Abstract
Purpose of Review
The world’s population is aging rapidly, with 40% of patients seen in US gastroenterology (GI) clinics being 60 years or older. Many gastrointestinal problems are more common or unique to the older adult because of progressive damage to the structure and function of the GI tract. Until recently, the epidemiology of disorders of gut-brain interaction (such as irritable bowel syndrome and functional dyspepsia) was not well-characterized.
Recent Findings
Forty percent of persons worldwide have disorders of gut-brain interaction (DGBI), with varying global patterns of incidence in older adults. There are multiple first-line approaches to managing DGBI which can also be combined including pharmacologic (e.g., neuromodulators) and nonpharmacologic approaches including dietary therapies and brain-gut behavioral therapies. However, there are considerations clinicians must account for when offering each approach related to unique biopsychosocial factors in the older adult population. In this review, we aim to critically review recent literature on the pathophysiology, epidemiology, and special considerations for diagnosing and managing DGBI in the older adult population.
Summary
There have been many advances in the management of DGBI over the past decades. Given the increase in the number of older adults in the USA and worldwide, there is an urgent need for evidence-based guidance to help providers guide comprehensive care for specifically our aging patient population with respect to DGBI.
Keywords: Disorders of gut-brain interaction, Geriatrics, Older adults
The Aging Global Population
Conventionally, “elderly” has been defined as a chronological age of 65 years old or older. The world’s population is rapidly aging with the number of adults aged 65 years or older projected to more than double by 2050, reaching ~ 1.5 billion people or 16% of the population [1]. In the USA, 40% of patients seen in gastroenterology (GI) clinics are 60 years or older [2].
Given this shift in demographics, there is a need for evidence-based guidance of the management of common GI disorders in this aging population. Disorders of gut-brain interaction (DGBI), also known as functional GI disorders, are highly prevalent, present in more than 40% of the population globally [3•]. DGBIs occur as a result of complex interactions between biological, psychological, and social factors. Specifically, these interactions include microbial dysbiosis within the gut, altered mucosal immune function, altered gut signaling (visceral hypersensitivity), and central nervous system dysregulation of the modulation of gut signaling and motor function [4]. DGBIs are diagnosed by Rome IV criteria and include irritable bowel syndrome (IBS) and functional dyspepsia which are the two most researched DGBI. There are multiple first-line approaches which can also be combined including pharmacologic (e.g., neuromodulators) and nonpharmacologic approaches including dietary therapies and brain-gut behavioral therapies [5].
In this review, we aim to critically review recent literature on the pathophysiology, epidemiology, and special considerations for diagnosing and managing DGBI in the older adult population.
Aging-Related Changes in Gastrointestinal Physiology and Enteric Nervous System
Gastrointestinal motor functions are regulated by the enteric neuromuscular system, the intrinsic nervous system of the GI tract [6]. An in-depth evaluation of the mechanisms of aging-related changes in GI physiologic and motor function is beyond the scope of this review. A recent review highlighted the many complex aging-associated degenerative changes to cells within the tunica muscularis including enteric neurons, smooth muscles, and interstitial cells which may be involved in the pathogenesis of age-related GI motor dysfunctions [7••]. Aging also changes macrophages’ functional phenotype from tissue-protective, anti-inflammatory to injurious, proinflammatory, causing a decline in enteric neural stem cells which are multipotent stem cells which can give rise to enteric neurons, non-myelinating peripheral enteric glial cells, and myofibroblasts.
An interesting phenomenon is that age-related neuronal declines are variable throughout the GI tract. For example, interstitial cells of Cajal (ICC) constitute the principal pacemaker machinery throughout the GI tract through generation of electrical slow-wave activity. Studies have shown that age-related decline of ICC density in the stomach, but not the small intestine and colon [7••].
A few studies have also investigated the age-related modifications in the neurotransmitter receptor systems including a recent study which showed age-related differences occur in GABAergic transmission in the human colon [8], with a more pronounced response in GABAA receptor-mediated contraction in older subjects with a greater potency and efficacy of a GABAA receptor agonist in the old compared to the young subjects. This may usher in the development of drugs potentiating the GABAAergic neurotransmission for the improvement of propulsive activity such as colonic pseudo-obstruction which are common in the elderly population.
