Abstract
Prophylaxis to prevent bleeding is highly recommended for hemophilia patients. The development of new drugs and tools for modeling personalized prophylaxis provides the means for people with hemophilia to lead active lives with a quality of life comparable to that of nonhemophilic individuals. The choice of regimens must be made on a highly individual basis. Unfortunately, reference guides neither always concur in their recommendations nor provide directions to cover all possible scenarios. In this review, a group of experts identify the significant limitations and unmet needs of prophylaxis, taking advantage of their clinical experience in the disease, and supported by a rigorous literature update. To perform a more systematic and comprehensive search for gaps, the main cornerstones that influence decisions regarding prophylactic patterns were first identified.
Bleeding phenotype, joint status, physical activity, pharmacokinetics/medication properties, and adherence to treatment were considered as the primary mainstays that should allow physicians guiding prophylaxis to secure the best outcomes. Several challenges identified within each of these topics require urgent attention and agreement. The scores to assess severity of bleeding are not reliable, and lead to no consensus definition of severe bleeding phenotype. The joint status is to be redefined in light of new, more efficient treatments with an agreement to establish one scale as the unique reference for joint health. Further discussion is needed to establish the appropriateness of high-intensity physical activities according to patient profiles, especially because sustaining trough factor levels within the safe range is not always warranted for long periods. Importantly, many physicians do not benefit from the advantages provided by the programs based on population pharmacokinetic models to guide individualized prophylaxis through more efficient and cost-saving strategies. Finally, ensuring correct adherence to long-term treatments may be time-consuming for practitioners, who often have to encourage patients and review complex questionnaires.
In summary, we identify five cornerstones that influence prophylaxis and discuss the main conflicting concerns that challenge the proper long-term management of hemophilia. A consensus exercise is warranted to provide reliable guidelines and maximize benefit from recently developed tools that should notably improve patients' quality of life.
Keywords: hemophilia, prophylaxis, bleeding phenotype, joint status, physical activity, pharmacokinetics, treatment adherence
Introduction
Prophylaxis with regular administration of therapeutic products to prevent bleeding is the recommended therapy in patients with severe hemophilia (SH) or moderate hemophilia with a severe phenotype. 1 In historical terms, prophylaxis with factor concentrates was first categorized according to intensity: Malmö, Dutch, and Canadian research groups opted for repeated high, intermediate, and tailored dose administrations, respectively. 2 3 4 Simultaneously, age and joint status at diagnosis also came to be considered. 5 Thus, primary prophylaxis was defined as continuous replacement treatment initiated prior to the second clinically evident large joint bleed, prior to clinically/radiologically documented joint disease, and before 3 years of age. Secondary and tertiary prophylaxis refer to treatment initiated after two or more joint bleeds, without or with proven joint disease, respectively. 6
To acknowledge the inclusion of the new extended half-life (EHL) factor concentrates and the nonfactor replacement treatments in the therapeutic arsenal, the 3rd edition of the World Federation of Hemophilia (WFH) Guidelines for the Management of Hemophilia has recently proposed a new definition of prophylaxis 1 as the regular administration of a hemostatic agent/agents with the goal of preventing bleeding while allowing patients to lead active lives and achieve a quality of life comparable to nonhemophiliac individuals.
In order to achieve this goal, it is important to bear in mind that interindividual variability makes it necessary to individualize prophylaxis on a patient-by-patient basis. Furthermore, it must be accepted that there will be physiological and lifestyle changes throughout the patient's life, which will compel us to make dose and/or dosing frequency adjustments to ensure optimized prophylaxis at any moment in the patient's lifetime.
Objectives
The first aim of this review article was to establish the main cornerstones that influence the guidelines for prophylaxis of hemophiliac patients. This was the required step to accomplish the second aim, namely the systematic identification of the limitations and unmet needs, that is, the unmet needs of patients with hemophilia concerning their proper prophylactic management in each of the different situations they have to face throughout their day-to-day life.
Methodology: Identification of Cornerstones, and Their Limitations and Unmet Needs
A multidisciplinary expert panel (seven health care professionals from five hemophilia treatment centers) convened across three virtual meetings.
First Meeting
The aim was to define the main cornerstones that clinicians should consider in the optimization of prophylaxis. Previously, each one of the experts had worked individually on this topic. They presented their suggestions in the course of the meeting. A discussion was then held to reach consensus. The experts agreed on the following cornerstones: bleeding phenotype, joint status, physical activity, pharmacokinetics (PK)/medication properties, and adherence ( Fig. 1 ). Once the five cornerstones had been defined, the authors divided up the tasks according to their fields of expertise. A comprehensive literature search was performed in the PubMed database, according to the following combination of keywords (also decided in the first meeting): (hemophil* OR haemophil*) AND (bleeding phenotype OR joint status OR physical activity OR pharmacokinetic* OR adherence OR telemedicine). The first search without limits retrieved 2,954 entries. A new, more stringent search was then performed, with the keywords restricted to Title/Abstract, the publication type restricted to Clinical trial, Registry or Review, and English as the only language allowed. The new search retrieved 532 entries: 52 of them regarding bleeding phenotype, 22 on joint status, 45 on physical activity, 318 on PK, and 95 on adherence.
Fig. 1.
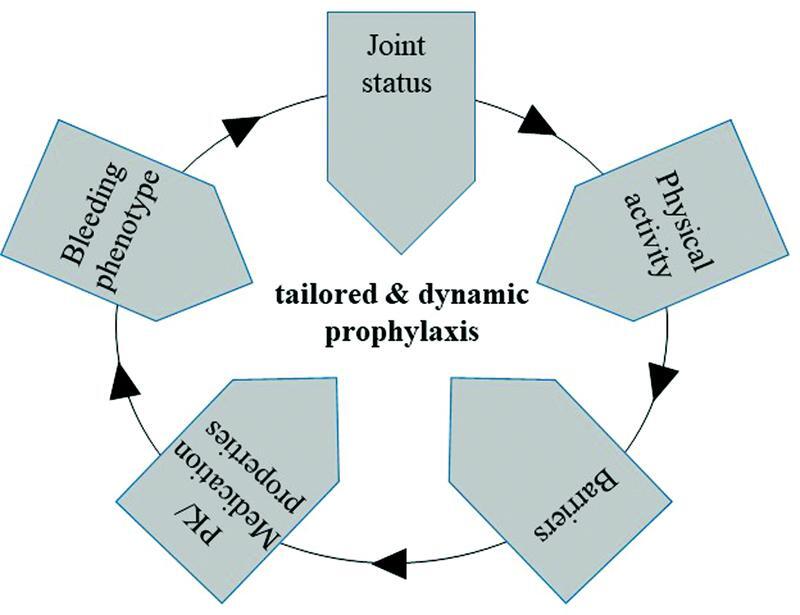
Determinants of prophylaxis. The five cornerstones that must be considered to decide the prophylaxis regimen in hemophilia A or B are shown. Assessing these determinants on a periodical basis warrants an optimized prophylaxis throughout the patient's life, thus improving his quality of life and saving costs.
Second Meeting
Taking advantage of their clinical experience and of a careful review of the literature, each author had previously identified the limitations and unmet needs that challenge the optimal management of each one of the five cornerstones. Such barriers were presented in the meeting. A first discussion was held.
