Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes coronavirus disease 2019 (COVID-19) and can be associated with serious complications, including acute respiratory distress syndrome. This condition is accompanied by a massive release of cytokines, also denominated cytokine storm, development of systemic oxidative stress and a prothrombotic state. In this context, it has been proposed a role for acetylcysteine (NAC) in the management of patients with COVID-19. NAC is a molecule classically known for its mucolytic effect, but it also has direct and indirect antioxidant activity as a precursor of reduced glutathione. Other effects of NAC have also been described, such as modulating the immune and inflammatory response, counteracting the thrombotic state, and having an antiviral effect. The pharmacological activities of NAC and its effects on the mechanisms of disease progression make it a potential therapeutic agent for COVID-19. NAC is safe, tolerable, affordable, and easily available. Moreover, the antioxidant effects of the molecule may even prevent infection and play an important role as a complement to vaccination. Although the clinical efficacy and dosing regimens of NAC have been evaluated in the clinical setting with small series of patients, the results are promising. In this article, we review the pathogenesis of SARS-CoV-2 infection and the current knowledge of the mechanisms of action of NAC across disease stages. We also propose NAC posology strategies to manage COVID-19 patients in different clinical scenarios.
Keywords: COVID-19, N-acetylcysteine, SARS-CoV-2, Antioxidant, Immunomodulation, Therapeutic role
Introduction
The current coronavirus disease 2019 (COVID-19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a lipid-enveloped, non-segmented, single-stranded RNA virus, widely distributed in humans and other mammals [1]. SARS-CoV-2 spreads primarily by inhalation of virus-laden aerosols and mainly infects the respiratory tract, although may cause damage to other systems. The clinical spectrum of COVID-19 is diverse: from asymptomatic or pauci-symptomatic to moderate or severe disease, with or without pneumonia. The most severe cases develop respiratory failure, septic shock, multi-organ failure and death [2]. Most of the complications experimented by patients with COVID-19 are due to the development of acute respiratory distress syndrome (ARDS). This condition is accompanied by an excessive release of pro-inflammatory mediators, referred to as “cytokine storm”, development of systemic oxidative stress and a prothrombotic state [3]. Thus, mitigating the exacerbated inflammatory response and the pro-oxidative mechanisms could hamper the progression of COVID-19 to severe/critical illness.
N-acetylcysteine (NAC) is a classic drug known for its mucolytic effect, but it also has a potent antioxidant activity. It stimulates glutathione biosynthesis, promotes detoxification, and acts directly as a scavenger of free radicals. Other actions have also been described, such as restoring the response of immune cells, modulating the inflammatory response, counteracting the thrombotic state, and exerting an antiviral effect [4]. Moreover, NAC has been associated with benefits on respiratory outcomes [5], [6] and other end-organ complications [7]. The pharmacological activities of NAC and its potential effects on disease progression make it a rational therapeutic option for COVID-19, as current treatments are limited and sometimes not effective enough. Accordingly, preliminary data indicates that NAC improves clinical outcomes in COVID-19 patients [8], [9], and may be considered as part of their multimodal management. In this paper, we review the pathogenic features of SARS-CoV-2 and the current knowledge of the relevant mechanisms of action of NAC across the infection phases. We also propose potential NAC posology strategies to manage COVID-19 patients in different clinical scenarios.
Pathophysiology of SARS-CoV-2 infection
After entering the respiratory tract, SARS-CoV-2 attaches to nasal and bronchial epithelial cells and pneumocytes through its spike (S) protein, which binds to the angiotensin-converting enzyme (ACE2) receptor present on the surface of the cell membranes. Subsequently, transmembrane serine protease type 2 (TMPRSS2), expressed in host cells, facilitates the virus entry by proteolytic cleaving [10]. ACE2 is also significantly expressed in other tissues like vascular endothelium, heart, kidney, intestine, and nervous system [11]. Viral replication and cell to cell transmission suppress ACE2 expression, which has an essential role in the renin angiotensin aldosterone system (RAAS), acting as a key counter-regulatory component. ACE2 breaks down angiotensin II to produce angiotensin (1−7), and to a lesser extent converts angiotensin I to angiotensin (1−9). These peptides have potent vasodilator, antioxidant, and anti-inflammatory properties, which attenuate the deleterious effects of angiotensin II. Thus, the suppression of ACE2 leads to a decrease in Ang (1−7) synthesis and elevated levels of angiotensin II. This triggers the activation of nicotinamide adenine dinucleotide phosphate oxidases (NOX), an important determinant of reactive oxygen species (ROS) generation and eventually endothelial damage [12]. In this sense, it has been shown that NOX2-derived oxidative stress is associated with severe clinical outcome and thrombotic events in COVID-19 patients [13].
