Abstract
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality. Besides traditional cardiovascular risk factors, arterial stiffness is a recognized predictor of cardiovascular risk.
Methods
We investigated the relationship between traditional cardiovascular risk factors, sex, and aortic pulse wave velocity in subjects living in a countryside area of Southern Switzerland. For this aim, we performed a cross-sectional analysis of data from adult participants of the Swiss Longitudinal Cohort Study, which, initiated in 2015, follows health status and disease risk factors in a Swiss countryside cohort at least 6 years of age.
Results
A total of 387 people (205 women and 182 men) were included. Hyperlipidemia, overweight, and obesity were more common (p ≤ 0.001) and LDL-cholesterol, triglycerides, and hemoglobin A1c were higher (p < 0.03) in men than women. Systolic and diastolic brachial and aortic blood pressures were higher in men (p < 0.02), whereas aortic pulse wave velocity and aortic pulse pressure were higher in women (p < 0.05). The aortic pulse wave velocity was significantly higher in subjects with hypertension, hyperlipidemia, diabetes, and obesity, and significantly increased with age (p < 0.0001). Multiple linear regression analysis showed a significant correlation between pulse wave velocity and age, female sex, brachial systolic blood pressure, and heart rate (p < 0.005).
Conclusion
Also in a countryside area, the aortic pulse wave velocity is higher in subjects with hypertension, hyperlipidemia, diabetes and obesity, and significantly increases with age. Furthermore, with advancing age, aortic pulse wave velocity is higher in women than men.
Trial Registration
ClinicalTrials.gov identifier, NCT02282748.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40119-022-00280-8.
Keywords: Pulse wave velocity, Arterial hypertension, Cardiovascular risk, Sex, Country, Rural
Key Summary Points
| Why carry out this study? |
| Urban and rural surroundings can impact cardiovascular risk. Arterial stiffness is a recognized predictor of cardiovascular risk. |
| We therefore investigated traditional cardiovascular risk factors, sex, and aortic pulse wave velocity in subjects living in a countryside area of Southern Switzerland. |
| What was learned from the study? |
| This study confirmed that also in the studied countryside cohort, aortic pulse wave velocity is higher in subjects with hypertension, hyperlipidemia, diabetes, and obesity, and significantly increases with age. |
| Interestingly, with advancing age, aortic pulse wave velocity was higher in women than men. |
| Pulse wave velocity correlated with age, female sex, brachial systolic blood pressure, and heart rate. |
Introduction
Cardiovascular disease is the leading cause of morbidity and mortality worldwide [1]. It is recognized that the individual risk of cardiovascular disease is dependent, in addition to age, on various factors, such as arterial hypertension, hyperlipidemia, diabetes mellitus, smoking, and excessive body weight [2–7]. Also genetic, inflammatory, and thrombotic factors play a pathogenic role in cardiovascular disease [2–7].
Interestingly, aortic stiffness is a good predictor of future cardiovascular events [8]. Indeed, great emphasis has recently been placed on aortic pulse wave velocity, a reliable hemodynamic marker of stiffness of large arteries, as an independent and attractive predictor of cardiovascular risk that may help in decision-making in several clinical settings [9]. Briefly, a healthy aorta exerts a cushioning function that limits arterial pulsatility and protects the microvasculature from potentially dangerous fluctuations in blood flow and pressure. Stiffening of large arteries, which occurs with aging and various pathologic states, impairs this cushioning function, and has relevant consequences on cardiovascular health [8–11]. Cross-sectional studies have shown a solid association of aortic stiffness not only with age but also with other cardiovascular risk factors such as elevated blood pressure, hyperlipidemia, diabetes, smoking, and excess body weight [8–11].
It is currently possible to non-invasively and accurately measure the aortic pulse wave velocity [10–14]. Recognizing that urban and rural surroundings can impact cardiovascular risk [15, 16], the aim of this study was to investigate the relationship between traditional cardiovascular risk factors, sex, and aortic pulse wave velocity in subjects living in a countryside area of Southern Switzerland.
