Abstract
Establishing the associations between magnetic resonance spectroscopy (MRS)-assessed gamma-aminobutyric acid (GABA) levels and transcranial magnetic stimulation (TMS)-derived ‘task-related’ modulations in GABAA receptor-mediated inhibition and how these associations change with advancing age is a topic of interest in the field of human neuroscience. In this study, we identified the relationship between GABA levels and task-related modulations in GABAA receptor-mediated inhibition in the dominant (left) and non-dominant (right) sensorimotor (SM) cortices. GABA levels were measured using edited MRS and task-related GABAA receptor-mediated inhibition was measured using a short-interval intracortical inhibition (SICI) TMS protocol during the preparation and premotor period of a choice reaction time (CRT) task in 25 young (aged 18–33 years) and 25 older (aged 60–74 years) adults. Our results demonstrated that GABA levels in both SM voxels were lower in older adults as compared to younger adults; and higher SM GABA levels in the dominant as compared to the non-dominant SM voxel pointed to a lateralization effect, irrespective of age group. Furthermore, older adults showed decreased GABAA receptor-mediated inhibition in the preparation phase of the CRT task within the dominant primary motor cortex (M1), as compared to young adults. Finally, results from an exploratory correlation analysis pointed towards positive relationships between MRS-assessed GABA levels and TMS-derived task-related SICI measures. However, after correction for multiple comparisons none of the correlations remained significant.
Keywords: Aging, GABA, MRS, TMS, SICI
1. Introduction
Healthy aging is characterized by a decline in motor control. At the behavioral level, age-related declines are evident in a variety of tasks including motor coordination (Boisgontier et al., 2018; Heuninckx et al., 2004; Maes et al., 2017; Marchini et al., 2017), cancelling a planned motor task (Bloemendaal et al., 2016; Smittenaar et al., 2015), and tasks requiring processing speed (Jordan and Rabbitt, 1977; Salthouse, 2000). These functional declines can be explained by a natural process of neurodegeneration and are linked with cortico-subcortical alterations (Seidler et al., 2010), including changes in structural (Boisgontier et al., 2018; Zivari Adab et al., 2018), functional (Heuninckx et al., 2005; King et al, 2018) andbiochemical (Cuypers et al., 2018; Hermans et al., 2018a; Maes, 2018) properties of the brain. These neurodegenerative processes appear to be associated with poorer regulation of cortical inhibition (Seidler et al., 2010; Levin et al., 2014). Along these lines, it has been argued that age-related motor declines can at least partly be explained by changes in cortical inhibitory function in the primary motor cortex (M1) (for a review see (Levin et al., 2014)).
The modulation of cortical inhibition in the human brain can be assessed with transcranial magnetic stimulation (TMS). Specifically, TMS can provide a measure of activation of gamma-aminobutyric acid type A (GABAA) and type B (GABAB) receptors, mediating inhibition at shorter [short-interval intracortical inhibition (SICI)] and longer [long-interval intracortical inhibition (LICI)] time scales, respectively (Ziemann et al., 2015). Furthermore, TMS is a suitable tool to investigate age-related inhibitory processes in combination with motor performance. However, while numerous TMS studies examined and identified age-related changes in the properties of the inhibitory system at rest (Hermans et al., 2018a; Marneweck et al., 2011; Peinemann et al., 2001), only few addressed age-related dynamic changes in GABAA- (Fujiyama et al., 2012a; Heise et al., 2013) and GABAB-ergic (Fujiyama et al., 2012b) inhibition during the preparation of a motor task. In the current study, we restrict the focus to the ‘fast-acting’ GABAA receptor-mediated inhibitory system. Previously, Heise et al (2013) investigated modulations in GABAA-mediated cortical inhibition during movement preparation in a visually triggered simple reaction time task and additionally tested the relations between these modulations and performance on tasks with graded dexterous demand. They reported a drastic reduction in event-related modulation of GABAA-ergic inhibition (i.e., reduced release of inhibition) in older adults, hence underscoring the particular importance of GABAA-ergic inhibition for the processing of motor actions. In another TMS study, Fujiyama et al. (2012) investigated age-related changes in modulation of GABAA-ergic inhibition during response preparation and generation of a go/no-go reaction time (RT) task (Fujiyama et al., 2012a). They reported that only young adults (and not older adults) were able to modulate GABAA-ergic inhibition (i.e. decreased inhibition) during response preparation. More importantly, both studies revealed that successful performance of a motor task in older adults is associated with the capacity to modulate cortical inhibition through GABAA-ergic neurotransmission systems.
Whereas TMS can be used to identify GABA-mediated inhibition, magnetic resonance spectroscopy (MRS) can be applied to reliably quantify in vivo GABA levels in specific regions of the brain (Puts and Edden, 2012; Mikkelsen et al, 2017, 2019; Mullins et al., 2014). Even though an age-related decrease in GABA levels in various regions of the aging brain (Cassady et al., 2019; Gao et al., 2013; Porges et al., 2017; Simmonite et al., 2019; Hermans et al., 2018b) has been identified in multiple studies, a minority reported no age-related differences (Hermans et al., 2018a; Mooney et al., 2017). On the one hand, methodological differences and tissue correction methods, in particular, may have contributed to this inconsistency in findings (Hermans et al., 2018a; Maes, 2018; Porges et al., 2017). On the other hand, it is likely that GABA levels in some brain regions are more prone to aging as compared to others (Hermans et al., 2018b).
Previous work reported no associations between sensorimotor (SM) GABA levels and ‘resting-state’ SICI (Hermans et al., 2018a; Mooney et al., 2017; Dyke et al., 2017; Tremblay et al., 2013) (targeting GABAA receptors). So far, associations between GABA levels and task-related modulations in GABAA receptor-mediated inhibition have not been investigated. In the present study, we used a multimodal approach (1) to unravel the age-related differences in GABAA receptor-mediated inhibitory processes in left and right M1’s during the preparation (period between warning and imperative signal) and premotor (period between imperative signal and movement initiation) phase of a choice reaction time (CRT) task; (2) to identify the age-related differences in the MRS measures of GABA levels in left and right SM cortices; and (3) to explore the relation between TMS measures of task-related GABAA receptor-mediated inhibition and MRS measures of GABA levels in the SM regions. We anticipated that age-related differences in GABA-ergic inhibition might be better revealed when using a more compelling motor paradigm [i.e. selecting the required effector(s) and active response inhibition of the non-selected effector(s)] as compared to resting-state GABAA receptor-mediated inhibition. Therefore, we presumed that a link between MRS GABA levels and task-related modulation in GABAA receptor-mediated inhibition would be unmasked as modulations in GABAA receptor-mediated inhibition might be dependent on MRS GABA levels in the corresponding SM brain region. More specifically, we tentatively predicted a positive relationship between GABA levels and GABAA receptor-mediated inhibition because higher GABA levels might be required to ensure successful modulation at the receptor level. Consequently, we predicted that lower SM GABA levels in older adults might limit task-related modulation in GABAA receptor-mediated inhibition.
2. Material and methods
2.1. Participants
A total of 50 healthy participants participated in this study: 25 young adults (aged 18–33 years, 22.08 ± 4.04 years (mean ± s.d.); 13 males) and 25 older adults (aged 60–74 years, 67.48 ± 4.37 years (mean ± s.d.); 12 males). All participants were right-handed according to the Edinburgh Handedness Inventory [lateralization quotient (LQ) young: 94.42 ± 10.15 (mean ± s.d.); LQ older adults: 87.64 ± 13.93 (mean ± s.d.) (Oldfield, 1971);] and had normal or corrected-to-normal vision. None of them reported a history of neurological, psychiatric, cardiovascular, or neuromuscular disorders. Participants were screened for Magnetic Resonance Imaging (MRI) (Dill, 2008) and TMS contraindications (Wassermann, 1998) and provided written informed consent prior to the start of the experiment. The protocol was approved by the local Medical Ethics Committee of KU Leuven (study number: S58333) and was conducted in accordance with the Declaration of Helsinki and its amendments (World-Medical-Association, 1964, 2008).
2.2. Experimental design
The study consisted of 3 experimental sessions. In the first session, high-resolution anatomical MRI and GABA-edited MRS data were acquired. In the second and the third session, TMS was applied to assess task-related measures of SICI in left and right M1, respectively. In each session, only one hemisphere was targeted. Stimulation order (left/right M1) was counterbalanced across participants. The timings between the MRS scan and the first TMS session, and between TMS session 1 and 2 were 9.19 ± 6.04 and 1.63 ± 2.04 weeks (mean ± s.d.), respectively.
2.3. Magnetic resonance spectroscopy
2.3.1. Data acquisition
A Philips 3T Achieva MR scanner (Philips Healthcare, The Netherlands) with a 32-channel receiver head coil was used for acquisition of a high-resolution 3D magnetization prepared rapid gradient echo (MPRAGE) T1-weighted anatomical image (TR = 9.6 ms; TE = 4.6 ms; 0.98 × 0.98 × 1.2 mm3 resolution; field of view = 256 × 256 mm2; 160 sagittal slices; flip angle = 8°). The edited MRS protocol was used to measure GABA with contribution from macromolecules (MM), commonly referred to as GABA+, using MEGA-PRESS: 14-ms editing pulses at 1.9 parts per million (ppm) of the proton frequency (edit-ON) and 7.46 ppm (edit-OFF); TR = 2000 ms; TE = 68 ms; 320 averages; 2048 points; 2 kHz spectral width; MOIST water suppression (Murdoch and Lampman, 1993). GABA + levels were measured in 3 × 3 × 3 cm3 voxels. The left and right M1 voxels were centered over the hand knob area (Yousry et al., 1997), parallel to the anterior and posterior axis. Due to overlap with the primary sensory area, we call this an SM voxel. The voxel was rotated to align with the cortical surface based on the coronal and sagittal views (see Fig. 1A).
Fig. 1.
