Abstract
Introduction
Malawi experienced two waves of COVID-19 between April 2020 and February 2021. A High negative impact of COVID-19 was experienced in the second wave, with increased hospital admissions that overwhelmed the healthcare system. This paper describes a protocol to implement a telephone-based syndromic surveillance system to assist public health leaders in the guidance, implementation, and evaluation of programs and policies for COVID-19 prevention and control in Malawi.
Study design
This is a serial cross-sectional telephonic-based national survey focusing on the general population and People living with HIV and AIDS.
Methods
We will conduct a serial cross-sectional telephone survey to assess self-reported recent and current experience of influenza-like illness (ILI)/COVID-19-like-illness (CLI), household deaths, access to routine health services, and knowledge related to COVID-19. Structured questionnaires will be administered to two populations: 1) the general population and 2) people living with HIV (PLHIV) on antiretroviral therapy (ART) at EGPAF-supported health facilities. Electronic data collection forms using secure tablets will be used based on randomly selected mobile numbers from electronic medical records (EMR) for PLHIV. We will use random digit dialing (RDD) for the general population to generate phone numbers to dial respondents. The technique uses computer-generated random numbers, using the 10-digit basic structure of mobile phone numbers for the two existing mobile phone companies in Malawi. Interviews will be conducted only with respondents that will verbally consent. A near real-time online dashboard will be developed to help visualize the data and share results with key policymakers.
Conclusion
The designed syndromic surveillance system is low-cost and feasible to implement under COVID-19 restrictions, with no physical contact with respondents and limited movement of the study teams and communities. The system will allow estimation proportions of those reporting ILI/CLI among the general population and PLHIV on ART and monitor trends over time to detect locations with possible COVID-19 transmission. Reported household deaths in Malawi, access to health services, and COVID-19 knowledge will be monitored to assess the burden and impact on communities in Malawi.
Keywords: Syndromic surveillance, COVID-19, Influenza-like illness, People living with HIV, Malawi
1. Introduction
Early in the COVID-19 pandemic, research and surveillance efforts have focused on describing the clinical course of infection with SARS-CoV-2, the virus that causes COVID-19, counting severe cases and deaths, and treating the sick. Experience with the Middle East respiratory syndrome (MERS), pandemic influenza, and other outbreaks has shown that as an epidemic spread, there is an urgent need to expand public health activities to elucidate the epidemiology of the pathogen and characterize its potential impact. The impact of an epidemic depends on the number of persons infected, the infection's transmissibility, and the spectrum of clinical severity. Especially critical is understanding the full spectrum of disease severity, which can range from asymptomatic and symptomatic-but-mild to severe, requiring hospitalization, and death [1].
People living with HIV (PLHIV) with advanced HIV disease, those with low cell counts and high viral loads, and those who are not taking antiretroviral therapy (ART) are immunocompromised and have an increased general risk of other infections and related complications [2]. In the early stages of the COVID-19 pandemic, modelling potential disruptions to HIV services for PLHIV and those at risk of acquiring HIV in sub-Saharan Africa showed possible negative impacts related to poor access, poor retention of clients in care, and increased mortality [2]. This has been proven in recent studies that have demonstrated that COVID-19 has brought additional challenges to the programming and provision of ART services, thereby negatively impacting ART adherence among PLHIV [[3], [4], [5]].
2. Malawi context
During the second wave of the COVID-19 pandemic in Malawi, from December 21st, 2020, to January 21st, 2021, the reproductive rate of the coronavirus increased from less than one to more than 2.1 in the 3rd week of January 2021, with an estimated COVID-19 positivity rate of about 30%. Health workers' infections increased from 642 cumulative totals in December 2020 to 1,061 in January 2021 [6].
Between April 2020 and March 15th, 2021, a total of 32,864 (15%) cases of SARS-CoV-2 were identified from 208,678 tests that were conducted in Malawi. Of the positive cases, 79% (25,045) were recorded in 2021 during and after the second wave. Since the beginning of the pandemic, 1,084 deaths have been reported (CFR = 3.3), of which 896 (82.7%) were registered in 2021. A total of 26,607 cases (81%) have recovered. Data analysis showed that 92% of the cumulative cases were locally transmitted, and cases were reported in all 28 districts nationwide [7].