Methods
Study Design
Cama and Lostallo are two neighboring countryside villages in Southern Switzerland with a population of approximately 1300 people and a low migration rate. Between April 2015 and June 2018, inhabitants of Cama, Lostallo, and surroundings were invited to enter the Swiss Longitudinal Cohort Study (SWICOS). This study aims to examine and longitudinally follow health status and disease risk factors in an aging Swiss countryside cohort. All residents were eligible for SWICOS. There were no exclusion criteria. Written informed consent was obtained from participants. In subjects unable to provide informed consent, it was obtained from a proxy. The study protocol was published in 2016 [17], and the SWICOS study is registered at ClinicalTrials.gov (NCT02282748). The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nordwest-und Zentralschweiz (protocol code EKNZ 2014-209).
A total of 496 participants entered the SWICOS study [18]. For the current cross-sectional analysis, we considered data from 474 subjects 18 years of age or more. The following information was collected: medical history including arterial hypertension, hyperlipidemia, diabetes mellitus, and tobacco smoking, and demographics including body weight, height, and waist circumference. Furthermore, blood was collected for the determination of LDL-cholesterol, triglycerides, and hemoglobin A1c. Brachial blood pressure, heart rate, and central hemodynamic parameters were also measured.
Medical history was assessed using a structured self-administered questionnaire. Weight was determined to the nearest 0.1 kg on a platform scale (TANITA SC-240 MA, Tanita Corporation, Tokyo, Japan), height to the nearest 0.1 cm with a stadiometer, and waist circumference to the nearest 0.5 cm with a non-stretching tape placed around the bell at the iliac crest level.
The following five traditional cardiovascular risk factors were considered for the present analysis: (1) history of arterial hypertension, (2) history of hyperlipidemia, (3) history of diabetes mellitus, (4) tobacco smoking, and (5) obesity (body mass index ≥ 30.0 kg/m2) [19].
Sitting brachial blood pressure and heart rate were measured twice after the participants had been seated for at least 5 min using an oscillometric device (OMRON 705CP-II) and the second value was retained [20]. Subsequently, an operator-independent, non-invasive device (Arteriograph®; Tensiomed, Budapest, Hungary) based on a validated oscillometric occlusive technique was applied for the determination of central hemodynamic parameters [12]. After 5 min of rest, supine brachial systolic and diastolic blood pressure, heart rate, and central hemodynamic parameters were simultaneously obtained. After two brachial blood pressure measurements, this device produces a cuff pressure over the brachial artery that is 35 mmHg in excess of the measured systolic blood pressure, which is employed to record the beat-to-beat pulse signals for 8 s. To calculate the aortic pulse wave velocity, the distance from the jugulum to the symphysis is measured in a straight line using a tape measure, as a surrogate value of the aortic length. The result is then divided by the difference in time between the beginning of the first wave and the beginning of the second (reflected) wave, resulting in an estimate of the aortic pulse wave velocity in meters/second (m/s) [11, 12]. To ensure quality of measured pulse wave velocity, measurements of aortic pulse wave velocity with a standard deviation of beat-to-beat variation greater than 1.0 m/s were excluded. Values above 10.0 m/s were considered as pathologically increased [12, 21]. All blood parameters were determined by means of an enzyme assay in the same accredited central laboratory (Unilabs Ticino, Breganzona, Switzerland) using an Architect ci8200 analyzer, as previously reported [18].
Analysis
Categorical variables are presented as percentages and were analyzed using the Fisher’s exact test. The Shapiro–Wilk test was used to test for normal distribution of continuous data. Continuous normally distributed variables are expressed as mean ± standard deviation (SD) and were compared using the Student’s two-tailed unpaired t test. Continuous non-normally distributed variables are expressed as median and interquartile range (IQR) and were analyzed using the Mann–Whitney U test. The association between pulse wave velocity with its possible determinants such as age, sex, heart rate, systolic and mean blood pressure, LDL-cholesterol and triglyceride levels, hemoglobin A1c, smoking, and obesity was explored with correlation analysis and simple linear regression, and subsequently analyzed using multiple linear regression. Sensitivity analyses integrating mean arterial pressure in multiple linear regression analysis, as well as using logistic regression (with pulse wave velocity greater than 10 m/s as an outcome) were also carried out. Analyses were performed using IBM SPSS Statistics software (version 25, IBM Corp., Armonk, NY, USA) and GraphPad Prism 9 for macOS (GraphPad Software, San Diego, CA, USA). p values less than 0.05 were considered significant.
