MVC—COV1901.
3.3. Immunogenicity
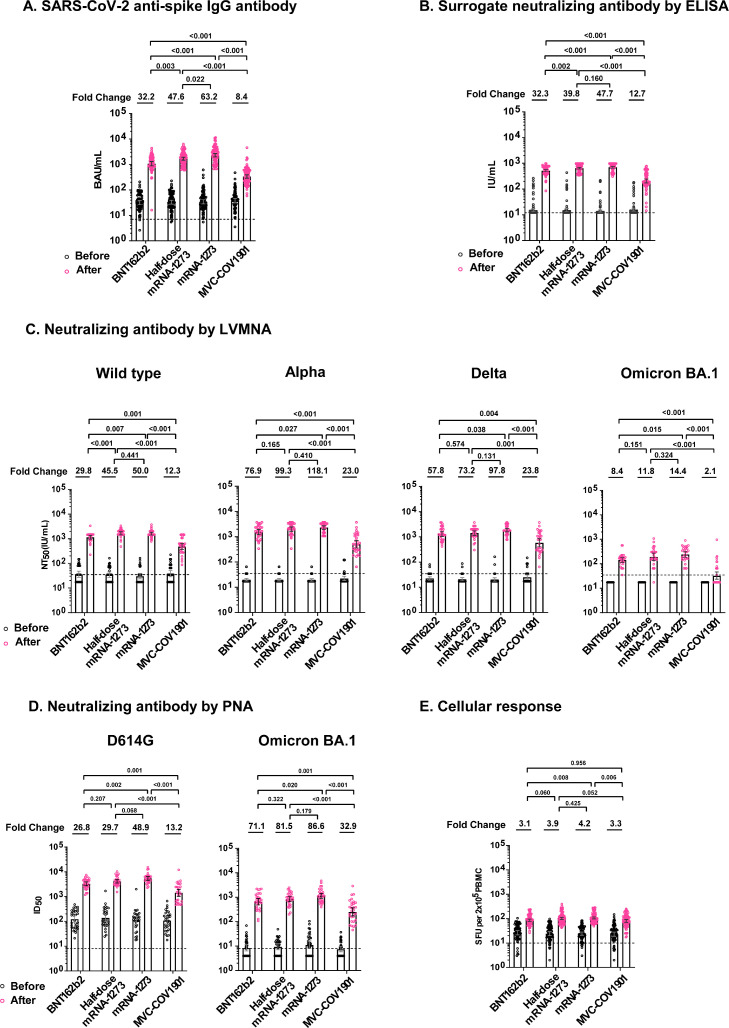
Compared with pre-boost, all study vaccines elicited significantly higher anti-spike IgG at 28 days post-boost (P < 0.001). The fold-rise ranged from 8.4 in MVC-COV1901 group to 63.2 in mRNA-1273 group (Table 2). The difference of immunogenicity between study vaccines was shown in Fig. 3 A. Antibody, assayed as surrogate neutralizing antibody by ELISA kit, was not detected in 262 (78%) of participants before receiving booster vaccine. After booster vaccination, the fold-rise ranged from 12.7 in MVC-COV1901 group to 47.7 in mRNA-1273 group (Fig. 3B). mRNA vaccines induced significantly higher anti-spike IgG and surrogate neutralizing antibody than protein vaccine (MVC-COV1901).
Fig. 3.
SARS-CoV-2 spike protein–specific immune responses before and after booster vaccination. A, Levels of SARS-CoV-2 spike protein-specific IgG antibodies at baseline (before booster vaccination) and after booster vaccination in the four groups. B, Levels of surrogate neutralizing antibodies by ELISA at baseline and after booster vaccination in the four groups. C, Levels of neutralizing antibodies at baseline and after booster vaccination, as assessed with a live virus microneutralization assay (LVMNA) in the four groups. D, Levels of neutralizing antibodies at baseline and after booster vaccination, as assessed with a SARS-CoV-2 pseudovirus neutralization assay (PNA) in the four groups. E, SARS-CoV-2 spike protein-specific T-cell response at baseline and after booster vaccination in the four groups, as measured by interferon-γ levels produced peripheral blood mononuclear cells after ex vivo stimulation. The P values on the top of figure were the comparison of the immune responses between groups. The dashed line indicated cutoff value.