Malawi has an estimated one million PLHIV, with an estimated HIV prevalence of 10.6% among 15 to 64-year-olds [8]. Malawi's rapid scale‐up of ART from 2004 to 2019 has contributed to a steady reduction in HIV incidence and HIV-related mortality [9]. The Department of HIV/AIDS in the Malawi Ministry of Health (MOH) estimated that by September 2019, 93% of PLHIV knew their HIV status, 83% of those who knew their HIV-positive status were on ART, and 92% of those on ART were virally suppressed. [10]. Despite this progress, there are still significant gaps in reaching the Joint United Nations Programme on HIV/AIDS (UNAIDS) 95-95-95 targets [11].
Malawi has made considerable progress towards achieving the 95-95-95 UNAIDS goals [11], and these gains must be maintained and safeguarded, especially in the context of COVID-19. In fulfilling its primary role of protecting the lives of its vulnerable citizens during disasters and reducing their exposure to risk through preparedness, the Government of Malawi (GoM) has led the development of a National Coronavirus Disease (COVID-19) Preparedness and Response Plan [12]. The plan establishes operational procedures for preparedness and response to the COVID-19 epidemic based on risks identified by the Malawi MOH, the World Health Organization (WHO), and other emerging context-based criteria [12].
3. Syndromic surveillance
Without a well-coordinated public health response that supports the rapid mobilization of resources where they are most needed, there was a high likelihood that the COVID-19 pandemic could overwhelm available healthcare resources, with hospitalizations far exceeding existing bed and intensive care unit (ICU) capacity. In addition, while Malawi has laboratory capacity to test for SARS-COV-2, with nine laboratories capable of SARS-COV-2 PCR testing, it was anticipated that testing large numbers could overwhelm the national laboratory system. Ensuring that a national surveillance system is in place, with the ability to monitor the spread of COVID-19 and detect locations with increased transmission, will be vital to guiding the public health response.
We, therefore, developed this protocol to implement an active phone-based syndromic surveillance system to monitor COVID-19-related symptoms and other indicators among the general population and PLHIV on ART in Malawi. The primary objectives of the survey are:
-
1.
To monitor trends and estimate the proportion of self-reported influenza-like illness (ILI)/COVID-19-like illness (CLI) among PLHIV on ART in Malawi by age, sex, pregnancy/breastfeeding status, and geographic location.
-
2.
To monitor trends and estimate the proportion of self-reported ILI/CLI among the general population in Malawi by age, sex, and geographic location.
-
3.
To monitor trends and estimate the proportion of self-reported household deaths in Malawi by age, sex, and geographic location.
-
4.
To assess perceptions, facilitators, and barriers to COVID-19 vaccination among respondents
4. Methods
4.1. Survey design
A serial cross-sectional study design will be employed to collect data in the general population and a subset of PLHIV clients on ART registered at select health facilities.
4.2. Survey setting and population
Data collection will include 1) structured primary data collection via telephone interviews among the general population aged 18 years and above; 2) structured primary data collection via telephone interviews among PLHIV aged 18 years and above receiving ART in facilities supported by the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) through the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. President's Emergency Plan for AIDS Relief.
4.3. General population
After obtaining verbal consent, we will administer a structured questionnaire to individuals by phone. The questionnaire includes COVID-like symptoms, recent household deaths, care-seeking behaviours, COVID-19 knowledge, and perceptions of COVID-19 vaccination. Trained staff will administer the questionnaires and enter data into a database using electronic tablets.
4.4. Inclusion criteria
In the general population, all adults aged 18 years or older who can speak either English or Chichewa, covering most of the population in Malawi, who answers the telephone call and verbally consent will be included in the survey. PLHIV age 18 years old or older, receiving services in EGPAF-supported sites, who own a phone number listed as PLHV on ART in the electronic medical record (EMR) system, and who confirm speaking English or Chichewa and provide verbal consent will be included in the survey.
