Abstract
Objective
To evaluate potential viral contamination on the surfaces of personal protective equipment (PPE) in COVID-19 wards.
Methods
Face shields, gloves, the chest area of PPE and shoe soles were sampled at different time points. The samples were tested for the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by PCR, and the cycle threshold (CT) values were recorded.
Results
The positive rate was 74.7% (239/320) for all PPE specimens. The CT values of the samples were ranked in the following order: face shields > chests > gloves > shoe soles (37.08±1.38, 35.48±2.02, 34.17±1.91 and 33.52±3.16, respectively; P for trend < .001). After disinfection, the CT values of shoe soles decreased compared with before disinfection (32.78±3.47 vs. 34.3±2.61, P = .037), whereas no significant effect of disinfection on the CT values of face shields, chests and gloves was observed. After disinfection, the CT values of specimens collected from shoe soles gradually increased; before disinfection, the CT values of shoe sole specimens were all less than 35.
Conclusions
SARS-CoV-2 can attach to the surfaces of the PPE of healthcare professionals in COVID-19 wards, especially the shoe soles and undisinfected gloves. Shoe soles had the highest SARS-CoV-2 loads among all tested PPE items.
Key Words: SARS-CoV-2, Nosocomial infection, Personal protective equipment
SARS-CoV-2; Nosocomial infectionThe omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has swept the world, and a large-scale outbreak occurred in Shanghai in early March 2022. To cope with the need to treat COVID-19, a number of designated hospitals have been established in various districts of Shanghai to specifically treat positive patients. According to the job characteristics of the health care personnel working in the wards for COVID-19-positive patients, their personal protective equipment (PPE) should provide multiple layers of protection1 and include caps, medical protective masks, protective clothing, isolation gowns, goggles, protective face shields, shoe (boot) covers, and gloves. Standardized skills for wearing and removing PPE are also critical.2 Because health care personnel have the closest contact with positive patients, the standardized use of PPE is an effective measure to reduce the risk of occupational exposure.3, 4, 5
However, some infectious viruses, including SARS-CoV-2, can survive on the surfaces of objects such as PPE for a certain period of time, which may allow its spread in the environment and thus pose a potential risk of infection.6 , 7 Studies have found that virus-contaminated objects can contaminate the skin.8 In addition to being in direct contact with a contaminated environment, the health care personnel of designated hospitals perform routine operations on patients and their objects, such as turning over and patting their backs, sputum excretion, sputum suction, bedding replacement, and venipuncture, that may increase the risk of PPE surface contamination with viruses and subsequent cross-infection.9 Therefore, virus infection control on the surfaces of PPE is particularly important.
The environment10 where health care personnel work, the frequency of contact with patients, the contact area, and the different operations on the patient performed by health care personnel may cause the surfaces of different PPE items to be contaminated with the virus. Differences in the viral loads on these surfaces may result in differences in the risk of infection.11 However, the risk of viral infection and the viral load on the surfaces of different PPE items are unclear. Therefore, this study aimed to investigate the potential for viral contamination on the surfaces of PPE of health care personnel working in a designated hospital.
Methods
Research data
Samples of the surfaces of PPE of health care personnel working in the positive ward were collected. The positive ward of the hospital had 100 beds and was a designated ward for the treatment of COVID-19-positive patients. The ward was a contaminated area, and the outside of the ward was a clean area.
Definition
According to the requirements of the Technical Guidelines for the Prevention and Control of COVID-19 in Medical Institutions (Third Edition),12 which issued by Medical Administration and Hospital Authority, National Health Commission of the People's Republic of China, routine environmental disinfection of the entire ward was performed at 8 AM and 4 PM every day. The disinfection period refers to the time period between 9 AM and 12 PM and the nondisinfection period refers to the time period between 1 PM and 4 PM. The disinfection area mainly included the surfaces and floor of the ward, the treatment preparation room, the treatment room, and public areas. The disinfectant was trichloroisocyanuric acid tablets (100 tablets/bottle, effective chlorine content 500±50 mg/tablet, Hangzhou Lionser Medical Disinfectant Co., Ltd.), which were dissolved in disinfectant fluid at a concentration of 1,000 mg/L for environmental disinfection. Each disinfection lasted approximately 1 hour. The glove surface is the surface of disposable surgical gloves that have not been disinfected after work; that is, the surface of the glove is in a contaminated state, which is referred to as “glove” in the text.