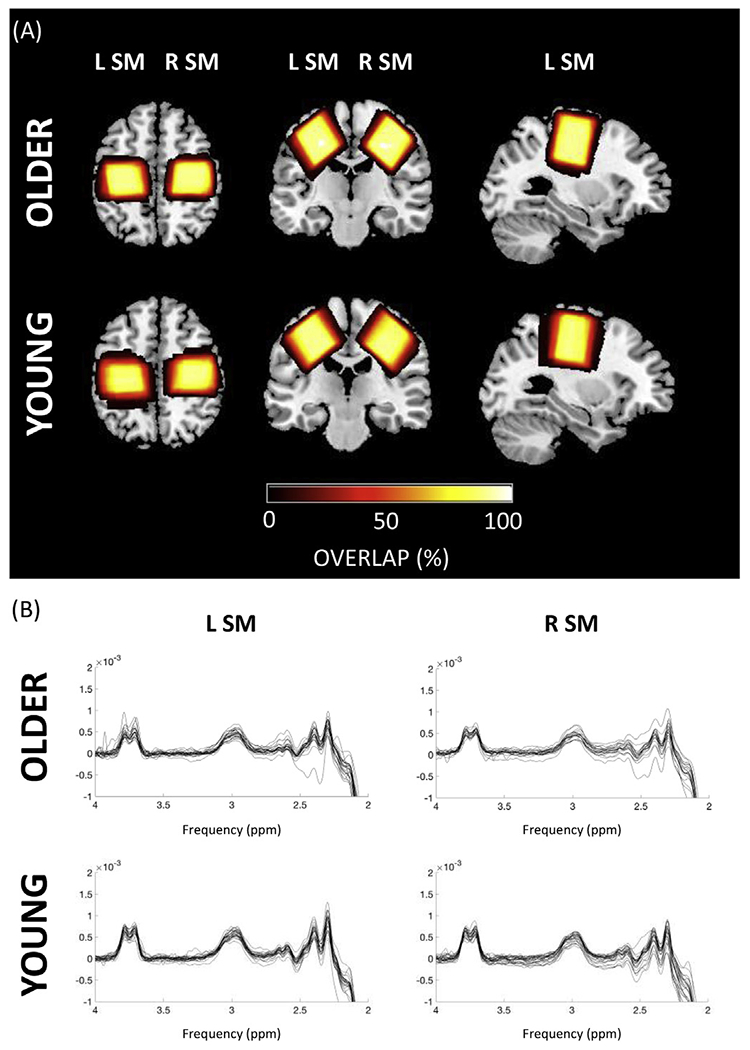
(A) Overview of the voxel positions in the left and right sensorimotor (SM) cortices for older and young adults in the axial, coronal and sagittal plane. The voxel overlap is expressed in percentage. Dark colors indicate low overlap, while bright colors indicate high overlap. (B) Spectra for left and right SM voxel for older and young adults. The GABA signal is expected at 3.0 ppm.
2.3.2. Data processing
Data were processed offline using the Gannet 3.0 toolbox (Edden et al., 2014) in MATLAB (R2016b, The MathWorks Inc., Natick, MA, 2000). Water was used as an internal concentration reference. Each dataset was frequency- and phase-corrected using spectral registration (Near et al., 2015) and filtered with 3-Hz exponential line broadening. The area under the edited GABA + signal at 3.0 ppm was estimated (see Fig. 1B). This editing scheme leads to a GABA signal that is approximately composed of 50% macromolecules, which are coupled to spins at 1.7 ppm that are also inverted by the 1.9 ppm editing pulses. Therefore, all GABA values are reported as GABA+ (i.e., GABA + macromolecules). GABA+ and unsuppressed water signals (sixteen unsuppressed water averages were acquired from each voxel) were modelled using a single Gaussian function with linear baseline parameters and a Gaussian-Lorentzian model (Edden et al., 2014), respectively. Next, MRS voxels were co-registered to the T1-weighted image and segmented to determine the different voxel tissue fractions [gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF)]. Based on these tissue fraction measurements, tissue-corrected GABA + values were obtained for each voxel (Edden et al., 2014). Tissue correction is necessary as it is assumed that GABA + levels are negligible in CSF and twice as high in GM as compared to WM (Harris et al., 2015). Additionally, tissue-specific relaxation and water visibility values were taken into account. Finally, GABA + levels from each participant were normalized to the average voxel composition of the corresponding age group (see (Harris et al., 2015); equation 6). This full tissue normalization thus results in a GABA + value taking into account the average voxel tissue composition for the cohort.
One older participant was excluded from the analyses as the data was not useable due to an error in the scanner settings. Before entering data into the statistical analysis, data quality was checked by examining the voxel placement, fit error (cut-off: 10%) of the GABA peak, the scanner frequency drift (expressed in Hz), lipid contamination, and water suppression. Subsequently, data from the left SM voxel for one older participant was excluded from the analyses due to a high fit error (fit error = 39.90%). For the right SM voxel, data from four older adults were excluded (two had fitting errors of 15.20% and 33.50%, voxel placement was incorrect for one participant, and one spectrum was compromised due to lipid contamination in another participant). Consequently, 23 datapoints from the left and 20 datapoints from the right SM for the older group and 25 datapoints from left and 25 datapoints from the right SM for the young group were included in the analyses.
2.4. Transcranial magnetic stimulation (TMS) protocol
2.4.1. Experimental task
The experimental setup, TMS protocol and an example of a single TMS trial are illustrated in Fig. 2 (panel A, B and C, respectively). Participants were seated on a comfortable chair with both forearms pronated and index fingers relaxed (EMG-controlled) resting on a platform, consisting of two pairs of micro switches that were positioned 30 cm in front of the participant. A signaling box was positioned at eye level, 1 m in front of the participant. The box consisted of a red light-emitting diode (LED) at the top and two green LEDs at the left and right corner at the bottom. The red LED served as the warning signal (WS). The two bottom green LEDs displayed the imperative signal (IS), either for a right (right move), a left index finger (left move) or a bimanual movement. For each trial, the red LED was lit for 0.5 s (preparatory period), then the red LED was switched off and finally the IS appeared by switching on one of the two green LEDs or both. The green LED was lit “on” for 1 s and then switched off. No-response trials were presented by keeping both green LEDs off until the onset of the next WS. Inter-trial intervals (i.e., time between two WS) were randomly varied between 4 and 6 s. Subjects were instructed to abduct and reposition the responding index finger as soon as possible following the IS. At the start of a trial, both index fingers were resting on their home button switches (Honeywell V-7-2B17D8-162, operating force 0.10 N, Honeywell, Charlotte, USA). These switches were embedded in the platform and the gravitational force applied to the index finger was sufficient to activate the switch. The target button switch (Omron Electronic Components D2FS-FL-N-T, operating force 0.25 N, Omron, Osaka, Japan) was set perpendicular to the platform as this orientation was most optimal to register the index finger abduction. The distance between the midpoint of the home buttons was 45 cm. Target buttons were positioned 1.5 cm medial and 1 cm lower with reference to the home button. Reaction time (RT) was defined as the time between the start of the IS and the release of the home button. For each trial, data collection was initiated 0.1 s prior to the onset of the WS and lasted for 1.5 s after WS onset. Only during the first TMS session, all participants performed an additional 40-trial practice block (12 right responses, 12 left responses, 12 bimanual responses, and 4 no-response trials) without TMS to familiarize them with the task and the setup. The same block was then repeated to calculate the timing of EMG onset. In the second session, this block was also presented, but without a familiarization block. The 75% time point of EMG onset was calculated for each participant individually and was defined as the average of the correct trials of the unimanual responses. For each trial, the 75% EMG onset was visually inspected and determined using a customized MATLAB script. The EMG signal preceding the release of the finger was rectified and onset was defined as the time point at which the EMG burst started to ramp up. We chose to include a time point for TMS application immediately prior to EMG (75% EMG) onset as we anticipated that task-related modulations in cortical excitability were present at this time point (Hinder et al., 2012).
Fig. 2.
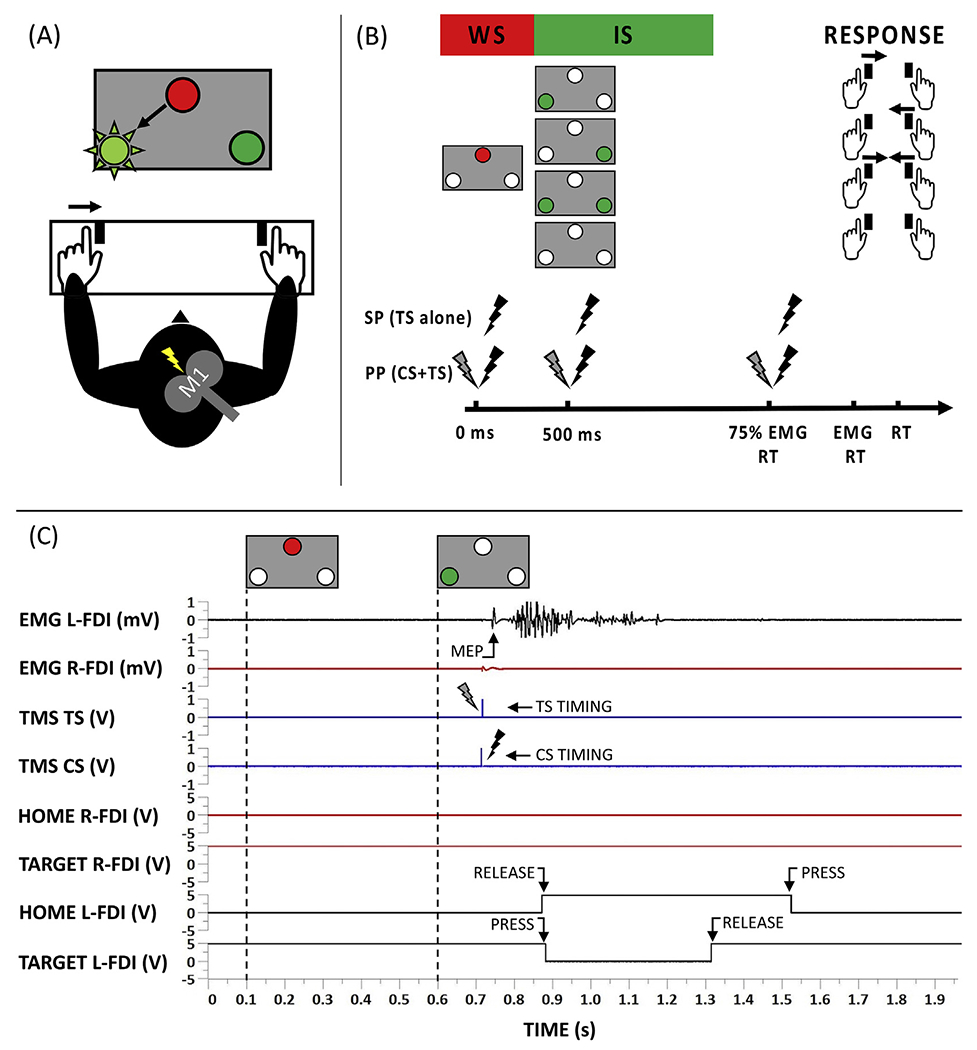
(A) Experimental setup. (B) TMS protocol. Single or paired TMS pulses were applied at 3 different time epochs: at the warning signal (WS), imperative signal (IS) or at 75% of the EMG onset. Participants were instructed to react correctly and as fast as possible to the light-up of one or two green LEDs, either for a right (right response), a left (left response) index finger movement or a bimanual response. If no LED was switched on, no reaction was required (no-response trials). (C) Example of a single TMS trial. After the WS, the IS (green led at the left side) was displayed. The participant had to react as fast as possible by releasing the corresponding home button (HOME L-FDI) and pressing the target button (TARGET L-FDI). During the trial, ppTMS was delivered to the right hemisphere at 75% of the EMG RT. Three ms after the conditioning stimulus (CS), the test stimulus (TS) was applied. Black traces correspond to the button data and EMG signal of the activated FDI, while red and blue traces correspond to the data of the non-active FDI and the TMS timings, respectively.