In parallel, the immune system is activated in response to the viral infection. The first events are mediated by the innate immune system, in which dendritic cells, NK cells and macrophages participate. SARS-CoV-2 is detected by the Toll-like receptor (TLR)− 3, TLR7 and TLR8, triggering a cascade of intracellular signals that ends up activating factors such as NF-kB and interferon (INF), which alter cellular defense mechanisms. These factors promote the recruitment of effector cells and the release of cytokines such as IL-6, TNF-α, IL-1β, and the activation of caspases. By their part, cytokines act locally and systemically, generating hemodynamic and metabolic changes that promote antimicrobial activity. These pro-inflammatory cytokines, acting on target cells, increase the activation of the transcription factor NK-kB, and generate a positive feedback that, if not controlled, will eventually produce a “cytokine storm” [14].
Recruitment of neutrophils and NK cells to the lung parenchyma is mediated by the chemokines, including CCL2, CCL5, CXCL8, and CXCL10. Neutrophils manifest by an increase in ROS generation and protease degranulation, leading to oxidative cell injury and potentiation of the hyperinflammation state. Neutrophil extracellular traps (NETs) might also contribute to cytokine release. This robust inflammatory reaction plays a key role in the ARDS pathogenesis [15], whereas NETs can trigger microvascular thrombosis, leading to damage in the lungs, heart, and kidneys.
Thus, the direct viral invasion, the oxidative stress, and the cytokine storm can provoke endothelial injury and the release of coagulation factors, leading to a pro-thrombotic state. Coagulation and platelet activation enhances the release of pro-inflammatory cytokines, generating a vicious cycle [16]. Disseminated intravascular coagulation and thromboembolic events can affect tissues sensitive to ischemic processes, such as cardiopulmonary and cerebrovascular tissues, which further worsen the prognosis. The infectious process evolves until the virus, as well as the infected cells, are eradicated or until the immune system cannot respond adequately, threatening patient`s life. Any therapeutic strategy that could attenuate hyperinflammation, oxidative stress, and the pro-thrombotic state could be helpful in preventing or slowing the progression to severe COVID and the development of complications.
Mechanisms of action of NAC relevant to SARS-CoV-2 infection pathophysiology
NAC has been used classically to improve the expectoration in patients with chronic bronchitis, bronchiectasis, or cystic fibrosis. The clinical applications of NAC cover diverse pathological conditions involving oxidative stress, ARDS, and certain cardiovascular diseases. Moreover, various mechanisms associate it with other benefits such as boosting the immune system, suppressing viral replication, and reducing inflammation [17]. For example, De Flora et al. demonstrated that the treatment with NAC tablets (600 mg) twice daily for six months vs. placebo had a robust and significant protective effect on local symptoms such a coryza, rhinorrhea, sore throat, catarrh and cough, and general symptoms, especially headache and myalgia arthralgia in patients with influenza and influenza-like episodes [18]. Only 25 % of virus-infected subjects in the NAC group developed flu symptoms, contrasting with 79 % of the subjects in the placebo group. The attenuation of influenza-like symptoms in subjects assigned to NAC treatment was accompanied by the modulation of cell production and release of cytokines. These mechanisms are particularly important in elderly subjects and patients affected by chronic pathological conditions well-known to make them vulnerable to viral respiratory diseases due to a general impairment of their defenses, including a loss of antioxidants and a reduction of immunocompetent system functionality [18].
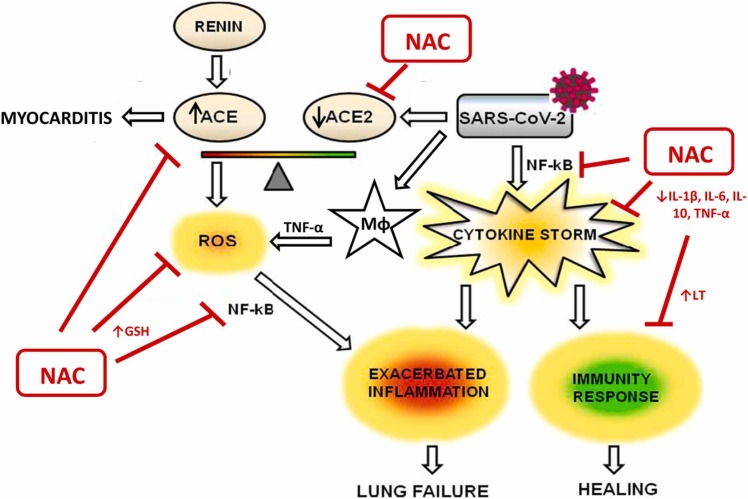
Thus, it is postulated that NAC, exerting its antioxidant and anti-inflammatory effects, could have a role in the prophylaxis and treatment of COVID-19 [19], [20]. Accordingly, many clinical trials have evaluated the efficacy of NAC administration, and many are still ongoing (714 studies, 349 completed, see https://www.clinicaltrials.gov). NAC could play a role in the treatment and prevention of COVID-19 through different mechanisms of action ( Fig. 1):
Fig. 1.
Schematic representation of potential mechanisms of NAC as antioxidant and anti-inflammatory agent in SARS-CoV2 infection.
Adapted from [27].
