Abstract
Coronavirus disease 2019 (COVID-19) is associated with pneumonia and has various pulmonary manifestations on computed tomography (CT). Although COVID-19 pneumonia is usually seen as bilateral predominantly peripheral ground-glass opacities with or without consolidation, it can present with atypical radiological findings and resemble the imaging findings of other lung diseases. Diagnosis of COVID-19 pneumonia is much more challenging for both clinicians and radiologists in the presence of pre-existing lung disease. The imaging features of COVID-19 and underlying lung disease can overlap and obscure the findings of each other. Knowledge of the radiological findings of both diseases and possible complications, correct diagnosis, and multidisciplinary consensus play key roles in the appropriate management of diseases. In this pictorial review, the chest CT findings are presented of patients with underlying lung diseases and overlapping COVID-19 pneumonia and the various reasons for radiological lung abnormalities in these patients are discussed.
Keywords: COVID-19 pneumonia, Computed tomography, Lung diseases
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with pneumonia and has various pulmonary manifestations on computed tomography (CT). Although the typical findings of COVID-19 pneumonia are predominantly peripheral ground-glass opacities with or without consolidations, throughout the course of the disease, atypical findings may be observed which resemble the imaging findings of other lung diseases.1 The sensitivity and specificity of chest CT for diagnosis of COVID-19 pneumonia vary according to the presence of typical and atypical findings. In addition to diagnosis of COVID-19, chest CT is used to evaluate the severity of the disease and prognosis, to manage treatment, to determine pulmonary complications in the acute phase, and to assess fibrotic lung diseases in the late phase.2., 3., 4. Furthermore, chest CT plays a vital role in the differentiation of COVID-19 pneumonia from other lung diseases.
Diagnosis of COVID-19 pneumonia is much more challenging for both clinicians and radiologists when there is a concomitant lung disease.5., 6. Chest CT findings may belong to both the pre-existing lung disease and COVID-19 pneumonia. Moreover, underlying lung diseases such as chronic obstructive pulmonary disease (COPD) and interstitial lung diseases may worsen COVID-19 pneumonia.7 Therefore, correct diagnosis with imaging and clinical findings is essential for the appropriate management. Knowledge about the chest CT findings of underlying lung diseases, possible complications, and COVID-19 pneumonia can lead the radiologist to the correct diagnosis.
The aim of this pictorial review was to present the chest CT findings of patients with underlying lung diseases and overlapping COVID-19 pneumonia and to discuss the various reasons for radiological lung lesions in these patients.
2. Interstitial lung diseases
Idiopathic interstitial pneumonias are classified as major idiopathic interstitial pneumonia, rare idiopathic interstitial pneumonia, and unclassifiable idiopathic interstitial pneumonia. Major idiopathic interstitial pneumonia has six subgroups; idiopathic interstitial fibrosis (IPF), nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), acute interstitial pneumonia (AIP) respiratory bronchiolitis (RP), associated interstitial lung disease (ILD), and desquamative interstitial pneumonia (DIP), while rare idiopathic interstitial pneumonia includes LIP and pleuroparenchymal fibroelastosis.8
IPF shows UIP patterns which are listed as macrocystic honeycombing, reticulation, traction bronchiectasis, architectural distortion, and focal ground-glass opacity with apicobasal gradient on chest CT. The main CT features of NSIP are ground-glass opacities, which are usually bilateral and symmetric with lower lobe predominance and subpleural sparing. Although COP may show diverse CT findings, the most common features are atoll sign, bilateral patchy and often migratory consolidative or ground-glass opacities with predominantly peripheral or peribronchovascular distribution.
AIP can be divided into two phases as exudative phase and the organizing phase. GGOs with or without air space consolidation are seen due to diffuse alveolar damage in the exudative phase. Traction bronchiectasis and architectural distortion occur due to fibrosis in the organizing phase.
RB-associated ILD and DIP are also known as smoking-related ILD. Centrilobular opacities, patchy GGOs, and bronchial wall thickening which show upper lobe predominance or diffuse distribution are observed on CT images of RB-ILD. Emphysema, GGOs, reticulation, and cysts with apicobasal gradient and peripheral predominance are seen in DIP.
LIP, which is usually associated with autoimmune diseases or AIDS, is characterized by GGOs, perivascular cysts, septal thickening, and centrilobular opacities with basilar predominance. Pleuroparenchymal fibroelastosis shows marked apical pleural thickening, subpleural consolidation with traction bronchiectasis, architectural distortion, and volume loss in the upper lobes.8., 9., 10.
2.1. Overlapping with COVID-19 and differentiation
All ILDs, particularly IPF, have acute exacerbations which have an increased mortality rate ranging from 35% to 70%.11 Acute exacerbations usually occur secondary to thoracic surgeries and viral infections, including COVID-19. Although the frequency of the COVID-19 in cases of acute exacerbations was very rare (1.4%), COVID-19 related acute exacerbations of IPF had a poorer prognosis than non-COVID-19-related patients.12 The lower frequency of COVID-19 in patients with underlying lung disease may be due to the fact that these patients are more afraid of getting COVID-19 than normal population and more cautious about hygiene and contact rules.
The differentiation of CT and clinical findings of ILDs from COVID-19 and the demonstration of the severity of the underlying ILD and COVID-19 pneumonia are very important for accurate patient management. However, it is not always easy to make a correct diagnosis both clinically and radiologically as most of the findings overlap with each other. Consolidations in AIP and COP, the atoll sign in COP, and GGOs in all other ILDs can mimic the acute phase of COVID-19 pneumonia. While round-shaped GGOs are suggestive for COVID-19 pneumonia, subpleural sparing is more in favor of NSIP, although it can be seen in COVID-19 pneumonia.13 On the other hand, traction bronchiectasis, reticulation, architectural distortion, and honeycombing in ILDs mimic the late phase of COVID-19 and worsen the course of the disease. Moreover, the long-term lung changes of COVID-19, including traction bronchiectasis, reticulation, and honeycombing, can aggravate ILD findings14., 15. (Fig. 1 ). Evaluation by comparing with previous CT images of patients with ILD will be very useful for differential diagnosis.
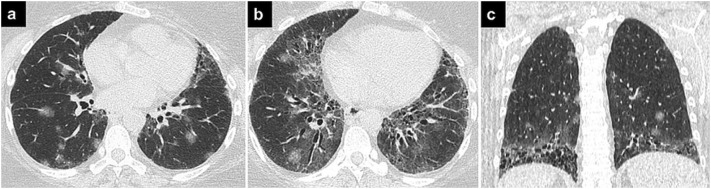
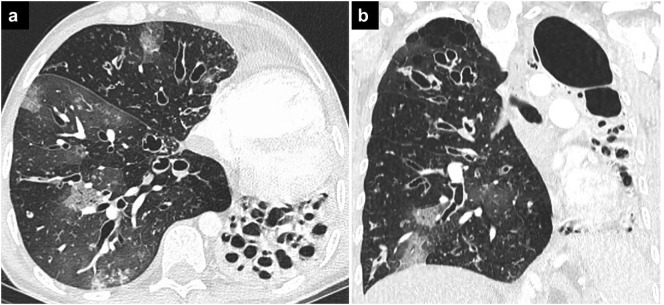
Fig. 1.
COVID-19 pneumonia and ILD.