The live virus neutralizing antibody against the wild type, alpha, delta and omicron BA.1 variants pre-booster were not detected in 48 (40%), 96 (80%), 87(73%) and 120 (100%) participants, respectively. The neutralizing activity increased significantly post booster (P < 0.001), the fold-rise ranged from 12.3 in MVC-COV1901 to 50.0 in mRNA-1273 against wild-type, 23.0 in MVC-COV1901 to 118.1 in mRNA-1273 against alpha variant, 23.8 in MVC-COV1901 to 97.8 in mRNA-1273 against delta variant and 2.1 in MVC-COV1901 to 14.4 in mRNA-1273 against omicron BA.1 variant (Fig. 3C). After each booster vaccine, the neutralization titers against alpha and delta variants were comparable with those of the wild type. The neutralization titers against omicron BA.1 variant were 6.4 to 13.5 times lower than those against the wild type. All except one participant who received mRNA vaccines as a booster had detectable neutralizing antibody against omicron BA.1 variant. Serum neutralizing antibody against omicron BA.1 was not detected in 60% of the participants who received MVC-COV1901 booster. The fold-rise of pseudovirus neutralization titers against D614G ranged from 13.2 in MVC-COV1901 to 48.9 in mRNA-1273 (Fig. 3D). The pseudovirus neutralization titers against omicron BA.1 variant were 4.6 to 5.2 times lower than that against D614G. In general, mRNA vaccines elicited significantly higher neutralizing antibody than protein vaccine (MVC-COV1901) did (Fig. 3C, D). There was no significant difference in neutralizing antibodies between BNT162b2, half-dose mRNA-1273 and mRNA-1273 (Fig. 3).
Level of interferon-γ was not detected in 10 (3%) participants before booster. All vaccines induced significant T-cell response by ELISpot (Table 2). All booster vaccines elicited at least 3-fold rise of interferon-γ (Fig. 3E). There was no significant difference in T cell response between study vaccines.
3.4. Correlations between immune responses
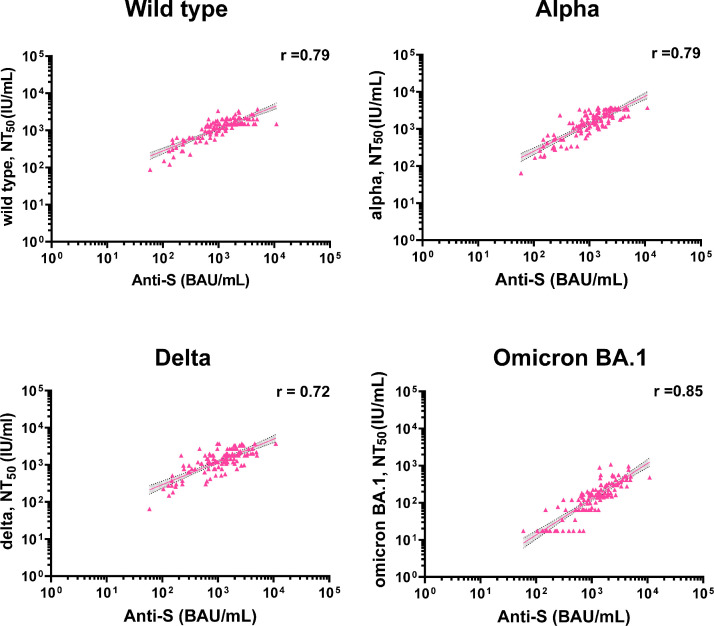
Strong correlations were found between anti-spike IgG and LVMNA against the wild type and VOCs post booster immunization (Spearman correlation coefficient ranged from 0.72 to 0.85) (Fig. 4 ). The correlations between different immunogenicity tests are provided in Figs. S2‒S4.
Fig. 4.
Correlation between anti-spike IgG and neutralizing antibodies after booster vaccination. The correlations between anti-spike IgG and neutralizing antibodies against the wild type by LVMNA (Spearman's correlation coefficient, 0.79, 95% CI 0.69–0.86; P < 0.001) (n = 120), neutralizing antibodies against the alpha variant by LVMNA (Spearman's correlation coefficient, 0.79, 95% CI 0.69–0.86; P < 0.001) (n = 120), neutralizing antibodies against the delta variant by LVMNA (Spearman's correlation coefficient, 0.72, 95% CI 0.59–0.81; P < 0.001), and neutralizing antibodies against the omicron BA.1 by LVMNA (Spearman's correlation coefficient, 0.85, 95% CI 0.79–0.89; P < 0.001) (n = 120) are demonstrated. The gray shaded areas indicate the 95% CI of the best-fit line. Each dot in the figure represents an individual participant.
4. Discussion
Data on protein vaccine as a booster and head-to-head comparison between different doses of mRNA vaccines remain scarce [23]. Our study addressed the immunogenicity, safety, and reactogenicity of currently recommended booster vaccines during the omicron pandemic. Furthermore, as the prevalence of SARS-CoV-2 infection in community in Taiwan was very low before this study, the result would unlikely be affected by the community-circulating viruses. It was likely the immunogenicity shown in this study truly represents the vaccination effect.
