Abstract
In infection with Schistosoma mansoni, hepatic granuloma formation is mediated by CD4+ T helper (Th) cells sensitized to schistosomal egg antigens. There is considerable variation among infected individuals with respect to both severity of disease and the T-cell response to egg antigens. In the BL/6 mouse, the egg granulomas are relatively small and the relevant sensitizing egg antigens are largely unknown. We investigated the CD4+ Th cell response of infected BL/6 mice to egg antigens fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and found a prominent lymphoproliferative response to be directed against a 62-kDa component. With the aid of a specific T-cell hybridoma, 4E6, the 62-kDa antigen was isolated; following partial digestion with endoproteinase Glu-C, an internal amino acid sequence was found to be identical with one present in the enzyme phosphoenolpyruvate carboxykinase (PEPCK) of the organisms Caenorhabditis elegans and Treponema pallidum and to differ by one residue from PEPCK of various other species. In CD4+ Th cells from 7.5- 8.5-week-infected BL/6 mice, the purified 62-kDa molecule elicited a potent proliferative response which, based on cytokine analysis, was of a mixed Th-1 and Th-2 type. Our results reveal a novel egg antigen of particular prominence in the BL/6 mouse and suggest that the immune response in schistosomiasis is a product of sensitization to egg antigens that may vary considerably in immunogenicity from strain to strain.
The immunopathological damage in schistosomiasis mansoni consists mainly of granulomatous inflammation around parasite eggs in the liver and intestines, which may lead to scarring, portal hypertension, hemorrhage, and death (2, 34). There is considerable variation in the overall severity of this disease, both in humans and in mice. For example, mice of the C3H and CBA strains develop significantly larger egg granulomas than do those of the BL/6 strain (5, 9).
Granulomatous inflammation is a complex cellular hypersensitivity reaction that involves recruitment and activation of several types of inflammatory cells, the interplay of numerous mediators, including cytokines, and the synthesis of matrix proteins. Granuloma formation is now known to be strictly dependent on CD4+ T helper (Th) cells specific for schistosomal egg antigens (SEA) (14, 23), and by virtue of signature cytokines, there is strong evidence that it can be mediated by Th-1 and Th-2-type CD4+ Th cells (7, 13, 28, 36, 46). The CD4+ Th cells become activated following specific recognition of accessory cell-bound major histocompatibility complex (MHC) class II molecules harboring selected SEA-derived peptides. However, identification of individual T-cell-sensitizing egg antigens and derived peptides is only at a very early stage.
We previously investigated the nature of the anti-SEA T-cell repertoire by using CD4+ Th cells from infected mice as well as panels of specific T-cell hybridomas. We found that C3H mice display strong polyclonal T-cell responses against the major egg antigen Sm-p40, whereas in BL/6 mice the response is much weaker (15). Moreover, the specificity analysis of the derived T-cell hybridomas suggested that in C3H mice, a significant proportion of the anti-SEA T-cell repertoire is directed against Sm-p40, whereas no hybridomas derived from BL/6 mice recognized this antigen (15). These findings imply that the immunopathology in the BL/6 strain is largely directed against antigens other than Sm-p40. They also raise the possibility that differences in egg antigen recognition patterns in mouse strains of different haplotypes may be reflected in the overall magnitude of the resulting immunopathology.
To gain insight into the basis of the immune response to schistosome eggs in BL/6 mice, we used specific T-cell hybridomas as monoclonal probes to discern which SEA components they recognize. We now report on the identification and characterization of a novel 62-kDa egg antigen, found to elicit a particularly strong response in CD4+ Th cells from infected mice of the BL/6 strain.
MATERIALS AND METHODS
Infection of mice.
Six- to eight-week-old female BL/6 (H-2b) and CBA (H-2k) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Some of the mice were infected intraperitoneally with 70 cercariae of Schistosoma mansoni (Puerto Rico strain). Cercariae were shed from infected Biomphalaria glabrata snails, provided to us by the Biomedical Research Institute, Rockville, Md.
Antigens.
SEA was prepared as described previously (1) at the Center for Tropical Diseases, University of Massachusetts at Lowell, and was obtained in part through the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Recombinant antigen Sm-p40 (amino acids 94 to 341) was prepared as previously described (15). Protein concentrations were determined by a modification of the Bradford method using the Coomassie Plus protein assay reagent (Pierce, Rockford, Ill.).
CD4+ Th cell responses.
CD4+ Th cells were isolated from mesenteric lymph node cells of mice after 7.5 to 8.5 weeks of infection. The cells were purified by negative selection as described elsewhere (15). The procedure involves nylon wool filtration and two cycles of incubation with monoclonal antibodies (MAbs) against I-Ab/I-Ek, heat-stable antigen and CD8, and complement. To assess proliferation, 1.5 × 105 CD4+ Th cells plus 4 × 105 syngeneic irradiated (with 3,000 rads) normal splenocytes were cultured in a volume of 200 μl for a total of 114 h in the presence of the indicated antigens. During the last 18 h of culture, 0.5 μCi of tritiated thymidine ([3H]dThd) was added to each well, and incorporation into DNA was measured by liquid scintillation spectroscopy; data presented represent means ± standard deviations (SD).
T-cell hybridoma 4E6.