Axial (a, b) and coronal (c) CT images showing bilateral round ground-glass opacities in a patient with COVID-19 pneumonia and ILD. Findings of UIP, such as peripheral and basal ground-glass opacities, traction bronchiectasis, and honeycombing can also be seen.
Patients with FVC of <80%, fibrotic changes, and obesity have been shown to have increased death rates in patients with both ILD and COVID-19. The data did not suggest any harm related to the treatment of ILD in patients with COVID-19, which could be because similar treatments, including steroids, immunomodulators, and antifibrotics, are used in both COVID-19 and ILD.16
3. Connective tissue diseases
Connective tissue diseases (CTDs) are a varied group of systemic inflammatory disorders that often affect the lungs. The CTDs that frequently have thoracic involvement are rheumatoid arthritis (RA), systemic sclerosis (SS), systemic lupus erythematosus (SLE), polymyositis (PM), dermatomyositis (DM), Sjogren syndrome, and mixed connective tissue disease. The main thoracic manifestations of CTDs are ILD and pulmonary arterial hypertension. In addition to pulmonary findings, pleural or pericardial effusions, esophageal dilatation, pulmonary artery enlargement, and mediastinal lymphadenopathies can be seen.17., 18. Although the radiological findings of CTDs are similar to each other, some clues can help in the differential diagnosis.
Pleural thickening and effusion is the most common thoracic manifestation of RA. Although UIP is the most common ILD pattern in RA, other ILD patterns including NSIP and OP can occur. Obliterative bronchiolitis with mosaic attenuation pattern and follicular bronchiolitis with multiple small nodules can also be seen. Rheumatoid pulmonary nodules are frequently peripherally located in the mid and upper lung regions and may cavitate.19., 20., 21.
The most common ILD pattern in SS is NSIP, but UIP is also frequently seen. Pulmonary artery enlargement and esophageal dilatation are usually observed whereas pleural involvement is uncommon.22., 23., 24. In contrast, pleural disease is the most common involvement in SLE. Acute lupus pneumonitis and diffuse alveolar hemorrhage are other rare but severe manifestations of SLE. Consolidations and GGOs are seen and may mimic infection on CT.25., 26.
The most common ILD patterns are NSIP and OP in DM/PM. Bilateral patchy often triangular or polygonal shaped consolidations with or without reverse halo sign are usually observed on CT.18
3.1. Overlapping with COVID-19 and differentiation
The frequency of CTDs was found 0.53% in 18,786 COVID-19 patients hospitalized in 23 centers. The lung involvement in CTDs may both mimic and worsen COVID-19 pneumonia..1., 27., 28. Bilateral GGOs predominantly located in the lower lobes can be confused in COVID-19 and NSIP. The late phase of COVID-19 with the interstitial disease can also mimic CTD-associated ILD with persistent GGOs or fibrous stripe. The concentration of fibrosis in the anterior aspects of the upper lobes also known as the anterior upper lobe sign and isolation of fibrosis to the lung bases with sharp demarcation in the craniocaudal image also known as the straight-edge sign can be helpful for the diagnosis of CTD-related ILD. But, fibrotic lung changes within anterior upper lobes in some COVID-19 related ARDS survivors should be kept in mind.29., 30.
Mediastinal lymphadenopathy and pleural involvement, which are rarely seen in COVID-19 pneumonia, are other frequent imaging findings in CTDs, especially SLE.18., 31. Acute lupus pneumonitis and diffuse alveolar hemorrhage, which are rare but severe complications of SLE, may resemble infectious pneumonia including COVID-19 pneumonia both radiologically and clinically.
Patients with CTDs may have more severe COVID-19 disease due to immune dysregulation. However, COVID-19 may cause the aggravation of underlying ILD. The NSIP pattern of CTDs may convert to fibrotic NSIP or UIP pattern with the late effects of COVID-19 pneumonia.14
In addition to the lung involvement of CTDs, opportunistic infections due to immunosuppressive treatment or secondary infectious diseases can occur and interfere with the imaging findings. For example, in a patient with COVID-19 pneumonia, cavitating rheumatoid lung nodules can be confused with secondary infections and vice versa. Clinical findings and knowledge about imaging findings of underlying lung diseases play a key role in correct diagnosis (Fig. 2 ).
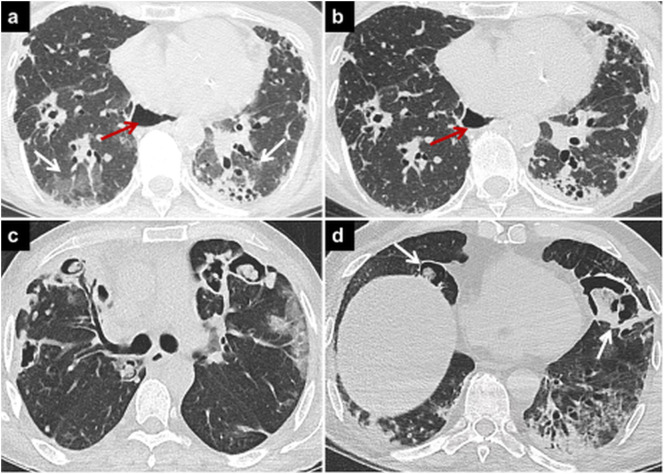
Fig. 2.
COVID-19 pneumonia and connective tissue diseases.
Axial CT image (a) of a patient with systemic sclerosis shows lung involvement findings such as peripheral opacities, reticulations, and traction bronchiectases. There are also bilateral GGOs in the lower lobes due to COVID-19 pneumonia (a, white arrows).The GGOs regressed in the follow-up CT image (b) obtained 3 months later. Esophageal dilatation due to scleroderma involvement can be seen on both CTs (a, b, red arrows).
Axial CT image (c) of a patient with RA and COVID-19 pneumonia showing bilateral cavitating rheumatoid nodules, and peripheral GGOs in the left upper lobe due to COVID-19 pneumonia. Cavitary rheumatoid lung nodules and cavitary superinfections may mimic each other in COVID-19 pneumonia. Axial CT image (d) of another patient with COVID-19 pneumonia and without CTDs, showing cavitary opacities in the right lower lobe and lingula (arrows). Deep tracheal aspiration and blood culture revealed Acinetobacter baumanii superinfection.
4. Sarcoidosis
Sarcoidosis is a multisystemic chronic inflammatory disorder characterized by non-caseating granulomas. Thoracic involvement is the most common finding and is responsible for most of the morbidity and mortality in patients with sarcoidosis. Typical radiological findings of pulmonary sarcoidosis are hilar and mediastinal lymphadenopathy, and parenchymal abnormalities predominantly located in the upper and middle zone.32 Micro/macronodules, GGOs, and airspace consolidation are suggestive of active and reversible disease whereas parenchymal distortion, honeycomb-like opacities, traction bronchiectasis, volume loss, and retraction of hila are indicators of the sequela and irreversible sarcoidosis.32., 33.
4.1. Overlapping with COVID-19 and differentiation
The estimated frequency of COVID-19 was reported as 5.1% in one of the largest multicenter clinical cohorts of sarcoidosis with a 9% overall mortality rate.34 In addition to typical findings, sarcoidosis can mimic various lung diseases, including COVID-19 pneumonia.1., 33. The imaging findings of active sarcoidosis and acute COVID-19 pneumonia may mimic each other, whereas irreversible sarcoidosis resembles the late findings of COVID-19. Pulmonary involvement of sarcoidosis may reduce normal lung parenchyma volume and cause severe disease in patients with COVID-19 pneumonia.