4.5. Sampling procedures
4.5.1. Sampling frame
4.5.1.1. General population
We will use a random digit dialing (RDD) technique, in which random numbers are computer generated using the basic structure of 10-digit mobile phone numbers in Malawi for the two existing mobile companies. The first three digits will be (099) for service provider X and (088) for service provider Y. The other digits will range between (000 0000) and (999 9999), resulting in 20 million unique numbers. The randomly selected mobile numbers will be entered into an electronic enrolment log for the general population clients. Approximately 8 million active users are registered between the two providers, resulting in an estimated 40% hit rate for active users.
4.5.1.2. PLHIV population cohort
We will include a sample of ART clients from the 179 EGPAF-supported facilities located in Blantyre, Chiradzulu, Dedza, Mchinji, Mwanza, Neno, Ntcheu, Thyolo, and Zomba districts. We estimate there are approximately 384,000 PLHIV in these districts, of which about 300,000 are ART clients in EGPAF-supported sites. We expect to reach about 60% (180,000) of PLHIV in EGPAF-supported facilities who are 18-year-old or older. The estimated sample population to be reached for the PLHIV group took into consideration the total number of HIV clients in care with mobile numbers in the EMR system, and stringent screening criteria based on clients' confirmation of receipt of ART in EGPAF-supported facilities.
4.5.1.3. Sample size calculation
This surveillance is descriptive and has no specific hypothesis. The sample size is an estimated number of respondents to be reached based on the number of data collectors to implement the protocol within the set period. We anticipate that we will be able to contact at least 2,000 individuals from the general population per week. We will also contact at least 1,000 PLHIV per week. We anticipate a refusal rate of about 10%. With a sample size of 1800 per week among the general population and 900 per week among PLHIV, we expect to estimate weekly proportions/rates of outcomes of interest, such as the proportion reported to have had ILI/CLI, with a precision of 2.3% for the general population and 3.3% for PLHIV.
4.5.1.4. Data collection procedures
Trained data collectors will capture data on electronic data collection forms using secure tablets. Data will be submitted to a secure online server at the end of each day or as soon as they are captured in Open Data Kit (ODK)1. Study supervisors will be stationed in the study office to monitor and troubleshoot common problems; COVID-19 prevention measures will be implemented in the office. For PLHIV, the data collectors will be healthcare workers with clinical knowledge to engage with ART clients.
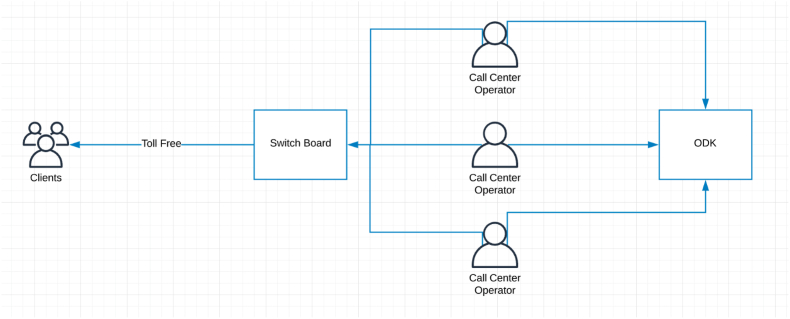
A common number will be used to dial out to the respondents through a call center-based system. (See Fig. 1).
Fig. 1.
Central Call Center System for calling respondents.
Each general population number will be dialed a maximum of three times within the same day, at different times, before it is classified as a non-response. In circumstances where the respondent is busy at the time of the call, the data collector will arrange an alternative day and time proposed by the respondent.
For PLHIV calls, the participant's name will be verified, as recorded in the EMR system, before the consenting process to ensure that the call respondent is the HIV-positive client enrolled on ART in the specified facility. Furthermore, the client will be asked if they are comfortable speaking about their health issues at the time of the call to better prepare the respondents for questions on their HIV-related care during the interview process.
4.5.1.5. Survey enrollment
Participant survey identifiers (SIDs) will be generated based on a random list of mobile numbers. The SID number will contain six digits; the first digit will identify the population: (1 = general population; 2 = PLHIV population); the following five digits will indicate a consecutive number of the survey participant. The SID will be used to identify participant data during the syndromic survey. No personal identifiers will be collected on the questionnaire to optimize the privacy protection of participants.
Electronic enrollment logs will be used for the general population and PLHIV groups. The electronic enrollment logs will show the response rate cascade from the randomly selected phone numbers to completed interviews.