Inhibiting ACE2 and decreasing the affinity of SARS-CoV-2 for its receptor, which is more abundant in the lungs. SARS-CoV-2 infects endothelial cells by binding to the ACE2 receptor to gain access to the interior of the cell. ACE2 receptors are found on the cell surface and compete for the same substrates, angiotensin II. The balance between angiotensin II and ACE2 is specific to each person, but if ACE2 is prevalent, there may be more inflammation. SARS-CoV-2 infection down-regulates the abundance of ACE2 on cell surfaces and results in an excessive and toxic accumulation of angiotensin II, which can cause respiratory failure and myocarditis [21], [22]. Thiols from NAC block the ACE2 thereby hampering penetration of SARS-CoV-2 into cells [23].
Maintenance of the redox equilibrium: Reduced glutathione (GSH), a ubiquitous tripeptide thiol, is a vital intracellular and extracellular protective which plays a number of key and/or crucial roles in the control of cellular redox signaling processes. GSH is considered to be one of the most important scavengers of reactive oxygen species (ROS), and its ratio with oxidized glutathione (GSSG) may be used as a marker of oxidative capacity. Intracellular levels of GSH and GSSG, as well as the GSH/GSSG ratio, are crucial parameters to maintain redox homeostasis and thus indirectly involved in redox signaling [24]. Excessive oxidative stress could be responsible for the alveolar damage, thrombosis, and red blood cell dysregulation observed in COVID-19 [25], [26]. Oral NAC increases GSH levels by providing the liver with an increased supply of cysteine and promoting GSH synthesis, and therefore reduces oxidative stress. This happens especially in older people, since the levels of cysteine in plasma decrease with age. GSH mediates the regulation of redox-dependent pro-inflammatory cytokines, such as IL-9 and TNF-α and also has vasodilator properties by increasing cyclic GMP levels and by contributing to the regeneration of endothelial-derived relaxing factor (EDRF), reducing the inflammatory state [27]. Treatment with NAC was able to attenuate the diminished ratio GSH/GSSG. The protective effect of NAC administration on a murine asthma model was demonstrated in two studies. Treatment with NAC was able to attenuate the diminished ratio GSH/GSSG in animals [28] and the repletion of the glutathione pool by NAC counteracted allergen induced airway reactivity/inflammation and restored oxidant-antioxidant balance [27]. Indeed, a decrease in GSH levels contributes to oxidative stress associated with aging and many pathological conditions, including neurodegeneration, inflammation and infections. Interestingly and as an example, a decline in the marker enzyme activity in human skeletal muscle biopsies and a slower recovery rate from exercise does indicate a significantly reduced capacity for oxidative phosphorylation which happens with aged muscle [29], [30]. NAC as a medicine for the management of COVID-19 infections may be a regulator of redox potential signaling where the primary attribute of redox signaling is its strict dependence on the kinetics and thermodynamics of electron transfer that up-regulates cellular functional metabolism [31], [32].
Increasing the proliferation of lymphocytes and the longevity of CD8 + cells and decreasing the production of various cytokines. The role that NAC may have in restoring normal levels of the inflammatory response is the result of a close interrelation between oxidative stress and inflammation, in whose response several mediators contribute, such as IL-1β, IL-6, IL-10, TNF-α, and NF-κB, which mediate the redox-dependent regulation of inflammatory cytokines [4].
Antiviral effect by activating TLR7 response to IFN-1: NAC could contribute to the prevention or control of infections caused by RNA viruses, since the drug amplifies the signaling functions of TLR7 and the mitochondrial antiviral signaling protein (MAVS) in the production of IFN-1. IFNs are able to inhibit the replication of the SARS-type coronavirus, so they can be useful in the treatment of COVID-19. Additionally, activation of TLR7 by single-stranded viral RNA, entrapped within endosomes, provides a key stimulus for the induction of IFN-1 by the RNA virus [33]. Moreover, some evidence indicates that hydrogen sulfide (H2S) acts as an antiviral host factor in patients with COVID-19; as the endogenous H2S production can be therapeutically augmented by administration of NAC, this strategy has the potential to provide clinical benefit [34].
Thus, endogenous failure of the main intracellular antioxidant, GSH, and increased GSH reductase may be the basis for severe forms and death from COVID-19. In addition, the redox imbalance in the cells of the alveolar epithelium, their apoptosis, the increase in inflammation and, consequently, the alteration of gas exchange, also cause a local increase in angiotensin II levels after the inactivation of the ACE2 receptor by the SARS-CoV-2. In the early phase of SARS-CoV-2 infection, NAC can restore normal levels of the inflammatory response in two ways. One focuses on restoring normal immune cell responses through inhibition of T-cell apoptosis, which can potentially reduce the incidence or severity of pneumonia associated with viral infection. The other is based on the fact that, given the low levels of GSH that have been seen in severe cases of the disease, it would be remarkable to seek a way to restore GSH levels in order to protect the most vulnerable people [35].