GGOs, alveolar consolidation, and crazy paving patterns may be seen in both sarcoidosis and COVID-19 pneumonia. However, upper and-middle zone predominance, perihilar predilection, and the presence of small nodules with perilymphatic distribution and peribronchovascular thickening are indicators of sarcoidosis.6 Also, mediastinal and hilar lymphadenopathies are typical features of sarcoidosis, but are not common in COVID-19 pneumonia. However, the presence of lympadenopathies in COVID-19 were related to disease severity.35
Furthermore, laboratory examinations do not help in differentiation as elevated C-reactive protein (CRP) and lymphopenia occur in both diseases.36 Although corticosteroid treatment is used for both acute flare-ups of sarcoidosis and COVID-19 pneumonia, the management of the two diseases changes according to different scenarios. Therefore, multidisciplinary management is required, with the consultation of rheumatology and pulmonology, as well as specialists of infectious diseases and radiology.
5. Other autoimmune diseases
Antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides are multisystem disorders that have three main clinicopathological variants including granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), and microscopic polyangiitis (MPA). ANCA-associated vasculitides may affect both airways and parenchyma and have secondary findings such as lymphadenopathy, and pleural and pericardial effusion. Although thoracic involvement of ANCA-associated vasculitides is varied and poorly specific, some imaging features can help in the differential diagnosis.37., 38., 39.
The most common CT finding of GPA is bilateral multiple pulmonary nodules or consolidations that may cavitate. The other imaging findings are GGOs frequently with peribronchovascular and perihilar distribution, airway thickening, bronchiectasis, and centrilobular nodules.37., 40.
The main imaging findings of EGPA are bilateral transient, patchy, non-segmental GGOs and consolidations without predilection of any lung lobe. Interlobular septal thickening because of edema and eosinophilic infiltration, bronchial dilatation, and wall thickening due to airway involvement can also occur.41
MPA is characterized by bilateral, diffuse, or patchy GGOs with consolidations due to diffuse alveolar hemorrhage or hemorrhagic diffuse alveolitis in the acute phase whereas reticular pattern and interstitial fibrosis may occur secondary to repetitive hemorrhage in the chronic phase.42., 43.
IgG4-related disease is another systemic disorder that is associated with elevated IgG4 levels and has recently attracted increasing attention. The CT findings are variable and consist of rounded consolidations and GGOs, thickening of bronchovascular bundles, interlobular septae, bronchiectasis, and honeycombing. In addition to parenchymal and airway involvement, pleural thickening, mediastinal lymphadenopathy, and fibrosing mediastinitis can occur. Pleural effusion is a rare finding in patients with IgG4-related disease.44., 45.
5.1. Overlapping with COVID-19 and differentiation
In a cohort of 102 ANCA-associated vasculitides, 29 patients had COVID-19 with a 27.6% severe disease rate and a 10.3% mortality rate.46 COVID-19 and vasculitides may have similar pathways and clinical characteristics such as neutrophil extracellular traps, high venous thromboembolism, hypo/anosmia, cough, and myalgia.47., 48., 49., 50. In addition to the clinical features, both diseases may show similar radiological characteristics including ground-glass opacities and consolidations.50., 51.
GGOs are seen in both EGPA and COVID-19 pneumonia. But, rounded GGOs are usually seen in COVID-19 pneumonia while patchy, migratory GGOs and usually coexisting small centrilobular nodules are suggestive of EGPA. MPA in the acute phase mimics the acute phase of COVID-19 pneumonia, and MPA in the chronic phase resembles the late phase of COVID-19 pneumonia. Moreover, all ANCA–associated vasculitides can worsen late fibrotic changes of COVID-19 pneumonia.
Tracheal wall thickening, especially in the subglottic region, and nodules with cavitation are suggestive of GPA while these imaging findings are not expected in COVID-19 pneumonia. However, cavitation especially within consolidation areas can occur in COVID-19 pneumonia. Nodules with cavitation can also mimic secondary infections including fungal and bacterial pneumonia or septic emboli in patients with COVID-19.52 Increased clinical symptoms such as fever, dyspnea, and cough would help the differentiation. Furthermore, tracheal stenosis can also occur in patients with COVID-19 after long-term intubation and tracheostomy. The location of tracheal stenosis on CT images guides the radiologist in the differential diagnosis. Subglottic stenosis suggests GPA whereas stenosis matching the location of the endotracheal tube on prior images is suggestive of prolonged intubation (Fig. 3 ).
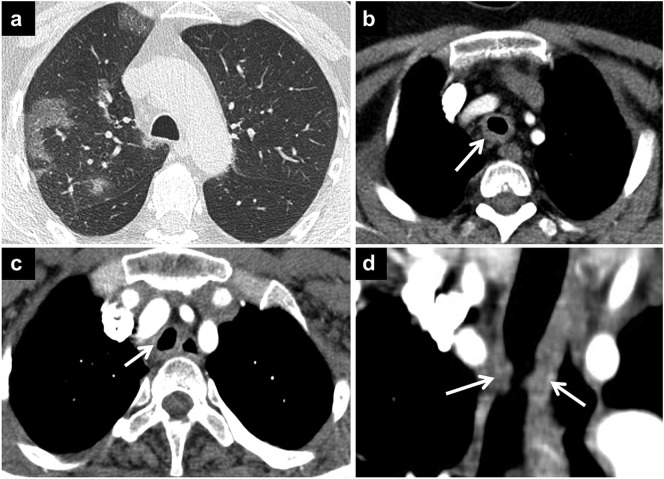
Fig. 3.
COVID-19 pneumonia and GPA.
Axial CT image of a patient with known GPA (a) shows GGOs with superimposed septal thickenings in the upper lobe compatible with COVID-19 pneumonia. Axial CT image (b) demonstrates circumferential tracheal wall thickening (arrow) due to GPA involvement. Tracheal stenosis can also be seen in COVID-19 pneumonia due to prolonged intubation. Axial (c) and coronal (d) CT images of another patient show post-intubation tracheal wall thickening and stenosis (arrows).
The presence of ANCA is the key in differentiating COVID-19 pneumonia from ANCA-associated vasculitis. When both diseases occur at the same time, the disease management is more challenging. COVID-19 has higher mortality rates in patients with ANCA-associated vasculitides than in the general population. Furthermore, the use of rituximab which is the most commonly used therapeutic agent in ANCA-associated vasculitides can cause a more severe hospital course of COVID-19 and re-infection.53 The hospitalization and management of the overlapping diseases should be arranged according to the clinical findings by multidisciplinary specialists.
IgG4-related disease can show poorer COVID-19 outcomes. Although consolidations and GGOs can be seen in both IgG4-related disease and COVID-19 pneumonia, fibrosing mediastinitis should suggest IgG4-related disease.