Other age-related physiologic changes such as changes in circadian rhythms which affect sleep quality and quantity (both decreasing with age) can also impact the DGBI [9]. A recent large prospective study found that sleep disorders are associated with DGBIs, especially in the presence of depressive symptoms [10], but this has not been well-characterized in the older adult population.
While many gastrointestinal problems are more common or unique to the older adult because of progressive damage to the structure and function of the GI tract (such as GI cancers, motility disorders such as dysphagia and constipation), the epidemiology of DGBI in older adults was not well characterized until recently.
Key Point
Many complex aging-associated degenerative changes occur in the enteric nervous symptoms including a shift of macrophages’ functional phenotype from tissue-protective, anti-inflammatory to injurious, proinflammatory, decrease in density of interstitial cells of Cajal in the stomach, and a more pronounced response in GABAA receptor-mediated contraction.
Epidemiology of DGBI in Older Adults
Physiologic changes in the GI tract associated with aging as highlighted above result in greater incidences of GI disorders across the spectrum of inflammatory, motor, and functional disorders [6].
In a recent large-scale multi-national study in 33 countries, the authors found that more than 40% of persons worldwide have disorders of gut-brain interaction. There were differing trends for DGBI prevalence based on survey methodology (via personal interviews in 9 household countries versus via internet survey in 26 countries) [3•]. In the Internet survey countries (including the USA and countries in Europe and South America), DGBI prevalence across the different anatomic regions decreased with age, but there was an opposite trend seen in household-survey countries (including countries in Asia and Africa). The authors offered several potential explanations for this variability, including cultural differences, social reporting sensitivity, ethnic diversity, genetics, and dietary habits.
As pain is a central feature to many Rome IV DGBIs including irritable bowel syndrome (IBS), there are some explanations to perhaps this decreased prevalence seen in the Internet survey countries. Several older studies using balloon distention in the esophagus and rectum have showed an increased visceral pain threshold in the elderly [11, 12]. The decreased sensitivity to visceral pain appears to translate to lower prevalence rates of IBS, as a meta-analysis showed that the odds of IBS in those aged 50 years and older are significantly lower than in those younger than 50 years (OR 0.75) [13]. A population-based study demonstrated that the virtual disappearance of abdominal pain with aging [14] and a more recent translational study found an age-related decrease in abdominal pain perception in IBS patients (elderly IBS patients were comparable to healthy controls), potentially related to a decrease in visceral pain-associated receptors transient receptor expression [15]. Conversely, a recent community survey based in the USA indicated that patients over 60 years of age were more likely to be diagnosed with IBS using Rome III criteria than those younger than 60 years [16].
Key Point
Conflicting trends exist for prevalence of DGBI globally in older adults; in the USA, a decrease in prevalence of DGBI was seen with age, potentially related to age-related decrease in abdominal pain perception.
Diagnostic Challenges in Diagnosis of DGBI in Older Adults
Given the increased prevalence of GI cancers and other inflammatory and motor disorders increase with age, new-onset gastrointestinal symptoms will likely be viewed with scrutiny. While historically DGBIs were considered a diagnosis of “exclusion,” it is now recommended that clinicians make a “positive” diagnosis in patients who meet the diagnostic criteria [17•] and do not have “alarm” symptoms and signs such as rectal bleeding or weight loss.
The Rome criteria have not specifically been validated for reliability in older patients, but at least for IBS has been shown to have longitudinal integrity (i.e., extensive testing is unlikely to uncover a new diagnosis) [18]. Understandably, the assessment of an older individual with new onset GI symptoms such as abdominal pain may constitute an “alarm” symptom and warrant further clinical work-up including an endoscopic evaluation if the patient is not up-to-date on colorectal cancer screening. Disorders which are especially prevalent in an older age group such as colon cancer and microscopic colitis should feature prominently in the differential diagnosis in the appropriate clinical context [19••]. It is also important to note that a diagnosis of DGBI can overlap with other GI conditions which may increase in prevalence in older adults. For example, IBS can overlap with inflammatory conditions (e.g., there is a second peak of inflammatory bowel diseases in older adults).
The assessment and diagnosis of DGBI in older adults requires clinical acumen and future research is needed to validate the Rome criteria in this population.
Key Point
The Rome criteria for diagnosing DGBIs are not specifically validated in older adults and given the increased prevalence of many gastrointestinal diseases in the elderly, acute, or new onset abdominal symptoms and/or red flags will require further evaluation.