The development of ARDS, recognized by the international medical community as the most common symptom in individuals with advanced-stage COVID-19, depends on the immune response of each subject. In high-responders, three fundamental processes are triggered in the body: mucus overproduction, exaggerated inflammatory response -cytokine storm-, and coagulopathies and endotheliopathies. The mucus plug, due to the infection, blocks the airways and prevents the passage of air, as it completely changes its consistency to a thicker and more viscous one that ends up adhering completely to the tissues. Additionally, hypoxemia secondary to ARDS and pulmonary edema may occur in the advanced stages of COVID-19, and NAC shows promise as an agent to modify the immune response and possibly reduce morbidity and mortality [36], as shown in similar conditions.
For example, Sharafkhah et al. demonstrated that about 37 % of mechanically ventilated patients develop pneumonia, namely, ventilator-associated-pneumonia (VAP); patients treated with NAC (600 mg in the feeding tube) twice daily developed significantly less clinically confirmed pneumonia compared with patients that received placebo (26.6 % vs. 46.6 %) There was also higher rates of recovery and reduced time spent in ICU in patients on mechanical ventilation when using NAC versus placebo [5].
In patients with mild-to-moderate acute lung injury due to a variety of underlying diseases, intravenous NAC treatment (40 mg/kg/day) for three days significantly improved systemic oxygenation, reduced the need for ventilatory support, and also slightly reduced the mortality rate [37]. In patients with early septic shock, NAC was able to attenuate production of IL-8, improve oxygenation and lung compliance and shorten the stay in the ICU [38], and even might reduce the 30-day mortality rate in ICU patients with carbapenem-resistant septic shock [39].
Clinical data on the use of NAC in COVID-19
When the COVID-19 pandemic began, it was already established that ROS play a central role in the inflammatory response and viral replication, and that antioxidants, which exert antiviral and anti-inflammatory effects, may also be effective for the treatment of cytokine storm. As stated above, the most serious complication of COVID-19 is ARDS, associated with high mortality rates despite current therapeutic efforts. Before the SARS-Cov-2 pandemic, the usefulness of NAC in ARDS was controversial. A systematic review and meta-analysis of controlled clinical trials evaluating the efficacy of NAC in patients with ARDS, included eight trials with a total of 289 patients [40]. Compared with the control group, NAC treatment did not reduce overall mortality (risk ratio: 0.83, 95 % confidence interval: 0.62–1.11; P = 0.21; I2 = 0 %), but it had a beneficial effect on the length of stay in the ICU. However, data on duration of mechanical ventilation, glutathione levels, and PaO2/FiO2 could not be pooled in the meta-analysis. Just before the declaration of COVID-19 as a pandemic, a Cochrane review of 48 controlled clinical trials, with 6299 patients treated with different drugs, including NAC, concluded that there was not enough evidence to establish with certainty whether corticosteroids, surfactants, NAC, statins and other drugs were effective in reducing mortality or the need/duration of mechanical ventilation in patients with ADRS [41].
Given that there was a rationale for the use of NAC, especially due to the lack of an effective treatment at the beginning of the pandemic, and its excellent safety profile, it was proposed to administrate NAC to the group of patients with higher risk of severe COVID-19 [17]. The first supporting evidence was mainly limited to descriptions of clinical cases. Subsequently, in a cohort study, Ibrahim et al. [8] demonstrated that intravenous NAC elicited clinical improvement in 10 patients from 38 to 71 years of age with severe COVID-19 on mechanical ventilation. This series included a patient with glucose-6 phosphate dehydrogenase deficiency. Intravenous NAC significantly reduced various inflammatory markers such as CRP and ferritin. Ultimately, eight patients were discharged, and the remaining two patients had clinical improvement at the time of publication.
At the same time, a single-center, double-blind, randomized, placebo-controlled trial conducted in Brazil reported negative results. However, this study evaluated the effect of NAC given as a single dose of 21 g (approximately 300 mg/Kg) for 20 h. The authors did not observe differences between the two groups in the primary end-point of needing invasive mechanical ventilation, or in secondary end-points such as mortality, ICU admission, or time on invasive mechanical ventilation [42]. Another study, which included 92 patients with COVID-19 and ARDS (47 assigned to NAC and 45 assigned to placebo), also found no difference in mortality at 28 days in the group treated with intravenous NAC at a dose of 40 mg/kg/day during 3 consecutive days [43].