The SEA specific T-cell hybridoma 4E6 was derived from BL/6 mice as described elsewhere (15). Antigen reactivity of this hybridoma was determined as described previously (15), with some modifications. Briefly, 106 4E6 hybridoma cells, together with 3 × 106 irradiated (with 3,000 rads) normal syngeneic splenocytes, were cultured either with graded concentrations of soluble proteins for 24 h or with proteins immobilized on nitrocellulose membranes (NC; Immobilon NC Pure; Millipore Corporation, Bedford, Mass.) for 42 h. Positive hybridoma responses were assessed by incubation of the culture supernatants with 9 × 103 HT-2 indicator cells for 22 h (44).
Determination of antigen-specific cytokine production.
CD4+ Th cells (106) plus 4 × 106 irradiated splenic antigen-presenting cells were cultured in 1-ml volumes together with graded concentrations of the indicated antigens in 48-well plastic plates. The cytokines gamma interferon (IFN)-γ, interleukin-2 (IL-2), IL-4, and IL-5 were measured in culture supernatants by enzyme-linked immunosorbent assay (ELISA), with corresponding cytokine-specific capture MAbs, detection MAbs, standard cytokines, and protocols from PharMingen (San Diego, Calif.). The cytokines were measured after optimal culture times, which were 24 h for IL-2 and 48 h for IFN-γ, IL-4, and IL-5.
SDS-PAGE and preparation of immunoblots.
Test materials were boiled in reducing sample buffer containing 2% sodium dodecyl sulfate (SDS) and 1% 2-mercaptoethanol (2-ME) and then separated by reduced sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (20). For preparation of immunoblots, 85 μg of protein of SEA was loaded on each lane. Proteins were transferred from gels to NC by electroblotting with 25 mM Tris–192 mM glycine buffer containing 10% methanol (pH 8.4) (41). The procedure was carried out overnight at a constant voltage of 15 V in a tank transfer system (Bio-Rad Laboratories, Hercules, Calif.). One lane of the blot was cut off, stained with amido black (0.1% in 5% acetic acid), and used as a reference to localize separated proteins on unstained NC. Each NC blot was cut into 30 sections corresponding to different molecular masses. Each section was cut further into smaller parts, sterilized by exposure to 15,000 rads of γ irradiation, and tested for the ability to stimulate CD4+ Th cell populations and the T-cell hybridoma. Corresponding pooled sections from two lanes were used for each culture well of hybridoma cells, and those from one half lane were used for the CD4+ Th cells. For preparation of controls strips, SEA (50 μg of protein) or phosphate-buffered saline (50 μl) was directly applied to 60 mm2 of NC.
Electroelution.
Protein fractions separated by SDS-PAGE were stained with a reversible copper stain (Bio-Rad Laboratories), excised from the gels, destained, and eluted for more than 24 h in an electroeluter (Bio-Rad Laboratories), using a volatile elution buffer containing 50 mM NH4HCO3, 0.1% SDS, and 0.1% 2-ME. Subsequent to elution, the SDS was removed by overnight electrodialysis in the same tank following replacement with fresh buffer made without SDS and 2-ME. Purity, molecular weight, and concentration of proteins isolated from gels were assessed on silver-stained gels with an image analyzing system (Molecular Analyst 2.1; Bio-Rad Laboratories).
Limited proteolytic digestion.
Materials isolated from reduced SDS-polyacrylamide gels were electroeluted and digested with 20 μg endoproteinase Glu-C (Staphylococcus aureus V8 protease; Promega, Madison, Wis.) per ml in the presence of 50 mM Tris-HCl buffer (pH 6.8), 20% glycerin, 0.01% bromophenol blue dye, 0.1% SDS, and 10 mM EDTA, essentially as described previously (18).
Protein sequencing.
After digestion with V8 protease, the proteolytic fragments were separated by SDS-PAGE as described above except that test materials were not reduced or boiled. For the SDS-PAGE, 0.1 mM sodium thioglycolate was added to the electrophoresis buffer in the upper chamber. Electrophoresis of V8 protease digests (10% gel) was typically run at 50 V for stacker gels and 120 V for separating gels at constant voltage. Fractions were electroeluted as described above in buffer without 2-ME and tested for the ability to stimulate hybridoma cells. After separation, V8 protease digests were also electroblotted to a polyvinylidene difluoride membrane (Immobilon-PSQ; Millipore) (24). The electroblot transfer was carried out for 70 min at 4°C at 50 V, using blotting buffer consisting of 25 mM Tris, 192 mM glycine, and 10% methanol. The blots were stained with 0.1% Coomassie blue G in 50% methanol and destained with 10% acetic acid in 50% methanol. After being washed with water, the blots were dried and the stimulatory fractions were excised for protein sequencing, which was carried out on an Applied Biosystems gas-phase sequencer (model 477A) at the Tufts Core Facility, Department of Physiology, Tufts University School of Medicine. The sequence was compared with sequences from known proteins with BLAST (Basic Local Alignment Search Tool) programs from the National Center for Biotechnology Information.
RESULTS
Identification of SEA components that stimulate BL/6 CD4+ Th cells.