After determining the 75% EMG onset timing, the main experiment started. This consisted of 3 blocks, each containing 54 trials. These 54 trials were combinations of the requested response (left, right, bimanual or no-response trial), timing of the TMS pulse (WS, IS or 75% EMG onset) and type of TMS [single-pulse (sp) or paired-pulse (pp)] (see Appendix, Table A1, for details). After administration of the 3 blocks, a maximum of 12 repetitions for each TMS condition and 6 repetitions for trials without TMS was obtained. Trials without TMS were collected for calculating RT, as the TMS pulse can influence RT (Pascual-Leone et al., 1992).
2.4.2. Electromyographic recordings (EMG)
EMG signals from the left and right first dorsal interosseous (FDI) muscles were continuously monitored and measured (Bagnoli-16, Delsys Inc, Boston, USA). After amplification (gain = 1000), bandpass filtering (4–1500 Hz) and 50/60 Hz noise elimination (Humbug, Quest Scientific, North Vancouver, Canada) the recorded EMG signals were digitized at 5000 Hz (CED Signal Version 4.11, Cambridge Electronic Design, Cambridge, UK) and stored on a computer for offline analysis.
2.4.3. TMS
TMS was performed using a figure-of-eight coil with an inner wing diameter of 50 mm, connected to a Magstim BiStim2 (Magstim, Whitland, Dyfed, UK). A monophasic TMS waveform was used to induce a posterior-anterior current in the brain. Prior to experimental measurements, spTMS was used to determine the optimal stimulation locations (hotspots) of left and right M1. For this purpose, each participant wore a swimming cap, containing an orthogonal 1 × 1 cm2 coordinate system, with references to anatomical landmarks (nasion, inion, and left and right auditory meatus). TMS was applied to the scalp with the coil rotated 45° away from the midsagittal line (Brasil-Neto et al., 1992). The hotspot was defined as the scalp location yielding the highest average motor evoked potential (MEP) after five consecutive stimulations of the relaxed FDI muscle. The coil position and orientation at the hotspot were co-registered to the individual anatomical MRI image using an MRI-based neuronavigation system (Brainsight, Rogue Research Inc, Montreal, Canada). For each hotspot, the resting motor threshold (rMT) was defined as the lowest stimulation intensity evoking MEPs with an amplitude larger than 50 μV peak-to-peak in at least five out of ten consecutive trials at rest (Rossini et al., 1999). Age-related inhibitory differences within each hemisphere were assessed using ppTMS. Specifically, a conditioning stimulus (CS) was followed by a test stimulus (TS) with an interstimulus interval of 3 ms to measure SICI. CS was set at 80% rMT (Hermans et al., 2018a; Ziemann et al., 1996) and TS was adjusted to elicit unconditioned MEP amplitudes ~1 mV peak-to-peak (Hermans et al., 2018a; Heise et al., 2013).
2.4.4. Data processing
MEPs were excluded from analysis based on a strictly standardized procedure, namely in case of either incorrect or premature responses, if these occurred after the onset of voluntary EMG activity in the FDI muscle, or if these did not appear within a 40-ms window starting 10 ms after the onset of TMS. In addition, MEPs were discarded if the root mean square of the EMG signal in one of the FDI muscles exceeded 20 μV during the 50-ms period immediately preceding the onset of the TMS pulse (i.e., high background EMG). Average MEPs were calculated based on at least 8 out of 12 repetitions for each TMS condition. Pulse timing at 75% of the EMG onset was recalculated after the experiment was finalized and resulted in an average timing of 71.88 ± 10.70% EMG onset (mean ± s.d.) and 68.53 ± 11.00% EMG onset (mean ± s.d.) for older and young adults, respectively. SICI was defined accordingly: (1 – (MEPpp/MEPsp)) * 100 (Motawar et al., 2016). In this respect, positive values indicate inhibition and negative values indicate disinhibition. Participants were excluded from the analysis if the overall error rate was >20%. After excluding 4 older and 4 younger participants, the statistical analysis was performed on the data of 21 young and 21 older participants. The average error rate was 9.02 ± 5.50% (mean ± s.d.) and 4.85 ± 3.57% (mean ± s.d.) for the older and the young group, respectively.
2.5. Statistics
Linear mixed-models (LMM) were performed using R 3.3.2 (Team and R.C., 2016) (R Core team 2016; lme4 package, version 1.1–15 (Bates et al., 2015)). Data were checked for normality of the residuals using the normal quantile plot and for homoscedasticity using the residual plot. If applicable, Box-Cox (MASS package, version 7.3–48 (Venables and Ripley, 2002)) transformations were applied to optimize model assumptions of the data. Tukey honestly significant difference (HSD) post-hoc pairwise comparisons were applied to explore significant main effects and/or interactions (multcomp package version 1.4–8 (Hothorn et al., 2008)).
Reaction time was analyzed using a full-factorial 2 [AGE GROUP: young and old] × 3 [RESPONSE: left, right and bimanual] LMM, with AGE GROUP and RESPONSE as fixed effects and SUBJECT as a random intercept.
GABA + levels were analyzed by a full-factorial 2 [AGE GROUP: young and old] × 2 [VOXEL: left SM and right SM] LMM, with AGE GROUP and VOXEL as fixed effects and SUBJECT as a random intercept. FREQUENCY DRIFT and FIT ERROR were modelled as covariates as these variables can be confounders (Maes, 2018).
SICI in the preparation period was analyzed using a full-factorial 2 [AGE GROUP: young and old] × 2 [HEMISPHERE: left and right] × 2 [TIMING: WS and IS] LMM, with AGE GROUP, HEMISPHERE and TIMING as fixed effects and SUBJECT as a random intercept.
SICI in the premotor period was analyzed using a full-factorial 2 [AGE GROUP: young and old] × 2 [HEMISPHERE: left and right] x 4 [RESPONSE: left, right, bimanual and no response] LMM, with AGE GROUP, HEMISPHERE and RESPONSE as fixed effects and SUBJECT as a random intercept.
A correlation analysis for exploring relationships between GABA + levels and SICI measures was performed using JMP Pro 14 (SAS Institute). Prior to analysis, data were checked for normality. If the data was normally distributed, the Pearson correlation coefficient was calculated; otherwise, the Spearman correlation coefficient was used. In this exploratory correlational analysis, a Bonferroni correction for multiple comparisons was applied.
Age group differences in tissue fractions (GM, WM, CSF) and quality metrics (frequency drift and fit error) were identified using independent t-tests or Mann-Whitney U tests.
The significance level was set to α = 0.05 for all analyses, unless specified otherwise.
3. Results
Detailed results of the LMMs are included in Appendix, Table A2 to A5.
3.1. Reaction time
An LMM including fixed effects for AGE GROUP (young and old), RESPONSE (left, right and bimanual) and a random intercept for SUBJECT revealed a significant main effect for AGE GROUP (F1,50 = 29.104, p < 0.001). There was no significant AGE GROUP × RESPONSE interaction effect (F2,100 = 1.521, p = 0.224), nor a main effect for RESPONSE (F2,100 = 2.776, p < 0.067). These results indicate that older adults were slower than younger adults and that RT values within age groups were similar for the required response (see Table 1 for RT values).
Table 1.
Reaction times in seconds (mean ± s.d.) for both age groups (old and young) and for each response condition (left, right and bimanual).
| REQUIRED RESPONSE |
|||
|---|---|---|---|
| BIMANUAL | LEFT | RIGHT | |
| OLD | 0.409 ± 0.059 | 0.433 ± 0.070 | 0.421 ± 0.062 |
| YOUNG | 0.336 ± 0.077 | 0.335 ± 0.065 | 0.330 ± 0.061 |
3.2. GABA + levels
An LMM including fixed effects for AGE GROUP (young and old) and VOXEL (left SM and right SM), FREQUENCY DRIFT and FIT ERROR modelled as covariates and a random intercept for SUBJECT revealed a significant main effect of AGE GROUP (F1,56.535 = 12.268, p < 0.001) but no AGE GROUP × VOXEL interaction (F1,46.214 = 0.025, p = 0.874, see Fig. 3) indicating lower GABA + levels in older as compared to young adults, irrespective of the voxel location. A significant main effect of VOXEL was obtained, indicating higher GABA + levels in the voxel positioned in the dominant (left) as compared to that in the non-dominant (right) hemisphere (F1,46.209 = 4.226, p = 0.045), irrespective of age group. Tissue compositions and quality metrics for the left and right SM voxels for both young and older adults are reported in Table 2.
Fig. 3.
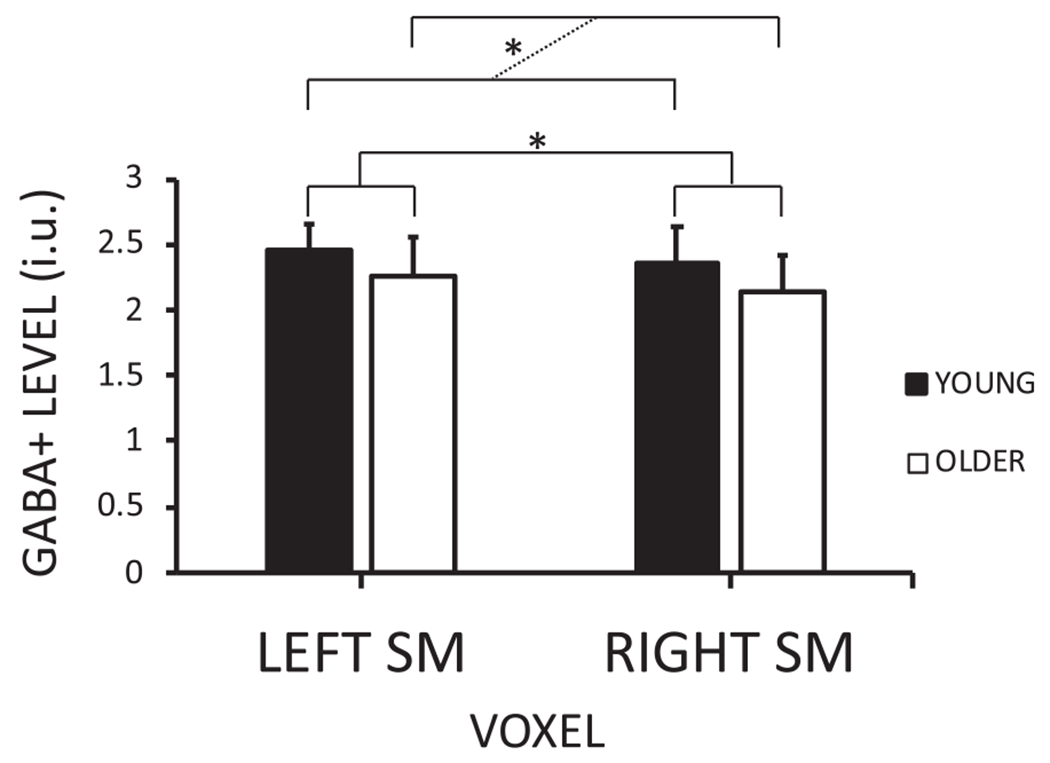
GABA + levels per voxel [left and right sensorimotor (SM)] for both age groups (young and older adults). The dashed line emphasizes the age group (young vs. older) comparison. P < 0.05 is indicated by an asterisk.