Third Meeting
In the final meeting, authors reached an agreement on the final set of practical issues that warrant further work to reach consensus among practitioners. These limitations and unmet needs are presented here, preceded by a brief update on each of the corresponding mainstays.
Bleeding Phenotype
Bleeding phenotype can be characterized by the severity, number, and spontaneity of bleeding episodes and does not follow a predictable pattern ( Table 1 ). In SH, the age at which the first joint bleeding occurs may vary, 7 there may be different bleeding phenotypes in patients sharing similar PK profiles, 8 and the development of hemophilic arthropathy does not always correlate with the bleeding phenotype. 9
Table 1. Bleeding phenotype.
| Bleeding phenotype |
|---|
| ➢ Defined by severity, number, and spontaneity of bleeds |
| ➢ Influenced by: |
| Genetic factors: |
| - Directly: F8 / F9 variants |
| - Indirectly: variants influencing procoagulant/anticoagulant pathways, joint bleeding-triggered inflammatory processes, and pharmacokinetics of factor concentrates |
| Nongenetic factors: - Physical activity (type, level) - Functional ability/physical coordination (e.g., strength, flexibility, stability, etc.) - Risk-taking behaviors - Muscle/joint status - Occurrence of trauma |
| ➢ Assessed by: |
| - HSS, a composite score of the sum of 3 components: 12 a |
| o Bleeding score: average value of annual incidence of joint bleeds in the last 10 years divided by 20 |
| o Joint score: last score obtained during the 10-year period divided by the maximum possible score of 86 (patient examined independently by one physician and one physiotherapist) b |
| o Factor score: average annual amount of factor used during the 10-year period divided by mean body weight in that period and divided by 6 kIU/kg (maximum consumption of regular prophylaxis) c |
| - Severity scoring system of the ISTH-SCC, whose severity criteria are: 13 d |
| o First spontaneous bleeding before age 6 months, 2 points |
| o Spontaneous joint bleeding before age 2 years, 2 points |
| o Unprovoked intracranial hemorrhage, 3 points |
| o Spontaneous s.c. hematomas: at least one palm-sized or multiple (> 3) coin-sized, 1 point |
| A phenotype is severe when a score > 3 is reached by the age of 3 years |
| - Global hemostasis assessment methods (validation pending) 14 |
| Limitations and unmet needs |
| ➢ There is no reliable score to assess severity of bleeding: HSS requires 10 years of data collection and does not use imaging techniques to assess joint status; ISTH-SSC score considers neither joint status nor physical activity and requires availability of bleeding data from earliest childhood |
| ➢ There is an urgent need to establish a consensus concept of severe bleeding phenotype in patients on prophylaxis so that specific guidelines for therapy adjustment can be developed |
Abbreviations: HSS, Hemophilia Severity Score; ISTH-SSC, Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis; ln, natural logarithm; s.c., subcutaneous.
Each component has a maximum value of 1. Worst composite value is 3.
Requires adjustment by the age at the start of prophylaxis: the joint score is multiplied by log(age at start of prophylaxis + 10) −1 . Age is set at 50 for those never in prophylaxis beforehand.
Requires adjustment for late start of prophylaxis: the factor score is multiplied by ln(age at start of prophylaxis + 1.72) −1 .
These criteria are the result of a consensus reached during the ISTH-SSC meeting that was held in Toronto (Canada) in 2015.
The bleeding phenotype has a genetic background, 10 is influenced by joint status and physical activity, and can condition the PK-guided prophylaxis design. 1 11 It should be clear that such genetic variations are not the only causes of bleeding phenotype variability. The presence of inhibitors could lead to higher bleeding risk, and the presence of several associated inherited prothrombotic risk factors such as factor V Leiden mutation could mitigate bleeding symptoms.
The assessment of the bleeding phenotype was initially performed using the Hemophilia Severity Score (HSS) 12 though its various limitations have led practitioners to ignore it. More recently, a consensus picture of what can be considered a SH phenotype has been defined by the Subcommittee on Factor VIII, Factor IX and Rare Coagulation Disorders, in the context of the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Haemostasis (ISTH). 13 Global hemostasis tests could allow us to detect phenotype differences between patients, though their clinical usefulness is not yet confirmed ( Table 1 ). 14
Achieving an annualized bleeding rate of zero using the least possible treatment burden is the ideal aim of prophylaxis. However, with the emergence of novel developments in hemophilia care such as EHL factor concentrates and the nonfactor replacement treatments, successful prevention of synovitis is evolving as the new, ambitious target.
Limitations and Unmet Needs
-
The aforementioned scores to assess severity of bleeding have some important limitations ( Table 1 ):
o The HSS requires no fewer than 10 years of data collection to classify patients, and, importantly, joint status is not evaluated using imaging techniques. 12
o The algorithm endorsed by the ISTH-SSC is suitable only for either those patients whose data regarding bleeding events in their earliest childhood are available, or those following on-demand therapy.
o Neither joint status nor physical activity is considered. 13
The lack of a reliable score means that there is no consensus definition of severe bleeding phenotype in a patient following prophylaxis with replacement therapy, which often leads to an undesirable disparity of criteria regarding treatment adjustment. Therefore, a fair number of patients might not obtain the maximum benefit from the therapeutic resources available.
Joint Status
Joint damage is determined by synovial membrane blood release into the joint, which is responsible for structural joint damage ( Table 2 ). 15 The target joint has been classically defined as one in which at least three or more spontaneous bleeding events have taken place within a 6-month period. 6 Nevertheless, the current improved prophylactic treatments have succeeded in reducing recurrent joint bleeds to such an extent that allowing the occurrence of more than two bleeds with no therapeutic intervention is rather uncommon. 5 16
Table 2. Joint status.
| Joint status |
|---|
| ➢ Determined by synovial membrane blood release into joint |
| ➢ Actions to assess joint damage (repeated periodically for prophylaxis adjustment): |
| - Annualized bleeding rate |
| - Physical exam: HJHS 22 a and/or Gilbert Score 23 |
| - Image analysis: US (HEAD-US 24 ) b , MRI (IPSG MRI Score 27 ) b , X-ray (Pettersson score) 28 |
| - Activity and functionality: FISH 29 and HAL 30 |
| Limitations and unmet needs |
| ➢ Terms describing joint health should be updated in order to clearly identify those situations requiring reconsideration of prophylaxis regimen |
| ➢ A comprehensive estimation of joint status in the context of each individual patient is not straightforward to achieve, since there is no agreement to establish one of the many assessment tools available as the preferred one |
| ➢ Further work required to determine the usefulness of physical activity questionnaires to predict bleeding risk |
| ➢ Cooperation of patient is a key factor to ensure optimized prophylaxis in the long-term |
Abbreviations: HJHS, Hemophilia Joint Health Score; US, ultrasound; HEAD-US, Haemophilia Early Arthropathy Detection with Ultrasound; IPSG, International Prophylaxis Study Group; MRI, magnetic resonance imaging; FISH, Functional Independence Score for Hemophilia; HAL, Haemophilia Activities List.
Preferred than Gilbert Score when joint damage is mild.
US and MRI are the only methods that are sensitive enough to diagnose synovitis.