Compared to these studies, with a very short administration period, there are positive results with longer dosages. In a randomized study that recruited 46 patients with COVID-19-associated pneumonia, Gaynitdinova et al. [44] analyzed the effect of adding NAC (1200–1500 mg intravenously) to the standard treatment. The group treated with NAC showed a significant increase in blood oxygen saturation and a decrease in CRP values and hospital stay. These findings were accompanied by a reduction in lung lesions assessed by computed tomography. The efficacy of NAC at a dose of 600 mg every 12 h orally for 14 days was retrospectively evaluated for the prevention of ARDS (PO2/FiO2 <150), the need of mechanical ventilation and mortality at 14 and 28 days [45]. In the group treated with NAC (42 patients out of 82), compared to the group without NAC, a statistically significant reduction was observed in the development of ARDS, the need for mechanical ventilation, and mortality. This improvement was accompanied by better oxygenation and a reduction in inflammatory markers, such as the number of leukocytes, CRP, or D-dimer values, assuming an anti-inflammatory effect as the cause of this clinical benefit [45]. Recently, Frades et al. [46] examined 274 patients admitted to an Intermediate Respiratory Care Unit with severe respiratory failure due to pneumonia caused by SARS-CoV-2. Their results were consistent with previous data, so that corticosteroids and anticoagulants were associated with better results. Although not statistically significant in the adjusted models, the authors found a benefit of NAC on survival, which they postulate could be due to the improvement in secretion density and its antioxidant effect. Another retrospective study did not show an impact of a five-day regimen of NAC on in-hospital mortality or ICU admission, but NAC shortened the length of hospital stay in patients with SARS-CoV-2 pneumonia [47]. Similarly, the case-control study conducted by Avdeev et al. [9] showed that an intravenous dose of 1200–1800 mg/day of NAC led to significant improvement in oxygenation parameters and reduction in CRP, clinical deterioration, and length of hospitalization in patients with COVID-19. Both in this study and in the previous ones ( Table 1), the level of evidence did not allow to establish categorical recommendations, which can only be made with data from controlled clinical trials.
Table 1.
Clinical studies evaluating NAC in patients with COVID-19.
| Study | Sample | Age | Clinical status | NAC dosage | Main effect |
|---|---|---|---|---|---|
| Ibrahim et al., 2020[8] | N = 9 | 38–71 years | Respirator-dependent | 1200 mg/day, IV, 2–9 days | Reduction in inflammatory markers (CRP and ferritin) |
| De Alencar et al., 2021 | N = 68 NAC; N = 67 placebo |
Median: 48 years | Severe disease | 21 g (∼300 mg/kg), IV, for 20 h | No significant differences in primary or secondary end-points. |
| Taher et al., 2021 | N = 47 NAC; N = 45 placebo |
57.6 ± 18.7 years | Mild-to-moderate COVID19-associated ARDS | 40 mg/kg/day, IV, for three consecutive days | No clinical benefit. |
| Gaynitdinova et al., 2021[44] | N = 24 NAC; N = 22, standard treatment |
57.90 ± 12.7 years | Moderate COVID-associated pneumonia | 1200–1500 mg/day, IV, during hospitalization | Improvement in oxygenation and inflammation parameters and reduction length of hospitalization. |
| Assimakopoulos et al., 2021[45] | N = 42 NAC; N = 40, standard treatment |
Mean, 61 – 64 years | Moderate or severe COVID-19 pneumonia | 1200 mg/day, orally for 14 days | Reduced risk for mechanical ventilation and mortality. |
| Faveiro et al., 2022[47] | N = 865 NAC; N = 321, no NAC |
Median, 63 years | Moderate or severe COVID-19 pneumonia | 900–1200 mg/day at least for 5 days | Shorter length of hospitalization. |
| Avdeev et al., 2022[9] | N = 24 NAC; N = 22, standard treatment |
Mean, 57 – 66 years | Moderate or severe COVID-19 pneumonia | 1200–1800 mg/day, IV, during hospitalization | Improvement in oxygenation and inflammation parameters and reduction length of hospitalization. |
ARDS, acute respiratory distress syndrome; CRP, C reactive protein; IV, intravenous; NAC, N-acetylcysteine.
To date, there have been few clinical trials evaluating NAC as a treatment in COVID-19 patients. In the first published study, Chavarría et al. [48] divided their study population (110 patients) into 5 groups with different treatment protocols. Treatment with antioxidant supplements such as vitamins C and E, NAC, melatonin and pentoxifylline improved survival scores and biological markers of inflammation and oxidative stress such as lipid peroxidation, IL-6, CRP, total systemic antioxidant capacity, and nitrites [48]. Using big-data and artificial intelligence tools, the usefulness of NAC, added to standard treatment, has recently been analyzed in a population of 19,208 hospitalized patients with COVID-19, of whom 2071 (10.8 %) received NAC orally at a dose of 600 mg every 8 h. In this study, treatment with NAC, systemic corticosteroids, and enoxaparin was associated with lower mortality [49]. Specifically for NAC, the reduction in mortality (odds ratio 0.56, 95 % confidence interval 0.47–0.67) remained significant in a multivariate analysis adjusted for baseline characteristics and concomitant use of corticosteroids. Thus, due to its favorable safety profile, and pending the results of currently ongoing controlled clinical trials, these data support the use of NAC in patients hospitalized with COVID-19 for severe pneumonia [49].
A small group of patients who experiment COVID-associated pneumonia or ARDS develop fibrotic lung lesions, the evolution and treatment of which are not yet well established. These lesions range from fibrotic changes in autopsy findings to severe forms of fibrosing organizing pneumonia. The use of antifibrotics, mainly pirfenidone and nintedanib, has recently been proposed, despite limited supporting evidence [50]. With the same approach, some authors have suggested the use of NAC for fibrotic consequences following SARS-CoV-2 infection. Although previous studies in idiopathic pulmonary fibrosis (IPF) showed negative results with NAC, this effect could vary substantially depending on the patient's genetic background. Accordingly, it has been showed that the polymorphism rs3750920 within the TOLLIP gene could influence the response to NAC in patients with IPF: although NAC did not provide a benefit in the whole trial IPF population, there was a positive effect in genetically predisposed individuals, specifically those with a TT rs3750920 genotype. This study demonstrated the importance of genetic factors as an element to examine in future studies that evaluate the usefulness of NAC in patients with lung fibrosing diseases [51].