Table 2.
Tissue fractions and quality metrics (mean ± s.d.) of the left (dominant) and right (non-dominant) sensorimotor voxel for both young and older adults.
| TISSUE FRACTION | DOMINANT (LEFT) SENSORIMOTOR VOXEL |
NON-DOMINANT (RIGHT) SENSORIMOTOR VOXEL |
||||
|---|---|---|---|---|---|---|
| YOUNG | OLDER | p value | YOUNG | OLDER | p value | |
| GRAY MATTER | 0.334 ± 0.030 | 0.257 ± 0.030 | <0.001 | 0.355 ± 0.031 | 0.267 ± 0.033 | <0.001 |
| WHITE MATTER | 0.605 ± 0.037 | 0.623 ± 0.042 | 0.051 | 0.573 ± 0.034 | 0.598 ± 0.040 | 0.035 |
| CEREBROSPINAL FLUID | 0.060 ± 0.015 | 0.119 ± 0.036 | <0.001 | 0.072 ± 0.019 | 0.134 ± 0.031 | <0.001 |
| QUALITY METRIC | YOUNG | OLDER | p value | YOUNG | OLDER | p value |
| FREQUENCY DRIFT (Hz) | 0.461 ± 0.263 | 0.743 ± 0.304 | <0.001 | 0.418 ± 0.242 | 0.779 ± 0.332 | <0.001 |
| FIT ERROR | 4.154 ± 1.337 | 4.513 ± 1.663 | 0.635 | 4.228 ± 1.050 | 4.469 ± 1.233 | 0.689 |
3.3. SICI
3.3.1. Preparation period
SICI profiles are presented in Fig. 4 and in Fig A1 (see appendix). An LMM including fixed effects for AGE GROUP (young and old), HEMISPHERE (left and right) and TIMING (WS and IS), and a random intercept for SUBJECT revealed a significant main effect of AGE GROUP (F1,40 = 4.661, p = 0.037) and a significant AGE GROUP × HEMISPHERE interaction (F1,120 = 4.846, p = 0.030). Tukey HSD contrasts revealed reduced inhibition in older as compared to younger adults in the dominant (left) hemisphere (z value = −2.914, p = 0.017). The AGE GROUP × HEMISPHERE × TIMING (F1,120 = 0.074, p = 0.787) and AGE GROUP × TIMING (F1,120 = 0.112, p = 0.727) interactions were not significant, indicating similar SICI values for both age groups from WS to IS.
Fig. 4.
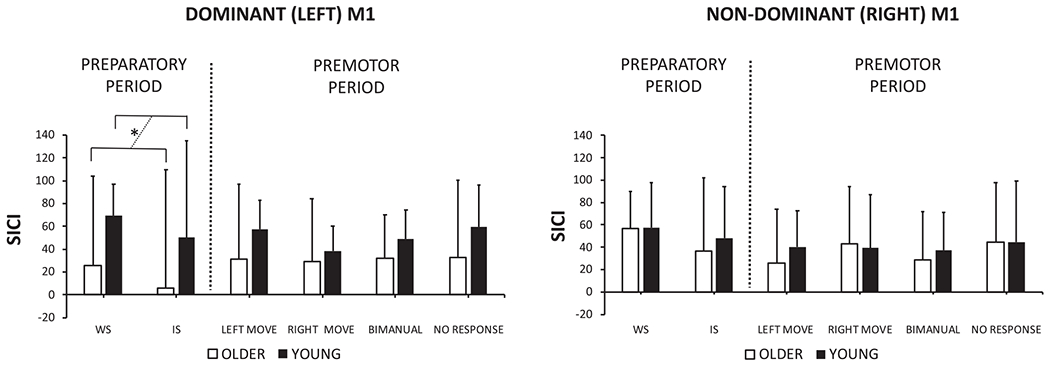
Short-interval intracortical inhibition (SICI) profiles for older and young adults for the left and right hemisphere at the warning signal (WS), imperative signal (IS) and at 75% of the EMG onset (left move, right move, bimanual and no response). SICI was defined as: (1 – (MEPpp/MEPsp)) * 100. Positive values indicate inhibition and negative values indicate disinhibition. The dashed line emphasizes the age group (young vs. older) comparison. P < 0.05 is indicated by an asterisk.
3.3.2. Premotor period
An LMM including fixed effects for AGE GROUP, HEMISPHERE and RESPONSE (left, right, bimanual and no-response), using SUBJECT as a random intercept and all possible interactions revealed a significant main effect of RESPONSE (F3,293.060 = 6.460, p < 0.001) and a RESPONSE × HEMISPHERE (F3,293.060 = 3.260, p = 0.022) interaction. These results will not be further discussed, as they fall outside the scope of this work. Tukey HSD contrasts are reported in Table A6 in the appendix. The AGE GROUP × HEMISPHERE × RESPONSE (F3,293.060 = 0.299, p = 0.826), AGE GROUP × RESPONSE (F3,293.060 = 1.523, p = 0.209) and AGE GROUP × HEMISPHERE (F1,293.061 = 1.793, p = 0.182) interactions were not significant, indicating that SICI profiles in the premotor period did not depend on AGE GROUP.
3.4. Relationship between GABA+ and SICI measures
An exploratory correlation analysis (see Table 3) revealed positive relationships between GABA + levels in the non-dominant (right) SM voxel and SICI in the non-dominant (right) M1 at the WS (r = 0.327; p = 0.017, see Fig. A2 in the appendix), and between GABA + level in the non-dominant (right) SM voxel and SICI over the non-dominant (right) M1 in the condition when a bimanual response was required (r = 0.347; p = 0.028, see Fig. A3 in the appendix) for older adults, suggesting that higher GABA + levels were related to stronger inhibition at these TMS timings. However, after Bonferroni correction none of the correlations remained significant (critical p-value for significance: 0.005/6 = 0.008).
Table 3.
Exploratory correlations (r) between GABA + levels and TMS measures of inhibition for older and young adults for the left and right hemisphere. Correlations are illustrated for each time point [at warning signal (WS), imperative signal (IS), left move, right move, bimanual move and no response].
| OLDER ADULTS |
YOUNG ADULTS |
|||||||
|---|---|---|---|---|---|---|---|---|
| DOMINANT (L) HEMI |
NON-DOMINANT (R) HEMI |
DOMINANT (L) HEMI |
NON-DOMINANT (R) HEMI |
|||||
| r | p | r | p value | r | p | r | p value | |
| PREPARATION PERIOD | ||||||||
| WS | −0.094 | 0.402 | 0.327 | 0.017 | −0.280 | 0.203 | 0.266 | 0.181 |
| IS | 0.022 | 0.806 | 0.326 | 0.076 | −0.149 | 0.767 | 0.259 | 0.278 |
|
| ||||||||
| PREMOTOR PERIOD | ||||||||
| LEFT MOVE | 0.091 | 0.336 | 0.129 | 0.388 | 0.000 | 0.992 | 0.047 | 0.841 |
| RIGHT MOVE | 0.061 | 0.724 | 0.189 | 0.520 | −0.049 | 0.832 | 0.103 | 0.266 |
| BIMANUAL MOVE | −0.151 | 0.525 | 0.347 | 0.028 | −0.041 | 0.860 | 0.192 | 0.404 |
| NO RESPONSE | −0.023 | 0.777 | 0.295 | 0.058 | −0.075 | 0.924 | 0.267 | 0.061 |
4. Discussion
The present study yielded three major findings. First, GABA + levels in both dominant and non-dominant SM voxels were found to be significantly lower in older adults compared to their younger counterparts. Additionally, a lateralization effect was identified, indicating significantly higher GABA + levels in the dominant as compared to the non-dominant SM voxel, irrespective of age group. Second, as compared to young adults, older adults showed a significant decrease of inhibition (as measured with SICI) in the preparation phase of the CRT task within the dominant M1. Finally, results from an exploratory correlation analysis pointed towards positive relationships between MRS-assessed GABA levels and TMS-derived task-related SICI measures. However, after correction for multiple comparisons none of the correlations remained significant.
4.1. Age-related differences in GABA + levels and lateralization between SM regions
GABA + levels in the SM voxels were significantly lower in older as compared to younger adults. This result is in line with other work (Cassady et al., 2019; Chalavi et al., 2018; Grachev et al., 2001) which also reported significantly lower SM GABA + levels in older as compared to young adults. In contrast, some studies reported no differences in SM GABA + levels between age groups (Hermans et al., 2018a; Mooney et al., 2017). As the field of GABA MRS is rapidly evolving, it is highly likely that differences in results can be accounted for by advancements in methodology and analysis methods. For example, Maes, 2018 reported that the identification of age-related changes in GABA + levels is dependent on whether brain structure alterations are considered in the quantification of GABA + levels (Maes, 2018). The study of GABA levels is of broader interest for the neuroscience of aging because GABA may play a role in compensation versus dedifferentiation mechanisms of aging. More specifically, brain dedifferentiation refers to reduced neural distinctiveness of neural representations in older adults and this may be a direct consequence of reduced inhibitory function. A decrease in neural specialization may be associated with increased brain activation and/or increased functional connectivity among the brain networks in older adults (King et al., 2018).