The study was approved by the hospital ethics committee, and all subjects attorney in the study were informed before enrollment.
Research methods
The surfaces of the PPE of the health care personnel in the contaminated area were sampled 1 day each week (dates: April 30, 2022, May 5, 2022, and May 14, 2022) for 3 consecutive weeks. The details are as follows:
Sampling personnel
The nosocomial infection monitoring personnel were uniformly sampled. On the day of sampling, 1 person was drawn at 8 AM and 12 PM into the ward for sampling. Samples were collected at 9 AM, 10 AM, 11 AM, 12 PM, 1 PM, 2 PM, 3 PM, and 4 PM.
Sampled personnel
-
1.
The personnel in the entire ward adopted a 24-hour work system. In the contaminated area, staff rotation was performed every 4 hours, and the number of people on duty was 7 (1 doctor + 3 nurses + 3 nursing assistants); the personnel in the clean area worked for 6 hours (clean area) + 2 hours (contaminated area), and rotation was performed every 8 hours. The number of people on duty was 3 (1 doctor + 2 nurses).
Randomization method: On the day of sampling, all workers who were in the contaminated area and met the sampling standards were numbered in advance to form a sampling population; using the random nonrepeated sampling method, 1 unit was selected from the pool each time, and the sampling was performed continuously 3-4 times to form a sampling population. Samples were collected from all health care personnel in the pool.
-
1.Inclusion criteria:
-
1)Health care personnel who entered the contaminated area for 4 hours each time.
-
2)Health care personnel performing routine medical operations.
-
1)
Exclusion criteria:
Workers who entered the contaminated area for less than 4 hours in an emergency situation.
-
1.
The sampling locations were the surface of the disposable medical face shield, the surface of the disposable medical surgical gloves, the surface of the chest area of the medical protective clothing, and the surface of the sole of the medical shoe cover. The specific location is shown in Figure 1 .
-
2.
Sampling method: A disposable sampling cotton swab was dipped in virus inactivation solution or saline to moisten it, and the surface of the PPE to be sampled was completely covered and evenly smeared horizontally and vertically 3 times using swab. The swab was then placed in a vial with virus inactivation solution, the portion of the swab that had been in contact with the hand was removed by cutting or folding, and the cap of the vial was tightened.
Fig 1.
The specific sampling locations of the sampled personnel.
Record operation
The activities of all sampled personnel during the hour before sampling were recorded, and the forms were completed by asking questions (Supplementary Table S1).
Specimen delivery
After each sampling, the specimens were placed in a special specimen bag, and sealed. The surface of the bag was sprayed and wiped with disinfectant containing 1,000 mg/L chlorine bag, and the bag was placed in a special transfer box, which was sent by the transporters to the hospital's PCR laboratory for testing, follow-up and recording of nucleic acid results. According to the “New coronavirus infected pneumonia laboratory Technical Guide to Detection (second Edition) ”,13 if the value of the N gene and/or open reading frame (ORF) gene (ie, CT value) was less than 40, the specimen was considered positive, and the values of both genes were recorded. The minimum value of the 2 genes was used for analysis.
Analysis of data
We analyzed the positive rate of all specimens and each type of specimen and compared the differences in the positive rates of each specimen type at different time periods. For the positive specimens, we compared the CT values of different specimen types at different time points.