NAC dosing according to the stage of SARS-CoV-2 infection
NAC was introduced as a mucolytic in the 1960 s, in patients with chronic respiratory diseases, at a dose of 600 mg per day. In addition to this effect, and being the antidote to paracetamol intoxication, NAC also has an antioxidant effect specially when used at higher doses, and its clinical benefit has been demonstrated in the prevention of COPD exacerbations, which frequently have a viral origin, especially respiratory viruses such as influenza type A and B, respiratory syncytial virus, and rhinovirus [52]. This beneficial effect has also been observed in other infectious processes such as community-acquired pneumonia [53]. Based on the long clinical experience and a solid pathophysiological basis, the use of NAC has been proposed in various pathological processes associated with oxidative stress, including COVID-19 [23], [35].
The mucolytic, antioxidant and anti-inflammatory effects of NAC produce a more effective response when administered parenterally to critically ill patients. NAC acting as a mucolytic dissolves the density of excess mucus, breaks the mucous plug lodged in the respiratory tract and therefore enables its expectoration and facilitates the passage of air. Thanks to its antioxidant effect, it acts directly TNF-α, IL-1, IL-6 and IL-8, decreasing the pro-inflammatory cytokines generated during the infection and, finally, reducing platelet aggregation and arterial thrombi thanks to the antithrombotic effect of the drug [32], [54]. Oral NAC administration (1200 mg/d) in patients with COVID-19 pneumonia reduces the risk for mechanical ventilation and mortality [45]. NAC is inexpensive, has very low toxicity, has been used in routine clinical practice for many years. NAC administered intravenously, orally, or inhaled, may suppress SARS-CoV-2 replication and improve outcomes if used timely. Table 2 shows our proposal for NAC dosing and treatment duration in the different COVID-19 clinical scenarios. NAC administration in combination with other antiviral agents may dramatically reduce the rates of hospital admission, mechanical ventilation, and mortality [17]. Moreover, it has been proposed that since copper exhibits strong virucidal effects, it could be combined with NAC at the early stages of the infection to decrease viral RNA levels [55].
Table 2.
Recommended NAC dosing as part of the therapeutic strategy for COVID-19.
| Clinical scenario | Dose (route of administration) | Reference |
|---|---|---|
| Infection prophylaxis Outpatient treatment Mild disease |
600 mg BID (oral) | De Flora et al., 2020[23] Shi & Puyo, 2020[17] |
| Inpatient treatment Mild to moderate disease |
600 mg TID (oral) | Izquierdo et al., 2022[49] |
| Inpatient treatment IRCU Moderate to severe disease |
300 mg TID (IV) | Frades et al., 2020[46] |
| Inpatient treatment ICU Severe disease |
300 mg 5–6 ID (IV) | Gaynitdinova et al., 2021 Shi[44] |
BID, twice a day; ICU, intensive care unit; IRCU, intermediate respiratory care unit, IV, intra-venous; TID, three times a day.
Conclusions
The potential mechanisms of NAC's beneficial actions have been investigated in several in vitro and in vivo studies. Treatment with this drug has shown a positive impact on health outcomes in patients with respiratory conditions such as community-acquired pneumonia, COPD, ARDS and, more recently, its potential suppressive action on the progression of COVID-19 makes it a very promising therapy against COVID-19. It is hypothesized that the mechanism of action of NAC may consisted of blocking the viral infection and the consequent cytokine storm. Although the level of evidence is limited and data from controlled clinical trials is needed, the information currently available supports the use of NAC in symptomatic patients with COVID-19 at a minimum dose of 1200 mg per day. In patients with severe disease and respiratory compromise, the use of intravenous NAC at a dose of 100 mg/kg for a minimum of three days may be indicated. This dose can be increased in the first 24 h to 150 mg/kg in situations of ARDS [56].