In addition to an age-related difference in SM GABA + levels, the current study identified, for the first time, an ‘asymmetry’ in SM GABA + levels between the dominant and non-dominant hemisphere, irrespective of age group. Our results differ from previous work of Puts et al (2018) and Grewal et al (2016) which reported hemispheric ‘symmetry’ in GABA distribution (Grewal et al., 2016; Puts et al., 2018). In the study of Puts et al (2018) left and right SM GABA + levels were assessed. The discrepancy in findings may be due to differences in sample composition, statistical power and hand preference. Although right-handed participants were investigated in both studies, degree of hand preference seemed to be different between studies (LQ Puts et al (2018): 67.38 ± 25.16 (mean ± s.d.) vs. LQ in the current study: young: 94.42 ± 10.15 (mean ± s.d.); older adults: 87.64 ± 13.93 (mean ± s.d.)). Since Puts et al (2018) reported a trend between GABA + levels in the dominant SM and hand preference (LQ) (Puts et al., 2018), we performed an additional analysis adding hand preference as a covariate into the model. However, statistical analysis did neither reveal a significant effect of hand preference on GABA + levels, nor did it change any conclusions. Grewal et al (2016) did not investigate left and right SM GABA + levels but reported hemispheric symmetry in left and right frontal, parietal and occipital regions (Grewal et al., 2016).
Yet, it is not clear why GABA + levels are significantly higher in the dominant as compared to the non-dominant hemisphere. This result may be consistent with the observation of a dominantly left-lateralized parietal-to-(pre)motor activation network (Swinnen et al., 2010), underscoring the dominance of the left hemisphere in various aspects of motor control (for a review see (Serrien et al., 2006)). There is also evidence that higher SM GABA + levels are related to better sensorimotor performance (for a review see (Stagg, 2014)). Moreover, it is likely that higher GABA + levels give rise to a better GABA modulation and that this modulatory capacity is stronger in the dominant hemisphere. In this respect, a study from Hammond et al (2004) showed that both inhibitory and excitatory intracortical circuits are more efficiently modulated in the dominant as compared to the non-dominant hemisphere (Hammond et al., 2004). Similarly, Civardi et al (2000) reported a significant enhancement of intracortical facilitation and inhibition in the dominant as compared to the non-dominant hemisphere (Civardi et al., 2000). Besides asymmetries in intracortical circuits, there is also evidence for asymmetry in interhemispheric inhibition (Baumer et al., 2007; Netz et al., 1995). Moreover, inhibition from the dominant to the non-dominant hemisphere is found to be stronger as compared to the opposite direction (Baumer et al., 2007; Netz et al., 1995). Therefore, we can speculate that higher GABA + levels in the dominant M1 could be related to a more efficient modulation of intra-and inter-cortical excitability which might result in better execution of more complex movements (requiring fine motor control) in the dominant hand. To clarify this idea, future research linking GABA + levels with a more extensive battery of neurophysiological measurements and motor tasks is recommended.
We also investigated whether asymmetry in GABA + levels could be explained by anatomical asymmetry in the motor cortex (Amunts et al., 1996; Foundas et al., 1998). However, the similarity in tissue fractions of the left and right SM voxels reported in the current study does not support the idea that the reported GABA + level asymmetry could be attributed to morphological asymmetry.
4.2. Age-related differences in task-related GABAA receptor-mediated inhibition
The age-related reduction in inhibition observed in the current study is in line with previous experiments that measured event-related SICI (Heise et al., 2013) or SICI at rest (Hermans et al., 2018a; Marneweck et al., 2011; Peinemann et al., 2001). This decrease of inhibition was observed only in the dominant M1 and only during the preparation phase of the CRT task. This observation is in agreement with the findings of our earlier work (Cuypers et al., 2013) in which a similar TMS-CRT paradigm was used, albeit without any ppTMS SICI recordings, focusing only on spTMS excitability. Here, Cuypers et al (2013) revealed that older adults showed significantly less MEP suppression as compared to young adults in the (left) dominant M1 during the preparation phase (Cuypers et al., 2013). In the premotor period, no significant age group effect was reported, consistent with our previous work (Cuypers et al., 2013) in which similar MEP levels for older and young adults were found towards movement onset of a CRT task.
4.3. Age-related associations between GABA + levels and task-related modulations in GABAA receptor-mediated inhibition
Recent studies showed no association between resting-state SICI, targeting GABAA receptors, and SM GABA + levels (Hermans et al., 2018a; Mooney et al, 2017; Dyke et al., 2017; Tremblay et al., 2013). Our exploratory correlation analysis, however, suggests relationships between task-related SICI and GABA + levels. Interestingly, these relationships were only found in older adults and were restricted to the non-dominant hemisphere. More specifically, there was a positive relationship between GABA + levels and SICI (both measured in the non-dominant hemisphere) at the warning signal of the preparatory period and in the bimanual response condition of the premotor period (see appendix, Fig. A2 & A3), suggesting that higher GABA + levels were related to stronger inhibition. Nonetheless, the results from this exploratory correlation analysis should be interpreted with caution as none of the tests survived correction for multiple comparisons. Future research is desirable to confirm these results and unravel the underlying mechanisms.
4.4. Limitations
A first limitation is the relatively large voxel size (3 × 3 × 3 cm3) used for GABA-edited MRS. Consequently, the volume measured with MRS exceeds the area targeted with TMS. Therefore, GABA levels measured in this study are originating not only from the M1 region, but also from the adjacent primary somatosensory cortex (S1). However, to ensure a sufficient signal-to-noise ratio and avoid long scanning times, a voxel of this size is commonly used for GABA-edited MRS and offers a realistic compromise between voxel size and signal quality (Mullins et al., 2014). A second limitation is the number of pulses applied per condition. Although, 12 repetitions per TMS condition were provided and at least 8 out of 12 MEPs were used for calculation of the average MEP per condition, Chang et al (2016) indicated that 21 and 20 repetitions are optimal for single-pulse TMS and SICI (Chang et al., 2016). Based on the data provided by Chang et al (2016), the Cronbach’s alpha and the probability of inclusion in the 95% confidence interval (CI) around the mean MEP amplitude for the current study ranges between 0.858 - 0.980 and 51.9%–83.3%, respectively. A third limitation concerns the variability of SICI. Even though we applied an established SICI protocol that is reported to result into robust and stable measures of inhibition (Chen et al., 1998; Kujirai et al., 1993), expressing the CS intensity as a percentage of the individual threshold for intracortical inhibition, as suggested by Orth et al. (2003), might further reduce the variability of the SICI measurement (Orth et al., 2003).
5. Conclusion
We have demonstrated that GABA + levels in both SM voxels were lower in older as compared to their younger counterparts and found a lateralization effect, indicating higher SM GABA + levels in the dominant as compared to the non-dominant hemisphere in both age groups. Further, older adults showed decreased SICI in the preparation phase of the CRT task within the dominant M1, as compared to young adults. Finally, associations between SM GABA + levels and task-related modulations in GABAA receptor-mediated inhibition were explored and pointed towards positive relationships. However, after correction for multiple comparisons none of the correlations remained significant.
Acknowledgments
The authors wish to thank Ir. P. Meugens and R. Clerckx for their crucial assistance in the development of the setup and the automatization of data processing, respectively. We additionally thank S. Vanuytven, K. Quadflieg and S. Denissen for their assistance with data collection.
Funding sources
This work was supported by the Research Fund KU Leuven (C16/15/070) and the Research Foundation Flanders grant (G089818N) and Excellence of Science grant (EOS 30446199, MEMODYN) awarded to SPS and DM and by the Hercules fund AUHL/11/01 (R-3987) awarded to RM and I005018N, awarded to DM and SPS. This work applies tools developed under NIH R01 EB016089 EB023693 and P41 EB015909; RAEE also receives salary support from the latter grants. The authors declare no competing financial interests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix A
Table A.1.
Overview of all combinations of TMS timing [warning signal (WS), imperative signal (IS) or at 75% EMG onset], type of TMS (single-pulse or paired-pulse) and requested response (left, right, bimanual or no-response trial).
| COMBINATION | TMS TIMING | TYPE OF TMS | REQUESTED RESPONSE | REPETITIONS |
|---|---|---|---|---|
| 1 | WS | SINGLE-PULSE | LEFT | 1 |
| 2 | WS | SINGLE-PULSE | RIGHT | 1 |
| 3 | WS | SINGLE-PULSE | BIMANUAL | 1 |
| 4 | WS | SINGLE-PULSE | NO RESPONSE | 1 |
| 5 | IS | SINGLE-PULSE | LEFT | 1 |
| 6 | IS | SINGLE-PULSE | RIGHT | 1 |
| 7 | IS | SINGLE-PULSE | BIMANUAL | 1 |
| 8 | IS | SINGLE-PULSE | NO RESPONSE | 1 |
| 9 | 75% EMG | SINGLE-PULSE | LEFT | 4 |
| 10 | 75% EMG | SINGLE-PULSE | RIGHT | 4 |
| 11 | 75% EMG | SINGLE-PULSE | BIMANUAL | 4 |
| 12 | 75% EMG | SINGLE-PULSE | NO RESPONSE | 4 |
| 13 | WS | PAIRED-PULSE | LEFT | 1 |
| 14 | WS | PAIRED-PULSE | RIGHT | 1 |
| 15 | WS | PAIRED-PULSE | BIMANUAL | 1 |
| 16 | WS | PAIRED-PULSE | NO RESPONSE | 1 |
| 17 | IS | PAIRED-PULSE | LEFT | 1 |
| 18 | IS | PAIRED-PULSE | RIGHT | 1 |
| 19 | IS | PAIRED-PULSE | BIMANUAL | 1 |
| 20 | IS | PAIRED-PULSE | NO RESPONSE | 1 |
| 21 | 75% EMG | PAIRED-PULSE | LEFT | 4 |
| 22 | 75% EMG | PAIRED-PULSE | RIGHT | 4 |
| 23 | 75% EMG | PAIRED-PULSE | BIMANUAL | 4 |
| 24 | 75% EMG | PAIRED-PULSE | NO RESPONSE | 4 |
| 25 | – | NO TMS | LEFT | 2 |
| 26 | – | NO TMS | RIGHT | 2 |
| 27 | – | NO TMS | BIMANUAL | 2 |
Table A.2.
