To the Editor: Outcomes of patients with cutaneous T-cell lymphoma (CTCL), a diverse group of non-Hodgkin lymphomas, during the COVID-19 pandemic are unknown. Patients with CTCL require active management and rely on various local and systemic therapies administered in-hospital or in-office, representing an increased risk for contracting COVID-19. There are no established maintenance protocols for cutaneous lymphomas. The COVID-19 pandemic served as an “experiment of nature” to understand the effects of reduced regimens and maintenance therapy. The professional societies developed emergency guidelines for the management of patients with cutaneous lymphomas during the pandemic to ensure patient safety.1 , 2 The International Society for Cutaneous Lymphomas created an International Cutaneous Lymphomas Pandemic Section to collect data to assess the impact of these guidelines. Using these data, we can determine if these measures were effective in preventing COVID-19 infection, what the impact was of maintenance therapy, and how delays in treatment affected disease outcomes in CTCL patients.
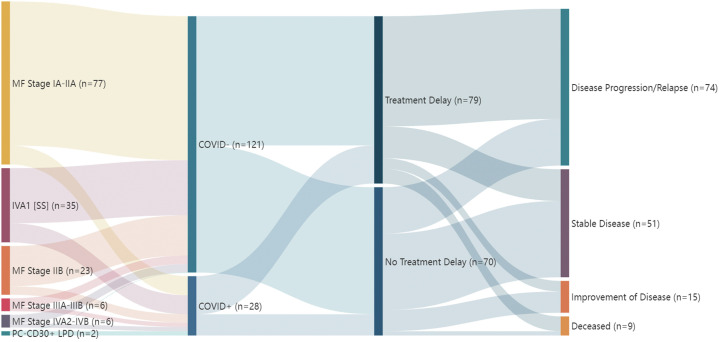
We performed a retrospective analysis of data from the electronic medical records at 9 international academic medical centers in 7 countries from March to October 2020, including 149 patients, actively managed with an established diagnosis of cutaneous lymphoma. Patient demographics, date of diagnosis, staging details, treatment details, delays or changes to treatment regimens, disease outcomes, and COVID-19 disease history including the source of transmission were recorded (Fig 1 ).
Fig 1.
Stratified clinical characteristics and outcomes in patients with cutaneous T-cell lymphoma during the COVID-19 pandemic.
Treatment was delayed for 53.0% of patients for a mean of 3.2 months (range: 10 days-10 months). Adjusting for age, race, biological sex, COVID-19 status, and disease stage, treatment delay was associated with a significant risk of disease relapse or progression across all stages (odds ratio 5.00; 95% confidence interval [2.38, 11.0], P value < .001) and, for each additional month that a patient experienced treatment delay, the odds of disease progression increased by 37.0% (odds ratio 1.37; 95% confidence interval [1.14, 1.70], P value < .001) (Table I ). A total of 28 (18.8%) patients with CTCL were diagnosed with COVID-19, none acquired from outpatient office visits. Patients who contracted COVID-19 did not have a statistically significant increase in odds of disease progression compared to COVID-19–negative patients (odds ratio 0.41; 95% confidence interval [0.15, 1.08], P value = .07).
Table I.
Demographic and outcomes by treatment delay status
| Treatment delay (n = 79) N (%) | No treatment delay (n = 70) N (%) | Total (N = 149) N (%) | |
|---|---|---|---|
| Age | |||
| Mean | 64 | 59 | |
| Median (IQR) | 65 (53, 78) | 62 (48, 72) | |
| Range | 28, 95 | 22, 88 | |
| Biological sex | |||
| Male | 43 (54.4) | 40 (57.1) | 83 (55.7) |
| Female | 36 (45.6) | 30 (42.9) | 66 (44.3) |
| Race | |||
| White | 60 (75.9) | 44 (62.9) | 104 (69.8) |
| Black/African American | 8 (10.1) | 19 (27.1) | 27 (18.1) |
| Other/unknown/declined | 11 (13.9) | 7 (10.0) | 18 (12.1) |
| Stage | |||
| IA-IIA | 40 (50.6) | 37 (52.9) | 77 (51.6) |
| IIB | 11 (13.9) | 12 (17.1) | 23 (15.4) |
| IIIA-IIIB | 4 (5.1) | 2 (2.8) | 6 (4.0) |
| IVA1 (SS) | 19 (24.1) | 16 (22.9) | 35 (23.5) |
| IVA2-IVB | 3 (3.8) | 3 (4.3) | 6 (4.0) |
| PC CD30+ LPD | 2 (2.5) | 0 (0.0) | 2 (1.3) |
| COVID-19 status | |||
| Positive | 18 (22.8) | 10 (14.3) | 28 (18.8) |
| Negative | 61 (77.2) | 60 (85.7) | 121 (81.2) |
| Length of treatment delay (mo) | |||
| Mean | 3.20 | 0.00 | |
| Median (IQR) | 3.00 [1.05, 4.00] | 0.00 [0.00, 0.00] | |
| Range | 0.30, 10.00 | 0.00, 0.00 | |
| Unknown | 2 | 0 | |
| Outcomes | |||
| Improvement of disease | 5 (6.3) | 10 (14.3) | 15 (10.1) |
| Stable disease | 15 (19.0) | 36 (51.4) | 51 (34.2) |
| Disease progression/relapse | 52 (65.8) | 22 (31.4) | 74 (49.7) |
| Deceased | 7 (8.9) | 2 (2.9) | 9 (6.0) |
Based on the results of this study, we conclude the following:
-
1.
Adequate safety measures and protocols can prevent transmission of COVID-19 at physicians' offices as no outpatient office-acquired infections were reported.
-
2.
Patients with cutaneous lymphomas who develop COVID-19 do not have worse outcomes than expected according to their risk factors.
-
3.
As the pandemic continues, our data reveals the need to re-evaluate the initial recommendations made by the USCLC and EORTC-CLTG, and it provides reassurance that our current COVID-19–conscious management practices are safe and effective.
-
4.
Delays in therapy lead to adverse clinical outcomes and should be avoided, as the risk of contracting COVID-19 in the outpatient setting did not outweigh the risk of morbidity and mortality due to disease progression.
-
5.
Ensuring continuity of treatment and maintenance therapy appears critical to avoiding disease progression, underscoring the importance of maintenance therapy for CTCL.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: This study was approved under Columbia IRB AAAT4244, and the institutional review boards and Institutional Protection of Human Subjects guidelines at each institution included.
Reprints not available from the authors.
References
- 1.Zic J.A., Ai W., Akilov O.E., et al. United States Cutaneous Lymphoma Consortium recommendations for treatment of cutaneous lymphomas during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:703–704. doi: 10.1016/j.jaad.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadavid E., Scarisbrick J., Ortiz Romero P., et al. Management of primary cutaneous lymphoma patients during COVID-19 pandemic: EORTC CLTF guidelines. J Eur Acad Dermatol Venereol. 2020;34:1633–1636. doi: 10.1111/jdv.16593. [DOI] [PMC free article] [PubMed] [Google Scholar]