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
JLIA has received honoraria for consultancy, projects, and talks from AstraZeneca, Bayer, Boehringer Ingelheim, Chiesi, Glaxo, Grifols, Smith Kline, Menarini, Novartis, Orion, Pfizer,Sandoz,Teva and Zambom; GPB has received grants, contracts and honoraria for talks from Zambon, Menarini, Chiesi, GSK, Boehringer Ingelheim, and Novartis. SPR and CGR declare no conflict of interest.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calzetta L., Matera M.G., Rogliani P., Cazzola M. Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2018;12:693–708. doi: 10.1080/17476348.2018.1495562. [DOI] [PubMed] [Google Scholar]
- 5.Sharafkhah M., Abdolrazaghnejad A., Zarinfar N., Mohammadbeigi A., Massoudifar A., Abaszadeh S. Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: a randomized, double blind, placebo-controlled clinical trial. Med Gas Res. 2018;8:19–23. doi: 10.4103/2045-9912.229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moradi M., Mojtahedzadeh M., Mandegari A., Soltan-Sharifi M.S., Najafi A., Khajavi M.R., et al. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med. 2009;103:434–441. doi: 10.1016/j.rmed.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Khan S.A., Campbell A.M., Lu Y., An L., Alpert J.S., Chen Q.M. N-acetylcysteine for cardiac protection during coronary artery reperfusion: a systematic review and meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.752939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim H., Perl A., Smith D., Lewis T., Kon Z., Goldenberg R., et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219 doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avdeev S.N., Gaynitdinova V.V., Merzhoeva Z.M., Berikkhanov Z.G.-M. N-acetylcysteine for the treatment of COVID-19 among hospitalized patients. J Infect. 2022;84:94–118. doi: 10.1016/j.jinf.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 12.Aleksova A., Ferro F., Gagno G., Cappelletto C., Santon D., Rossi M., et al. COVID-19 and renin-angiotensin system inhibition: role of angiotensin converting enzyme 2 (ACE2) – is there any scientific evidence for controversy? J Intern Med. 2020;288:410–421. doi: 10.1111/joim.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Violi F., Oliva A., Cangemi R., Ceccarelli G., Pignatelli P., Carnevale R., et al. Nox2 activation in Covid-19. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdaca G., Paladin F., Tonacci A., Isola S., Allegra A., Gangemi S. The potential role of cytokine storm pathway in the clinical course of viral respiratory pandemic. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norooznezhad A.H., Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19) Micro Res. 2021;137 doi: 10.1016/j.mvr.2021.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Z., Puyo C.A. N-Acetylcysteine to combat COVID-19: an evidence review. Ther Clin Risk Manag. 2020;16:1047–1055. doi: 10.2147/TCRM.S273700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Flora S., Grassi C., Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997;10:1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- 19.Elhidsi M., Fachrucha F., Irawan R.Y. N-acetylcysteine for COVID-19: a potential adjuvant therapy. J Heal Sci. 2021;11:1–6. doi: 10.17532/jhsci.2020.1156. [DOI] [Google Scholar]
- 20.Jorge-Aarón R.-M., Rosa-Ester M.-P. N-acetylcysteine as a potential treatment for COVID-19. Future Microbiol. 2020;15:959–962. doi: 10.2217/fmb-2020-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scialo F., Daniele A., Amato F., Pastore L., Matera M.G., Cazzola M., et al. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198:867–877. doi: 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babajani F., Kakavand A., Mohammadi H., Sharifi A., Zakeri S., Asadi S., et al. COVID-19 and renin angiotensin aldosterone system: pathogenesis and therapy. Heal Sci Rep. 2021;4 doi: 10.1002/hsr2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Flora S., Balansky R., La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020;34:13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee R. Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. J Biol Chem. 2012;287:4397–4402. doi: 10.1074/jbc.R111.287995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39:644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanfar A., Al Qaroot B. Could glutathione depletion be the Trojan horse of COVID-19 mortality? Eur Rev Med Pharm Sci. 2020;24:12500–12509. doi: 10.26355/eurrev_202012_24046. [DOI] [PubMed] [Google Scholar]
- 27.Silvagno F., Vernone A., Pescarmona G.P. The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19. Antioxidants. 2020;9:624. doi: 10.3390/antiox9070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J., Yao L., Shi J., Li J., Xu C. Protective effects of N-acetylcysteine on a chemical-induced murine model of asthma. J Asthma. 2021;58:1208–1215. doi: 10.1080/02770903.2020.1781166. [DOI] [PubMed] [Google Scholar]