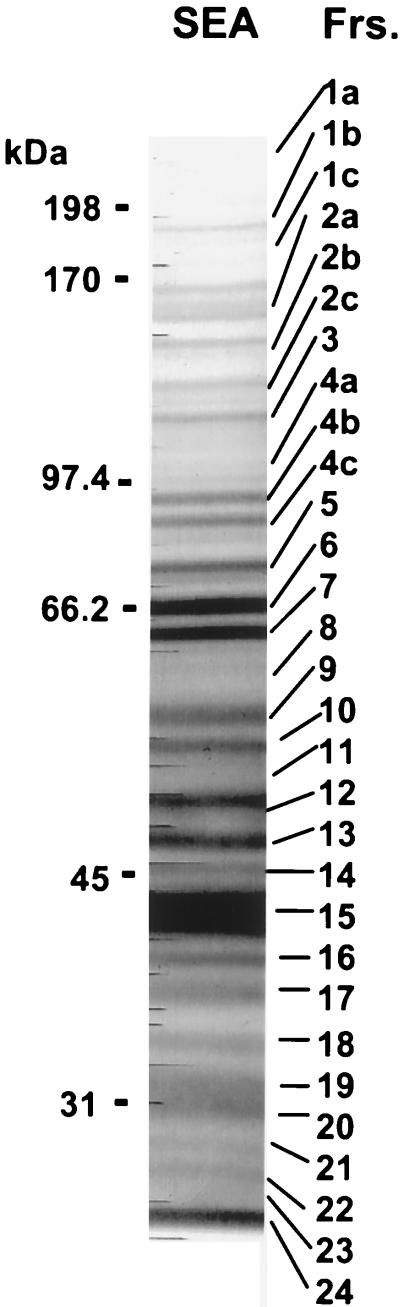
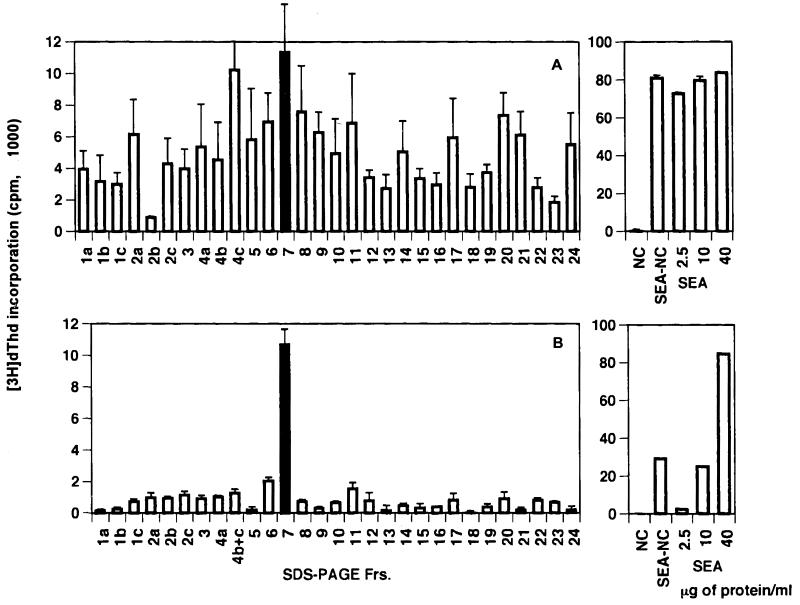
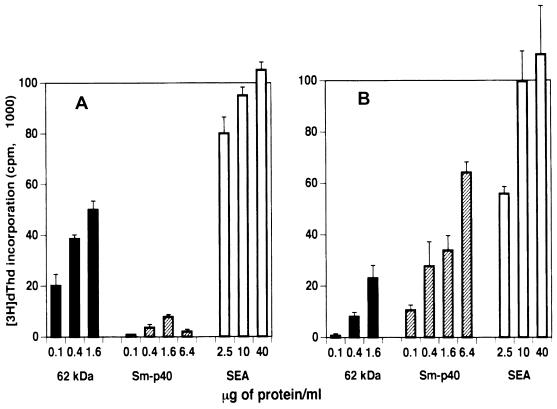
An initial experiment was conducted to examine the relative immunogenicity of the various egg antigens in schistosome-infected BL/6 mice. For this purpose, SEA was fractionated by SDS-PAGE; this procedure resulted in the separation of 30 discernible components. Results of a representative experiment depicting the various molecules with their apparent molecular masses are shown in Fig. 1. Individual components were subsequently excised from the immunoblots and tested for the ability to stimulate CD4+ Th cells from infected BL/6 mice. Results from a representative experiment shown in Fig. 2A indicate that the CD4+ Th cells reacted against several fractionated SEA components; however, the response to fraction 7 was among the strongest.
FIG. 1.
Representative SDS-PAGE (7.5% gel) profile of SEA on immunoblots stained with amido black. Molecular weight marker standards are indicated on the left. The prominent band of fraction 15 represents the Sm-p40 antigen. Frs., fractions.
FIG. 2.