Statistical method
Statistical analysis was performed using IBM SPSS 19.0 software. Continuous variable data with normal distribution was expressed as mean ± standard deviation (Mean±SD), and was analyzed by t test. Categorical variable data was expressed as rate, and X 2 test was used for comparison. The variation tendency of CT values was analyzed by Jonckheere-Terpstra test. The differences were statistically significant at P < .05.
Results
Overall analysis
There were a total of 320 specimens, of which 239 (74.7%) were positive. The positive rates of face shields, chests, gloves and soles were 32.5% (26/40), 77.50% (62/80), 96.25% (77/80) and 92.50% (74/80), respectively. Compared with the disinfection period, the positive rates of face shields (25% vs 40%), chests (65%), and shoe soles (90% vs 95%) increased in the nondisinfection period, whereas the positive rate of gloves decreased (100% vs 92.5%). The average CT value of the 239 positive specimens was 34.63±2.60, and the CT values of the face shields (n = 26), chests (n = 62), gloves (n = 77), and shoe soles (n = 74) were successively lower (37.08±1.38, 35.48±2.02, 34.17±1.91 and 33.52±3.16, respectively; P for trend <.001).
Comparison of the positive rate of each time period
-
1.
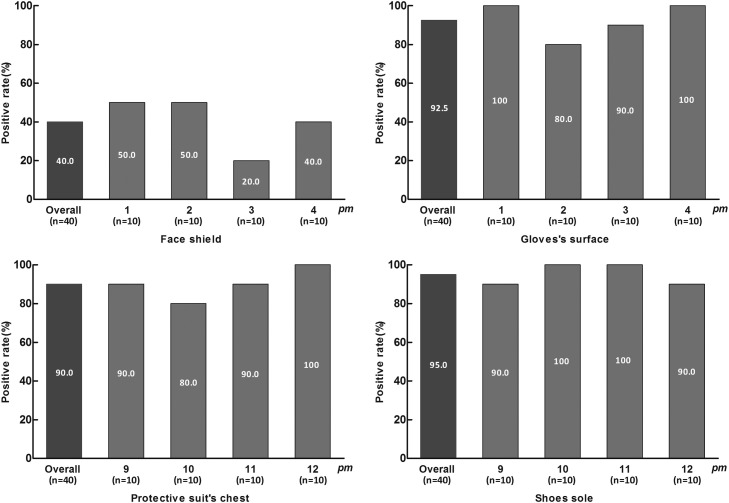
There were a total of 160 specimens during the disinfection period, of which 112 (70%) were positive. The positive rates of face shields, chests, gloves, and soles were 25% (10/40), 65% (26/40), 100% (40/40), and 90% (36/40), respectively (Fig 2 ).
-
2.
There were a total of 160 specimens in the nondisinfection period, of which 127 (79.4%) were positive. The positive rates of face shields, chests, gloves, and soles were 40% (16/40), 90% (36/40), 92.5% (37/40), and 95% (38/40), respectively (Fig 3 ).
Fig 2.
Comparison of the positive rate of each time period in the forenoon.
Fig 3.
Comparison of the positive rate of each time period in the afternoon.
Comparison of the CT values of positive specimens
-
1.
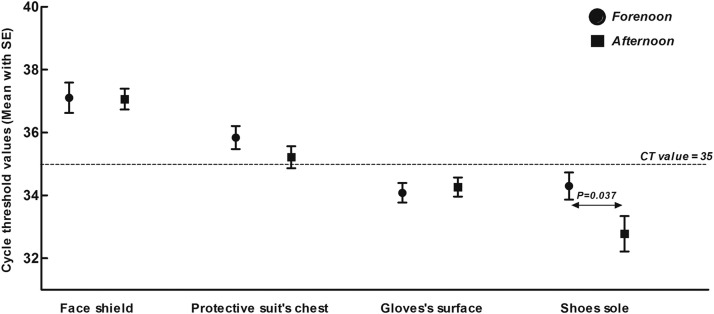
Compared with the disinfection period, the CT value of shoe soles decreased significantly during the nondisinfection period (34.3±2.61 vs 32.78±3.47, P = .037), whereas the average CT values of the positive specimens (34.44±2.80 vs 34.83±2.35, P = .252), face shields (37.06±1.33 vs 37.11±1.53, P = .939), chests (35.22±2.10 vs 35.84±1.88, P = .232), and gloves (34.08±1.99 vs 34.27±1.85, P = .675) did not change significantly (Fig 4 ).