Joint status is also a marker of long-term efficacy of prophylaxis. Image analysis-based studies have shown that classical prophylaxis, although efficient, is unable to fully prevent joint damage. 17 18 Accordingly, more recent studies have detected subclinical synovitis and early changes in joint cartilage in hemophiliac patients, irrespective of age, severity, number of bleeds, or treatment regimen. 19 Furthermore, some findings suggest that around 37% of severe or moderate hemophilia patients with a low incidence of hemarthrosis may undergo deterioration of joint health within the following 5 to 10 years. 20 This is due to subclinical bleeds that are noticed neither by patients nor physicians because they do not cause symptoms but that progressively damage joint structure in the synovial membrane, cartilage, and subchondral bone. 21
With the aim of quantifying damage, expert groups recommend assessment of the annualized bleeding rate and a physical exam including the assessment of the Hemophilia Joint Health Score and/or Gilbert Score. 22 23 Image analysis procedures are advisable. Ultrasound (US) is particularly recommended for the following reasons: easy availability, test repeats allowed, high sensitivity and specificity to allow diagnosis of synovitis in joints with normal radiographic imaging, and clinical examination. US should preferably be used in the point-of-care modality, so that it is available to all patients. US can be used to identify acute bleeds and detect chronic synovitis and osteochondral damage. At present, the most widespread point-of-care US protocol is Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US). 24 On the other hand, magnetic resonance imaging (MRI) may not always be available and does not allow test repeats. 25 26 Nevertheless, it provides very detailed information and allows the assessment of joint status by the International Prophylaxis Study Group MRI score. 27 The X-ray-based Pettersson score is particularly useful to appraise bone condition. 28 Finally, the assessment of patient activity and functionality by using tools such as the Functional Independence Score for Hemophilia 29 or the Haemophilia Activities List 30 is also recommended. Although there are other scores and questionnaires, those outlined here are not only the most widely used but also the ones currently recommended by reference guides. 1 31
It is important to note that establishing point-of-care methods such as HEAD-US allows easy follow-up of patients to detect early changes in joint status. Therefore, provided that these procedures are repeated periodically from early childhood, the appropriate adjustment of prophylaxis regimen, when required, will guarantee optimized treatment throughout the whole lifetime and, importantly, will aid the preservation of joint health. 32 33 Finally, it must be remarked that the treatment guidelines may change in the coming years to adapt to new therapeutic tools, such as EHL factors or nonfactor products, and to PK-guided personalized therapies that are enabling more efficient prophylaxis and, accordingly, increasingly good joint health.
Limitations and Unmet Needs
-
In consonance with the new, more efficient hemostatic therapies, there is a need to redefine concepts such as target joint or annual joint bleeding rate ( Table 2 ). This is a relevant issue, since this would allow us to introduce changes in the prophylaxis patterns only in those situations where they are really required.
o Since chronic synovitis is indirect evidence of repeated exposure to blood in the joint, the definition of the target joint could be updated based on the presence/absence of chronic synovitis. 34
o In any case, consensus effort regarding this topic is mandatory.
Achieving a comprehensive assessment of joint health may not be straightforward. A large number of tools is currently available to be used for this purpose. However, there is no consensus to establish one of the scores as the preferred one, so that the status of the same patient could be considered differently in accordance with the method used.
The role of the questionnaires that address physical activity to predict bleeding risk must be clarified.
The involvement of the patient is mandatory to allow appropriate follow-up in the long term.
Physical Activity
Physical activity is the collection of routine physical activities performed throughout one person's everyday life ( Table 3 ). These are undoubtedly beneficial since they increase joint stability, strength, and mobility range, prevent joint deterioration, increase patient ambulation, prevent obesity, and improve physical fitness, thus increasing self-confidence. 35 36 37 Nevertheless, hemophiliac patients and/or persons in their home environment are concerned about some physical activities that are usually considered to increase bleeding risk. However, absolute risk associated with doing physical activity may be low. 38
Table 3. Physical activity.
| Physical activity |
|---|
| ➢ Defined by day-to-day physical activities throughout life |
| ➢ Recommended FVIII/FIX trough levels according to comprehensive patient profiles: 39 |
| - < 1% for those: |
| o < 2 years of age with no bleeds |
| o With poor adherence associated with complex venous access |
| o With no bleeds despite low-frequency dosing prophylaxis |
| - 1–3% for those: |
| o Sedentary with no recurrent bleeds in same joint, moderate/mild arthropathy, and no pro-bleeding comorbidities |
| o With moderate hemophilia and spontaneous bleeding in spite of basal factor at 1–2% |
| - 3–5% for those: |
| o On mild physical activity |
| - 5–15% for those: |
| o On high-risk physical activity |
| Limitations and unmet needs |
| ➢ Setting up individualized programs of physical activity is time consuming for the practitioner; interdisciplinary teams are sometimes unavailable |
| ➢ No agreement among guides regarding suitability of high-intensity physical activity according to patient profile |
| ➢ Risk of unrealistic feeling of safety associated with the use of EHL factors |
| ➢ Lack of evidence regarding efficacy of nonfactor products to cover high-intensity physical activity |
Abbreviations: FVIII, factor VIII; FIX, factor IX; EHL, extended half-life.
Unveiling the optimal factor requirement for prophylaxis in each patient according to physical activity, joint status, and other individual hallmarks would be highly desirable. An increasing body of new evidence challenges the established prophylaxis target level of factor VIII (FVIII) or FIX activity of 1%, suggesting higher target values. Recent expert recommendations suggest the following trough values 39 :
< 1%: children under 2 years with no bleeds, with the purpose of delaying as much as possible the placement of a central access device; patients who either are not comfortable with prophylaxis or have poor adherence due to complicated venous access; patients who do not experience joint bleeds despite following a low-frequency dosing prophylactic regimen.
1 to 3%: sedentary patients or those whose physical activity is low, with no recurrent bleeds in the same joint, with moderate or mild arthropathy and no comorbidities increasing bleeding risk; patients with moderate hemophilia experiencing spontaneous bleeding with factor levels of 1 to 2%.
3 to 5% in patients with mild physical activity.
5 to 15% in patients with high-risk physical activity.
There are many guidelines addressing physical activity practice in persons with hemophilia (PwH). The guidelines of the National Hemophilia Foundation “Playing it Safe” use a bleeding risk scoring system that considers activities to be low (swimming), low-moderate (hiking), moderate (running), moderate-high (mountain biking), or high risk (boxing). 40 On the other hand, the WFH recommends the regular practice of physical activity, avoiding close contact sports. A prophylactic regimen that guarantees appropriate factor levels must be followed by those doing contact or speed sports (motorcycling). 1 The choice of physical activity must take into consideration the patient's physical status, skills, and preferences, as well as local habits. The advice of the patient's doctor must be sought before starting up a program of physical activity, and the patient, family, and doctor must participate in the decision-making process, considering the peak and trough levels that will be required for the physical activity chosen.
Limitations and Unmet Needs
Individualized programs of physical activity matching unique personal characteristics are highly desirable. For this purpose, consensus among several specialists is usually required. However, such teams are not always available, and it is not infrequent for practitioners not to have enough time to set up programs and/or follow-up patients.
There is no consensus among guidelines concerning appropriateness of high-risk sports according to patient profiles.
The guidelines do not address the differences concerning physical condition and joint status among adult and pediatric patients.
The availability of EHL factors allows the achievement of a higher circulating factor level, thus making it possible to practice more intense physical activities. However, this advantage may constitute a problem associated with a spurious feeling of safety. Those patients with established arthropathy should be particularly aware of this risk.
Although EHL products allow a high trough factor level, securing such safety values unfailingly is not straightforward. Thus, patients should be cautious, particularly when performing high-risk activities for prolonged periods of time.