CD4+ Th cell responses (A) and 4E6 T-cell hybridoma responses (B) to SDS-PAGE fractions on immunoblots of SEA. CD4+ Th cells were isolated from mesenteric lymph nodes of 8.5-week-infected BL/6 mice. Cell culture conditions in each case were as described in Materials and Methods. [3H]dThd incorporation was assessed by liquid scintillation spectroscopy. Data are expressed as means ± 1 SD. Background radioactivity from cultures in the absence of antigen is subtracted. Panels on the right reflect corresponding CD4+ Th cell or hybridoma stimulation in the presence of NC, NC coated with 50 μg of SEA (SEA-NC), or the indicated amounts of SEA. Experiments with CD4+ Th cells as well as with the T-cell hybridoma were repeated twice, with similar results. Frs., fractions.
T-cell hybridoma 4E6 recognizes a strongly stimulatory SEA component.
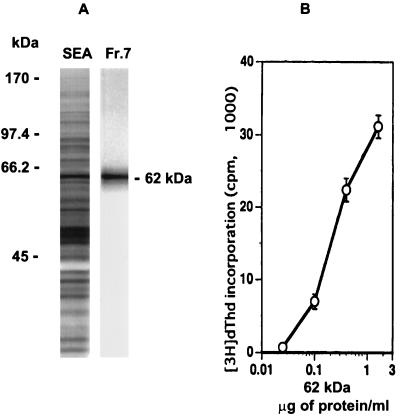
In addition to the CD4+ Th cells, a panel of SEA-specific BL/6 T-cell hybridomas was screened with the fractionated SEA components. One of these T-cell hybridomas, 4E6, responded to a potent antigen contained in fraction 7 (Fig. 2B). Following excision of the relevant area and electroelution from gels, the stimulatory component appeared as a single band on silver-stained gels, with an apparent molecular mass of 62 kDa (Fig. 3A). The identified 62-kDa antigen elicited a dose-dependent stimulation of hybridoma 4E6, as determined by the proliferation of HT-2 indicator cells; a protein concentration of 100 ng/ml was sufficient to elicit a significant response (Fig. 3B).
FIG. 3.
Identification of a 62-kDa antigen recognized by T-cell hybridoma 4E6. (A) Eluted SDS-PAGE gel fraction 7 (Fr.7) (Fig. 1) from 20 μg of SEA, examined for purity on a 10% silver-stained SDS-polyacrylamide gel, shown next to total SEA and molecular weight marker standards. (B) Dose response of T-cell hybridoma 4E6 to 62-kDa antigen as measured by HT-2 indicator cell proliferation. Data are expressed as mean ± 1 SD. Background radioactivity from hybridoma cultures in the absence of antigen is subtracted.
Partial amino acid sequence of the 62-kDa antigen.
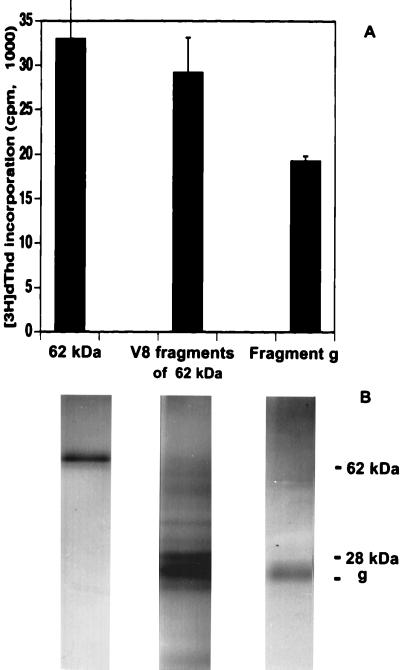
When initially tested for the purpose of amino acid sequencing, the 62-kDa antigen was found to have a blocked amino terminus. For this reason it was subjected to limited proteolytic digestion with V8 protease, which cleaves peptides at the carboxyl end of Glu residues (45). This treatment yielded several different fragments. Discernible fragments were electroeluted and used to stimulate hybridoma 4E6; the fragments were also reexamined for purity on silver-stained SDS-polyacrylamide gels under nonreducing conditions. Figure 4A shows 62-kDa fragment g, which displayed stimulatory activity 55 to 71% greater than those of the intact and digested molecules; Fig. 4B shows the migration on SDS-PAGE of fragment g, which has an apparent molecular mass of 26 to 27 kDa. The V8 protease of 28 kDa displayed an electrophoretic mobility similar to that of fragment g but, as expected, had no stimulatory activity (not shown).
FIG. 4.
Stimulation of the 4E6 T-cell hybridoma with V8 protease fragments of the 62-kDa antigen. (A) Stimulation of T-cell hybridoma 4E6 by, from left to right, the intact 62-kDa antigen, the fragments of the 62-kDa antigen derived from digestion with 2 μg of V8 protease, and the most stimulatory fragment g. Each of these stimulatory activities was obtained from an initial 450 μg of SEA. Data are expressed as mean ± 1 SD. Background radioactivity from hybridoma cultures in the absence of antigen is subtracted. (B) Corresponding silver-stained SDS-PAGE profile of, from left to right, the 62-kDa antigen, the combined V8 protease fragments of the 62-kDa antigen, and fragment g. Five percent of each antigen preparation used for stimulation was examined on the SDS-polyacrylamide gel. Part of the V8 protease migrated close to fragment g but had no stimulatory activity (not shown). The position of intact V8 protease (28 kDa) is shown.
Identification of the 62-kDa antigen.