6. Pneumoconioses
Pneumoconioses are a broad group of lung diseases caused by the inhalation of particles. Silicosis, coal worker pneumoconiosis, and asbestosis are the most common types of pneumoconiosis. Multiple, well-defined, uniform nodules predominantly located in upper lobes and hilar/mediastinal lymphadenopathies commonly with calcification are the main imaging features in silicosis and coal-worker pneumoconiosis. Fibrotic, focal soft-tissue masses with irregular margins, calcification, and associated surrounding pericicatricial emphysema in upper zones are seen as complicated forms of silicosis and coal-worker pneumoconiosis. While pleural plaques, pleural effusion, subpleural lines, and reticulation are the most common CT findings of asbestosis, traction bronchiectasis, honeycombing, and parenchymal bands can occur in the later phase of the disease.54., 55.
6.1. Overlapping with COVID-19 and differentiation
COVID-19 rates among mineworkers exceed the population rates in South Africa. Mineworkers are at risk for both pneumoconiosis and COVID-19 pneumonia because of working in crowded and poorly ventilated places. COVID-19 may also cause severe outcomes, especially in patients with interstitial lung disease due to pneumoconiosis.56 Although imaging features of silicosis and coal worker pneumoconiosis are not usually confused with COVID-19, subpleural lines and interlobular linear opacities in asbestosis can mimic the recovery phase of COVID-19 pneumonia. Associated linear opacities with pleural plaques in asbestosis can help the differential diagnosis. Furthermore, interstitial fibrosis in pneumoconiosis may not only mimic long-term persistent lung disease after COVID-19 but may also cause severe COVID-19 disease and progression to chronicity of lung abnormality. Therefore, vaccination and self-isolation become more important in these patients15., 16. (Fig. 4 ).
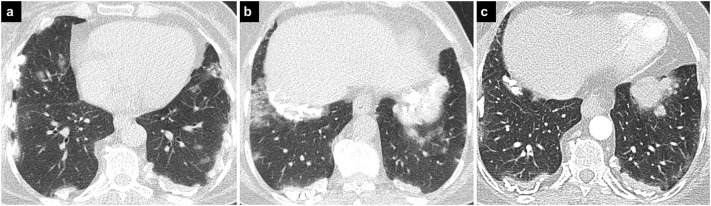
Fig. 4.
COVID-19 pneumonia and asbestosis.
Axial CT images (a, b) demonstrate bilateral calcified pleural plaques secondary to asbestosis and bilateral GGOs due to COVID-19 pneumonia. In the previous CT image (c) (8 months earlier) there are reticular densities and mild GGOs adjacent to the pleural plaques compatible with asbestosis, which may be confused with COVID-19 pneumonia.
7. Cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive inherited disease resulting from defective mucociliary clearance. The most common pulmonary CT features of CF are upper lobe predominant bronchiectasis, bronchial wall thickening, mosaic attenuation pattern, tree-in-bud pattern, consolidation, and atelectasis.57 The tree-in-bud pattern may occur due to the involvement of small airways or endobronchial spread of infection. Consolidation is usually seen secondary to infection in exacerbations of CF. In addition to infection, consolidation and GGO can also be seen due to hemoptysis caused by enlarged bronchial arteries.58., 59.
7.1. Overlapping with COVID-19 and differentiation
Although infectious agents commonly cause acute exacerbations in individuals with CF, the incidence of COVID-19 pneumonia (0.07%) and severe disease appear not to be higher than in the general population. Lower incidence may be the result of higher awareness of infection and control practices in patients with CF. However, post-transplant individuals experience more severe disease.60 Consolidation and GGO on CT images can be seen in patients with CF due to pulmonary hemorrhage and pneumonia. While clinical findings may help in differential diagnosis, an enlarged bronchial artery on CT can help the diagnosis and guide the interventional treatment of hemoptysis. Bacterial infections can mimic COVID-19 pneumonia both clinically and radiologically in individuals with CF. Laboratory findings such as high neutrophil count and some imaging features including tree-in-bud pattern or lobar pulmonary opacities with bulging fissures suggest bacterial pneumonia. Co-infections can occur especially in hospitalized patients with CF and COVID-19 pneumonia. The co-existence of COVID-19, co-infection and CF in the same patient can cause decreased pulmonary function and increased morbidity and mortality (Fig. 5 ).
Fig. 5.
COVID-19 pneumonia and cystic fibrosis.
Axial (a) and coronal (b) CT images show widespread bronchiectases and bronchial wall thickenings in both lungs due to cystic fibrosis. In the left lung, multiple cystic bronchiectases have destroyed the lung parenchyma causing significant volume loss. GGOs can be seen in the right lung due to COVID-19 pneumonia.
8. Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a spectrum of obstructive airway diseases including two major subtypes of chronic bronchitis-small airway disease and emphysema. The main CT findings can be classified as emphysema, airway disease, and associated features. Emphysema can occur as centrilobular and paraseptal. Airway disease can be seen as bronchial wall thickening, centrilobular opacities, and air trapping. Saber sheath trachea, tracheobronchomalacia, tracheobronchial diverticula, bronchiectasis, patchy ground-glass abnormality, mild subpleural reticulation, and pulmonary arterial enlargement can be observed as associated findings.61
8.1. Overlapping COVID-19 and differentiation
The pooled prevalence rates of COPD patients in COVID-19 cases were 2%.62 Patients with COPD may suffer severe COVID-19 disease and require intensive care unit (ICU) admission, although mortality rates are no higher than the ordinary risk of death from other causes. The use of glucocorticoids in the treatment of both COPD and COVID-19 may be the reason why patients with both diseases do not have worse outcomes.7., 63. Although emphysema is not expected to occur in patients with COVID 19, reticulation, bronchial wall thickening, and ground-glass opacities may be seen in both COPD and COVID-19 pneumonia.61., 64. Furthermore, traction bronchiectasis may occur in the late phase of COVID-19 pneumonia and mimic the findings of COPD. Underlying COPD in patients with COVID-19 might also result in a more severe, long-term, persistent lung disease and interstitial fibrosis.
GGOs of COVID-19 within emphysema may resemble a crazy-paving pattern which can be an indicator of severe COVID-19.4 However, air cysts predominantly located in the anterior part of the lungs can be seen secondary to COVID-19 pneumonia itself or prolonged ventilation and mimic emphysema52., 65. (Fig. 6 ). COPD patients with emphysema and COVID-19 may be at high risk of pneumothorax.66 Early diagnosis of pneumothorax and definition of CT findings of both diseases would be helpful for clinicians and provide appropriate management of the diseases.
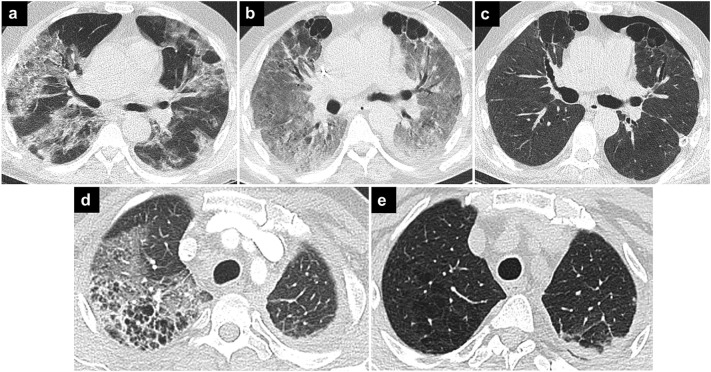
Fig. 6.
COVID-19 pneumonia and emphysema.