- 29.Damiano S., Muscariello E., La Rosa G., Di Maro M., Mondola P., Santillo M. Dual role of reactive oxygen species in muscle function: can antioxidant dietary supplements counteract age-related sarcopenia? Int J Mol Sci. 2019;20 doi: 10.3390/ijms20153815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lian D., Chen M.-M., Wu H., Deng S., Hu X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants. 2022;11 doi: 10.3390/antiox11040755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominari A., Hathaway Iii D., Kapasi A., Paul T., Makkar S.S., Castaneda V., et al. Bottom-up analysis of emergent properties of N-acetylcysteine as an adjuvant therapy for COVID-19. World J Virol. 2021;10:34–52. doi: 10.5501/wjv.v10.i2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong K.K., Lee S.W.H., Kua K.P. N-acetylcysteine as adjuvant therapy for COVID-19 – a perspective on the current state of the evidence. J Inflamm Res. 2021;14:2993–3013. doi: 10.2147/JIR.S306849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarty M.F., DiNicolantonio J.J. Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis. 2020;63:383–385. doi: 10.1016/j.pcad.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourgonje A.R., Offringa A.K., van Eijk L.E., Abdulle A.E., Hillebrands J.-L., van der Voort P.H.J., et al. N-acetylcysteine and hydrogen sulfide in coronavirus disease 2019. Antioxid Redox Signal. 2021;35:1207–1225. doi: 10.1089/ars.2020.8247. [DOI] [PubMed] [Google Scholar]
- 35.Di Marco F., Foti G., Corsico A. Where are we with the use of N-acetylcysteine as a preventive and adjuvant treatment for COVID-19? Eur Rev Med Pharmacol Sci. 2022;26:715–721. doi: 10.26355/eurrev_202201_27898. [DOI] [PubMed] [Google Scholar]
- 36.Assimakopoulos S.F., Marangos M. N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suter P.M., Domenighetti G., Schaller M.D., Laverrière M.C., Ritz R., Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest. 1994;105:190–194. doi: 10.1378/chest.105.1.190. [DOI] [PubMed] [Google Scholar]
- 38.Spapen H., Zhang H., Demanet C., Vleminckx W., Vincent J.L., Huyghens L. Does N-acetyl-L-cysteine influence cytokine response during early human septic shock? Chest. 1998;113:1616–1624. doi: 10.1378/chest.113.6.1616. [DOI] [PubMed] [Google Scholar]
- 39.Oliva A., Bianchi A., Russo A., Ceccarelli G., Cancelli F., Aloj F., et al. Effect of N-acetylcysteine administration on 30-day mortality in critically Ill patients with septic shock caused by carbapenem-resistant klebsiella pneumoniae and acinetobacter baumannii: a retrospective case-control study. Antibiotics. 2021;10 doi: 10.3390/antibiotics10030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X., Ma Y., He J., Li Y., Zhu H., Yu X. N-acetylcysteine for adults with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Hong Kong J Emerg Med. 2019;26:288–298. doi: 10.1177/1024907918794559. [DOI] [Google Scholar]
- 41.Lewis S.R., Pritchard M.W., Thomas C.M., Smith A.F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Alencar J.C.G., Moreira C.L., Müller A.D., Chaves C.E., Fukuhara M.A., da Silva E.A., et al. Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2021;72:e736–e741. doi: 10.1093/cid/ciaa1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taher A., Lashgari M., Sedighi L., Rahimi-Bashar F., Poorolajal J., Mehrpooya M. A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome. Pharmacol Rep. 2021;73:1650–1659. doi: 10.1007/s43440-021-00296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaynitdinova V.V., Avdeev S.N., Merzhoeva Z.M., Berikkhanov Z.G.-M., Medvedeva I.V., Gorbacheva T.L. N-acetylcysteine as a part of complex treatment of moderate COVID-associated pneumonia. Pulmonologiya. 2021;31:21–29. doi: 10.18093/0869-0189-2021-31-1-21-29. [DOI] [Google Scholar]
- 45.Assimakopoulos S.F., Aretha D., Komninos D., Dimitropoulou D., Lagadinou M., Leonidou L., et al. N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study. Infect Dis. 2021;53:847–854. doi: 10.1080/23744235.2021.1945675. [DOI] [PubMed] [Google Scholar]
- 46.Frades S., Miguel M., Prieto A., Ormaechea I., Blas F., López Yeste P., et al. The role of intermediate respiratory care units in preventing ICU collapse during the COVID pandemic. Int J Respir Pulm Med. 2020;7:147. doi: 10.21203/rs.3.rs-52228/v1. [DOI] [Google Scholar]
- 47.Faverio P., Rebora P., Rossi E., Del Giudice S., Montanelli F., Garzillo L., et al. Impact of N-Acetylcysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study. ERJ Open Res. 2022;8:542–2021. doi: 10.1183/23120541.00542-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chavarría A.P., Vázquez R.R.V., Cherit J.G.D., Bello H.H., Suastegui H.C., Moreno-Castañeda L., et al. Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput Struct Biotechnol J. 2021;19:1379–1390. doi: 10.1016/j.csbj.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izquierdo J.L., Soriano J.B., González Y., Lumbreras S., Ancochea J., Echeverry C., et al. Use of N-Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19. Sci Prog. 2022;105 doi: 10.1177/00368504221074574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oldham J.M., Ma S.-F., Martinez F.J., Anstrom K.J., Raghu G., Schwartz D.A., et al. TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192:1475–1482. doi: 10.1164/rccm.201505-1010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanguinetti C.M. N-acetylcysteine in COPD: why, how, and when? Multidiscip Respir Med. 2016;11:8. doi: 10.1186/s40248-016-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q., Ju Y., Ma Y., Wang T. N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial. Medicine. 2018;97 doi: 10.1097/MD.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou N., Yang X., Huang A., Chen Z. The potential mechanism of N-acetylcysteine in Treating COVID-19. Curr Pharm Biotechnol. 2021;22:1584–1590. doi: 10.2174/1389201021999201228212043. [DOI] [PubMed] [Google Scholar]
- 55.Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. Vivo (Brooklyn) 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cazzola M., Rogliani P., Salvi S.S., Ora J., Matera M.G. Use of thiols in the treatment of COVID-19: current evidence. Lung. 2021;199:335–343. doi: 10.1007/s00408-021-00465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]