Because of its strong stimulatory activity, fragment g (Fig. 4B) was subjected to amino acid sequencing. This procedure yielded a 10-amino-acid peptide with the sequence AGFFGVAPGT (Fig. 5). Computer search of GenBank disclosed that this sequence, together with a deduced Glu residue (the site of V8 protease cleavage), is identical to one found in phosphoenolpyruvate carboxykinase (PEPCK-GTP; EC 4.1.1.32). PEPCK is an enzyme of the gluconeogenic pathway and has been described for a variety of species, including helminths, bacteria, fungi, vertebrates, and arthropods. In the species listed in Fig. 5, PEPCK is composed of 608 to 651 amino acids. The sequence obtained from our 62-kDa antigen is typically located in the midportion of the molecule and is completely identical to that in PEPCK of the organisms Caenorhabditis elegans and Treponema pallidum; moreover, it varies by only one residue (Ala replaced with either Ser, Asn, Arg, Tyr, or Phe) with respect to PEPCK of other species listed in Fig. 5. A secondary sequence was also found in proteolytic fragment g; this corresponded to the V8 protease.
FIG. 5.
Internal amino acid sequence obtained from fragment g and related sequences in other species corresponding to PEPCK. The 10-amino-acid sequence is shown at the top. Vertical lines indicate identical residues; points indicate mismatched residues. The location (∗) of the 10-mer together with the deduced Glu (E) residue (the site of V8 protease cleavage) within PEPCK is indicated for each species. The total number of amino acids (aa) in each PEPCK is indicated in parentheses.
Polyclonal CD4+ Th cell responses to the 62-kDa antigen.
To ascertain its relative immunogenicity, the 62-kDa antigen was used to stimulate proliferative and cytokine responses in polyclonal CD4+ Th cells from infected mice. Figure 6A shows that in BL/6 mice, low concentrations of the 62-kDa component elicited a potent dose-dependent proliferative response compared to unfractionated SEA; this response was vastly stronger than that induced by Sm-p40, which is a demonstrated major egg immunogen in mice of the H-2k haplotype (4, 15, 16). By comparison, the 62-kDa component also induced a significant proliferative response in the CBA (H-2k) mouse, although in this case it was considerably smaller than that elicited by the Sm-p40 antigen (Fig. 6B).
FIG. 6.
Proliferative responses of CD4+ Th cells from BL/6 (A) and CBA (B) mice to the 62-kDa antigen. CD4+ Th cells were isolated from mesenteric lymph nodes of 8.5-week-infected mice. Culture conditions were as described in Materials and Methods. [3H]dThd incorporation was assessed by liquid scintillation spectroscopy. Data are expressed as mean ± 1 SD. Also shown for comparison are responses to Sm-p40 and SEA. Background radioactivity from cultures in the absence of antigen is subtracted. The same pattern of stimulation was observed when cells from 7.5-week-infected mice were used (not shown).
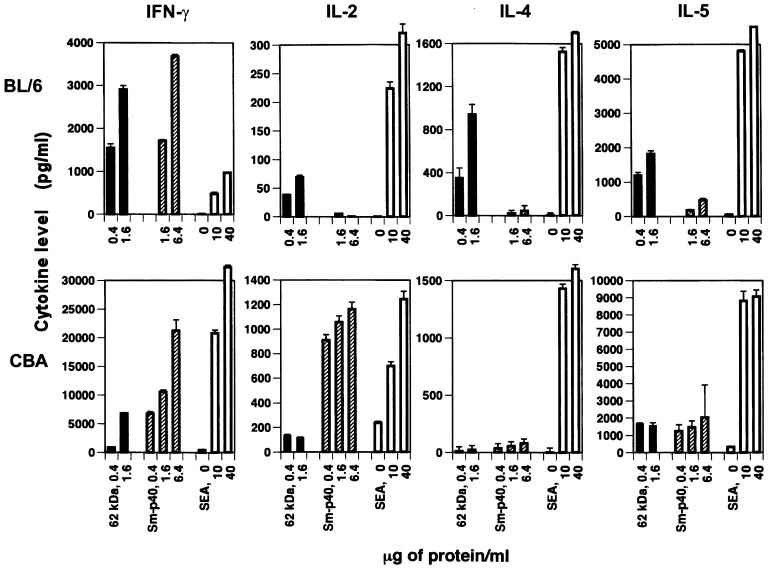
Examination by ELISA of culture supernatants from CD4+ Th cells obtained from 8.5-week-infected BL/6 mice revealed that stimulation with the 62-kDa antigen elicited significant secretion of IFN-γ, IL-4, and IL-5 but smaller amounts of IL-2 (Fig. 7). Interestingly, the 62-kDa antigen by itself stimulated more IFN-γ than unfractionated SEA; however, production of IL-2, IL-4, and IL-5 was substantially lower than that elicited by unfractionated SEA. Moreover, except for IFN-γ, there was virtually no cytokine production in response to Sm-p40. In CBA mice, the 62-kDa antigen elicited relatively little cytokine secretion in comparison with total SEA, even though, except for IL-4, overall cytokine production in response to SEA was markedly higher than in the BL/6 strain. Another striking attribute of the CBA strain is the elevated IFN-γ and IL-2 response to Sm-p40, which contrasted sharply with little IL-5 production and virtually no IL-4.