Axial CT images in the upper row show lung parenchymal changes in a patient with COVID-19 pneumonia. Bilateral opacities that are seen on the first CT image (a) progressed over time to ARDS, and bilateral subpleural bullae are seen in the anterior part of upper lobes in follow-up CT image (b). The last CT image (c) shows that the opacities have regressed, but there are persisting subpleural bullae leading to pneumothorax on the left.
Axial CT image of another patient (d) shows opacities due to COVID-19 pneumonia in the right upper lobe superimposed on emphysematous parenchyma. The previous CT image (e) of the same patient better delineates the emphysematous parenchyma.
9. Malignant diseases
Thoracic malignancies consist of metastases and primary malignancies such as lung, airway, pleural, and chest wall tumors. Lung metastases are usually seen as well-circumscribed, rounded solid lesions of variable sizes, while primary lung tumors are often characterized by sub-solid density, air bronchogram, and lobulated or spiculated margin.67 In addition to solitary nodules and mass, primary lung tumors particularly adenocarcinomas can also appear as consolidation and GGOs and resemble infectious diseases. Pleural effusion, pleural thickening, and mediastinal lymphadenopathy can accompany both primary and secondary cancers.68
9.1. Overlapping with COVID-19 and differentiation
An underlying malignant disease can worsen the outcome of COVID-19. The pooled incidence of cancer in COVID-19 patients was 6%.
The incidence of COVID-19 in cancer patients is highest in hematological and lung cancers. Patients with lung cancer have been associated with a near doubling of severe COVID-19.7., 69. Potential causes of higher rates of severe disease and death are impaired immunity due to chemotherapy, especially in hematological malignancies and lung involvement, particularly in lung cancer. Receiving chemotherapy 4 weeks before the symptoms of COVID-19 leads to a high risk of death.69 Vaccination before chemotherapy and self-isolation during chemotherapy is very important to prevent COVID-19 in cancer patients.
COVID-19 may mimic lung cancer by showing atypical chest CT findings including isolated upper lobe involvement, the presence of a solitary lesion, and pleural effusion.13 On the other hand, the presence of cystic change, vessel convergence sign and pleural retraction are mostly seen in lung cancer.70 In addition to solitary lesions, multiple GGOs and consolidations may resemble mucinous lung adenocarcinomas and hemorrhagic metastases such as angiosarcoma.1
New GGOs can be confused with new foci of lung adenocarcinoma in patients with both diseases. In addition, interlobular septal thickening and crazy paving pattern in COVID-19 pneumonia may imitate lymphangitic carcinomatosis in cancer patients. Follow-up chest CT may be useful in differentiation, as previously reported dynamic changes of COVID-19 pneumonia occur in a shorter period of time. GGOs and consolidations of COVID-19 pneumonia can also obscure metastases in cancer patients, so the investigation of lung metastases and disease progression can be performed after the resolution of COVID-19 pneumonia.
Besides COVID-19, cancer patients are prone to other opportunistic infectious diseases which can be confused with COVID-19 pneumonia. Anamnesis, increased tumor markers, and clinical findings suggesting viral infection can help to establish the correct diagnosis (Fig. 7 ).
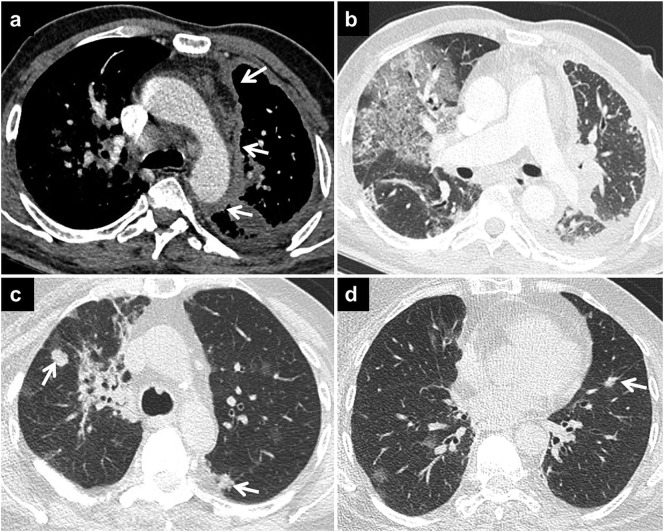
Fig. 7.
COVID-19 pneumonia and malignancy.
Axial CT image in the mediastinal window (a) shows irregular mediastinal and costal pleural thickenings (arrows) on the left in a patient with malignant mesothelioma and COVID-19 pneumonia. Axial CT image in the lung window (b) of the same patient shows GGOs with superimposed septal thickenings in the right lung due to COVID-19 pneumonia.
Axial CT images (c, d) of another patient with metastatic breast carcinoma and COVID-19 pneumonia demonstrate both round GGOs due to COVID-19 pneumonia and metastatic nodules (arrows) in both lungs.
10. Pulmonary edema
Pulmonary edema is caused by the increased accumulation of fluid in the pulmonary interstitium and alveoli due to the increased hydrostatic pressure, permeability, or both. Although the most common reasons for pulmonary edema are heart failure and acute respiratory distress syndrome (ARDS), several other reasons such as acute mitral valve insufficiency, pulmonary embolus, re-expansion, reperfusion, and neurogenic injuries can cause pulmonary edema. GGOs, consolidations, bronchovascular thickening, and septal thickening are the most common imaging features of pulmonary edema.
The distribution of the imaging abnormalities provides clues to the etiology of pulmonary edema. Unilateral right lung, especially upper lobe, involvement suggests acute mitral valve insufficiency, while pulmonary edema secondary to neurogenic injury usually affects lung apices. Gravitational gradient usually appears in ARDS. Bilateral perihilar opacities, also known as the batwing sign, are often seen in heart failure. Pleural effusion and cardiomegaly usually accompany the parenchymal findings of pulmonary edema due to decompensated heart failure.71., 72.
10.1. Overlapping with COVID-19 and differentiation
COVID-19 can cause pulmonary edema because of increased pulmonary venous pressure and alveolar capillary membrane permeability. In COVID-19, cytokine storm and inflammation induce increased alveolar capillary membrane permeability while dysregulation of the renin angiotensin aldosterone system (RAAS) causes increased hydrostatic pressure and volume overload. Subsequently, ARDS may develop due to secondary inflammation and fibrosis.73 Therefore, COVID-19 can worsen underlying pulmonary edema and cause decompensated heart failure.
However, imaging findings of pulmonary edema and COVID-19 pneumonia show high similarity with GGOs, consolidations, septal thickening. Although differentiating CT findings can be very challenging, the distribution of GGOs, and the presence of pleural effusion can help the radiological differential diagnosis. The presence of fever also suggests accompanying COVID-19 in patients with pulmonary edema.74 In addition to treatment for COVID 19 pneumonia, the drugs that decrease the fluid in the lung can contribute to reducing lung damage75 (Fig. 8 ).