The current study revealed that mRNA vaccines elicited more adverse events than protein vaccine and the reactogenicity was similar to previous reports [13]. Compared to COV-BOOST, [13] using BNT162b2 or mRNA-1273 as a booster caused slightly more local and systemic adverse events in persons primed with 2 doses of ChAdOx1 nCov-19 in this study. MVC-COV1901 booster showed similar adverse event patterns to NVX-CoV2373 booster, except for local swelling and myalgia [13]. MVC-COV1901 booster elicited more frequent local swelling and myalgia than NVX-CoV2373.
The antibody response of BNT162b2 and mRNA-1273 booster was generally consistent with previous reports from the UK and USA [13,14,24]. The serum level of anti-spike IgG and live virus neutralization activity induced by MVC-COV1901 was approximately 31% to 47% of the response by BNT162b2. The observation was also found in COV-BOOST study when the antibody response by NVX-CoV2373 booster was compared with that by BNT162b2 booster [13]. Although all vaccines induced substantial neutralizing antibody titers against alpha and delta variants, significantly lower neutralization against omicron BA.1 variant was observed in all groups of heterologous vaccine boosting, in line with findings of previous studies [25,26]. The largest reduction was observed in MVC-COV1901. While mRNA booster vaccination could regain the neutralization ability against the wild type and major VOCs, post-booster serum by the protein-based vaccine failed to neutralize the omicron BA.1 variant. Evidence regarding deficient neutralization against immune-evasion VOCs caused by protein-based vaccine booster remains limited [27]. Why protein-based vaccines failed to stimulate humoral immunity as efficiently as mRNA vaccines is unknown. A development failure in another mRNA vaccine–CVnCoV (CureVac AG) –had been implicated due to the insufficient vaccine dosage design [28]. Therefore, insufficient vaccine antigen may be one of the reasons for the scarcity in neutralization of omicron BA.1 variant from protein-based vaccine booster.
Accumulated evidence supports that booster vaccination would increase the neutralization breadth against SARS-CoV-2. A previous study showed that in individuals receiving mRNA vaccine one year after natural infection, the vaccination strengthened all components of the B cell response and provoked the serum neutralizing activity against VOCs as well as, or even higher than the wild type strain [29]. Another study also showed similar findings: compared to sera obtained from those two-dose vaccines, sera from individuals after mRNA vaccine booster had a better and more robust correlation of wild-type virus neutralization with delta and omicron, indicating a better cross-neutralization ability [30]. Our study also found that regardless of the vaccine type, the neutralization titer against alpha and delta was comparable to the wild type. The finding re-assured the increased cross-neutralization activity after booster vaccination, and also reinforced the importance of the booster vaccination during the pandemic when new variants continue to emerge.
In this study, neutralizing antibody against omicron BA.1 variant could not be detected in 60% of participants who received MVC-COV1901 as a booster by LVMNA. However, all participants boosted with MVC-COV1901 had detectable neutralizing antibody against omicron by PNA. Although there was a correlation between LVMNA and PNA, PNA itself could over-express or under-express binding receptors to influence neutralization. On the other hand, the shift of cell entry in the omicron variant may explain this discordance. Omicron variant has been proven to shift the main cell entry route from the TMPRSS2 dependent pathway to the endocytic pathway, which is significantly different from other VOCs [31], [32], [33] Therefore, the neutralization titer obtained from LVMNA may better reflect omicron's overall immune evasion performance, including antibody relevant and antibody independent immune evasion.
Although humoral immunity after a booster varied, T cell response against different VOCs remained relatively consistent. A study assessing the cross-reactivity of T cell response to the omicron variant showed that T cell reactivity to omicron is preserved in most prior infected and vaccinated individuals [34]. Apart from the primary series, the T cell response after a booster mainly arises from memory T cell proliferation. Our study suggested that all the vaccines used in this study could generate efficient and equal T cell response in HCW primed by the ChAdOx1 nCov-19 in the heterologous booster design.
The study identified no significant difference in neutralizing antibody response between BNT162b2 and half-dose mRNA-1273 booster. The effectiveness of the two mRNA vaccines against symptomatic disease by omicron variant in England was also similar [35]. The study revealed that antibody response of half-dose mRNA-1273 was in between BNT162b2 and mRNA-1273. With less local and systemic adverse events and comparable immunogenicity, the study supports the recommendation to use a half-dose rather than full-dose mRNA-1273 as a booster. Our study showed that mRNA vaccines were better than a protein vaccine (MVC-COV1901) in neutralizing antibody response to VOCs.