Research is needed to assess the suitability of some new drugs, for example, bispecific antibodies, that are an alternative to replacement therapy products, regarding the coverage of physical activities requiring high hemostatic protection.
Pharmacokinetics/Medication Properties
Monoclonal antibodies such as emicizumab, whose ability to bring together FIXa and FX obviates the need for FVIII, or concizumab, targeting tissue factor pathway inhibitor, are emergent therapies which, together with gene therapy, are going to improve the management of hemophilia in the near future. 41 Nevertheless, replacement therapy with FVIII or FIX is still considered to be the gold-standard treatment in most cases. The advances that have been made in the field of PK of FVIII and FIX have been noticeable. For this reason, this work will focus on how the new PK tools can optimize the adjustment of treatment and benefit patients. The group will address the emergent therapies in future reviews.
The PK profile of a drug is obtained through calculating a series of parameters related to its plasma concentration over time ( Table 4 ). PK analyses of coagulation factors are of paramount importance since PwH exhibit a relatively high variability among individuals. The low intrasubject variability and the high interpatient variability regarding PK parameters are well known and it is essential to take them into account when designing personalized therapies. This is the reason why there is no prophylactic regimen able to fit all hemophiliac patients, and, indeed, prompts us to carry out a careful PK study in every single patient to optimize prophylaxis on an individual basis.
Table 4. Pharmacokinetics.
| Pharmacokinetics |
|---|
| ➢ Aimed to describe factor plasma concentration over time and the differences between individuals |
| ➢ PK analysis procedures: |
| - Traditional multiple sampling analyses: limited by need for washout period and high number of blood collections after a single infusion |
| - Population PK models: no washout period, low number of collections, consider patient's covariates |
| ➢ PopPK tools: NONMEM-7, 69 myPKFiT, 46 WAPPS-HEMO (McMaster Pop-PK), 47 Hemotik 50 (see Table 5 ) |
| Limitations and unmet needs |
| ➢ Lack of awareness of PopPK models to guide personalized prophylaxis leads to misconceptions: |
| - Too complex to be used in daily practice |
| - Benefit is not superior |
| - Saving factor/doses rarely occurs |
| - Poor adherence precludes from obtaining benefit |
| ➢ Time required to achieve expertise and use on a regular basis not suitable for rushed physicians |
| ➢ Overuse |
Abbreviations: PopPK, population pharmacokinetics.
PK behavior of FVIII is mostly unpredictable. Although variables like age, blood group, and von Willebrand factor are known to influence circulating FVIII levels, the uncertainty about FVIII PK is generally regarded as very high. PK study has been largely simplified in recent years with newer tools, although some degree of training is required. Currently, there are tools available that are accurate enough to provide objective data, 42 and, therefore, to adjust prophylaxis according to each patient's individual requirements. The savings achieved in bleed prevention, FVIII consumption, and medical assistance make PK studies a very cost-effective strategy.
Recommended methods for individual PK estimation can be categorized into two distinct groups. The first one consists of direct individual analyses, as proposed by the ISTH in 2001. 43 These studies had important limitations, such as the requirement for a washout period, which could increase the bleeding risk, or the need to collect up to 10 or 11 blood samples over a short period, which may not be feasible in all patients, especially in children and those with poor venous access. The second group consists of predictive population models based on a Bayesian approach. These methods do not require a washout period; blood needs to be collected only two or three times after factor infusion, and independent PK studies after different infusions can be combined to draw a more complete PK profile, thus avoiding the need for long stays in hospital to complete a PK profile after a single, unique factor infusion. PK parameter estimation is performed according to mathematical models that also consider each patient's individual covariates such as weight, age, or body mass index. 44 Once a population model is available for each coagulation factor, the individual population PK (PopPK) can be obtained just by providing individual covariates as well as biometrics and FVIII or FIX values in the two or three samples, commonly 4 hours after infusion and 24 to 48 hours later. 45 There are several user-friendly PopPK tools that are currently easily accessible, such as myPKFiT 46 and WAPPS-HEMO (McMaster Pop-PK) ( Table 5 ). 47 Both of them are free to use after registration, and are fully accepted by the hemophilia community. The latter can run analyses with any factor product at any dose, while myPKFiT can be used only with Advate or Adynovate and is limited to approved doses. The result of analyses is immediately available with myPKFiT, while a short validation period is required with WAPPS-HEMO (McMaster Pop-PK). Both of them also have mobile applications available for the patients and have been approved as medical devices. Interestingly, both platforms are able to calculate doses at irregular intervals, which is a very common practice in the real world. Only WAPPS-HEMO (McMaster Pop-PK) has developed links for integration of data with other systems such as Florio or Haemoassist, 48 49 or with international registries of coagulation disorders such as those of the American Thrombosis and Hemostasis Network ( https://news.wapps-hemo.org/press-release/ ). Finally, Hemotik is a PopPK tool that can be used with Nuwiq. 50
Table 5. Comparison between different modern approaches to PopPK calculation in hemophilia.
| PopPK tool | Expertise required | Type/Cost | Product | PK estimation | Restricted to approved doses | Mobile app | Medical device | Dose calculator | Integration |
|---|---|---|---|---|---|---|---|---|---|
| NonMem 69 | ++ + ++ | Desktop/$$$ | All | Immediate | No | No | No | No | No |
| MyPKFiT 46 | ++ | Web app/free | Advate, Rixubis, or Adynovate |
Immediate | Yes | Yes | Yes | Yes | No |
|
WAPPS-Hemo
47
(McMaster PopPK) |
++ | Web app/free | All | > 24 h | No | Yes | Only MyPopPK version | Yes | Yes: WBDR, Florio, Haemoassist |
| Hemotik 50 | ++ | Web app | Nuwiq | Immediate | No | No | No | Yes | No |
Abbreviations: PopPK, population pharmacokinetics; PK, pharmacokinetics; WBDR, World Bleeding Disorders Registry; $$$, payment required.
When prophylaxis is guided by a PK study its efficacy increases even though the costs are not necessarily higher. 51 These analyses provide objective and accurate parameters, and last over time, with a close correspondence between observed and predicted results. 52 The design of a PK-guided individualized prophylaxis protocol has also been shown to improve adherence, 53 allows specialists to objectively decide when the switch to other products is a suitable option, 54 and saves factor consumption-related costs. 55
In the establishment of an optimization process, PK studies should not be used alone but together with patient information concerning the other prophylaxis cornerstones, to ensure the best treatment personalization and thus aspire to excellence in the management of the PwH.
Limitations and Unmet Needs
Many of the barriers are related to the widespread lack of awareness regarding the functioning and potential of the relatively new PopPK models ( Table 4 ). This lack of awareness leads to a series of misconceptions that raise practical issues.
It falsely magnifies the complexity of the technical procedures and, thus, invites clinicians to shift responsibility to others.
It precludes the adequate perception of the real benefits derived from the application of these models to establish personalized prophylaxis patterns, which thus are not seen as superior to those obtained with the former PK methods.
The reduction in the number of factors used and/or the number of weekly doses that can be achieved by applying PopPK models is not perceived as important. Some physicians still believe that there are situations where the number of factor/doses used is even higher when using PopPK. In fact, although personalizing treatments via PopPK is a suitable way to save costs, 55 some clinicians may have the concern that the pharmaceutical industry promotes the use of PK tools to encourage the expenditure related to replacement therapy products.