Special Considerations in Management of DGBI in Older Adults
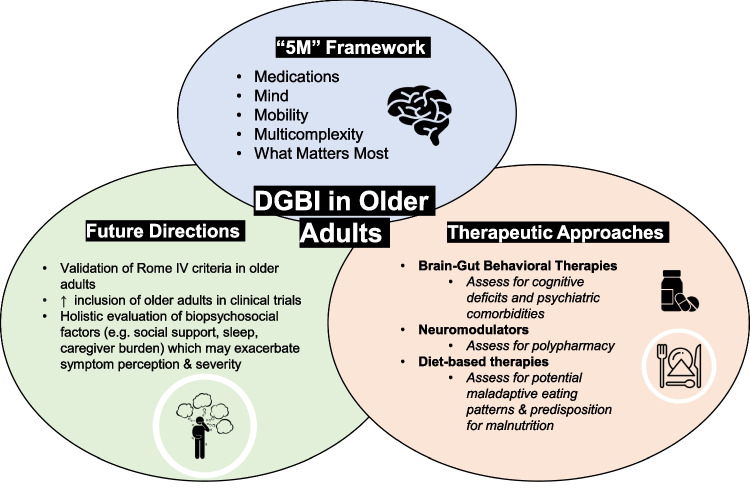
No studies have focused on the safety and efficacy of treatment strategies for DGBI in the older population, and no guidelines that have been developed specifically for the older population. An analysis of 130 non-pediatric DGBI trials found that older adults were included in only 55 of the 130 trials [20]. A recent article highlighted several guiding principles on the management of two gastroenterology conditions, inflammatory bowel disease, and cirrhosis in older adults [21••]. The authors adapted the “5 M framework” from geriatrics for age-friendly healthcare; the 5Ms are medications, mind, mobility, multicomplexity, and what matters most. We aim to highlight special considerations applying this same framework to the most common approaches in the management of DGBI.
Brain-Gut Behavioral Therapy
Brain-gut behavioral therapies (BGBT) are clinician-administered, short-term, non-pharmacologic interventions which target central or gut-brain pathways to ameliorate GI symptoms [22•]. There are five classes of BGBT (discussed in detail in a recent Rome working team report) including self-management programs, GI focused cognitive-behavioral therapy (CBT), gut-directed hypnotherapy, mindfulness-based interventions, and psychodynamic-interpersonal therapy. The advantages of BGBT are that they can be highly personalized and be delivered in conjunction with pharmacologic interventions. Patient selection is paramount for effectiveness of BGBT and ideal for patients who are empowered for self-management, have insight into the role of brain-gut axis into their illness, and do not have significant psychiatric or cognitive comorbidities.
For older adults, cognition is of greater concern; studies of community dwelling elderly populations have shown significant rates of mental disorders (including anxiety disorders, affective and substance-related disorders, and dementia) with estimated prevalence of 8.5–25% [23, 24]. Psychiatric comorbidities are significant in the older adult population with about 10–15% of community-dwelling adults experiencing depressive symptoms, and higher rates of depression in the hospital (12–45%) and long-term care facilities (about 40%) [25]. Older adults with cognitive impairment and significant psychiatric comorbidities are not ideal candidates for BGBT, and clinicians should assess for cognitive impairment with brief screening tests such as the Mini-Mental State Examination as well as the clock-drawing test [26] prior to recommendation of BGBT.
The advent of digital therapeutics also highlights some barriers for the older adult population. Recently, the US Food and Drug Administration (FDA) granted authorization for mobile apps delivering remote gut-directed hypnotherapy for patients with abdominal pain associated with IBS [27] as well as another mobile app utilizing the principles of CBT with proven durable benefits in relief of bloating and abdominal pain for patients with IBS [28]. There is now a plethora of digital tools that can improve the care of patients with DGBI through improved symptom tracking, physiologic monitoring, direct provision of care, and patient support [29]. While it is a common belief that older adults are aversive to new technology, a 2019 national survey of older adults revealed that most older adults were interested in using telehealth [30]. However, a “digital gap” has emerged during the rapid adoption of telehealth during the coronavirus disease (COVID-19) pandemic with one recent survey finding that GI patients aged 60 years and older were significantly more likely to have a telephone visit and significantly less likely to have a virtual video visit than those younger than 60 years [2]. This highlights the importance of developing strategies to improve access to digital therapeutics and telehealth for older adults and the integration of patient portals so family and caregivers to help overcome technological barriers.