There are limitations of the trial. First, this is not a “mix-and-match” trial. We enrolled only HCWs primed with 2 doses of ChAdOx1 nCov-19 vaccines such that the design cannot evaluate the priming effect of different COVID-19 vaccine. Second, our participants are HCWs, and they are generally young without comorbidities. The immunogenicity in old age, the most vulnerable group, was not evaluated in this study. No direct conclusions can be drawn on the extent of responses in the elderly. However, previous studies showed similar booster effect on humoral and cellular responses between younger and older adults [13]. Third, our data does not include more detailed T-cell immune response evaluations, particularly responses to more recent omicron variants. One prior study revealed T-cell cross-recognition of omicron variant even when induced by the vaccine based on the progenitor strain [36]. The BA.4/BA.5 omicron variants contain more altered sequences and are more evasive of antibody induced by non-omicron strain, so more data are need for these newest omicron VOCs.
The study indicated that LVMNA is a better method to evaluate neutralization activity of immune sera against immune evasion omicron variant. Third dose booster not only increases neutralizing antibody titer but also enhance antibody breadth against SARS-CoV-2 variants. Heterologous booster vaccination with mRNA vaccines is recommended for those who have received 2 doses of ChAdOx1 nCov-19.
Funding
This work was supported by Medigen Vaccine Biologics Corporation (award number: XMRPG3L0101) and Chang Gung Memorial Hospital (award numbers: CORPG3K0311, CORPG3L0371, CORPG3L0481, and CORPG3K0242), Taiwan. The study was also supported in part by the Research Center for Epidemic Prevention Science, Chang Gung University (award number: MOST 109–2327-B-182–002), Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Trial registration
ClinicalTrials.gov NCT05132855.
CRediT authorship contribution statement
Chih-Hsien Chuang: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft. Chung-Guei Huang: Data curation, Formal analysis, Methodology, Resources, Validation, Writing – original draft. Ching-Tai Huang: Data curation, Formal analysis, Methodology, Resources, Validation, Writing – original draft. Yi-Ching Chen: Data curation, Formal analysis, Visualization, Writing – original draft. Yu-An Kung: Data curation, Formal analysis, Investigation, Writing – original draft. Chih-Jung Chen: Data curation, Formal analysis, Investigation. Tzu-Chun Chuang: Data curation, Formal analysis, Investigation, Project administration, Visualization. Ching-Chi Liu: Data curation, Formal analysis, Investigation, Project administration, Visualization. Po-Wei Huang: Data curation, Formal analysis, Investigation. Shu-Li Yang: Data curation, Formal analysis, Investigation. Po-Wen Gu: Data curation, Investigation. Shin-Ru Shih: Data curation, Funding acquisition, Methodology, Resources, Validation, Writing – review & editing. Cheng-Hsun Chiu: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We would like to acknowledge the assistance from Clinical Trial Center of Chang Gung Memorial Hospital. Moreover, we would like to acknowledge the volunteers for their willingness to participate in this trial. Additionally, we would like to acknowledge Taiwan Centers for Disease Control and Taiwan Food and Drug Administration, Ministry of Health and Welfare for their scientific input and approval needed to implement this trial. All vaccines were acquired from Taiwan CDC.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105328.