There is a common misconception related to adherence. It is sometimes thought that patients would not benefit from PK-guided individualized prophylaxis because they would not adhere to treatment as closely as advisable, while published evidence demonstrates the opposite. 53
Although to a lesser extent than commonly perceived, achieving expertise and the daily use of these tools does require an investment of time that is often not available for most physicians.
There is a risk of overuse of PopPK-based procedures to personalize prophylaxis, which may not be required in low complexity cases.
Adherence
Prophylaxis reduces the number of breakthrough bleedings and improves joint health. However, there are important barriers to prophylaxis that should be taken into account before choosing a treatment regimen for each patient. In fact, benefits associated with prophylaxis are directly related to trough levels, which should always be within the recommended range according to patient profile (see above), for which an appropriate adherence to treatment must be achieved ( Table 6 ). Adherence is described as the patient's active and voluntary involvement in his own caregiving, in collaboration with his health care providers and with the purpose of achieving a predetermined therapeutic target. In PwH, it can be assessed by using validated methods such as the Veritas-Pro questionnaire or the Haemo-Adhaesione scale. 56 57
Table 6. Adherence.
| Adherence |
|---|
| ➢ Patient's involvement in own caregiving together with health care providers to achieve his therapeutic target |
| ➢ Assessed by validated methods: Veritas-Pro questionnaire 56 |
| ➢ Influenced by factors related to: |
| - Patient: misconceptions, age (problematic in adolescence, young adulthood) |
| - Disease: bleeding phenotype |
| - Treatment: venous access, prophylactic regimen |
| - Health care system, socioeconomic condition, availability, and access to different treatments |
| ➢ Procedures to improve adherence: |
| - Educational programs in hemophilia treating centers/patient associations |
| - Transition programs between pediatric and adult units |
| - Web/app-based training |
| - Reinforcement of emotional coping skills |
| - Establishing routines, encouraging participation in sport/social activities |
| - In nonadherent or elderly patients: extended half-life or subcutaneously administered factors |
| - In the elderly: continuing education, social worker/psychologist involvement, home care services |
| ➢ In order to encourage shared decisions, patient must be: |
| - Educated/informed about expected results according to individual profile |
| - Encouraged to access online reliable scientific information |
| - Encouraged to share experiences in patient associations |
| - Offered (by health provider) an easy-to-access communication channel |
| ➢ Telemedicine allows the control of adherence, even in complex scenarios, and offers other relevant benefits: |
| - Particularly suitable for those living away from treatment center |
| - Control of physical activity, stock supply, number/severity of bleeds |
| - Communication of results, discussion of therapeutic strategies, identification of need for in-person visit |
| - Valid communication channel in pandemic scenarios |
| Limitations and unmet needs |
| ➢ Dependence on health literacy, only achieved when both patient and doctor are encouraged |
| ➢ Time-consuming questionnaires, which require doctor's expertise |
| ➢ Time-consuming visits to share decisions |
| ➢ Mobile apps may stress patients |
| ➢ Non-in-person visits may result in loss of interest |
A variety of factors and barriers to prophylaxis influences adherence 58 :
Patient-related: misconceptions about the disease; lack of adherence during the adolescent years, when patients challenge parental control, is not unusual; young adulthood, when the patient has to start taking responsibility for his treatment.
Disease-related: bleeding phenotype.
Treatment-related: venous access, regimen (required needs).
Factors related to the health care system and socioeconomic conditions.
A careful analysis of these factors via a multidisciplinary approach is advisable to address lack of adherence, especially in adolescents and young adults, and to identify its main causes. There are tools and procedures that can be useful to improve patient involvement and compliance with treatment: educational programs conducted by health professionals or peers in hemophilia treating centers or/and in patient associations 58 59 ; transition programs between pediatric and adult units 60 ; Web-/app-based training with same-age or somewhat older patients; reinforcement of skills concerning emotional coping, since the adolescent patient is insecure and seeks acceptance and self-acceptance; establishing routines; encouraging participation in sport and social activities; use of EHL factor concentrates to decrease the dosing frequency 59 61 62 ; and use of EHL factor concentrates or subcutaneously administered factors in nonadherent patients.
Focusing on elderly patients, fluent communication is particularly important to improve their adherence. Their needs and expectations have to be acknowledged. They should be informed about tertiary prophylaxis benefits, such as pain decrease after reduction of subclinical bleeding or decrease of fatal bleeding risk. Psychologists and social workers should assist in identifying barriers. In patients with advanced age, the risk versus benefit balance should be carefully assessed to make a decision about switching to regimens involving lower dosing frequencies or the use of subcutaneously administered products. Close follow-up including onsite visits and running programs of home care services with the assistance of skilled nursing professionals are also highly advisable. 63 Generally speaking, things should be made easier for these patients. The ideal treatment choice would rely on the use of EHL factor concentrates or subcutaneously administered products. A close monitoring of adherence should be performed. Some authors claim that, when advisable, prophylaxis should be administered in hospital. 58
To facilitate treatment compliance in patients of all ages, they, or their caregivers, must be empowered through making shared decisions ( Table 6 ). To accomplish this purpose, the patient must be educated and informed about the efficacy, safety, and posology of the available treatment options. Indeed, he must be extensively informed about the expected results considering his bleeding phenotype, lifestyle, physical activity, PK parameters, and joint status for which the Haemophilia Joint Visualizer may be useful. 64 Patients can be advised to browse Web pages with reliable scientific contents and can also be encouraged to contact patient associations to share experiences with others who may be routinely following the same therapy regimen. These actions could help him to solve queries and concerns that may not have emerged in the visit to the hospital. Health care providers should also give the patient an e-mail address to be permanently in touch and discuss any issues, and, if deemed advisable, should prompt him to go to the hospital for a second visit. 58
The increasing use of telemedicine in daily clinical practice can result in positive effects on adherence. Telemedicine is a useful tool for the following profiles ( Table 6 ): young patients, caregivers with an adequate sociocultural background who are familiar with new technology, and patients living away from a comprehensive hemophilia treatment center and without access to hemophilia care-trained multidisciplinary teams. By this means, pharmacological assistance and control of physical activity, adherence, and stock supply, are guaranteed. Importantly, the number and severity of bleeds can also be reported. All this information thus allows for adjustment of factor dosing or switching to another product in real time. 65 Telemedicine also allows the communication of results of analytical tests, the discussion of therapeutic strategies, follow-up of patients recruited for clinical trials, and delivery of educational programs to encourage self-treatment and home-based recovery. Of note, telephone triage consultations to discern those patients who require an in-person visit for joint examination, accurate bleed assessment or whatever, from those who do not, will make it possible to alleviate health care pressure. In summary, telemedicine provides a chance to carry the treatment center to the patient. Furthermore, it becomes an essential tool in pandemic scenarios ( Fig. 2 ), 66 67 even where hemophilic patient management involves more than one medical speciality. 68 Indeed, the ease of contacting the patient make it possible to monitor adherence effectively.
Fig. 2.