Pharmacologic approaches
Polypharmacy, characterized by the simultaneous use of multiple medications, including prescription drugs, over-the-counter drugs, and supplements, is also frequent in older persons. Polypharmacy increases the risk of adverse drug reactions in older adults specifically due to aging changes that impact both pharmacokinetics and pharmacodynamics [19••]. Drug-drug interactions and medication errors also place older adults at increased risk of adverse drug events. A thorough medication reconciliation including all over-the-counter medications such as supplements and herbal medications is key to identify potentially harmful drug-drug interactions. Given the risk of polypharmacy in older adults, this is a population where we need to consider (if appropriate) the combination with non-pharmacologic approaches.
Neuromodulators form the foundation of pharmacologic management of persistent pain in DGBI. The most utilized classes of medications include tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and others, such as mirtazapine [31]. However, these medications have well-documented anticholinergic effects, even at low doses. The American Geriatrics Society (AGS) provides an updated Beers Criteria every 3 years which aims to provide a comprehensive, systematic review and grading of the evidence on drug-related problems and adverse events in older adults [32]. Antidepressants, which include TCAs, SSRIs, and SNRIs, are recommended by the AGS to be avoided in older adults as they increased risk of falls; “unless safer alternatives are not available.” The recommendation also mentions that “data for antidepressants are mixed but [there is] no compelling evidence that certain antidepressants confer less fall risk than others.” In general, SSRIs are considered to have the best safety profile in the elderly with the lowest potential for drug-drug interactions [33]; however, they confer less pain benefit in patients. Some experts favor the use of SNRIs over TCAs for chronic abdominal pain due to a more favorable side effect profile (and in cases where constipation or comorbid depression limits use of a TCA) and may be a consideration in older adults [34]. Overall, the maxim of “start low, go slow” for initiating neuromodulators applies especially for older adults.
Other classes of medications commonly used in the management of DGBI which are recommended to be avoided or at reduced dosages include antispasmodics, alpha-2-delta ligands such as gabapentin and pregabalin, atypical antipsychotics, tramadol and opioids. In general, the AGS recommends avoiding a total of three or more CNS-active drugs.
Diet-Based Therapies
Diet-based therapies are a component of management of certain DGBIs and even considered first-line for some DGBIs such as IBS (e.g., low FODMAP diet) [35]. However, older persons are predisposed to undernutrition and malnutrition via a multitude of mechanisms. Physiologic changes such as reduction in taste, salivation, smell, and hormonal changes can contribute to earlier satiety and decreased appetite [19••]. Eating patterns also fluctuate with age including a reduction in food intake as well as an alteration in the type of food that is consumed [19••]. Oral disease is common in older adults (related to tooth loss, poor oral hygiene, defective or absence of prosthetic appliances, hyposalivation, and chewing deficiencies related to cognitive decline) and can also predispose to malnutrition [36]. Socioeconomic factors such as food insecurity and limited control over food sources related to mobility and place of residence (e.g., long-term facility) also must be considered when considering diet-based therapies [37].
There is increasing recognition of the importance of screening for and preventing the development of maladaptive dietary restriction in patients with DGBI as patients may develop orthorexia nervosa or avoidant/ restrictive food intake disorder (ARFID) [38]. The prevalence of ARFID in the older adult population has not been studied specifically but prevalence of disordered eating and ARFID ranges between 13 and 55% and 40% respectively in patients with DGBI [39, 40]. Given the physiologic changes described above, older adults may be more predisposed to developing disordered eating habits when prescribed diet-based therapies. Thus, the involvement of registered dietitians who are experts in nutritional assessment becomes paramount when recommending and executing diet-based therapies in the older adult to prevent nutritional deficiencies and the development of restrictive eating behaviors.
Social and Lifestyle Factors
Given the biopsychosocial framework to understanding the development of DGBI, the impact of certain aging-related lifestyle factors such as social support, financial wellness, and caregiver burden on DGBI prevalence and severity must be considered. Extensive literature has shown that having positive social engagement and interactions are the highest predictor of living a long life [41]. Stressors such as weak social support infrastructure compounded by mobility concerns and the COVID-19 pandemic can erode social connectedness, worsening depression, and anxiety.