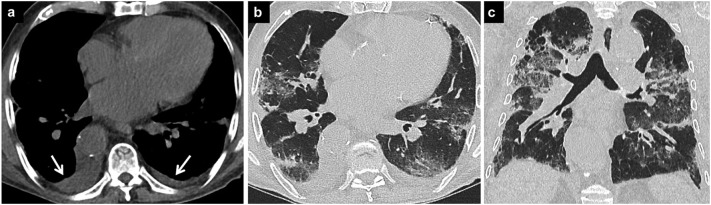
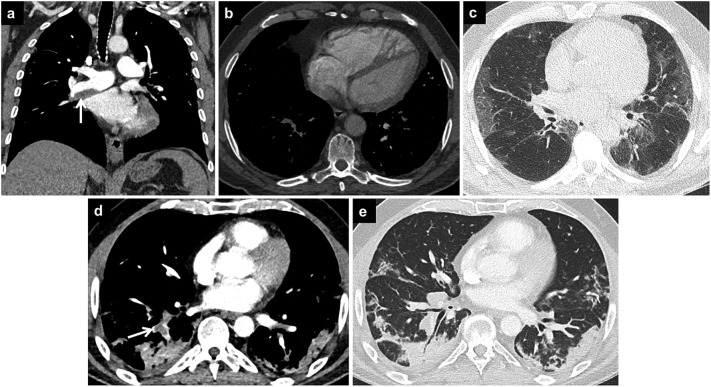
Fig. 8.
COVID-19 pneumonia and pulmonary edema.
Axial CT image (a) of a patient with subacute COVID-19 pneumonia shows cardiomegaly and bilateral pleural effusions (arrow). CT image in lung window (b) demonstrates bilateral peripheral opacities due to COVID-19 pneumonia. Coronal CT image (c) shows that there are also perihilar opacities due to accompanying pulmonary edema.
11. Pulmonary thromboembolism
Pulmonary thromboembolism (PTE) can occur due to coagulation abnormalities which are provoked by various factors including surgery, trauma, chemotherapy, cancer, immobilization, and COVID-19.76 Although pulmonary CT angiography is very sensitive in the demonstration of PTE, knowledge of accompanying parenchymal findings is the cornerstone, especially in patients with COVID-19 who usually undergo non-contrast chest CT. Acute pulmonary embolism is characterized by wedge-shaped pleural-based opacification or consolidation with air bubbles. The halo sign may be seen secondary to adjacent hemorrhage. Mosaic attenuation and peripheral irregular linear densities due to residual scars from infarcts can be observed in patients with chronic PTE. Enlargement of the pulmonary artery and the right ventricle can also help the diagnosis of PTE.77
11.1. Overlapping with COVID-19 and differentiation
The incidence of PTE in hospitalized patients with COVID-19 has been found to be approximately 1.9–8.9%. Critically ill COVID-19 patients requiring ICU show a higher incidence for PTE.76 Also, underlying chronic PTE may worsen with COVID-19 and vice versa.
Predominantly peripheral GGOs and consolidations may obscure accompanying or underlying infarct. Furthermore, imaging findings of pulmonary infarct may overlap with COVID-19 pneumonia. Wedge-shaped and bubbly consolidation can be clues for PTE. Pulmonary CT angiography can be performed upon suspicious radiological and clinical findings. The use of therapeutic anticoagulants and balancing the risk of increased bleeding play a vital role in the management of patients with COVID-19 and accompanying PTE (Fig. 9 ).
Fig. 9.
COVID-19 and pulmonary thromboembolism.
Coronal CT image of a patient with previously diagnosed CTEPH shows chronic PTE in the right interlobar artery (arrow). Right ventricular dilatation and interventricular septal flattening due to increased right ventricular pressure is seen on axial CT image (b). Axial CT image (c) of the same patient shows bilateral peripheral GGOs compatible with COVID-19 pneumonia.
Axial CT image (d) of another patient with COVID-19 pneumonia demonstrates thrombi in the right lower pulmonary artery branches (arrow). The CT image in the lung window (e) shows bilateral peripheral opacities of subacute COVID-19 pneumonia, and pleura-based opacity is seen in right lower lobe representing pulmonary infarction.
12. Conclusion
The evaluation of lung involvement in patients with COVID-19 and underlying lung disease is challenging both clinically and radiologically. The imaging features of COVID-19 and underlying lung disease can overlap and cause difficulties in the differential diagnosis. Although a multidisciplinary approach is essential for appropriate disease management, radiologists play an important role in facilitating the diagnosis by knowing the imaging findings of COVID-19 and existing lung disease.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Duzgun S.A., Durhan G., Demirkazik F.B., Akpinar M.G., Ariyurek O.M. COVID-19 pneumonia: the great radiological mimicker. Insights Imaging. 2020;11(1):118. doi: 10.1186/s13244-020-00933-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machnicki S., Patel D., Singh A., Talwar A., Mina B., Oks M., et al. The usefulness of chest CT imaging in patients with suspected or diagnosed COVID-19: a review of literature. Chest. 2021;160(2):652–670. doi: 10.1016/j.chest.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccarese F., Coppola F., Spinelli D., Galletta G.L., Lucidi V., Paccapelo A., et al. Diagnostic accuracy of North America expert consensus statement on reporting CT findings in patients suspected of having COVID-19 infection: an italian single-center experience. Radiol Cardiothorac Imaging. 2020;2(4) doi: 10.1148/ryct.2020200312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durhan G., Ardali Duzgun S., Basaran Demirkazik F., Irmak I., Idilman I., Gulsun Akpinar M., et al. Visual and software-based quantitative chest CT assessment of COVID-19: correlation with clinical findings. Diagn Interv Radiol. 2020;26(6):557–564. doi: 10.5152/dir.2020.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katal S., Aghaghazvini L., Gholamrezanezhad A. Chest-CT findings of COVID-19 in patients with pre-existing malignancies; a pictorial review. Clin Imaging. 2020;67:121–129. doi: 10.1016/j.clinimag.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tana C., Mantini C., Cipollone F., Giamberardino M.A. Chest imaging of patients with sarcoidosis and SARS-CoV-2 infection. Current evidence and clinical perspectives. Diagnostics (Basel) 2021;11(2) doi: 10.3390/diagnostics11020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aveyard P., Gao M., Lindson N., Hartmann-Boyce J., Watkinson P., Young D., et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9(8):909–923. doi: 10.1016/S2213-2600(21)00095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller-Mang C., Grosse C., Schmid K., Stiebellehner L., Bankier A.A. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27(3):595–615. doi: 10.1148/rg.273065130. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson E.C., Berkowitz E.A. Lung CT: part 2, the interstitial pneumonias–clinical, histologic, and CT manifestations. AJR Am J Roentgenol. 2012;199(4):W464–W476. doi: 10.2214/AJR.10.7309. [DOI] [PubMed] [Google Scholar]
- 11.Collard H.R., Ryerson C.J., Corte T.J., Jenkins G., Kondoh Y., Lederer D.J., et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 12.Kondoh Y., Kataoka K., Ando M., Awaya Y., Ichikado K., Kataoka M., et al. COVID-19 and acute exacerbation of interstitial lung disease. Respir Investig. 2021;59(5):675–678. doi: 10.1016/j.resinv.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceylan N., Cinkooglu A., Bayraktaroglu S., Savas R. Atypical chest CT findings of COVID-19 pneumonia: a pictorial review. Diagn Interv Radiol. 2021;27(3):344–349. doi: 10.5152/dir.2020.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X., Fan Y., Alwalid O., Li N., Jia X., Yuan M., et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon J.J., Heyman B., Ko J.P., Condos R., Lynch D.A. CT of post-acute lung complications of COVID-19. Radiology. 2021;301(2):E383–E395. doi: 10.1148/radiol.2021211396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake T.M., Docherty A.B., Harrison E.M., Quint J.K., Adamali H., Agnew S., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med. 2020;202(12):1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kligerman S.J., Groshong S., Brown K.K., Lynch D.A. Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. Radiographics. 2009;29(1):73–87. doi: 10.1148/rg.291085096. [DOI] [PubMed] [Google Scholar]