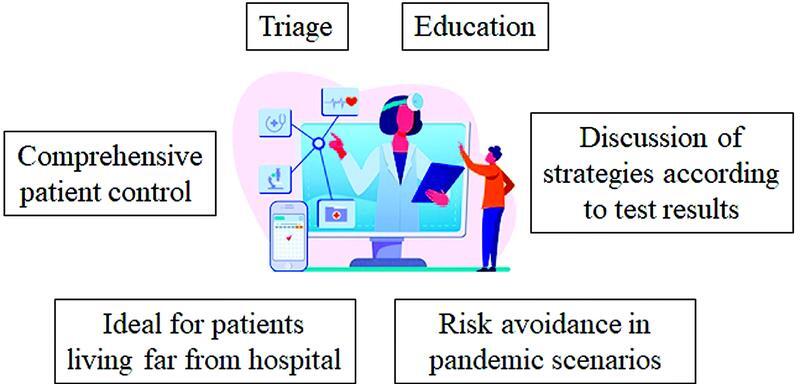
Advantages of telemedicine associated with the hemophilic patient management. Illustration courtesy of www.freepik.es ( https://www.freepik.es/vectores/personas ).
Limitations and Unmet Needs
The success of adherence relies upon the patient's good health literacy, and this is only achieved when both doctor and patient are encouraged enough ( Table 6 ).
The patient or caregiver needs to have the ability to understand the basic concepts regarding the hallmarks of the disease and its course.
Completion and review of questionnaires are time-consuming for patients and physicians, respectively. Importantly, the latter need a certain expertise to interpret the answers correctly.
Sharing decisions with patients/caregivers, while highly beneficial for adherence, may also be extremely time-consuming for doctors and patients/caregivers.
Older patients or those with limited cognitive abilities may not benefit from telemedicine.
In some cases, using mobile apps, if this is possible for the patient, may exert a negative stressful effect.
Too many non-in-person visits may sometimes result in discouragement and loss of interest.
Limitations and Strengths
Some issues regarding patient care still require further exploration to achieve consensus views on their optimal management. However, the recognition of the five cornerstones on which treatment relies, and the identification of the limitations and unmet needs in each one of them, represent an advance since they highlight where our efforts should be focused.
Concluding Remarks
It is important to recognize that the individual prophylactic regimen will likely need to be modified with time as circumstances change. An ideal prophylaxis program should take into account all of the aforementioned items and also be able to prevent bleeding while allowing patients to lead active lives and achieve a quality of life comparable to that of nonhemophiliac individuals. To date, the cornerstones of personalized prophylaxis are focused on bleeding phenotype, joint status, physical activity, PK/medication properties, and adherence. A consensus exercise is required to provide unambiguous guidelines to handle the conflicting concerns identified here regarding the management of people with hemophilia A or B.
Acknowledgments
The authors thank Ramón Montes Díaz, from Ambos Marketing Services S.L., for the writing assistance, and Oscar Herrero, Sonia Llorente, and Juan Gracia, from Novo Nordisk, for their helpful technical assistance.
Funding Statement
Funding This study was funded by Novo Nordisk.
Conflict of Interest None declared.
Authors' Contributions
R.N., M.T.A., S.B., J.R.G.P., H.C.R., R.B., and V.J.Y. designed this article, researched the literature, prepared the contents of all sections in the paper, and co-wrote and reviewed this collaborative research paper.
Disclosures
The authors disclose financial activities outside the submitted work. R.N. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting and/or funds for research from Takeda, Bayer, CSL-Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma, Sanofi, and Pfizer. M.T.A. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, CSL-Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma, Biomarin, Sanofi, Pfizer, Novartis, and Amgen. S.B. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting and/or funds for research from Takeda, Bayer, CSL-Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma, Biomarin, Sanofi, and Pfizer. J.R.G.P. has received fees for consulting services by Amgen, Novartis, SOBI, Grifols, and CSL Behring and speaking honoraria from Novo Nordisk, Shire, SOBI, Roche, Daiichi Sankyo, Pfizer, Rovi, Amgen, and Novartis. H.C.R. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or consulting and/or funds for research from Pfizer, Roche, Sobi, NovoNordisk, Takeda, and Bayer. R.B. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting and/or funds for research from Takeda, Bayer, CSL-Behring, Novo Nordisk, Sobi, Roche, Boehringer-Ingelheim, and Pfizer. V.J.Y. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting and/or funds for research from Takeda, Bayer, CSL-Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma, Biomarin, Sanofi, and Pfizer. The preparatory meetings have been financed by Novo Nordisk.
These two authors contributed equally to the work.
References
- 1.WFH Guidelines for the Management of Hemophilia panelists and co-authors Srivastava A, Santagostino E, Dougall A.WFH Guidelines for the Management of Hemophilia, 3rd edition Haemophilia 202026(Suppl 6):1–158. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson I M, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232(01):25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 3.van den Berg H M, Fischer K, Mauser-Bunschoten E P. Long-term outcome of individualized prophylactic treatment of children with severe haemophilia. Br J Haematol. 2001;112(03):561–565. doi: 10.1046/j.1365-2141.2001.02580.x. [DOI] [PubMed] [Google Scholar]
- 4.Association of Hemophilia Clinic Directors of Canada Prophylaxis Study Group . Feldman B M, Pai M, Rivard G E. Tailored prophylaxis in severe hemophilia A: interim results from the first 5 years of the Canadian Hemophilia Primary Prophylaxis Study. J Thromb Haemost. 2006;4(06):1228–1236. doi: 10.1111/j.1538-7836.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- 5.Berntorp E, Astermark J, Björkman S.Consensus perspectives on prophylactic therapy for haemophilia: summary statement Haemophilia 20039(Suppl 1):1–4. [DOI] [PubMed] [Google Scholar]
- 6.Subcommittee on Factor VIII, Factor IX and Rare Coagulation Disorders of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis . Blanchette V S, Key N S, Ljung L R, Manco-Johnson M J, van den Berg H M, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk K, Fischer K, van der Bom J G, Grobbee D E, van den Berg H M. Variability in clinical phenotype of severe haemophilia: the role of the first joint bleed. Haemophilia. 2005;11(05):438–443. doi: 10.1111/j.1365-2516.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk K, van der Bom J G, Lenting P J. Factor VIII half-life and clinical phenotype of severe hemophilia A. Haematologica. 2005;90(04):494–498. [PubMed] [Google Scholar]
- 9.Wyseure T, Mosnier L O, von Drygalski A. Advances and challenges in hemophilic arthropathy. Semin Hematol. 2016;53(01):10–19. doi: 10.1053/j.seminhematol.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PedNet and the Rodin Study Group Carcao M D, van den Berg H M, Ljung R, Mancuso M E.Correlation between phenotype and genotype in a large unselected cohort of children with severe hemophilia A Blood 2013121193946–3952., S1 [DOI] [PubMed] [Google Scholar]
- 11.Rendo P, Shafer F, Korth-Bradley J M, Sivamurthy K, Korin J. Factors that influence the bleeding phenotype in severe hemophilic patients. Blood Coagul Fibrinolysis. 2013;24(07):683–690. doi: 10.1097/MBC.0b013e3283614210. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S, Eelde A, Holmström M, Ståhlberg G, Odeberg J, Blombäck M. Validation of a composite score for clinical severity of hemophilia. J Thromb Haemost. 2008;6(07):1113–1121. doi: 10.1111/j.1538-7836.2008.03001.x. [DOI] [PubMed] [Google Scholar]