Results
Among the 474 subjects at least 18 years of age considered for the study, we excluded 87 participants with standard deviation of aortic pulse wave velocity greater than 1.0 m/s, as shown in Fig. 1. The baseline characteristics of the remaining 387 participants (205 women and 182 men) are given in Table 1. Included subjects (male to female, M:F, ratio 1.08) had a comparable sex distribution to that of the whole population (M:F ratio 0.89, p = 0.095) living in Cama and Lostallo. Male and female participants did not differ with respect to age and prevalence of history of arterial hypertension, diabetes, and current tobacco smoking. Hyperlipidemia, overweight, and obesity were more common in men as compared to women. Circulating LDL-cholesterol, triglycerides, and hemoglobin A1c were slightly but significantly higher in men than in women (Table 1).
Fig. 1.
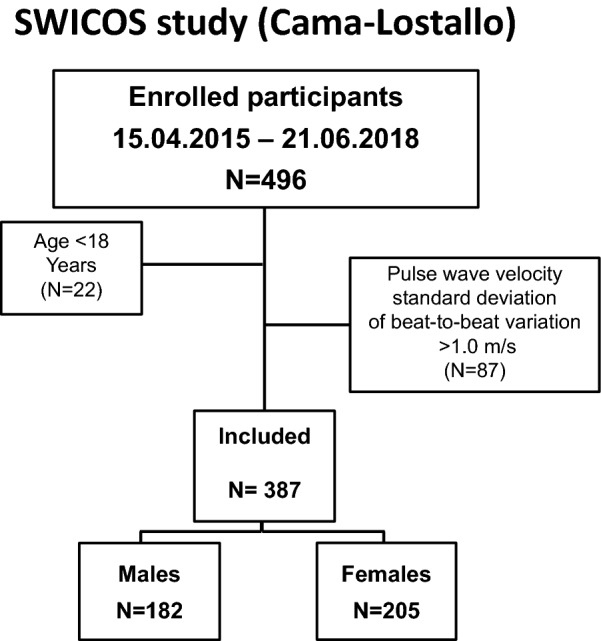
Flowchart of study participants
Table 1.
Baseline characteristics of the SWICOS study participants
| All, N = 387 | Men, N = 182 | Women, N = 205 | p value | |
|---|---|---|---|---|
| Age, years (mean ± SD) | 48.1 ± 15.7 | 48.5 ± 15.4 | 47.7 ± 16.0 | 0.58 |
| Medical history | ||||
| Arterial hypertension | ||||
| N (%) | 50 (13) | 30 (17) | 20 (9.8) | 0.68 |
| Treated, N | 38 | 21 | 17 | |
| Dyslipidemia | ||||
| N (%) | 62 (16) | 41 (23) | 21 (10) | < 0.001 |
| Treated, N | 34 | 23 | 11 | |
| Diabetes mellitus | ||||
| N (%) | 17 (4.4) | 9 (4.9) | 8 (3.9) | 0.63 |
| Treated, N | 17 | 9 | 8 | |
| Tobacco smoking, N (%) | 77 (20) | 41 (23) | 36 (18) | 0.25 |
| General data | ||||
| Weight, kg (mean ± SD) | 71.2 ± 14.7 | 81.0 ± 12.4 | 62.5 ± 10.4 | < 0.001 |
| Height, m (mean ± SD) | 1.684 ± 0.089 | 1.747 ± 0.066 | 1.629 ± 0.067 | < 0.001 |
| Body mass index, kg/m2 (mean ± SD) | 25.0 ± 4.1 | 26.6 ± 3.7 | 23.6 ± 3.9 | < 0.001 |
| Overweighta, N (%) | 135 (35) | 90 (49) | 45 (22) | < 0.001 |
| Obesityb, N (%) | 46 (12) | 32 (18) | 14 (6.8) | 0.001 |
| Waist circumference, cm (mean ± SD) | 89.6 ± 12.1 | 95.0 ± 11.0 | 84.8 ± 11.0 | < 0.001 |
| Waist to height ratio (mean ± SD) | 0.53 ± 0.07 | 0.54 ± 0.06 | 0.52 ± 0.07 | 0.001 |
| Laboratory datac | ||||
| LDL-cholesterol, mmol/L (median IQR) | 3.3 [2.6–4.0] | 3.5 [2.8–4.1] | 3.2 [2.5–3.8] | 0.025 |
| Triglycerides, mmol/L (median IQR) | 1.2 [0.8–1.7] | 1.5 [1.0–2.2] | 1.0 [0.7–1.3] | < 0.001 |