Reaction time: Output of the linear mixed model including parameter estimates and Type III tests. An asterisk indicates a significant P value (P < 0.05).
| REACTION TIME | |||||
|---|---|---|---|---|---|
|
| |||||
| Fixed effect estimates | ESTIMATE | STD. ERROR | DF | t VALUE | P (>|t|) |
| INTERCEPT | −1.07852 | 0.04814 | 61.29 | −22.405 | <2e-16 * |
| AGE GROUP = YOUNG | −0.31183 | 0.06808 | 61.29 | −4.581 | 2.33e-05 * |
| RESPONSE = LEFT | 0.07397 | 0.02631 | 100.00 | 2.812 | 0.00593 * |
| RESPONSE = RIGHT | 0.03901 | 0.02631 | 100.00 | 1.483 | 0.14126 |
| AGE GROUP = YOUNG x RESPONSE = LEFT | −0.06165 | 0.03720 | 100.00 | −1.657 | 0.10062 |
| AGE GROUP = YOUNG x RESPONSE = RIGHT | −0.04834 | 0.03720 | 100.00 | −1.299 | 0.19684 |
| Type III tests of fixed effects | SS | NUM DF | DEN DF | F VALUE | P (>F) |
|
| |||||
| AGE GROUP | 0.251787 | 1 | 50 | 29.1041 | 1.876e-06 * |
| RESPONSE | 0.048039 | 2 | 100 | 2.7764 | 0.06707 |
| AGE GROUP x RESPONSE | 0.026313 | 2 | 100 | 1.5208 | 0.22355 |
Table A.3.
GABA + levels: Output of the linear mixed model including parameter estimates and Type III tests. An asterisk indicates a significant P value (P < 0.05).
| GABA + LEVELS | |||||
|---|---|---|---|---|---|
|
| |||||
| Fixed effect estimates | ESTIMATE | STD. ERROR | DF | t VALUE | P(>|t|) |
| INTERCEPT | 2.29946 | 0.12692 | 91.94 | 18.117 | <2e-16 * |
| AGE GROUP = YOUNG | 0.22666 | 0.07746 | 86.00 | 2.926 | 0.00439 * |
| VOXEL = RIGHT SM | −0.09710 | 0.06468 | 47.41 | −1.501 | 0.13995 |
| FREQUENCY DRIFT | 0.10491 | 0.09627 | 88.00 | 1.090 | 0.27880 |
| FIT ERROR | −0.02839 | 0.01980 | 92.93 | −1.434 | 0.15500 |
| AGE GROUP = YOUNG x VOXEL = RIGHT | 0.01400 | 0.08790 | 46.21 | 0.159 | 0.87413 |
| Type III tests of fixed effects | SS | NUM DF | DEN DF | F VALUE | P (>F) |
|
| |||||
| AGE GROUP | 0.53700 | 1 | 56.535 | 12.2675 | 0.0009081 * |
| VOXEL | 0.18501 | 1 | 46.209 | 4.2264 | 0.0454751 * |
| FREQUENCY DRIFT | 0.05199 | 1 | 88.002 | 1.1876 | 0.2787965 |
| FIT ERROR | 0.08999 | 1 | 92.929 | 2.0557 | 0.1549981 |
| AGE GROUP x VOXEL | 0.00111 | 1 | 46.214 | 0.0254 | 0.8741312 |
Table A.4.
SICI preparatory period: Output of the linear mixed model including parameter estimates and Type III tests. An asterisk indicates a significant P value (P < 0.05). Note that for modeling purposes SICI values were defined as MEPpp / MEPsp, instead of (1 - (MEPpp / MEPsp)) * 100. This was done because negative values cannot be transformed using box-cox transformations (λ = 0.010).
| SICI - preparatory period | |||||
|---|---|---|---|---|---|
|
| |||||
| Fixed effect estimates | ESTIMATE | STD. ERROR | DF | t VALUE | P (>|t|) |
| INTERCEPT | −0.6286 | 0.2123 | 102.19 | −2.960 | 0.00382 * |
| AGE GROUP = YOUNG | −0.6704 | 0.3003 | 102.19 | −2.232 | 0.02777 * |
| HEMISPHERE = RIGHT | −0.2407 | 0.2259 | 120.00 | −1.066 | 0.28871 |
| TIMING = WARNING SIGNAL | −0.1011 | 0.2259 | 120.00 | −0.448 | 0.65524 |
| AGE GROUP = YOUNG x HEMISPHERE = RIGHT | 0.436 | 0.3194 | 120.00 | 1.365 | 0.17482 |
| HEMISPHERE = RIGHT x TIMING = WARNING SIGNAL | −0.1002 | 0.3194 | 120.00 | −0.314 | 0.75441 |
| AGE GROUP = YOUNG x TIMING = WARNING SIGNAL | −0.1402 | 0.3194 | 120.00 | −0.439 | 0.66143 |
| AGE GROUP = YOUNG x HEMISPHERE = RIGHT x TIMING = WARNING SIGNAL | 0.1225 | 0.4517 | 120.00 | 0.271 | 0.78680 |
| Type III tests of fixed effects | SS | NUM DF | DEN DF | F VALUE | P (>F) |
|
| |||||
| AGE GROUP | 2.49664 | 1 | 40 | 4.6605 | 0.03692 * |
| HEMISPHERE | 0.07467 | 1 | 120 | 0.1394 | 0.70954 |
| TIMING | 1.52720 | 1 | 120 | 2.8508 | 0.09392 |
| AGE GROUP x HEMISPHERE | 2.59615 | 1 | 120 | 4.8463 | 0.02962 * |
| HEMISPHERE x TIMING | 0.01591 | 1 | 120 | 0.0297 | 0.86345 |
| AGE GROUP x TIMING | 0.06555 | 1 | 120 | 0.1224 | 0.72710 |
| AGE GROUP x HEMISPHERE x TIMING | 0.03937 | 1 | 120 | 0.0735 | 0.78680 |
Table A.5.
SICI premotor period: Output of the linear mixed model including parameter estimates and Type III tests. An asterisk indicates a significant P value (P < 0.05). Note that for modeling purposes SICI values were defined as MEPpp / MEPsp, instead of (1 - (MEPpp / MEPsp)) * 100. This was done because negative values cannot be transformed using box-cox transformations (λ = 0.127).
| SICI - premotor period | |||||
|---|---|---|---|---|---|
|
| |||||
| Fixed effect estimates | ESTIMATE | STD. ERROR | DF | t VALUE | P (>|t|) |
| INTERCEPT | −0.550478 | 0.145877 | 132.31 | −3.774 | 0.000242 * |
| AGE GROUP = YOUNG | −0.183110 | 0.206301 | 132.31 | −0.888 | 0.376373 |
| HEMISPHERE = RIGHT | 0.068666 | 0.150368 | 293.02 | 0.457 | 0.648257 |
| RESPONSE = LEFT | −0.107971 | 0.152464 | 293.30 | −0.708 | 0.479400 |
| RESPONSE = NO | −0.161524 | 0.150368 | 293.02 | −1.074 | 0.283622 |
| RESPONSE = RIGHT | 0.003577 | 0.150368 | 293.02 | 0.024 | 0.981040 |
| AGE GROUP = YOUNG X HEMISPHERE = RIGHT | 0.074862 | 0.212653 | 293.02 | 0.352 | 0.725060 |
| HEMISPHERE = RIGHT x RESPONSE = LEFT | 0.129753 | 0.214140 | 293.17 | 0.606 | 0.545032 |
| HEMISPHERE = RIGHT x RESPONSE = NO | −0.216634 | 0.212653 | 293.02 | −1.019 | 0.309174 |
| HEMISPHERE = RIGHT x RESPONSE = RIGHT | −0.299863 | 0.212653 | 293.02 | −1.410 | 0.159569 |
| AGE GROUP = YOUNG x RESPONSE = LEFT | −0.116089 | 0.214140 | 293.17 | −0.542 | 0.588151 |
| AGE GROUP = YOUNG x RESPONSE = NO | −0.191718 | 0.212653 | 293.02 | −0.902 | 0.368033 |
| AGE GROUP = YOUNG x RESPONSE = RIGHT | 0.201932 | 0.212653 | 93.02 | 0.950 | 0.343105 |
| AGE GROUP = YOUNG x HEMISPHERE = RIGHT x RESPONSE = LEFT | 0.046625 | 0.301790 | 293.10 | 0.154 | 0.877325 |
| AGE GROUP = YOUNG x HEMISPHERE = RIGHT x RESPONSE = NO | 0.237951 | 0.300736 | 293.02 | 0.791 | 0.429450 |
| AGE GROUP = YOUNG x HEMISPHERE = RIGHT x RESPONSE = RIGHT | 0.013500 | 0.300736 | 293.02 | −0.045 | 0.964226 |
| Type III tests of fixed effects | SS | NUM DF | DEN DF | F VALUE | P (>F) |
|
| |||||
| AGE GROUP | 0.1992 | 1 | 42.026 | 0.8390 | 0.3649170 |
| HEMISPHERE | 0.1569 | 1 | 293.061 | 0.6609 | 0.4168903 |
| RESPONSE | 4.5997 | 3 | 293.060 | 6.4582 | 0.0003017 * |
| AGE GROUP x HEMISPHERE | 0.4257 | 1 | 293.061 | 1.7932 | 0.1815743 |
| HEMISPHERE x RESPONSE | 2.3216 | 3 | 293.060 | 3.2597 | 0.0219303 * |
| AGE GROUP x RESPONSE | 1.0843 | 3 | 293.060 | 1.5225 | 0.2087618 |
| AGE GROUP x HEMISPHERE x RESPONSE | 0.2132 | 3 | 293.060 | 0.2993 | 0.8259178 |
Table A.6.