- 13.subcommittee on factor viii, factor ix and rare coagulation disorders . Mancuso M E, Bidlingmaier C, Mahlangu J N, Carcao M, Tosetto A. The predictive value of factor VIII/factor IX levels to define the severity of hemophilia: communication from the SSC of ISTH. J Thromb Haemost. 2018;16(10):2106–2110. doi: 10.1111/jth.14257. [DOI] [PubMed] [Google Scholar]
- 14.Tarandovskiy I D, Balandina A N, Kopylov K G. Investigation of the phenotype heterogeneity in severe hemophilia A using thromboelastography, thrombin generation, and thrombodynamics. Thromb Res. 2013;131(06):e274–e280. doi: 10.1016/j.thromres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Brecelj J, Bole V, Benedik-Dolnicar M, Grmek M. The co effect of prophylaxis and radiosynovectomy on bleeding episodes in haemophilic synovitis. Haemophilia. 2008;14(03):513–517. doi: 10.1111/j.1365-2516.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- 16.Carcao M, Lambert T.Prophylaxis in haemophilia with inhibitors: update from international experience Haemophilia 201016(Suppl 2):16–23. [DOI] [PubMed] [Google Scholar]
- 17.Manco-Johnson M J, Abshire T C, Shapiro A D. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(06):535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 18.Cross-sectional MRI study investigators . Oldenburg J, Zimmermann R, Katsarou O. Controlled, cross-sectional MRI evaluation of joint status in severe haemophilia A patients treated with prophylaxis vs. on demand. Haemophilia. 2015;21(02):171–179. doi: 10.1111/hae.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De la Corte-Rodriguez H, Rodriguez-Merchan E C, Alvarez-Roman M T, Martin-Salces M, Martinoli C, Jimenez-Yuste V. The value of HEAD-US system in detecting subclinical abnormalities in joints of patients with hemophilia. Expert Rev Hematol. 2018;11(03):253–261. doi: 10.1080/17474086.2018.1435269. [DOI] [PubMed] [Google Scholar]
- 20.Kuijlaars I AR, Timmer M A, de Kleijn P, Pisters M F, Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017;23(06):934–940. doi: 10.1111/hae.13327. [DOI] [PubMed] [Google Scholar]
- 21.van Meegeren M E, Roosendaal G, Jansen N W, Lafeber F P, Mastbergen S C. Blood-induced joint damage: the devastating effects of acute joint bleeds versus micro-bleeds. Cartilage. 2013;4(04):313–320. doi: 10.1177/1947603513497569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro T, Abad A, Feldman B M. Developing a new scoring scheme for the Hemophilia Joint Health Score 2.1. Res Pract Thromb Haemost. 2019;3(03):405–411. doi: 10.1002/rth2.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert M S.Prophylaxis: musculoskeletal evaluation Semin Hematol 199330(3, Suppl 2):3–6. [PubMed] [Google Scholar]
- 24.Martinoli C, Della Casa Alberighi O, Di Minno G. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) Thromb Haemost. 2013;109(06):1170–1179. doi: 10.1160/TH12-11-0874. [DOI] [PubMed] [Google Scholar]
- 25.Foppen W, van der Schaaf I C, Beek F JA, Mali W PTM, Fischer K. Diagnostic accuracy of point-of-care ultrasound for evaluation of early blood-induced joint changes: comparison with MRI. Haemophilia. 2018;24(06):971–979. doi: 10.1111/hae.13524. [DOI] [PubMed] [Google Scholar]
- 26.Poonnoose P M, Hilliard P, Doria A S. Correlating clinical and radiological assessment of joints in haemophilia: results of a cross sectional study. Haemophilia. 2016;22(06):925–933. doi: 10.1111/hae.13023. [DOI] [PubMed] [Google Scholar]
- 27.International Prophylaxis Study Group . Lundin B, Manco-Johnson M L, Ignas D M. An MRI scale for assessment of haemophilic arthropathy from the International Prophylaxis Study Group. Haemophilia. 2012;18(06):962–970. doi: 10.1111/j.1365-2516.2012.02883.x. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson H, Ahlberg A, Nilsson I M. A radiologic classification of hemophilic arthropathy. Clin Orthop Relat Res. 1980;(149):153–159. [PubMed] [Google Scholar]
- 29.Poonnoose P M, Manigandan C, Thomas R. Functional Independence Score in Haemophilia: a new performance-based instrument to measure disability. Haemophilia. 2005;11(06):598–602. doi: 10.1111/j.1365-2516.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 30.van Genderen F R, Westers P, Heijnen L. Measuring patients' perceptions on their functional abilities: validation of the Haemophilia Activities List. Haemophilia. 2006;12(01):36–46. doi: 10.1111/j.1365-2516.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 31.Participants of the International Symposium on Outcome Measures in Hemophilic Arthropathy . Fischer K, Poonnoose P, Dunn A L. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia. 2017;23(01):11–24. doi: 10.1111/hae.13088. [DOI] [PubMed] [Google Scholar]
- 32.Fischer K, Van den Berg H M, Thomas R.Dose and outcome of care in haemophilia–how do we define cost-effectiveness? Haemophilia 200410(Suppl 4):216–220. [DOI] [PubMed] [Google Scholar]
- 33.Feldman B M, Rivard G E, Babyn P. Tailored frequency-escalated primary prophylaxis for severe haemophilia A: results of the 16-year Canadian Hemophilia Prophylaxis Study longitudinal cohort. Lancet Haematol. 2018;5(06):e252–e260. doi: 10.1016/S2352-3026(18)30048-6. [DOI] [PubMed] [Google Scholar]
- 34.O'Hara J, Walsh S, Camp C. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16(01):84. doi: 10.1186/s12955-018-0908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negrier C, Seuser A, Forsyth A. The benefits of exercise for patients with haemophilia and recommendations for safe and effective physical activity. Haemophilia. 2013;19(04):487–498. doi: 10.1111/hae.12118. [DOI] [PubMed] [Google Scholar]
- 36.Mulvany R, Zucker-Levin A R, Jeng M. Effects of a 6-week, individualized, supervised exercise program for people with bleeding disorders and hemophilic arthritis. Phys Ther. 2010;90(04):509–526. doi: 10.2522/ptj.20080202. [DOI] [PubMed] [Google Scholar]
- 37.Czepa D, von Mackensen S, Hilberg T. Haemophilia & Exercise Project (HEP): the impact of 1-year sports therapy programme on physical performance in adult haemophilia patients. Haemophilia. 2013;19(02):194–199. doi: 10.1111/hae.12031. [DOI] [PubMed] [Google Scholar]
- 38.Broderick C R, Herbert R D, Latimer J. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308(14):1452–1459. doi: 10.1001/jama.2012.12727. [DOI] [PubMed] [Google Scholar]
- 39.Iorio A, Iserman E, Blanchette V. Target plasma factor levels for personalized treatment in haemophilia: a Delphi consensus statement. Haemophilia. 2017;23(03):e170–e179. doi: 10.1111/hae.13215. [DOI] [PubMed] [Google Scholar]
- 40.Anderson A, Forsyth A. Vol. 44 New York, NY: National Hemophilia Foundation; 2005. Playing it Safe: Bleeding Disorders, Sports and Exercise. [Google Scholar]
- 41.Mancuso M E, Mahlangu J N, Pipe S W.The changing treatment landscape in haemophilia: from standard half-life clotting factor concentrates to gene editing Lancet 2021397(10274):630–640. [DOI] [PubMed] [Google Scholar]