| Hemoglobin A1c, % (median IQR) | 5.2 [5.0–5.4] | 5.3 [5.1–5.5] | 5.2 [5.0–5.4] | 0.002 |
LDL low density lipoprotein
aBody mass index ≥ 25.0–30.0 kg/m2
bBody mass index > 30.0 kg/m2
cN = 380 (202 women and 178 men)
In the age groups up to 60 years, systolic and diastolic brachial blood pressures as well as aortic systolic blood pressure were higher in men as compared to women, whilst this difference disappeared in the older cohort (Table 2). On the other hand, after 40 years of age, aortic pulse pressure and pulse wave velocity were higher in women than in men (Table 2).
Table 2.
Peripheral and central hemodynamics in the SWICOS study population according to age groups and sex
| All (n = 387) | Age < 40 years | p value | Age 40–60 years | p value | Age > 60 years | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (n = 49) | Women (n = 54) | Men (n = 91) | Women (n = 111) | Men (n = 42) | Women (n = 40) | |||||
| Peripheral hemodynamics | ||||||||||
| Systolic, mmHg (mean ± SD) | 129 ± 17 | 130 ± 12 | 114 ± 12 | < 0.001 | 135 ± 15 | 124 ± 17 | < 0.001 | 144 ± 21 | 140 ± 18 | 0.33 |
| Diastolic, mmHg (mean ± SD) | 80 ± 11 | 78 ± 9 | 73 ± 8 | 0.004 | 85 ± 10 | 79 ± 10 | < 0.001 | 85 ± 10 | 82 ± 12 | 0.25 |
| Mean, mmHg (mean ± SD) | 97 ± 12 | 96 ± 9 | 95 ± 11 | 0.66 | 95 ± 13 | 96 ± 13 | 0.64 | 104 ± 14 | 100 ± 11 | 0.15 |
| Heart rate, beat/min (mean ± SD) | 66 ± 11 | 65 ± 10 | 69 ± 9 | 0.028 | 67 ± 11 | 67 ± 10 | 0.78 | 68 ± 10 | 65 ± 11 | 0.02 |
| Central hemodynamics | ||||||||||
| Aortic systolic blood pressure mmHg (median, IQR) | 123 (112, 137) | 117 (110, 124) | 111 (102, 119) | < 0.001 | 127 (118, 136) | 122 (112, 133) | 0.016 | 139 (124, 155) | 144 (124, 155) | 0.19 |
| Aortic pulse pressure, mmHg (median, IQR) | 45 (39, 53) | 40 (38, 46) | 39 (36, 45) | 0.31 | 42 (37, 49) | 45 (39, 52) | 0.021 | 55 (42, 61) | 59 (55, 70) | 0.019 |
| Aortic pulse wave velocity m/s (median ± SD) | 7.7 (6.8, 8.8) | 6.5 (6.0, 6.2) | 6.6 (6.1, 7.2) | 0.80 | 7.7 (7.1, 8.4) | 8.1 (7.3) | 0.031 | 8.8 (8.0, 11.0) | 10.5 (8.9, 12.0) | 0.014 |
In univariate analyses, the aortic pulse wave velocity was significantly higher in subjects with hypertension, hyperlipidemia, diabetes, and obesity as compared to subjects without these cardiovascular risk factors, while tobacco smoking was not associated with a significant increase in aortic pulse wave velocity (Table 3).
Table 3.