FIG. 7.
Cytokine production by CD4+ Th cells from BL/6 and CBA mice stimulated with the 62-kDa antigen. CD4+ Th cells were isolated from mesenteric lymph nodes of 8.5-week-infected mice. Culture conditions were as described in Materials and Methods. The cytokines IFN-γ, IL-2, IL-4, and IL-5 were measured in culture supernatants by ELISA. Data are expressed as mean ± 1 SD. Also shown for comparison are responses to Sm-p40 and SEA. The same pattern of cytokine production was observed when cells from 8-week-infected mice were used (not shown).
DISCUSSION
Hepatointestinal granuloma formation and the ensuing potentially lethal pathologic events in schistosomiasis mansoni are strictly dependent on, and mediated by, CD4+ Th cells specific for egg antigens. Although previous reports described several T-cell-stimulatory activities (3, 12, 21), the precise identity of most sensitizing egg antigens remains largely unknown. Given the potential of modifying the immunopathology in experimental schistosomiasis by manipulating the underlying egg antigen-specific CD4+ Th cell response, our laboratory has developed monoclonal T-cell hybridomas from sensitized mice as probes to identify individual egg components. The reasoning behind this approach is the likelihood that the majority of such clones will have specificities for the most immunogenic of the egg components, which then could be tracked down and identified.
This paper reports on such an event in which the highly sensitive T-cell hybridoma 4E6 was instrumental in the identification of a strongly stimulatory 62-kDa egg component in the BL/6 mouse. Limited proteolytic digestion of this component yielded stimulatory fragment g which, in turn, facilitated internal amino acid sequencing of a peptide identical or similar to one contained in PEPCK of various species. It is likely that hybridoma 4E6 recognizes the same epitope in the intact 62-kDa molecule as in fragment g and that the difference in stimulation is due to a concentration effect. Although not previously recognized as an egg antigen, PEPCK has been identified in adult worms and sporocysts of S. mansoni (39, 40). Moreover, a cDNA clone from a genomic library of S. mansoni has been partially sequenced (bases 1 to 304) and shown to be homologous with PEPCK (29).
In contrast to an impressive number of cloned S. mansoni worm antigens (11, 27, 30, 31, 35, 38), the list of identified S. mansoni antigens preferentially expressed in eggs is still in its beginning stage, although Fig. 1 and 2A suggest several potential immunogenic egg components possibly representing previously reported candidate antigens (17, 25, 37). Of these, undoubtedly the most abundant component (fraction 15 in Fig. 1) has been identified as Sm-p40, which is a small heat shock protein with homologies to alpha-crystallins (26). Sm-p40 is a potent egg immunogen which elicits a strong Th-1-type response in C3H and CBA mice, which develop large egg granulomas (4, 15, 16). Sm-p40 has been fully sequenced (26) and found to have at least three T-cell epitopes, of which one has been found to be dominant in the C3H and CBA strains (6, 16).
Sm-p40 and the novel 62-kDa antigen lend themselves to interesting and important comparisons. A strong T-cell response to Sm-p40 is restricted by H-2k, and only a minor reactivity is detected in BL/6 (H-2b) mice as well as mice of the H-2d and H-2q haplotypes (16). In contrast, the 62-kDa antigen elicits the major T-cell response in BL/6 mice, although CBA mice do react to it albeit with a response significantly weaker than that directed against Sm-p40. Another contrast is that Sm-p40 preferentially stimulates Th-1-type cytokine production, whereas the cytokines elicited in the BL/6 strain by the 62-kDa antigen appear to be of a mixed Th-1 and Th-2 type. Of interest, however, is that while in BL/6 mice the overall cytokine response is markedly lower, the 62-kDa antigen elicits a stronger IFN-γ response by itself than when included in SEA, surprisingly at a time of infection (8.5 weeks) when the anti-SEA cytokine response in this strain is known to shift to the Th-2 type (28, 36).
Even though the reason for the difference in severity of schistosomal infection among individual humans and different mouse strains is not known, there is strong evidence of an underlying genetic control. In humans, a more pronounced disease course has been associated with genes within (19, 33, 43) or outside (22) the MHC complex. In mice, both kinds of genes have been similarly implicated (5). Non-MHC genes could influence the severity of infection by controlling a variety of factors involved in the inflammatory and repair processes, including cytokines, costimulatory and adhesion molecules, and extracellular matrix components. On the other hand, MHC control could be exerted by determining the number of epitopes, if any, that are recognized in each antigen and dictating the type of ensuing T-cell response, as suggested herein. For example, it has been shown that a Plasmodium falciparum circumsporozoite epitope is restricted by I-Ab (8, 10); in contrast, BL/6 mice do not respond to an 86-kDa antigen from adult schistosome worms (32), nor do they make immunoglobulin G antibodies to the Sm-p40 antigen (42).
An important question that remains to be answered is to what extent the genetically determined recognition and response to an antigen influences the outcome of immunopathology and clinical disease. Our findings suggest that the relative immunogenicity of different schistosomal egg antigens varies from strain to strain and, extrapolating to outbred populations, possibly from individual to individual. However, the identification of egg antigens potentially has important practical implications because the most pathogenesis-related responses could be targeted for specific down-regulation.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant AI 18919 and by the UNDP/World Bank WHO Special Program for Research and Training in Tropical Diseases. B. glabrata cercariae were provided by the Biomedical Research Institute, Rockville, Md., under NIH-NIAID contract N01 AI-55260.