-
2.
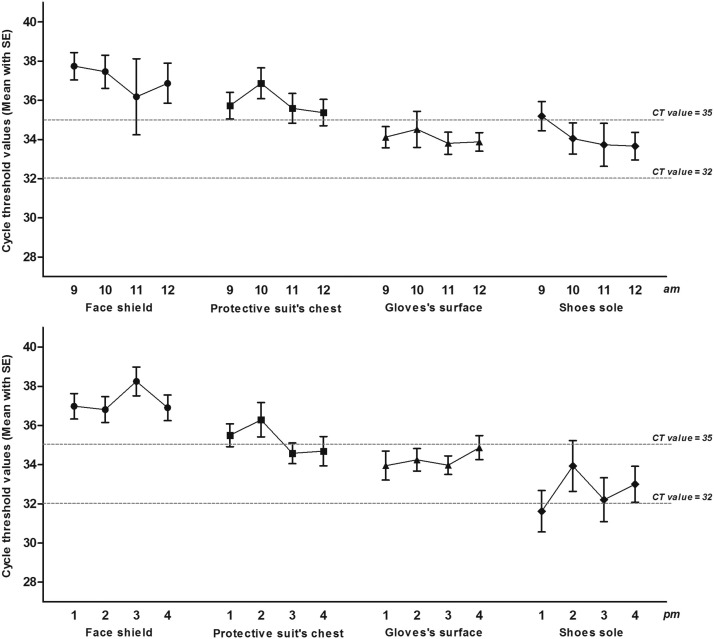
At the different time points, the CT values of the glove and shoe sole samples were almost all lower than 35, while the CT values of the face shield and chest samples were almost all higher than 35. Specifically, between 9 AM and 12 PM, the CT values each hour were as follows: glove surfaces (34.12±1.71, 34.52±2.91, 33.81±1.77, and 33.88±1.5, respectively) and sole surfaces (35.32±2.36, 34.8±2, 33.73±3.47 and 33.66±2.23, respectively). Between 1 PM and 4 PM, the CT values each hour were as follows: glove surfaces (33.95±2.33, 34.25±1.63, 33.97±1.42, and 34.87±1.93, respectively) and sole surfaces (31.62±3.16, 33.93±4.09, 32.48±3.79, and 33±2.74, respectively; P = .745). Figure 5 shows the change trend.
Fig 4.
Comparison of the CT values of positive specimens.
Fig 5.
The variation tendency of CT values of each specific sampling locations.
Discussion
This study found that SARS-CoV-2 can attach to the surfaces of PPE in designated hospitals, especially the soles of shoes and nondisinfected gloves. Among the sampled PPE items, the soles of shoes had the highest viral load.
Influenza virus, coronavirus, respiratory syncytial virus, and parainfluenza virus are the most common types of viruses that cause respiratory tract infections through droplets and aerosols,14 and a large number of SARS-CoV-2 droplets and aerosols float in the air in the wards of COVID-19 designated hospitals.15 Although multiple variants of SARS-CoV-2 have emerged, their route of transmission is basically the same, and there is a risk of infection through this route when personnel remove their PPE.2 Consequently, SARS-CoV-2 contamination on the surfaces of the PPE of health care personnel in the hospital or ward requires attention. Previous studies have found that after 4 hours of wearing PPE in the contaminated area, shoe soles are more susceptible to contamination than other PPE surfaces such as the neck, wrist and abdomen.16 The results of this study further support this conclusion. The positive rates varied among the different PPE surfaces, and the positive rates of shoe soles and nondisinfected gloves were highest. Further analysis showed that although the positive rates of the different PPE surfaces did not differ significantly among the different time points, the overall positive rate of each PPE surface was higher during the nondisinfection period in the afternoon than during the disinfection period in the morning. This difference, although not significant, may indirectly suggest a role for routine environmental abatement.