Unadjusted aortic pulse wave velocity and cardiovascular risk factors in the SWICOS study participants
| Subjects with risk factor | Subjects without risk factor | p value | |||
|---|---|---|---|---|---|
| N | Median [IQR] | N | Median [IQR] | ||
| Arterial hypertension | 50 | 9.1 [8.0–10.9] | 337 | 7.6 [6.8–8.6] | < 0.0001 |
| Dyslipidemia | 62 | 9.2 [8.2–11.0] | 325 | 7.5 [6.8–8.5] | < 0.0001 |
| Diabetes mellitus | 17 | 9.6 [8.3–11.9] | 370 | 7.7 [6.8–8.7] | < 0.0001 |
| Smoking | 77 | 7.4 [6.7–8.6] | 309 | 7.8 [6.9–7.8] | 0.061 |
| Obesitya | 46 | 9.0 [8.1–10.9] | 341 | 7.6 [6.8–8.7] | < 0.0001 |
aBody mass index > 30.0 kg/m2
The aortic pulse wave velocity significantly increased with age (p < 0.0001) both in men and women (Fig. 2, Supplementary Table S1). This central hemodynamic variable did not differ between men and women in subjects less than 40 years of age but was significantly higher in women as compared to men in subjects 40–60 and greater than 60 years of age (Fig. 2).
Fig. 2.
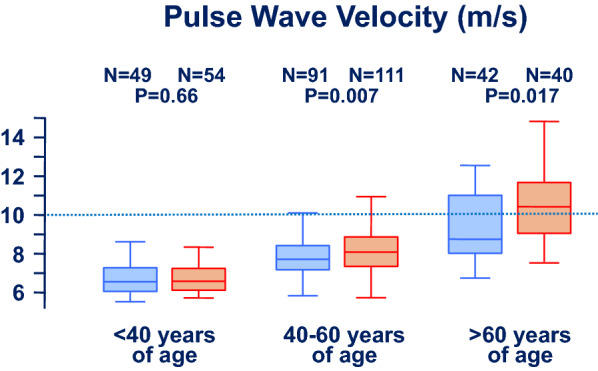
Impact of age and sex on unadjusted aortic pulse wave velocity. Men (blue rectangles) and women (orange rectangles) are given different symbols. Results are presented as boxplots
Women and men did not significantly differ in age when stratified according to the number of cardiovascular risk factors (i.e., without, with one, and with two or more) (Table 4). In subjects without and in those with two or more traditional cardiovascular risk factors, pulse wave velocity was higher in women than in men (Table 4).
Table 4.
Age, aortic pulse wave velocity, and mean arterial pressure according to numbers of cardiovascular risk factors in the SWICOS study participants without, with 1 or with ≥ 2 cardiovascular risk factors
| Risk factor (N) | All | Men | Women | p value |
|---|---|---|---|---|
| 0 | ||||
| N | 217 | 83 | 134 | |
| Age, years mean ± SD | 45.0 ± 14.8 | 44.1 ± 15.2 | 45.9 ± 14.6 | 0.38 |
| Pulse wave velocity, m/s median [IQR] | 7.4 [6.8–8.3] | 7.2 [6.5–7.7] | 7.7 [6.9–8.7] | < 0.001 |
| Mean arterial pressure, mmHg median (IQR) | 93 (85, 100) | 91 (84, 98) | 94 (87, 102) | 0.011 |
| 1 | ||||
| N | 112 | 61 | 51 | |
| Age, years mean ± SD | 46.5 ± 15.8 | 47.5 ± 14.6 | 45.4 ± 17.3 | 0.47 |
| Pulse wave velocity, m/s median [IQR] | 7.8 [6.8–8.5] | 7.8 [6.8–8.5] | 7.9 [6.8–10.8] | 0.12 |
| Mean arterial pressure, mmHg median (IQR) | 99 (92, 109) | 103 (95, 110) | 96 (89, 105) | 0.017 |
| ≥ 2 | ||||
| N | 57 | 37 | 20 | |
| Age, years mean ± SD | 62.2 ± 10.5 | 60.7 ± 10.1 | 65.0 ± 10.0 | 0.14 |
| Pulse wave velocity, m/s median [IQR] | 9.3 [8.4–11.1] | 9.1 [8.2–10.8] | 10.5 [8.7–12.5] | < 0.02 |
| Mean arterial pressure, mmHg median [IQR] | 104 [93, 113] | 102 [90, 114] | 104 [97, 112] | 0.70 |
Each of the considered cardiovascular risk factors was significantly correlated with pulse wave velocity and, in simple linear regression, the slope of that relationship was significantly different from zero (Supplementary Table S1). The results of multiple linear regression analysis confirmed a significant association between pulse wave velocity and age, female sex, systolic blood pressure, and heart rate (Table 5). In sensitivity analysis, very similar results were found while integrating mean instead of systolic blood pressure in the model, with a significant association between pulse wave velocity and age, female sex, mean arterial blood pressure, heart rate, and body mass index (Supplementary Table S2). In logistic regressions looking at predictors for the outcome of pulse wave velocity greater than 10 m/s, the same results were mostly confirmed, with age, sex, body mass index, and systolic but not mean blood pressure being significantly associated with that outcome (Supplementary Tables S3 and S4).