Tukey HSD contrasts for all combinations of hemisphere (left and right) and action (left move, right move, bimanual move and no response). SICI values were defined as MEPpp / MEPsp. P values in bold indicate a significant difference between contrasts.
| CONTRAST | z | p value | ||
|---|---|---|---|---|
| LEFT HEMI - LEFT MOVE | vs | LEFT HEMI - BIMANUAL MOVE | −1.534 | 0.789 |
| LEFT HEMI - NO RESPONSE | vs | LEFT HEMI - BIMANUAL MOVE | −2.362 | 0.260 |
| LEFT HEMI - RIGHT MOVE | vs | LEFT HEMI - BIMANUAL MOVE | 0.960 | 0.980 |
| RIGHT HEMI - BIMANUAL MOVE | vs | LEFT HEMI - BIMANUAL MOVE | 0.974 | 0.978 |
| RIGHT HEMI - LEFT MOVE | vs | LEFT HEMI - BIMANUAL MOVE | 0.855 | 0.990 |
| RIGHT HEMI - NO RESPONSE | vs | LEFT HEMI - BIMANUAL MOVE | −2.285 | 0.302 |
| RIGHT HEMI - RIGHT MOVE | vs | LEFT HEMI - BIMANUAL MOVE | −0.881 | 0.988 |
| LEFT HEMI - NO RESPONSE | vs | LEFT HEMI - LEFT MOVE | −0.813 | 0.993 |
| LEFT HEMI - RIGHT MOVE | vs | LEFT HEMI - LEFT MOVE | 2.487 | 0.201 |
| RIGHT HEMI - BIMANUAL MOVE | vs | LEFT HEMI - LEFT MOVE | 2.501 | 0.195 |
| RIGHT HEMI - LEFT MOVE | vs | LEFT HEMI - LEFT MOVE | 2.383 | 0.250 |
| RIGHT HEMI - NO RESPONSE | vs | LEFT HEMI - LEFT MOVE | −0.736 | 0.996 |
| RIGHT HEMI - RIGHT MOVE | vs | LEFT HEMI - LEFT MOVE | 0.659 | 0.998 |
| LEFT HEMI - RIGHT MOVE | vs | LEFT HEMI - NO RESPONSE | 3.322 | 0.020 |
| RIGHT HEMI - BIMANUAL MOVE | vs | LEFT HEMI - NO RESPONSE | 3.336 | 0.019 |
| RIGHT HEMI - LEFT MOVE | vs | LEFT HEMI - NO RESPONSE | 3.217 | 0.029 |
| RIGHT HEMI - NO RESPONSE | vs | LEFT HEMI - NO RESPONSE | 0.077 | 1.000 |
| RIGHT HEMI - RIGHT MOVE | vs | LEFT HEMI - NO RESPONSE | 1.482 | 0.818 |
| RIGHT HEMI - BIMANUAL MOVE | vs | LEFT HEMI - RIGHT MOVE | 0.014 | 1.000 |
| RIGHT HEMI - LEFT MOVE | vs | LEFT HEMI - RIGHT MOVE | −0.105 | 1.000 |
| RIGHT HEMI - NO RESPONSE | vs | LEFT HEMI - RIGHT MOVE | −3.245 |
0.026
|
| CONTRAST | z | p value | ||
| RIGHT HEMI - RIGHT MOVE | vs | LEFT HEMI - RIGHT MOVE | −1.840 | 0.592 |
| RIGHT HEMI - LEFT MOVE | vs | RIGHT HEMI - BIMANUAL MOVE | −0.119 | 1.000 |
| RIGHT HEMI - NO RESPONSE | vs | RIGHT HEMI - BIMANUAL MOVE | −3.259 | 0.025 |
| RIGHT HEMI - RIGHT MOVE | vs | RIGHT HEMI - BIMANUAL MOVE | −1.855 | 0.582 |
| RIGHT HEMI - NO RESPONSE | vs | RIGHT HEMI - LEFT MOVE | −3.140 | 0.036 |
| RIGHT HEMI - RIGHT MOVE | vs | RIGHT HEMI - LEFT MOVE | −1.736 | 0.664 |
| RIGHT HEMI - RIGHT MOVE | vs | RIGHT HEMI - NO RESPONSE | 1.404 | 0.856 |
Fig. A.1.
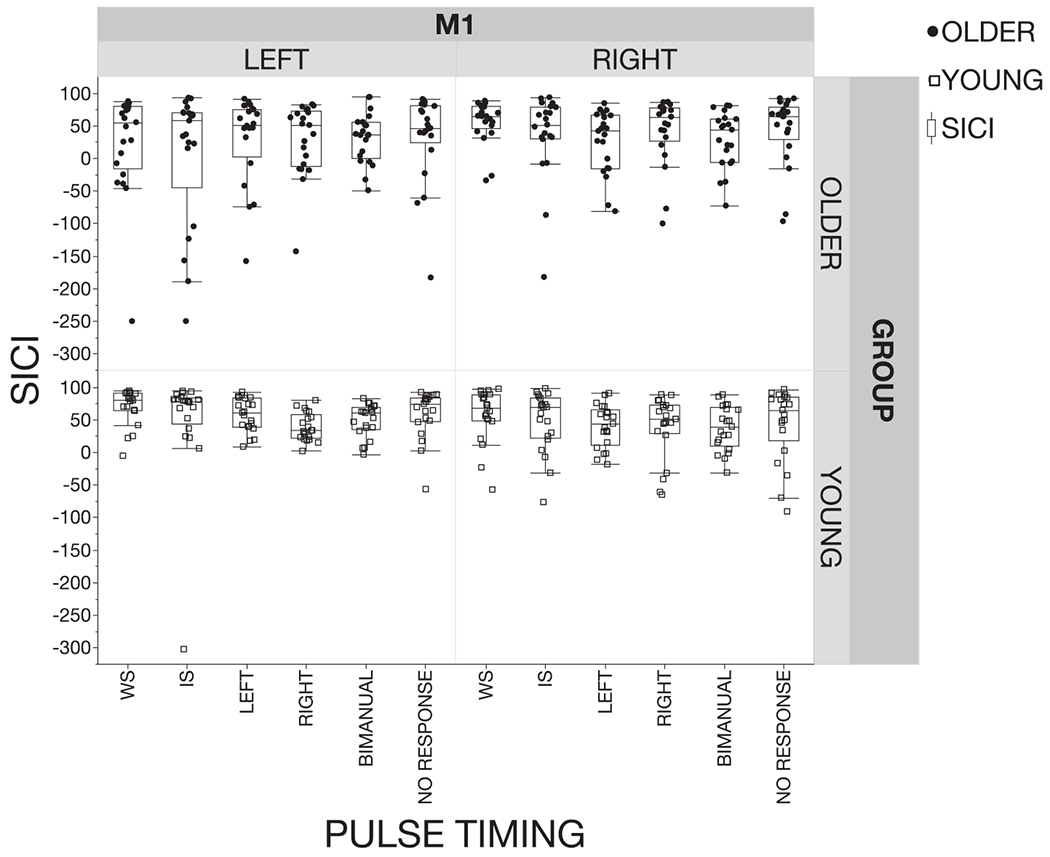
Individual averaged data for short-interval intracortical inhibition (SICI) profiles for older and young adults for the left and right hemisphere at the warning signal (WS), imperative signal (IS) and at 75% of EMG onset (left move, right move, bimanual and no response). SICI was defined as: (1 – (MEPpp / MEPsp)) * 100. Positive values indicate inhibition and negative values indicate disinhibition. The box plot represents the median (50th percentile), the 25th and the 75th percentile. Whiskers represent the minima and maxima. Note that some datapoints are extreme. However, this is inherent to the nature of TMS and therefore, it was decided to include all MEP’s resulting from non-erroneous trials in the data-analysis.
Fig. A.2.
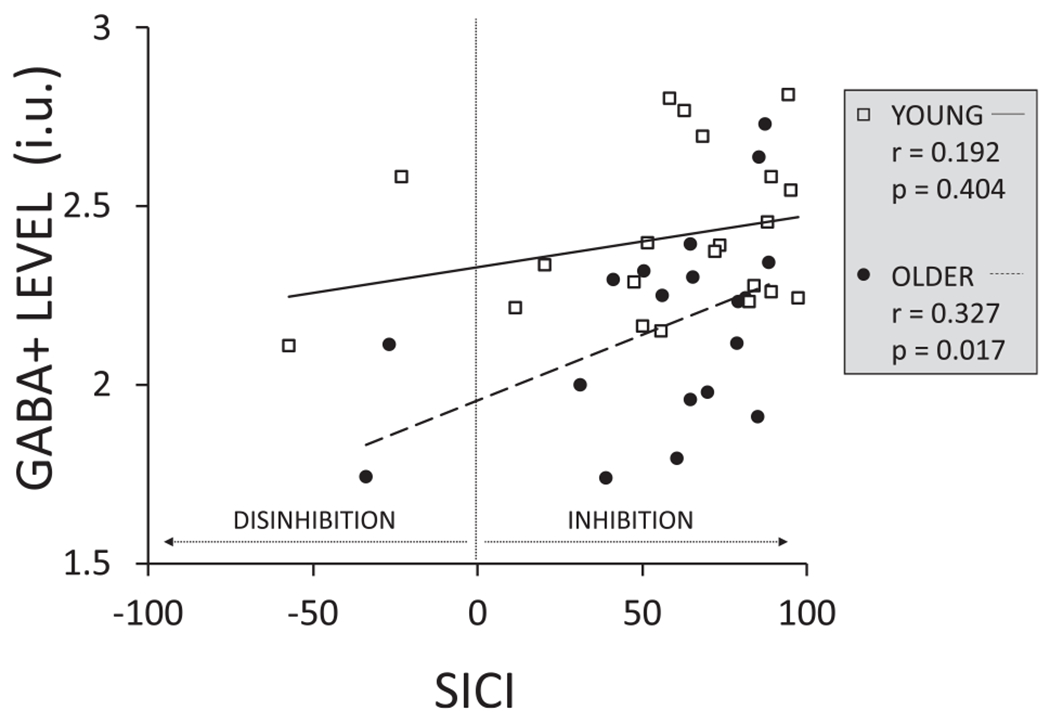
Exploratory correlations between GABA + level for the right sensorimotor (SM) voxel and short-interval intracortical inhibition (SICI) over the right M1 at the warning signal (preparation period) for older and younger adults. SICI was defined as: (1 - (MEPpp / MEPsp)) * 100.
Fig. A.3.
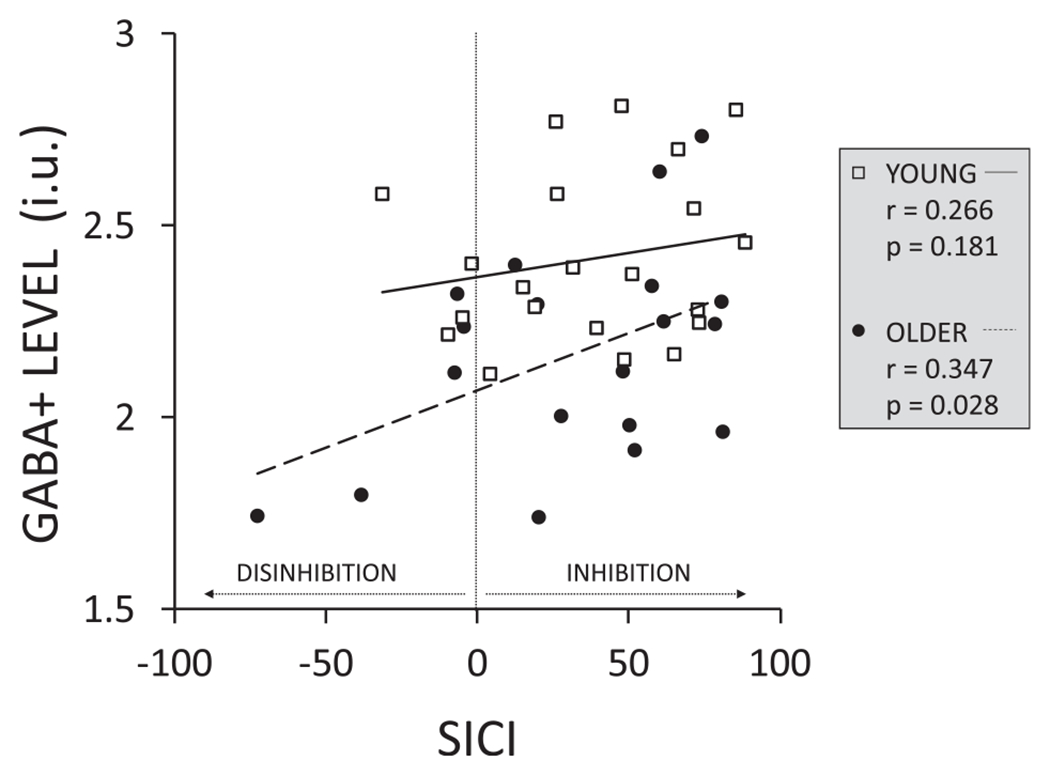
Exploratory correlations between GABA + level for the right sensorimotor (SM) voxel and short-interval intracortical inhibition (SICI) over the right M1 at 75% of the EMG onset (premotor period) when participants prepare initiation of a bimanual response (bimanual move) for older and younger adults. SICI was defined as: (1 – (MEPpp / MEPsp)) * 100.