The electronic enrollment log for the general population will not include the names of the respondents, phone numbers, and any other identifiable data; only the randomly selected phone numbers and SID numbers will be recorded. For the PLHIV population, the randomly selected mobile numbers, SID numbers, and the clients' names will be entered into the electronic enrolment log so that data collectors can verify the name of the PLHIV respondent before proceeding with the consenting process. The logs will be stored in the secure, encrypted, password-protected server at the study office in Lilongwe, separate from the study database. Access will be limited only to designated survey staff. Once the interview data are cleaned and verified, the names will be removed from the PLHIV enrolment log.
4.5.1.6. Data flow and linkage with informatics system
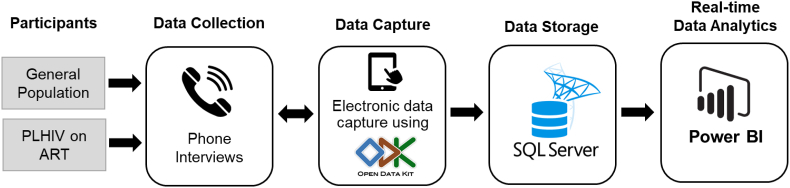
During the phone interview, data will be entered into data collection tablets using the ODK platform. They will be remotely transmitted through a secure Virtual Private Network (VPN) and stored in a secure Microsoft SQL server database. Microsoft PowerBI will have a live connection with ODK/KOBO using the KOBO PowerBI Connector configuration. The integration of KOBO/ODK and PowerBI will provide near real-time data analytics that will help the survey team track progress, analyze data, and make key decisions (See Fig. 2).
Fig. 2.
Informatics Infrastructure for survey data flow.
4.5.1.7. Data quality assurance and management
In order to ensure that the data collected are accurate and valid, study staff will conduct a series of data quality checks. Electronic data entry forms with internal data consistency checks and coded responses will be the primary tool used to maintain the quality of survey data. Periodic frequencies and cross-tabulation of select variables will be used to monitor the data quality. Data collectors will be trained on standard operating procedures (SOPs), and close supervision will be provided during data collection, management, cleaning, and analysis. In case of any major irregularities identified through daily data queries and verification, data cleaning will be done close to real time.
Survey teams will be trained on using tablets and mobile phones for data collection and how to administer informed consent and the survey questionnaire verbally. The training will build interview skills and focus on data accuracy, data entry, familiarity with the systems for collecting and securing data, and the protection of human subjects in research. Further, the entire survey team will be trained on survey requirements prior to data collection, including data security policies and patient privacy and confidentiality requirements. All staff will sign a confidentiality agreement prior to accessing any source data documents.
4.5.1.8. Supervision and monitoring
For every set of five data collectors, one team supervisor will be assigned to monitor the quality of the interviews and data entry. The team supervisor will monitor around 5% of the daily interviews, provide immediate feedback to the data collectors, and assist them in quality improvement. In case of missing data due to the data collector's error, if possible, a second call will be made to the same respondent to correct the error.
4.5.1.9. Data analysis
We will develop automated scripts to process and analyze the data as they are received. This process will be used to analyze trends in ILI, CLI, deaths, suspected COVID-19 cases, COVID-19 testing, preventive behaviours, access to healthcare services, and knowledge about COVID-19 through the use of tables, maps, or graphs which the GoM can use to inform response interventions. Each week, survey data will be analyzed by COVID-19 symptom to (1) estimate the proportion of participants reporting that symptom; (2) develop geographic mappings based on the participant's self-reported location; and (3) graphically display geographic location and interview times to show time trends.
Data will be disaggregated by age groups, gender, and geographical areas for both PLHIV and the general population. On a weekly or monthly basis, the proportion of households reporting a death will be estimated for the PLHIV and general population and will be presented graphically. COVID-19 suspected cases and reported testing, preventive behaviours, access to healthcare services, and knowledge variables will be summarized as frequencies, proportions, and trends in knowledge over time. Knowledge variables will also be disaggregated by gender, age group, and regional area. For the analysis, ILI and CLI will be defined as a respondent reporting to have an acute respiratory illness (at least one sign/symptom of respiratory disease, e.g., sore throat, cough, difficulties in breathing, fever) or other COVID-19-related symptoms (headache, fatigue, general body pains, loss of smell and taste, diarrhea).