- 18.Capobianco J., Grimberg A., Thompson B.M., Antunes V.B., Jasinowodolinski D., Meirelles G.S. Thoracic manifestations of collagen vascular diseases. Radiographics. 2012;32(1):33–50. doi: 10.1148/rg.321105058. [DOI] [PubMed] [Google Scholar]
- 19.Hakala M., Paakko P., Sutinen S., Huhti E., Koivisto O., Tarkka M. Association of bronchiolitis with connective tissue disorders. Ann Rheum Dis. 1986;45(8):656–662. doi: 10.1136/ard.45.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aquino S.L., Webb W.R., Golden J. Bronchiolitis obliterans associated with rheumatoid arthritis: findings on HRCT and dynamic expiratory CT. J Comput Assist Tomogr. 1994;18(4):555–558. doi: 10.1097/00004728-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Ahuja J., Arora D., Kanne J.P., Henry T.S., Godwin J.D. Imaging of pulmonary manifestations of connective tissue diseases. Radiol Clin North Am. 2016;54(6):1015–1031. doi: 10.1016/j.rcl.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Coral-Alvarado P., Pardo A.L., Castano-Rodriguez N., Rojas-Villarraga A., Anaya J.M. Systemic sclerosis: a world wide global analysis. Clin Rheumatol. 2009;28(7):757–765. doi: 10.1007/s10067-009-1144-9. [DOI] [PubMed] [Google Scholar]
- 23.Wells A.U., Steen V., Valentini G. Pulmonary complications: one of the most challenging complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii40–iii44. doi: 10.1093/rheumatology/kep109. [DOI] [PubMed] [Google Scholar]
- 24.Fujita J., Yoshinouchi T., Ohtsuki Y., Tokuda M., Yang Y., Yamadori I., et al. Non-specific interstitial pneumonia as pulmonary involvement of systemic sclerosis. Ann Rheum Dis. 2001;60(3):281–283. doi: 10.1136/ard.60.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheema G.S., Quismorio F.P., Jr. Interstitial lung disease in systemic lupus erythematosus. Curr Opin Pulm Med. 2000;6(5):424–429. doi: 10.1097/00063198-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Zamora M.R., Warner M.L., Tuder R., Schwarz M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997;76(3):192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ye C., Zhong J., Cai S., Dong L., Li C., Hou X., et al. COVID-19 infection in patients with connective tissue disease: a multicity study in Hubei province, China. MedComm. 2021;2(1):82–90. doi: 10.1002/mco2.56. (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dourmishev L., Guleva D., Pozharashka J., Drenovska K., Miteva L., Vassileva S. Autoimmune connective tissue diseases in the COVID-19 pandemic. Clin Dermatol. 2021;39(1):56–63. doi: 10.1016/j.clindermatol.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung J.H., Cox C.W., Montner S.M., Adegunsoye A., Oldham J.M., Husain A.N., et al. CT features of the usual interstitial pneumonia pattern: differentiating connective tissue disease-associated interstitial lung disease from idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 2018;210(2):307–313. doi: 10.2214/AJR.17.18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gulati A., Lakhani P. Interstitial lung abnormalities and pulmonary fibrosis in COVID-19 patients: a short-term follow-up case series. Clin Imaging. 2021;77:180–186. doi: 10.1016/j.clinimag.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Zeng W., Li X., Chen H., Shi L., Li X., et al. CT imaging changes of corona virus disease 2019(COVID-19): a multi-center study in Southwest China. J Transl Med. 2020;18(1):154. doi: 10.1186/s12967-020-02324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Criado E., Sanchez M., Ramirez J., Arguis P., de Caralt T.M., Perea R.J., et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567–1586. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 33.AA Önder Ö G Durhan MG Akpinar F Demirkazık OM Ariyürek. Mimickers of “The Great Mimicker”: Differential Diagnosis of Pulmonary Sarcoidosis Based Upon Imaging Findings. ECR 2020/C-06511. Doi:10.26044/ecr2020/C-06511.
- 34.Brito-Zeron P., Gracia-Tello B., Robles A., Alguacil A., Bonet M., De-Escalante B., et al. Characterization and outcomes of SARS-CoV-2 infection in patients with sarcoidosis. Viruses. 2021;13(6) doi: 10.3390/v13061000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valette X., du Cheyron D., Goursaud S. Mediastinal lymphadenopathy in patients with severe COVID-19. Lancet Infect Dis. 2020;20(11):1230. doi: 10.1016/S1473-3099(20)30310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tana C., Giamberardino M.A., Di Gioacchino M., Mezzetti A., Schiavone C. Immunopathogenesis of sarcoidosis and risk of malignancy: a lost truth? Int J Immunopathol Pharmacol. 2013;26(2):305–313. doi: 10.1177/039463201302600204. [DOI] [PubMed] [Google Scholar]
- 37.Chung M.P., Yi C.A., Lee H.Y., Han J., Lee K.S. Imaging of pulmonary vasculitis. Radiology. 2010;255(2):322–341. doi: 10.1148/radiol.10090105. [DOI] [PubMed] [Google Scholar]
- 38.Feragalli B., Mantini C., Sperandeo M., Galluzzo M., Belcaro G., Tartaro A., et al. The lung in systemic vasculitis: radiological patterns and differential diagnosis. Br J Radiol. 2016;89(1061) doi: 10.1259/bjr.20150992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durhan G. Thorax radiology in AAV. Acta Medica. 2021;52:21–26. [Google Scholar]
- 40.Martinez F., Chung J.H., Digumarthy S.R., Kanne J.P., Abbott G.F., Shepard J.A., et al. Common and uncommon manifestations of Wegener granulomatosis at chest CT: radiologic-pathologic correlation. Radiographics. 2012;32(1):51–69. doi: 10.1148/rg.321115060. [DOI] [PubMed] [Google Scholar]
- 41.Worthy S.A., Muller N.L., Hansell D.M., Flower C.D. Churg-Strauss syndrome: the spectrum of pulmonary CT findings in 17 patients. AJR Am J Roentgenol. 1998;170(2):297–300. doi: 10.2214/ajr.170.2.9456932. [DOI] [PubMed] [Google Scholar]
- 42.Lauque D., Cadranel J., Lazor R., Pourrat J., Ronco P., Guillevin L., et al. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P) Medicine (Baltimore) 2000;79(4):222–233. doi: 10.1097/00005792-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Eschun G.M., Mink S.N., Sharma S. Pulmonary interstitial fibrosis as a presenting manifestation in perinuclear antineutrophilic cytoplasmic antibody microscopic polyangiitis. Chest. 2003;123(1):297–301. doi: 10.1378/chest.123.1.297. [DOI] [PubMed] [Google Scholar]
- 44.Inoue D., Zen Y., Abo H., Gabata T., Demachi H., Kobayashi T., et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology. 2009;251(1):260–270. doi: 10.1148/radiol.2511080965. [DOI] [PubMed] [Google Scholar]
- 45.Ryu J.H., Sekiguchi H., Yi E.S. Pulmonary manifestations of immunoglobulin G4-related sclerosing disease. Eur Respir J. 2012;39(1):180–186. doi: 10.1183/09031936.00025211. [DOI] [PubMed] [Google Scholar]