We thank David Stollar and Peter Brodeur for critically reading the manuscript and Philip LoVerde for helpful discussions.
REFERENCES
- 1.Boros D L, Warren K S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boros D L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown A P, Remold H G, Warren K S, David J R. Partial purification of antigens from eggs of Schistosoma mansoni that elicit delayed hypersensitivity. J Immunol. 1977;119:1275–1278. [PubMed] [Google Scholar]
- 4.Cai Y, Langley J G, Smith D I, Boros D L. A cloned major Schistosoma mansoni egg antigen with homologies to small heat shock proteins elicits Th1 responsiveness. Infect Immun. 1996;64:1750–1755. doi: 10.1128/iai.64.5.1750-1755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheever A W, Duvall R H, Hallack T A, Jr, Minker R G, Malley J D, Malley K G. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Boros D L. Identification of the immunodominant T cell epitope of p38, a major egg antigen, and characterization of the epitope-specific Th responsiveness during murine schistosomiasis mansoni. J Immunol. 1998;160:5420–5427. [PubMed] [Google Scholar]
- 7.Chikunguwo S M, Kanazawa T, Dayal Y, Stadecker M J. The cell-mediated response to schistosomal antigens at the clonal level. In vivo functions of cloned murine egg antigen-specific CD4+ T helper type 1 lymphocytes. J Immunol. 1991;147:3921–3925. [PubMed] [Google Scholar]
- 8.Del Giudice G, Cooper J A, Merino J, Verdini A S, Pessi A, Togna A R, Engers H D, Corradin G, Lambert P H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986;137:2952–2955. [PubMed] [Google Scholar]
- 9.Fanning M M, Peters P A, Davis R S, Kazura J W, Mahmoud A A. Immunopathology of murine infection with Schistosoma mansoni: relationship of genetic background to hepatosplenic disease and modulation. J Infect Dis. 1981;144:148–153. doi: 10.1093/infdis/144.2.148. [DOI] [PubMed] [Google Scholar]
- 10.Good M F, Berzofsky J A, Maloy W L, Hayashi Y, Fujii N, Hockmeyer W T, Miller L H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986;164:655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grezel D, Capron M, Grzych J M, Fontaine J, Lecocq J P, Capron A. Protective immunity induced in rat schistosomiasis by a single dose of the Sm28GST recombinant antigen: effector mechanisms involving IgE and IgA antibodies. Eur J Immunol. 1993;23:454–460. doi: 10.1002/eji.1830230223. [DOI] [PubMed] [Google Scholar]
- 12.Harn D A, Danko K, Quinn J J, Stadecker M J. Schistosoma mansoni: the host immune response to egg antigens. I. Partial characterization of cellular and humoral responses to pI fractions of soluble egg antigens. J Immunol. 1989;142:2061–2066. [PubMed] [Google Scholar]
- 13.Henderson G S, Lu X, McCurley T L, Colley D G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-gamma mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J Immunol. 1992;148:2261–2269. [PubMed] [Google Scholar]
- 14.Hernandez H J, Wang Y, Tzellas N, Stadecker M J. Expression of class II, but not class I, major histocompatibility complex molecules is required for granuloma formation in infection with Schistosoma mansoni. Eur J Immunol. 1997;27:1170–1176. doi: 10.1002/eji.1830270518. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez H J, Trzyna W C, Cordingley J S, Brodeur P H, Stadecker M J. Differential antigen recognition by T cell populations from strains of mice developing polar forms of granulomatous inflammation in response to eggs of Schistosoma mansoni. Eur J Immunol. 1997;27:666–670. doi: 10.1002/eji.1830270314. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez H J, Edson C M, Harn D A, Ianelli C J, Stadecker M J. Schistosoma mansoni: genetic restriction and cytokine profile of the CD4+ T helper cell response to dominant epitope peptide of major egg antigen Sm-p40. Exp Parasitol. 1998;90:122–130. doi: 10.1006/expr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch C, Almeida C A, Doughty B L, Goes A M. Characterization of Schistosoma mansoni 44.7/56.8 kDa egg antigens recognized by human monoclonal antibodies which induce protection against experimental infection and proliferation of peripheral blood mononuclear cells from schistosomiasis patients. Vaccine. 1997;15:948–954. doi: 10.1016/s0264-410x(96)00305-2. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy T E, Gawinowicz M A, Barzilai A, Kandel E R, Sweatt J D. Sequencing of proteins from two-dimensional gels by using in situ digestion and transfer of peptides to polyvinylidene difluoride membranes: application to proteins associated with sensitization in Aplysia. Proc Natl Acad Sci USA. 1988;85:7008–7012. doi: 10.1073/pnas.85.18.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima S, Yano A, Sasazuki T, Ohta N. Associations between HLA and immune responses in individuals with chronic schistosomiasis japonica. Trans R Soc Trop Med Hyg. 1984;78:325–329. doi: 10.1016/0035-9203(84)90109-3. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lukacs N W, Boros D L. Splenic and granuloma T-lymphocyte responses to fractionated soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991;59:941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, Feingold J, Weissenbach J, Dessein A J. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat Genet. 1996;14:181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- 23.Mathew R C, Boros D L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986;54:820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 25.Moser D, Doenhoff M J, Klinkert M Q. A stage-specific calcium-binding protein expressed in eggs of Schistosoma mansoni. Mol Biochem Parasitol. 1992;51:229–238. doi: 10.1016/0166-6851(92)90073-s. [DOI] [PubMed] [Google Scholar]