Statistical tests will be conducted at the 5% (two-sided) significance level. Estimates along with 95% confidence intervals and associated p values will be reported. Comparisons of participants between and within the groups will be undertaken for some measures of interest such as sex, age, access to healthcare services, knowledge of COVID-19, death of household members from any cause, and other characteristics of interest. Continuous variables will be summarized through means, standard deviations, or medians, while categorical data will be by numbers and percentages. Statistically significant tests (χ2, t-test) will be computed to compare groups for each of the characteristics of interest. Multivariable analyses will be conducted to assess associations between the selected outcome variables by selected covariates.
4.5.1.10. Ethical considerations
This survey protocol will be reviewed and approved by the National Health Sciences Research Committee (NHSRC) in Malawi; Advarra Institutional Review Board, as contracted by EGPAF; and the Centers for Disease Control and Prevention (CDC) Center for Global Health Associate Director for Science. The joint recommendations from each of these committees will be combined into a unified protocol and standards of practice.
We will obtain verbal consent from potential survey participants using a standard script that the data collectors will read. The scripts were developed in English, translated into Chichewa, and back-translated into English. The informed consent includes information on who is carrying out the survey, the survey purpose, how the findings will be used, what will be asked of participants, risks and benefits of participation, the possibility to opt out at any time, and who to contact for more information. Participants will also be able to ask questions if there are any concerns.
4.5.1.11. Potential risks and benefits
Participation in the survey involves minimal risk. A potential risk of the surveillance system is that some of the interview questions maybe sensitive, such as a history of household illness or death, and may cause minor emotional distress. We will provide detailed training and practice with interviewing standards to reduce such effects. Several steps have been taken to prevent and mitigate any potential risk by:1) ensuring that all survey staff are trained in the protection of human subjects in research and sign a confidentiality agreement; 2) the phone numbers and other personally identifiable data will be kept securely and separate from the database; and 3) using health care workers to be the data collectors. The data collectors will first check with the respondents whether they are in a place where they can properly answer the questions. There is a possibility that the interview might be overhead by family members or other person/s in proximity of the client during the interview. In this regard, we have included a verification in the informed consent process that requires study participants to confirm that they are in a place where they can speak freely. The survey launch will be accompanied by appropriate publicity and communication to advise the public about the survey and encourage citizens to participate fully; this should help reduce non and partial responses.
The benefits of this surveillance system include providing relatively providing data inexpensively to enable the timely analysis of trends and geographic areas with observed incidences of ILI and CLI. Telephone interviews are less expensive and time-consuming than face-to-face interviews and may solicit a higher response rate. Telephone interviews may allow a larger sample size and minimize contact risks of being exposed to SARS-CoV-2.
5. Discussion
This syndromic surveillance protocol proposes the collection of self-reported data on COVID-19-related indicators, including clinical signs and symptoms, as proxy measures. These data will be disseminated through dashboards allowing for real-time collection, analysis, interpretation, and dissemination to identify potential public health threats [13].
A key challenge that will need to be considered in implementing this telephone COVID-19 syndromic surveillance protocol is HIV clients' willingness to disclose their HIV and ART status over the phone. We hope to mitigate the concern by engaging expert clients to be the data collectors. In the context of Malawi, expert clients are treatment-experienced adults living with HIV who work as lay cadre in the ART clinic. Their main tasks include assisting with individual and group treatment literacy sessions, individual adherence counseling, and tracing ART patients who have missed appointments and those classified as defaulters. They provide a unique approach that can only be offered by a peer within the same community, using their own experience of living with HIV as a tool to establish credibility and rapport. Perhaps most importantly, this cadre frees up skilled healthcare workers, enabling them to focus on the more complex, technically challenging patients in HIV service areas and the general population. In the context of our study, we see this as an opportunity to leverage existing expert clients for improved rapport, which may improve response rates and disclosure among the PLHIV group. Additionally, the survey launch will be accompanied by appropriate publicity and communication to advise the public about the survey and encourage citizens to participate fully; this will reduce non- and partial responses.