- 46.Hocevar A.T.M., Rotar Z. COVID-19 in patients with ANCA associated vasculitis – single center experience [abstract] Arthritis Rheumatol. 2022;74(suppl 9) https://acrabstracts.org/abstract/covid-19-in-patients-with-anca-associated-vasculitis-single-center-experience/ [Google Scholar]
- 47.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Minno A., Ambrosino P., Calcaterra I., Di Minno M.N.D. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Semin Thromb Hemost. 2020;46(7):763–771. doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srouji I., Lund V., Andrews P., Edwards C. Rhinologic symptoms and quality-of-life in patients with Churg-Strauss syndrome vasculitis. Am J Rhinol. 2008;22(4):406–409. doi: 10.2500/ajr.2008.22.3204. [DOI] [PubMed] [Google Scholar]
- 50.Qurratulain Q., Ahmed A., Jones Q. Lesson of the month: severe granulomatosis with polyangiitis (GPA): a diagnostic challenge during the COVID-19 pandemic. Clin Med (Lond) 2021;21(1):79–80. doi: 10.7861/clinmed.2020-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duran E., Kilic L., Durhan G., Inkaya A.C., Guven G.S., Karakaya G., et al. Vital corner of diagnostic challenge: eosinophilic granulomatosis with polyangiitis or COVID-19 pneumonia? Ann Rheum Dis. 2020:1–3. doi: 10.1136/annrheumdis-2020-218533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal A., Tandon A., Bhatt S., Aggarwal A., Dagar S., Bansal H. COVID19 pneumonia with cavitation and cystic lung changes: multi-detector computed tomography spectrum of a gamut of etiologies. BJR Open. 2021;3(1) doi: 10.1259/bjro.20210007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronbichler A., Geetha D., Smith R.M., Egan A.C., Bajema I.M., Schonermarck U., et al. The COVID-19 pandemic and ANCA-associated vasculitis - reports from the EUVAS meeting and EUVAS education forum. Autoimmun Rev. 2021;20(12) doi: 10.1016/j.autrev.2021.102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chong S., Lee K.S., Chung M.J., Han J., Kwon O.J., Kim T.S. Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics. 2006;26(1):59–77. doi: 10.1148/rg.261055070. [DOI] [PubMed] [Google Scholar]
- 55.Remy-Jardin M., Remy J., Farre I., Marquette C.H. Computed tomographic evaluation of silicosis and coal workers' pneumoconiosis. Radiol Clin North Am. 1992;30(6):1155–1176. [PubMed] [Google Scholar]
- 56.Naidoo R.N., Jeebhay M.F. COVID-19: a new burden of respiratory disease among South African miners? Curr Opin Pulm Med. 2021;27(2):79–87. doi: 10.1097/MCP.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Averill S., Lubner M.G., Menias C.O., Bhalla S., Mellnick V.M., Kennedy T.A., et al. Multisystem imaging findings of cystic fibrosis in adults: recognizing typical and atypical patterns of disease. AJR Am J Roentgenol. 2017;209(1):3–18. doi: 10.2214/AJR.16.17462. [DOI] [PubMed] [Google Scholar]
- 58.Aziz Z.A., Davies J.C., Alton E.W., Wells A.U., Geddes D.M., Hansell D.M. Computed tomography and cystic fibrosis: promises and problems. Thorax. 2007;62(2):181–186. doi: 10.1136/thx.2005.054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wielputz M.O., Eichinger M., Biederer J., Wege S., Stahl M., Sommerburg O., et al. Imaging of cystic fibrosis lung disease and clinical interpretation. Rofo. 2016;188(9):834–845. doi: 10.1055/s-0042-104936. [DOI] [PubMed] [Google Scholar]
- 60.Mathew H.R., Choi M.Y., Parkins M.D., Fritzler M.J. Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm Med. 2021;21(1):173. doi: 10.1186/s12890-021-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynch D.A., Austin J.H., Hogg J.C., Grenier P.A., Kauczor H.U., Bankier A.A., et al. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner society. Radiology. 2015;277(1):192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marron R.M., Zheng M., Fernandez Romero G., Zhao H., Patel R., Leopold I., et al. Impact of chronic obstructive pulmonary disease and emphysema on outcomes of hospitalized patients with coronavirus disease 2019 pneumonia. Chronic Obstr Pulm Dis. 2021;8(2):255–268. doi: 10.15326/jcopdf.2020.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu K., Zeng Y., Xie P., Ye X., Xu G., Liu J., et al. COVID-19 with cystic features on computed tomography: a case report. Medicine (Baltimore) 2020;99(18) doi: 10.1097/MD.0000000000020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zantah M., Dominguez Castillo E., Townsend R., Dikengil F., Criner G.J. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir Res. 2020;21(1):236. doi: 10.1186/s12931-020-01504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J.E., Jeong W.G., Kim Y.H. Differentiation of primary lung cancer from solitary lung metastasis in patients with colorectal cancer: a retrospective cohort study. World J Surg Oncol. 2021;19(1):28. doi: 10.1186/s12957-021-02131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Purandare N.C., Rangarajan V. Imaging of lung cancer: implications on staging and management. Indian J Radiol Imaging. 2015;25(2):109–120. doi: 10.4103/0971-3026.155831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y.-J., Yang W.-J., Liu D., Cao Y.-Q., Zheng Y.-Y., Han Y.-C., et al. COVID-19 and early-stage lung cancer both featuring ground-glass opacities: a propensity score-matched study. TranslLung Cancer Res. 2020;9(4):1516–1527. doi: 10.21037/tlcr-20-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barile M. Pulmonary edema: a pictorial review of imaging manifestations and current understanding of mechanisms of disease. Eur J Radiol Open. 2020;7 doi: 10.1016/j.ejro.2020.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gluecker T., Capasso P., Schnyder P., Gudinchet F., Schaller M.D., Revelly J.P., et al. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19(6):1507–1531. doi: 10.1148/radiographics.19.6.g99no211507. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 73.Santos J.L.F., Zanardi P., Alo V., Rodriguez M., Magdaleno F., De Langhe V., et al. Pulmonary edema in COVID-19 treated with furosemide and negative fluid balance (NEGBAL): a different and promising approach. J Clin Med. 2021;10(23) doi: 10.3390/jcm10235599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suciadi L.P., William Y., Jorizal P., Tarigan V.N., Santoso A.H., Henrina J., et al. Comparing lung CT in COVID-19 pneumonia and acute heart failure: an imaging conundrum. Cureus. 2021;13(5) doi: 10.7759/cureus.15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui X., Chen W., Zhou H., Gong Y., Zhu B., Lv X., et al. Pulmonary edema in COVID-19 patients: mechanisms and treatment potential. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.664349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakr Y., Giovini M., Leone M., Pizzilli G., Kortgen A., Bauer M., et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaptein F.H.J., Kroft L.J.M., Hammerschlag G., Ninaber M.K., Bauer M.P., Huisman M.V., et al. Pulmonary infarction in acute pulmonary embolism. Thromb Res. 2021;202:162–169. doi: 10.1016/j.thromres.2021.03.022. [DOI] [PubMed] [Google Scholar]