There are about 17.7 million caregivers of persons aged 65 or older (reported by the National Health and Aging Trends Study and the National Survey of Caregivers), but it is unclear what portion of these caregivers are same-generation caregivers (usually an older adult’s spouse) who have different physical and cognitive capabilities to caregiving than next-generation caregivers (usually an older adult’s children) [41]. Caregiving tasks can be labor and time intensive and stressful leading to wide-ranging biopsychosocial impact on the caregivers. Same-generation caregivers have not been studied specifically, but rates of psychologic distress among caregivers versus non-caregiver groups and caregivers are also at risk for adverse physical health outcomes as measured by global health status indicators [42]. Increased caregiver burden may impact symptom perception and severity in patients diagnosed with DGBI; for example, caregivers for chronically ill patients have a high prevalence of IBS [43].
An evaluation of the biopsychosocial factors contributing to a patient’s illness experience will help clinicians deliver more holistic care and perhaps be able to understand and mitigate factors which may exacerbate symptom perception and severity.
Key Point
The “5 M” framework for age-friendly care (medications, mind, mobility, multicomplexes, and what matters most) should be applied to therapeutic approaches for DGBI including pharmacologic (i.e., neuromodulators, complementary, and alternative medicines) and non-pharmacologic modalities such as brain-gut behavioral therapies and diet-based therapies (see Fig. 1).
Fig. 1.
Special considerations for the most common approaches in the management of disorders of gut-brain interaction in older adults.
Future Directions
Given the increase in the number of older adults in the USA and worldwide, there is an urgent need for guidelines to help providers guide comprehensive care for our aging patient population. Specifically, with respect to DGBI, below are top priorities:
High-quality, prospective studies validating the “positive” approach to the diagnosis of DGBI.
Clinical trials, retrospective, and prospective studies studying the epidemiology and management of DGBI should be enriched with older patients as this reflects a “real-world” population.
Adoption of patient-reported outcomes in prospective studies which are important to older adult including side-effect profiles and physical and cognitive function.
Conscientiously developing and expanding access to digital therapeutics with the needs of older adults in mind.
Holistic evaluation of older adults to assess for biopsychosocial factors which may exacerbate symptom perception and severity when DGBI diagnoses are being considered.
There have been many advances in the management of DGBI over the past decades. Our next goals should be to equip our clinicians with the evidence-based guidance they need to care for this population as they age.
Author Contribution
All authors have reviewed and approved the final draft to be submitted.
Declarations
Competing Interests
Yuying Luo has received consulting fees from Mahana Therapeutics. Laurie Keefer is a consultant to Pfizer and Abbvie and is a co-founder/equity owner for Trellus Health.
Footnotes
This article is part of the Topical Collection on Geriatrics
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.United Nations. World population prospects 2019. Volume II: Demographic profiles. 2019. https://population.un.org/wpp. Accessed 20 July 2020.
- 2.Kochar B, Ufere NN, Nipp et al. Video-based telehealth visits decrease with increasing age. Am J Gastroenterol. 2020;10.14309 [DOI] [PMC free article] [PubMed]
- 3.• Sperber A, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160(1):99–114. Apr 12. This article discusses the current global epidemiology of disorders of gut-brain interaction. [DOI] [PubMed]
- 4.Van Oudenhove L, Crowell MD, Drossman DA, et al. Biopsychosocial Aspects of Functional Gastrointestinal Disorders. Gastroenterology. 2016;S0016–5085(16):00218–223. doi: 10.1053/j.gastro.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keefer L, Ko CW, Ford AC. AGA Clinical practice update on management of chronic gastrointestinal pain in disorders of gut-brain interaction: expert review. Clin Gastroenterol Hepatol. 2021;19(12):2481–2488.e1. doi: 10.1016/j.cgh.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20(4):418–429.39 [DOI] [PubMed]
- 7.•• Nguyen VTT, Taheri N, Chandra A, Hayashi Y. Aging of enteric neuromuscular systems in gastrointestinal tract. Neurogastroenterol Motil. 2022;34(6):e14352. This is a scoping review of the multitude of mechanisms involved in the aging of the gastrointestinal system. [DOI] [PMC free article] [PubMed]