References
- Amunts K, et al. , 1996. Asymmetry in the human motor cortex and handedness. Neuroimage 4 (3 Pt 1), 216–222. [DOI] [PubMed] [Google Scholar]
- Bates D, et al. , 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw 67, (1) 1–48. [Google Scholar]
- Baumer T, et al. , 2007. Laterality of interhemispheric inhibition depends on handedness. Exp. Brain Res 180 (2), 195–203. [DOI] [PubMed] [Google Scholar]
- Bloemendaal M, et al. , 2016. Contrasting neural effects of aging on proactive and reactive response inhibition. Neurobiol. Aging 46, 96–106. [DOI] [PubMed] [Google Scholar]
- Boisgontier MP, et al. , 2018. Cerebellar gray matter explains bimanual coordination performance in children and older adults. Neurobiol. Aging 65, 109–120. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, et al. , 1992. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol 9 (1), 132–136. [PubMed] [Google Scholar]
- Cassady K, et al. , 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalavi S, et al. , 2018. The neurochemical basis of the contextual interference effect. Neurobiol. Aging 66, 85–96. [DOI] [PubMed] [Google Scholar]
- Chang WH, et al. , 2016. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin. Neurophysiol 127 (8), 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, et al. , 1998. Intracortical inhibition and facilitation in different representations of the human motor cortex. J. Neurophysiol 80 (6), 2870–2881. [DOI] [PubMed] [Google Scholar]
- Civardi C, et al. , 2000. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin. Neurophysiol 111 (4), 624–629. [DOI] [PubMed] [Google Scholar]
- Cuypers K, et al. , 2013. Age-related differences in corticospinal excitability during a choice reaction time task. Age (Dordr) 35 (5), 1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers K, Maes C, Swinnen SP, 2018. Aging and GABA. Aging, Albany NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill T, 2008. Contraindications to magnetic resonance imaging: non-invasive imaging. Heart 94 (7), 943–948. [DOI] [PubMed] [Google Scholar]
- Dyke K, et al. , 2017. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage 152, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, et al. , 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40 (6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, et al. , 1998. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry Neuropsychol Behav Neurol 11 (2), 65–71. [PubMed] [Google Scholar]
- Fujiyama H, et al. , 2012. Age-related differences in corticomotor excitability and inhibitory processes during a visuomotor RT task. J. Cogn. Neurosci 24 (5), 1253–1263. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, et al. , 2012. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol. Aging 33 (7), 1484 e1–14. [DOI] [PubMed] [Google Scholar]
- Gao F, et al. , 2013. Magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachev ID, et al. , 2001. Aging alters the multichemical networking profile of the human brain: an in vivo (1)H-MRS study of young versus middle-aged subjects. J. Neurochem 77 (1), 292–303. [DOI] [PubMed] [Google Scholar]
- Grewal M, et al. , 2016. GABA quantitation using MEGA-PRESS: regional and hemispheric differences. J. Magn. Reson. Imaging 44 (6), 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G, et al. , 2004. Transcranial magnetic stimulation reveals asymmetrical efficacy of intracortical circuits in primary motor cortex. Exp. Brain Res 155 (1), 19–23. [DOI] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA, 2015. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 42 (5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise KF, et al. , 2013. The aging motor system as a model for plastic changes of GABA-mediated intracortical inhibition and their behavioral relevance. J. Neurosci 33 (21), 9039–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans L, et al. , 2018. GABA levels and measures of intracortical and interhemispheric excitability in healthy young and older adults: an MRS-TMS study. Neurobiol. Aging 65, 168–177. [DOI] [PubMed] [Google Scholar]
- Hermans L, et al. , 2018. Brain GABA levels are associated with inhibitory control deficits in older adults. J. Neurosci 38 (36), 7844–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S, et al. , 2004. Ipsilateral coordination deficits and central processing requirements associated with coordination as a function of aging. J Gerontol B Psychol Sci Soc Sci 59 (5), P225–P232. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, et al. , 2005. Neural basis of aging: the penetration of cognition into action control. J. Neurosci 25 (29), 6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinder MR, Fujiyama H, Summers JJ, 2012. Premotor-motor interhemispheric inhibition is released during movement initiation in older but not young adults. PLoS One 7 (12), e52573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P, 2008. Simultaneous inference in general parametric models. Biom. J 50 (3), 346–363. [DOI] [PubMed] [Google Scholar]
- Jordan TC, Rabbitt PM, 1977. Response times to stimuli of increasing complexity as a function of ageing. Br. J. Psychol 68 (2), 189–201. [DOI] [PubMed] [Google Scholar]
- King BR, et al. , 2018. Age-related declines in motor performance are associated with decreased segregation of large-scale resting state brain networks. Cerebr. Cortex 28 (12), 4390–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, et al. , 1993. Corticocortical inhibition in human motor cortex. J. Physiol 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, et al. , 2014. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci. Biobehav. Rev 43, 100–117. [DOI] [PubMed] [Google Scholar]
- Maes C, et al. , 2017. Two hands, one brain, and aging. Neurosci. Biobehav. Rev 75, 234–256. [DOI] [PubMed] [Google Scholar]
- Maes C, et al. , 2018. Age-related differences in GABA levels are driven by bulk tissue changes. Hum. Brain Mapp 39, 3652–3662. 10.1002/hbm.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini A, et al. , 2017. Age-associated differences in motor output variability and coordination during the simultaneous dorsiflexion of both feet. Somatosens. Mot. Res 34 (2), 96–101. [DOI] [PubMed] [Google Scholar]
- Marneweck M, Loftus A, Hammond G, 2011. Short-interval intracortical inhibition and manual dexterity in healthy aging. Neurosci. Res 70 (4), 408–414. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, et al. , 2017. Big GABA: edited MR spectroscopy at 24 research sites. Neuroimage 159, 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, et al. , 2019. Big GABA II: water-referenced edited MR spectroscopy at 25 research sites. Neuroimage 191, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Cirillo J, Byblow WD, 2017. GABA and primary motor cortex inhibition in young and older adults: a multimodal reliability study. J. Neurophysiol 118 (1), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motawar B, et al. , 2016. Delayed grip relaxation and altered modulation of intracortical inhibition with aging. Exp. Brain Res 234 (4), 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, et al. , 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JB, Lampman DA, 1993. Beyond WET and DRY: optimized pulses for water suppression In: Proceedings of the SMRM 12th Annual Meeting (New York). [Google Scholar]
- Near J, et al. , 2015. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med 73 (1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V, 1995. Hemispheric asymmetry of transcallosal inhibition in man. Exp. Brain Res 104 (3), 527–533. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9 (1), 97–113. [DOI] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC, 2003. The variability of intracortical inhibition and facilitation. Clin. Neurophysiol 114 (12), 2362–2369. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, et al. , 1992. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain 115 (Pt 4), 1045–1059. [DOI] [PubMed] [Google Scholar]
- Peinemann A, et al. , 2001. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci. Lett 313 (1–2), 33–36. [DOI] [PubMed] [Google Scholar]
- Porges EC, et al. , 2017. Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults. Biol Psychiatry Cogn Neurosci Neuroimaging 2 (1), 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Edden RA, 2012. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog. Nucl. Magn. Reson. Spectrosc 60, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, et al. , 2018. GABA levels in left and right sensorimotor cortex correlate across individuals. Biomedicines 6 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, et al. , 1999. Applications of magnetic cortical stimulation. The international federation of clinical neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl 52, 171–185. [PubMed] [Google Scholar]
- Salthouse TA, 2000. Aging and measures of processing speed. Biol. Psychol 54 (1–3), 35–54. [DOI] [PubMed] [Google Scholar]
- Seidler RD, et al. , 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev 34 (5), 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP, 2006. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci 7 (2), 160–166. [DOI] [PubMed] [Google Scholar]
- Simmonite M, et al. , 2019. Aug. Age-related declines in occipital GABA are associated with reduced fluid processing ability. Acad. Radiol 26 (8), 1053–1061. 10.1016/j.acra.2018.07.024. Epub 2018 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smittenaar P, et al. , 2015. Proactive and reactive response inhibition across the lifespan. PLoS One 10 (10) e0140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, 2014. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage 86, 19–27. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, et al. , 2010. Shared neural resources between left and right interlimb coordination skills: the neural substrate of abstract motor representations. Neuroimage 49 (3), 2570–2580. [DOI] [PubMed] [Google Scholar]
- Team, R.C., 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. [Google Scholar]
- Tremblay S, et al. , 2013. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+glutamine. J. Neurophysiol 109 (5), 1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD, 2002. Modern Applied Statistics with S, fourth ed. Springer, New York. [Google Scholar]
- Wassermann EM, 1998. In: Risk and Safety of Repetitive Transcranial Magnetic [Google Scholar]
- Stimulation: Report and Suggested Guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996, 108. Electroencephalogr Clin Neurophysiol, pp. 1–16, 1. [DOI] [PubMed] [Google Scholar]
- Yousry TA, et al. , 1997. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120 (Pt 1), 141–157. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC, 1996. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol 496 (Pt 3), 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, et al. , 2015. TMS and drugs revisited 2014. Clin. Neurophysiol 126 (10), 1847–1868. [DOI] [PubMed] [Google Scholar]
- Zivari Adab H, et al. , 2018. White matter microstructural organisation of interhemispheric pathways predicts different stages of bimanual coordination learning in young and older adults. Eur. J. Neurosci 47 (5), 446–459. [DOI] [PubMed] [Google Scholar]