- 42.Hermans C, Mahlangu J, Booth J. Pharmacokinetic modelling and validation of the half-life extension needed to reduce the burden of infusions compared with standard factor VIII. Haemophilia. 2018;24(03):376–384. doi: 10.1111/hae.13483. [DOI] [PubMed] [Google Scholar]
- 43.Lee M, Morfini M, Schulman S, Ingerslev J.The Factor VIII/Factor IX Scientific and Standardization Committee of the International Society for Thrombosis and Haemostasis, The design and analysis of pharmacokinetic studies of coagulation factorsISTH Website. 2001. Available at:https://cdn.ymaws.com/www.isth.org/resource/group/d4a6f49a-f4ec-450f-9e0f-7be9f0c2ab2e/official_communications/fviiipharmaco.pdf
- 44.Iorio A, Blanchette V, Blatny J, Collins P, Fischer K, Neufeld E. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(12):2461–2465. doi: 10.1111/jth.13867. [DOI] [PubMed] [Google Scholar]
- 45.Subcommittee on Factor VIII, Factor IX, and Rare Bleeding Disorders . Ragni M V, Croteau S E, Morfini M, Cnossen M H, Iorio A. Pharmacokinetics and the transition to extended half-life factor concentrates: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16(07):1437–1441. doi: 10.1111/jth.14153. [DOI] [PubMed] [Google Scholar]
- 46.Reininger A. Optimizing prophylaxis: development of an advate PK calculator and dosing medical device based on a Bayesian population PK model: OR07. Haemophilia. 2014;•••:20. [Google Scholar]
- 47.WAPPS-Hemo co-investigator network . Iorio A, Keepanasseril A, Foster G. Development of a Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo): study protocol. JMIR Res Protoc. 2016;5(04):e239. doi: 10.2196/resprot.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.https://florio.com/es/ https://florio.com/es/
- 49.Mondorf W, Siegmund B, Mahnel R. Haemoassist–a hand-held electronic patient diary for haemophilia home care. Haemophilia. 2009;15(02):464–472. doi: 10.1111/j.1365-2516.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 50.Delavenne X, Dargaud Y, Ollier E, Négrier C. Dose tailoring of human cell line-derived recombinant factor VIII simoctocog alfa: using a limited sampling strategy in patients with severe haemophilia A. Br J Clin Pharmacol. 2019;85(04):771–781. doi: 10.1111/bcp.13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Megías-Vericat J E, Bonanad S, Haya S. Bayesian pharmacokinetic-guided prophylaxis with recombinant factor VIII in severe or moderate haemophilia A. Thromb Res. 2019;174:151–162. doi: 10.1016/j.thromres.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 52.Stemberger M, Kallenbach F, Schmit E. Impact of adopting population pharmacokinetics for tailoring prophylaxis in haemophilia A patients: a historically controlled observational study. Thromb Haemost. 2019;119(03):368–376. doi: 10.1055/s-0039-1677700. [DOI] [PubMed] [Google Scholar]
- 53.Nagao A, Yeung C HT, Germini F, Suzuki T. Clinical outcomes in hemophilia A patients undergoing tailoring of prophylaxis based on population-based pharmacokinetic dosing. Thromb Res. 2019;173:79–84. doi: 10.1016/j.thromres.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 54.WAPPS co-investigators . Yu J K, Iorio A, Edginton A N. Using pharmacokinetics for tailoring prophylaxis in people with hemophilia switching between clotting factor products: a scoping review. Res Pract Thromb Haemost. 2019;3(03):528–541. doi: 10.1002/rth2.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pasca S, Milan M, Sarolo L, Zanon E. PK-driven prophylaxis versus standard prophylaxis: when a tailored treatment may be a real and achievable cost-saving approach in children with severe hemophilia A. Thromb Res. 2017;157:58–63. doi: 10.1016/j.thromres.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Bonanad S, García-Dasí M, Aznar J A. Adherence to prophylaxis in adult patients with severe haemophilia A. Haemophilia. 2020;26(05):800–808. doi: 10.1111/hae.14039. [DOI] [PubMed] [Google Scholar]
- 57.Torres-Ortuño A, Cuesta-Barriuso R, Nieto-Munuera J, Castiello-Munuera Á, Moreno-Moreno M, López-Pina J A. Haemo-adhaesione: a new measure of adherence for adolescent and adult patients with haemophilia. Patient Prefer Adherence. 2020;14:455–465. doi: 10.2147/PPA.S233601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thornburg C D, Duncan N A. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686. doi: 10.2147/PPA.S139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.British Society for Haematology . Rayment R, Chalmers E, Forsyth K. Guidelines on the use of prophylactic factor replacement for children and adults with haemophilia A and B. Br J Haematol. 2020;190(05):684–695. doi: 10.1111/bjh.16704. [DOI] [PubMed] [Google Scholar]
- 60.Lee Mortensen G, Strand A M, Almén L. Adherence to prophylactic haemophilic treatment in young patients transitioning to adult care: a qualitative review. Haemophilia. 2018;24(06):862–872. doi: 10.1111/hae.13621. [DOI] [PubMed] [Google Scholar]
- 61.Schrijvers L H, Schuurmans M J, Fischer K. Promoting self-management and adherence during prophylaxis: evidence-based recommendations for haemophilia professionals. Haemophilia. 2016;22(04):499–506. doi: 10.1111/hae.12904. [DOI] [PubMed] [Google Scholar]
- 62.García-Dasí M, Aznar J A, Jiménez-Yuste V. Adherence to prophylaxis and quality of life in children and adolescents with severe haemophilia A. Haemophilia. 2015;21(04):458–464. doi: 10.1111/hae.12618. [DOI] [PubMed] [Google Scholar]
- 63.Italian Association Of Haemophilia Centres (AICE) . von Mackensen S, Gringeri A, Siboni S M, Mannucci P M. Health-related quality of life and psychological well-being in elderly patients with haemophilia. Haemophilia. 2012;18(03):345–352. doi: 10.1111/j.1365-2516.2011.02643.x. [DOI] [PubMed] [Google Scholar]
- 64.Takedani H, Solimeno L, Saxena K, Kalweit L, Mathew P. The Haemophilia Joint Visualizer: development of a personalized, interactive, web-based tool to help improve adherence to prophylaxis. Haemophilia. 2017;23(02):e155–e158. doi: 10.1111/hae.13164. [DOI] [PubMed] [Google Scholar]
- 65.Boccalandro E A, Dallari G, Mannucci P M. Telemedicine and telerehabilitation: current and forthcoming applications in haemophilia. Blood Transfus. 2019;17(05):385–390. doi: 10.2450/2019.0218-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banchev A, Goldmann G, Marquardt N. Impact of telemedicine tools on record keeping and compliance in haemophilia care. Hamostaseologie. 2019;39(04):347–354. doi: 10.1055/s-0038-1676128. [DOI] [PubMed] [Google Scholar]
- 67.Hollander J E, Carr B G. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 68.Álvarez-Román M T, De la Corte-Rodríguez H, Rodríguez-Merchán E C. COVID-19 and telemedicine in haemophilia in a patient with severe haemophilia A and orthopaedic surgery. Haemophilia. 2021;27(01):e137–e139. doi: 10.1111/hae.14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauer R J. NONMEM Tutorial Part II: estimation methods and advanced examples. CPT Pharmacometrics Syst Pharmacol. 2019;8(08):538–556. doi: 10.1002/psp4.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]