- 8.Zizzo MG, Cicio A, Raimondo S, Alessandro R, Serio R. Age-related differences of γ-aminobutyric acid (GABA)ergic transmission in human colonic smooth muscle. Neurogastroenterol Motil. 2022;34(3):e14248. doi: 10.1111/nmo.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen-Michael VH, Vecchierini MF. Exploration of sleep disorders in the elderly: which particularities? Geriatr Psychol Neuropsychiatr Vieil. 2016;14(4):429–437. doi: 10.1684/pnv.2016.0634. [DOI] [PubMed] [Google Scholar]
- 10.Bouchoucha M, Mary F, Bon C, Bejou B, Airinei G, Benamouzig R. Sleep quality and functional gastrointestinal disorders. A psychological issue J Dig Dis. 2018;19(2):84–92. doi: 10.1111/1751-2980.12577. [DOI] [PubMed] [Google Scholar]
- 11.Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272(1 Pt 1):G1–3. doi: 10.1152/ajpgi.1997.272.1.G1. [DOI] [PubMed] [Google Scholar]
- 12.Lagier E, Delvaux M, Vellas B, Fioramonti J, Bueno L, Albarede JL, Frexinos J. Influence of age on rectal tone and sensitivity to distension in healthy subjects. Neurogastroenterol Motil. 1999;11(2):101–107. doi: 10.1046/j.1365-2982.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 13.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Talley NJ. The effects of ageing on the onset and disappearance of unexplained abdominal pain: a population-based study. Aliment Pharmacol Ther. 2014;39(2):217–225. doi: 10.1111/apt.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckers AB, Wilms E, Mujagic Z, et al. Age-related decrease in abdominal pain and associated structural- and functional mechanisms: an exploratory study in healthy individuals and irritable bowel syndrome patients. Front Pharmacol. 2021;16(12):806002. doi: 10.3389/fphar.2021.806002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayuk GS, Wolf R, Chang L. Comparison of symptoms, healthcare utilization, and treatment in diagnosed and undiagnosed individuals with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2017;112(6):892–899. doi: 10.1038/ajg.2016.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.• Drossman DA, Tack J. Rome foundation clinical diagnostic criteria for disorders of gut-brain interaction. Gastroenterology. 2022;162(3):675–679. This article discusses the background and rationale for the Rome IV diagnostic criteria for disorders of gut-brain interaction. [DOI] [PubMed]
- 18.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376(26):2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 19.•• Pitchumoni CS, Dharmarajan TS. Geriatric Gastroenterology. 2nd ed. Springer International Publishing AG; 2021. This is a seminal textbook (now in its 2nd edition) featuring a compendium of reviews on management of various gastrointestinal conditions in the older adult.
- 20.Bar N, Surjanhata B, Weeks I, Silver JK, Murray HB. Analysis of age, race, ethnicity, and sex of participants in clinical trials focused on disorders of gut-brain interaction [published online ahead of print, 2022 Jun 7]. Gastroenterology. 2022;S0016–5085(22)00595–9. [DOI] [PMC free article] [PubMed]
- 21.•• Kochar B, Ufere NN, Ritchie CS, Lai JC. The 5Ms of geriatrics in gastroenterology: the path to creating age-friendly care for older adults with inflammatory bowel diseases and cirrhosis. Clin Transl Gastroenterol. 2022;13(1):e00445. This article adopts a “5 M framework” from geriatrics for age-friendly healthcare to two common gastrointestinal conditions; this framework can be applied widely to all chronic GI conditions. [DOI] [PMC free article] [PubMed]
- 22.• Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome working team report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan;162(1):300–315. This article discusses the broad applicability of brain-gut behavior therapies for managing disorders of gut-brain interaction. [DOI] [PubMed]
- 23.Andreas S, Schulz H, Volkert J, Dehoust M, Sehner S, Sulling A, Ausin B, et al. Prevalence of mental disorders in elderly people: the European MentDis_ICF65+ study. Br J Psychiatry. 2017;210(2):125–131. doi: 10.1192/bjp.bp.115.180463. [DOI] [PubMed] [Google Scholar]
- 24.Baladon L, Fernandez A, Rubio-Valera M, Cuevas-Esteban J. Prevalence of mental disorders in non-demented elderly people in primary care. Int Psychogeriatr. 2015;27(5):757–768. doi: 10.1017/S1041610214002841. [DOI] [PubMed] [Google Scholar]
- 25.Kok RM, Reynolds CF., 3rd Management of depression in older adults: a review. JAMA. 2017;317(20):2114–2122. doi: 10.1001/jama.2017.5706. [DOI] [PubMed] [Google Scholar]
- 26.Jin J. Screening for cognitive impairment in older adults. JAMA. 2020;323(8):800. doi: 10.1001/jama.2020.0583. [DOI] [PubMed] [Google Scholar]
- 27.PRWeb. metaMe Health receives FDA clearance for Regulora®, the First FDA-authorized treatment specifically for abdominal pain due to irritable bowel syndrome (IBS). Accessed February 1, 2022. https://www.prweb.com/releases/metame_health_receives_fda_clearance_for_regulora_the_first_fda_authorized_treatment_specifically_for_abdominal_pain_due_to_irritable_bowel_syndrome_ibs/prweb18363959.htm
- 28.Fierce Biotech. Mahana Therapeutics gears up to launch IBS treatment app with $61 M series B. Accessed February 14, 2022.