Appendix. Supplementary materials
References
- 1.Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., Feng W., et al. Booster of mRNA-1273 strengthens SARS-CoV-2 Omicron neutralization. medRxiv. 2021 doi: 10.1101/2021.12.15.21267805. 2021.12.15.21267805. [DOI] [Google Scholar]
- 2.Falsey A.R., Frenck R.W., Jr, Walsh E.E., Kitchin N., Absalon J., Gurtman A., et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 2021;385(17):1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385(7):585–594. doi: 10.1056/nejmoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rössler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N. Engl. J. Med. 2022;386(7):698–700. doi: 10.1056/nejmc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejnirattisai W., Zhou D., Supasa P., Liu C., Mentzer A.J., Ginn H.M., et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184(11):2939–2954. doi: 10.1016/j.cell.2021.03.055. .e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L., Mok B.W., Chen L.L., Chan J.M., Tsang O.T., Lam B.H., et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2021:ciab1041. doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heterologous Primary and Booster COVID-19 Vaccination—Evidence Based Regulatory Considerations (13 December 2021) European Medicines Agency; 2021. Biological health threats and vaccine strategy office European medicines agency pandemic task force for COVID-19 (COVID-ETF) pp. 1–26.https://www.ema.europa.eu/en/documents/report/heterologous-primary-booster-covid-19-vaccination-evidence-based-regulatory-considerations_en.pdf EMA/349565/2021Available at. (accessed 01 August 2022) [Google Scholar]
- 9.Coronavirus (COVID-19) vaccination information for public health professionals . UKHSA gateway number 2020300; 2022. Guidance. COVID-19: the Green book, Chapter 14a.https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a?UNLID=37746918202111120157 (accessed 01 August) [Google Scholar]
- 10.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/nejmoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaxman A., Marchevsky N.G., Jenkin D., Aboagye J., Aley P.K., Angus B., et al. Oxford COVID vaccine trial group. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398(10304):981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 Vaccine effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/s0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous Covid-19 booster vaccinations. N. Engl. J. Med. 2022;386(11):1046–1057. doi: 10.1056/nejmoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Food and Drug Administration . Guidance for industry; 2022. Toxicity Grading Scale For Health Adults and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials.https://www.fda.gov/media/73679/download September 2007Available at. (accessed 01 August. [Google Scholar]
- 16.Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A., et al. Performance characteristics of the abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020;58(8) doi: 10.1128/jcm.00941-20. e00941‒20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riester E., Findeisen P., Hegel J.K., Kabesch M., Ambrosch A., Rank C.M., et al. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J. Virol. Methods. 2021;297 doi: 10.1016/j.jviromet.2021.114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C.Y., Liu K.T., Shih S.R., Ye J.J., Chen Y.T., Pan H.C., et al. Neutralization assessments reveal high cardiothoracic ratio and old age as independent predictors of low neutralizing antibody titers in hemodialysis patients receiving a single dose of COVID-19 Vaccine. J. Pers. Med. 2022;12(1):68. doi: 10.3390/jpm12010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferré V.M., Lebourgeois S., Chenane H.R., Menidjel R., Masson C., Collin G., et al. Vaccine Ab neutralization against Omicron and SARS-CoV-2 variants using neutralization and specific ELISA assays. J. Infect. 2022;84(6):863–865. doi: 10.1016/j.jinf.2022.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 21.Huang C.G., Dutta A., Huang C.T., Chang P.Y., Hsiao M.J., Hsieh Y.C., et al. Relative COVID-19 viral persistence and antibody kinetics. Pathogens. 2021;10(6):752. doi: 10.3390/pathogens10060752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K.T., Gong Y.N., Huang C.G., Huang P.N., Yu K.Y., Lee H.C., et al. Quantifying neutralizing antibodies in patients with COVID-19 by a two-variable generalized additive model. mSphere. 2022;7(1) doi: 10.1128/msphere.00883-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australian Technical Advisory Group on Immunisation (ATAGI) recommendations on the use of a booster dose of COVID-19 vaccine. March 1, 2022. Available at: https://www.health.gov.au/sites/default/files/documents/2022/03/atagi-recommendations-on-the-use-of-a-booster-dose-of-covid-19-vaccine.pdf (accessed 01 August 2022).
- 24.Sablerolles R.S.G., Rietdijk W.J.R., Goorhuis A., Postma D.F., Visser L.G., Geers D., et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N. Engl. J. Med. 2022;386(10):951–963. doi: 10.1056/nejmoa2116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N. Engl. J. Med. 2022;386(11):1088–1091. doi: 10.1056/nejmc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemet I., Kliker L., Lustig Y., Zuckerman N., Erster O., Cohen C., et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N. Engl. J. Med. 2022;386(5):492–494. doi: 10.1056/nejmc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ai J., Zhang H., Zhang Y., Lin K., Zhang Y., Wu J., et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022;11(1) doi: 10.1080/22221751.2021.2022440. 337‒3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolgin E. CureVac COVID vaccine let-down spotlights mRNA design challenges. Nature. 2021;594(7864):483. doi: 10.1038/d41586-021-01661-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457–466. doi: 10.1016/j.cell.2021.12.033. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pia L., Rowland-Jones S. Omicron entry route. Nat. Rev. Immunol. 2022;22(3):144. doi: 10.1038/s41577-022-00681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui K.P.Y., Ho J.C.W., Cheung M.C., Ng K.C., Ching R.H.H., Lai K.L., et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 33.Meng B., Abdullahi A., Ferreira I.A.T.M., Goonawardane N., Saito A., Kimura I., et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706–714. doi: 10.1038/s41586-022-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naranbhai V., Nathan A., Kaseke C., Berrios C., Khatri A., Choi S., et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell. 2022;185(6):1041–1051. doi: 10.1101/2022.01.04.21268586. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022;386(16):1532–1546. doi: 10.1056/nejmoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185(5):847–859. doi: 10.1016/j.cell.2022.01.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.