Another issue is that the interpretation of the study data might be affected by increasing influenza trends during the winter season. To assist with interpreting the reported prevalence of ILI and CLI found in this study, we will triangulate results with historical data on influenza trends in the country. Furthermore, during our data collection and analysis, any respondent classified as a suspected COVID-19 case will be anyone who will report having had any COVID-19-like signs and symptoms as defined by CDC[14] and WHO[15]. This will be one of the limitations as most of the clinical conditions like fever or chills, cough, shortness of breath or difficulty breathing, fatigue, headache, loss of taste, or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea are nonspecific symptoms and this may result in false classification of suspected COVID-19 cases. Further analysis will be done on the data using multivariable analysis of selected outcomes to provide a programmatic response of public health interventions targeted to those of a specific group as indicated by the findings.
In conclusion, since the first COVID-19 case was identified, Malawi has faced challenges ensuring that all suspected cases and their contacts are tested for COVID-19. Without a well-coordinated public health response that supports the rapid mobilization of resources where they are most needed, it is anticipated that the COVID-19 pandemic could overwhelm existing healthcare resources, with hospitalizations far exceeding existing bed and intensive care unit capacity. Ensuring that a cost-effective national syndromic surveillance system is in place, with the ability to monitor the spread of COVID-19 and detect locations with increased transmission, will be vital to driving down new infections through well-placed interventions.
Consent for publication
Not applicable.
Funding
This surveillance work is supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement Number U2UGH002010. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of PEPFAR or CDC. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of their supporting or funding agencies.
Author contributions
All listed study investigators contributed to the conception of the idea and contributed to the design of the protocol. T.M. produced the draft manuscript, and all other investigators supported it with reviews and contributions to the manuscript report.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The author team is grateful to the Government of Malawi for providing the platform for this syndromic surveillance system. We also gratefully acknowledge support from the Malawi Ministry of Health Department of HIV/AIDS, the PEPFAR/CDC team in Malawi and Atlanta, and Elizabeth Glaser Pediatric AIDS Foundation Malawi for their technical role and leadership in establishing the syndromic surveillance system.
Footnotes
ODK: Is a free, open-source suite of tools that allows data collection using Android mobile devices and data submission to an online server, even without an Internet connection or mobile carrier service at the time of data collection.
References
- 1.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of covid-19 - studies needed. N. Engl. J. Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 2.Jewell B.L., et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7(9):e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booton R.D., et al. The impact of disruptions due to COVID-19 on HIV transmission and control among men who have sex with men in China. J. Int. AIDS Soc. 2021;24(4) doi: 10.1002/jia2.25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linnemayr S., et al. HIV care experiences during the COVID-19 pandemic: mixed-methods telephone interviews with clinic-enrolled HIV-infected adults in Uganda. AIDS Behav. 2021;25(1):28–39. doi: 10.1007/s10461-020-03032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mistler C.B., et al. J Community Health; 2021. The Impact of COVID-19 on Access to HIV Prevention Services Among Opioid-dependent Individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNICEF, Malawi COVID-19 Situation Report January 2021, UNICEF: Malawi.
- 7.UNICEF, Malawi COVID-19 Situation Report 1-15 March 2021, UNICEF: Malawi.
- 8.Ministry of Health, Centers for Disease Control and Prevention (CDC), and ICAP at Columbia University . December. Lilongwe, Malawi; Atlanta, Georgia and New York, New York, USA: 2016. Malawi population-based HIV impact assessment (MPHIA) 2015-16: summary sheet, C.a.I. Ministry of health. [Google Scholar]
- 9.UNAIDS . 2019. UNAIDS Special Analysis. 2019. [Google Scholar]
- 10.Ministry of Health . HIV Programme Performance, D.o.H.a. AIDS. MOH: Lilongwe, Malawi; 2020. [Google Scholar]
- 11.UNAIDS, Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030 2015.
- 12.Government of Malawi . GoM; 2020. National Covid-19 Preparedness and Response Plan (March - June 2020) [Google Scholar]
- 13.Mostashari F., Hartman J. Syndromic surveillance: a local perspective. J. Urban Health. 2003;80(2 Suppl 1):i1–i7. doi: 10.1093/jurban/jtg042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC); Symptoms of COVID-19 Symptoms of COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
- 15.WHO Coronavirus disease. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/coronavirus