- 29.Pathipati MP, Shah ED, Kuo B et al. Digital health for functional gastrointestinal disorders [published online ahead of print, 2021 Nov 19]. Neurogastroenterol Motil. 2021;e14296. [DOI] [PMC free article] [PubMed]
- 30.Kurlander JK, Singer J, Solway D, et al. National poll on healthy aging. Ann Arbor, MI: University of Michigan; 2019. [Google Scholar]
- 31.Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): a Rome foundation working team report. Gastroenterology. 2018;154(4):1140–1171.e1. doi: 10.1053/j.gastro.2017.11.279. [DOI] [PubMed] [Google Scholar]
- 32.Fixen DR. 2019 AGS Beers criteria for older adults. Pharm Today. 2019;25(11):42–54. doi: 10.1016/j.ptdy.2019.10.022. [DOI] [Google Scholar]
- 33.Baldwin RC, Chiu E, Katona C, et al. Guidelines on depression in older people: Practising the evidence. London: Martin Dunitz; 2002. [Google Scholar]
- 34.Törnblom H, Drossman DA. Psychotropics, antidepressants, and visceral analgesics in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2018;20(12):58. doi: 10.1007/s11894-018-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray H, Doerfler B, Harer K, Keefer L. Psychological considerations in the dietary management of patients with DGBI. Am J Gastroenterol. 2022;117(6):985–994. doi: 10.14309/ajg.0000000000001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kossioni AE. The association of poor oral health parameters with malnutrition in older adults: a review considering the potential implications for cognitive impairment. Nutrients. 2018;10(11):1709. doi: 10.3390/nu10111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, Freund-Levi Y, Fielding RA, Cheng FW, Jensen GL, Wu D, Meydani SN. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr. 2017;8(1):17–26. doi: 10.3945/an.116.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuck CJ, Sultan N, Tonkovic M, Biesiekierski JR. Orthorexia nervosa is a concern in gastroenterology: a scoping review [published online ahead of print, 2022 Jul 10]. Neurogastroenterol Motil. 2022;e14427. 10.1111/nmo.14427 [DOI] [PMC free article] [PubMed]
- 39.Burton MurrayH, Riddle M, Rao F, et al. Eating disorder symptoms, including avoidant/restrictive food intake disorder, in patients with disorders of gut-brain interaction. Neurogastroenterol Motil. 2020;e14258. [DOI] [PubMed]
- 40.Peters JE, Basnayake C, Hebbard GS, Salzberg MR, Kamm MA. Prevalence of disordered eating in adults with gastrointestinal disorders: a systematic review. Neurogastroenterol Motil. 2021;e14278. [DOI] [PubMed]
- 41.Van Orden KA, Bower E, Lutz J, Silva C, Gallegos AM, Podgorski CA, Santos EJ, Conwell Y. Strategies to promote social connections among older adults during "social distancing" restrictions. Am J Geriatr Psychiatry. 2021;29(8):816–827. doi: 10.1016/j.jagp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz R, Beach SR, Czaja SJ, Martire LM, Monin JK. Family caregiving for older adults. Annu Rev Psychol. 2020;4(71):635–659. doi: 10.1146/annurev-psych-010419-050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remes-Troche JM, Torres-Aguilera M, Montes-Martínez V, Jiménez-García VA, Roesch-Dietlen F. Prevalence of irritable bowel syndrome in caregivers of patients with chronic diseases. Neurogastroenterol Motil. 2015;27(6):824–831. doi: 10.1111/nmo.12556. [DOI] [PubMed] [Google Scholar]