- 26.Nene V, Dunne D W, Johnson K S, Taylor D W, Cordingley J S. Sequence and expression of a major egg antigen from Schistosoma mansoni. Homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- 27.Pearce E J, James S L, Hieny S, Lanar D E, Sher A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc Natl Acad Sci USA. 1988;85:5678–5682. doi: 10.1073/pnas.85.15.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce E J, Caspar P, Grzych J M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabelo, E. M. L., G. R. Franco, V. Azevedo, H. B. Pena, T. M. Santos, W. S. F. Maria, N. A. Rodrigues, J. M. Ortega, and S. D. J. Pena. 1996. Analysis of cDNA libraries from different developmental stages of Schistosoma mansoni with the aim of producing expressed sequence Tags. GenBank DNA sequence A140576. [DOI] [PubMed]
- 30.Reynolds S R, Shoemaker C B, Harn D A. T and B cell epitope mapping of SM23, an integral membrane protein of Schistosoma mansoni. J Immunol. 1992;149:3995–4001. [PubMed] [Google Scholar]
- 31.Reynolds S R, Dahl C E, Harn D A. T and B epitope determination and analysis of multiple antigenic peptides for the Schistosoma mansoni experimental vaccine triose-phosphate isomerase. J Immunol. 1994;152:193–200. [PubMed] [Google Scholar]
- 32.Schweitzer A N. Alternative patterns of MHC-restricted antibody responsiveness following intraperitoneal immunization of inbred mice with different preparations of an 86 kilodalton antigen of Schistosoma mansoni. Parasite Immunol. 1992;14:267–277. doi: 10.1111/j.1365-3024.1992.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Secor W E, del Corral H, dos Reis M G, Ramos E A, Zimon A E, Matos E P, Reis E A, do Carmo T M, Hirayama K, David R A, David J R, Harn D A., Jr Association of hepatosplenic schistosomiasis with HLA-DQB1*0201. J Infect Dis. 1996;174:1131–1135. doi: 10.1093/infdis/174.5.1131. [DOI] [PubMed] [Google Scholar]
- 34.Smithers S R, Doenhoff M J. Schistosomiasis. In: Cohen S, Warren K S, editors. Immunology of parasitic diseases. Oxford, England: Blackwell Scientific; 1982. pp. 527–607. [Google Scholar]
- 35.Soisson L M, Masterson C P, Tom T D, McNally M T, Lowell G H, Strand M. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J Immunol. 1992;149:3612–3620. [PubMed] [Google Scholar]
- 36.Stadecker M J, Hernandez H J. The immune response and immunopathology in infection with Schistosoma mansoni: a key role of major egg antigen Sm-p40. Parasite Immunol. 1998;20:217–221. doi: 10.1046/j.1365-3024.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 37.Sung C K, Dresden M H. Cysteinyl proteinases of Schistosoma mansoni eggs: purification and partial characterization. J Parasitol. 1986;72:891–900. [PubMed] [Google Scholar]
- 38.Tendler M, Brito C A, Vilar M M, Serra-Freire N, Diogo C M, Almeida M S, Delbem A C, Da Silva J F, Savino W, Garratt R C, Katz N, Simpson A S. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci USA. 1996;93:269–273. doi: 10.1073/pnas.93.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tielens A G, Van der Meer P, van den Heuvel J M, van den Bergh S G. The enigmatic presence of all gluconeogenic enzymes in Schistosoma mansoni adults. Parasitology. 1991;102:267–276. doi: 10.1017/s0031182000062582. [DOI] [PubMed] [Google Scholar]
- 40.Tielens A G, Horemans A M, Dunnewijk R, van der Meer P, van den Bergh S G. The facultative anaerobic energy metabolism of Schistosoma mansoni sporocysts. Mol Biochem Parasitol. 1992;56:49–57. doi: 10.1016/0166-6851(92)90153-b. [DOI] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trzyna W C, Cordingley J S. Schistosoma mansoni: genetic non-response to p40, the major protein antigen of the egg, reveals a novel mechanism enhancing IgM production during infection. Parasite Immunol. 1993;15:601–611. doi: 10.1111/j.1365-3024.1993.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 43.Waine G J, Ross A G, Williams G M, Sleigh A C, McManus D P. HLA class II antigens are associated with resistance or susceptibility to hepatosplenic disease in a Chinese population infected with Schistosoma japonicum. Int J Parasitol. 1998;28:537–542. doi: 10.1016/s0020-7519(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 44.Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979;150:1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson J M. Fragmentations of polypeptides by enzymatic methods. In: Darbre A, editor. Practical protein chemistry: a handbook. New York, N.Y: John Wiley & Sons; 1986. pp. 122–148. [Google Scholar]
- 46.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]