Further viral load analysis showed that shoe soles not only had the highest positive rate but also had the highest viral load. Each decrease in the CT value of SARS-CoV-2 by 1 unit means that the risk of viral infectivity is significantly increased.17 The high viral load of shoe soles may reflect the effects of gravity and airflow, which cause the virus to fall on the ground16; shoe soles may then become contaminated as personnel in the ward walk frequently to complete routine medical work tasks. In addition, this study found that the viral load of shoe soles was significantly higher during the nondisinfection period in the afternoon than during the disinfection period in the morning. This finding may suggest that the effects of conventional environmental disinfection are limited in duration. In addition, the virus may accumulate on the surfaces of PPE, which also means that it is necessary to reduce transmission of SARS-CoV-2 from the surfaces of PPE to personnel. More attention should be given to the disinfection of shoe soles.
Previous studies have shown that in only 5 seconds of contact time, 31.6% of influenza A viral particles can attach to the surface of a glove.18 In COVID-19-positive wards, gloves are the most contaminated surface.19 The results further support these conclusions. The positive rate of the surfaces of nondisinfected gloves was very high, which may be related to the fact that the virus easily attaches to the surface of gloves after contact with the body fluids and belongings of COVID-19-positive patients. The viral load on the surfaces of nondisinfected gloves was relatively high, thus reinforcing the importance of hand hygiene and hand sanitation supervision from the point of view of nosocomial infection.
Viral attachment on the face shield and chest area of the PPE is closely related to the medical operations of health care personnel. For example, during tracheal intubation, the surrounding area of the human head is easily contaminated.20 Although the positive rate was high, the viral load was significantly low (the CT values were almost all over 35), which is consistent with the results of previous studies.16 We further found that the viral load did not change significantly over time. The risk of infection from these 2 PPE surfaces may be relatively low.21 , 22
This study has certain limitations. First, this study monitored the virus on PPE surfaces, but the laboratory could only detect the viral load and could not determine viral activity. Thus, the risk of virus infection and transmission from PPE surfaces could not be determined. After disinfection in the morning, the viral load of the shoe soles showed a continuous increase with time, suggesting that it may be necessary to increase the frequency of environmental disinfection or the concentration of disinfectant. However, this study cannot provide supporting data for the effectiveness of such measures. The study was a single-center, small-scale study. For dynamic observation and monitoring, the number of samples that met the requirements was small. Therefore, multicenter, large-sample studies are still needed for further confirmation.
Conclusion
SARS-CoV-2 easily adheres to the surfaces of PPE in designated hospitals. In addition to hand hygiene, it is necessary to pay attention to the infectivity of SARS-CoV-2 adhered to the soles of shoes. Consent for publication
Not applicable.
Acknowledgments
We thank all the participants for assisting in the completion of this study while treating the patients in the COVID-19 wards.
Footnotes
Conflict of interests: None to report.
Authors' contributions: P.L.H., C.Y.J. and Y.S.Y. carried out the studies, participated in collecting data, and drafted the manuscript. G.Y.H., S.B.L., L.L.Z., X.R.X., L.X.H., L.S.Y. and D.Z.N. participated in collecting data and helped to draft the manuscript. W.G.J. performed the statistical analysis. W.F. and H.J.R. design, review and editing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate: The study protocol was approved by the Ethics Review Board of Jiading District Central Hospital (2022K10), an affiliated teaching hospital of Shanghai University of Medicine & Health Sciences. All subjects attorney in the study were informed before enrollment.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2022.10.017.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Zhou B, Zhang J, Wu AH. Key points of occupational protection in designated hospitals for COVID-19 treatment. Chin Hosp Manage. 2022;42:58–60. [Article in Chinese] [Google Scholar]
- 2.Tian Z, Stedman M, Whyte M, Anderson SG, Thomson G, Heald A. Personal protective equipment (PPE) and infection among healthcare workers - What is the evidence? Int J Clin Pract. 2020;74:e13617. doi: 10.1111/ijcp.13617. [DOI] [PubMed] [Google Scholar]
- 3.Casanova LM, Erukunuakpor K, Kraft CS, et al. Assessing viral transfer during doffing of ebola-level personal protective equipment in a biocontainment unit. Clin Infect Dis. 2018;66:945–949. doi: 10.1093/cid/cix956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomas ME, Kundrapu S, Thota P, et al. Contamination of health care personnel during removal of personal protective equipment. JAMA Intern Med. 2015;175:1904–1910. doi: 10.1001/jamainternmed.2015.4535. [DOI] [PubMed] [Google Scholar]
- 5.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seif F, Noorimotlagh Z, Mirzaee SA, et al. The SARS-CoV-2 (COVID-19) pandemic in hospital: an insight into environmental surfaces contamination, disinfectants' efficiency, and estimation of plastic waste production. Environ Res. 2021;202 doi: 10.1016/j.envres.2021.111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh M, Sadat A, Abdi R, et al. Detection of SAR-CoV-2 on surfaces in food retailers in Ontario. Curr Res Food Sci. 2021;4:598–602. doi: 10.1016/j.crfs.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behzadinasab S, Chin AWH, Hosseini M, Poon LLM, Ducker WA. SARS-CoV-2 virus transfers to skin through contact with contaminated solids. Sci Rep. 2021;11:22868. doi: 10.1038/s41598-021-00843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doos D, Barach P, Alves NJ, et al. The dangers of reused personal protective equipment: healthcare workers and workstation contamination. J Hosp Infect. 2022;127:59–68. doi: 10.1016/j.jhin.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantú VJ, Belda-Ferre P, Salido RA, et al. Implementation of practical surface SARS-CoV-2 surveillance in school settings. mSystems. 2022;7:e0010322. doi: 10.1128/msystems.00103-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barajas-Carrillo VW, Covantes-Rosales CE, Zambrano-Soria M, et al. SARS-CoV-2 transmission risk Model in an urban area of Mexico, based on GIS analysis and viral load. Int J Environ Res Public Health. 2022;19:3840. doi: 10.3390/ijerph19073840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medical Administration and Hospital Authority, National Health Commission of the People's Republic of China. Technical Guidelines for the Prevention and Control of COVID-19 in Medical Institutions (Third Edition). Accessed September 8, 2021. http://www.nhc.gov.cn/yzygj/s7659/202109/c4082ed2db674c6eb369dd0ca58e6d30.shtml
- 13.National Health Commission of the People's Republic of China. New Coronavirus Infected Pneumonia Laboratory Technical Guide to Detection (Second Edition) Accessed January 22, 2020. http://www.nhc.gov.cn/xcs/zhengcwj/202001/c67cfe29ecf1470e8c7fc47d3b751e88.shtml
- 14.Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 16.Jung J, Kim JY, Bae S, et al. Contamination of personal protective equipment by SARS-CoV-2 during routine care of patients with mild COVID-19. J Infect. 2020;81:e165–e167. doi: 10.1016/j.jinf.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9:573–586. doi: 10.1007/s40121-020-00324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH., Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Ye G, Lin H, Chen S, et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman O, Meir M, Shavit D, Idelman R, Shavit I. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091–2093. doi: 10.1001/jama.2020.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Bayat S, Mundodan J, Hasnain S, et al. Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? J Infect Public Health. 2021;14:1201–1205. doi: 10.1016/j.jiph.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabaan AA, Tirupathi R, Sule AA, et al. Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19. Diagnostics (Basel) 2021;11:1091. doi: 10.3390/diagnostics11061091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.