Table 5.
Multiple regression analysis investigating the association between arterial stiffness (as assessed by pulse wave velocity) and several cardiovascular risk factors in 387 study participants
| Pulse wave velocity (m/s) | |||
|---|---|---|---|
| Linear regression coefficient | 95% CI | p value | |
| Age | 0.06 | 0.04–0.07 | < 0.001* |
| Female sex | 1.03 | 0.72–1.35 | < 0.001* |
| Brachial systolic blood pressure | 0.31 | 0.21–0.04 | < 0.001* |
| LDL-cholesterol | − 0.02 | − 0.18 to 0.13 | 0.75 |
| Triglycerides | 0.39 | − 0.12 to 0.20 | 0.63 |
| Hemoglobin A1c | 0.04 | − 0.20 to 0.28 | 0.74 |
| Smoking | 0.12 | − 0.23 to 0.47 | 0.50 |
| Body mass index | 0.31 | 0.01–0.07 | 0.15 |
| Heart rate | 0.02 | 0.01–0.03 | 0.005* |
LDL low density lipoprotein
*Statically significant association
Discussion
The results of this study, collected in two countryside villages in Southern Switzerland, confirm the correlation between aortic pulse wave velocity and age, arterial hypertension, hyperlipidemia, diabetes mellitus, and obesity, and further emphasize a cumulative impact of a series of cardiovascular risk factors on wall properties of large arteries. Importantly, the study demonstrates that traditional cardiovascular risk factors are important modulators of arterial stiffness [8–11, 13] also in rural surroundings [15]. The investigated countryside area, known to have a favorable cardiovascular health profile [19], is characterized by a low migration rate, therefore offering ideal candidates to explore the prognostic importance of the observed associations over a longitudinal follow-up, as foreseen in the SWICOS project.
The novelty of this study is the focus on a countryside area and the differential attention paid to male and female participants in different age groups. Indeed, in this analysis, traditional cardiovascular risk factors were more prevalent among men as compared to women. Interestingly, however, the aortic pulse wave velocity was more often pathologically altered in women over 40 years of age than in men of the same age. These data are basically in line with data obtained in urban areas. In women, the arterial stiffness is lower than in age-matched men from puberty to menopause [22]. However, this trend reverses during and after menopause, with a steeper increase of aortic pulse wave velocity in women compared to men [23]. The mechanisms underlying this increase in aortic pulse wave velocity in postmenopausal women are unknown. It has been speculated that this evolution might reflect hormonal changes occurring after menopause, given that estrogens exert a beneficial effect on cardiovascular health. However, estrogen supplementation does not decelerate progression of arterial stiffness in postmenopausal women [22]. Alternatively, it has been suggested that increased arterial stiffness in older women results from an elevation in proinflammatory cytokines, which follows the decline in ovarian function with menopause [24]. Interestingly, some previous works concluded that no sex difference in pulse wave velocity exists after correcting for other cardiovascular risk factors [25]. The finding of a significant difference between sexes in our study may therefore appear, at first look, surprising. However, looking at the age subgroups (Fig. 2, Table 2), it becomes clear that age has a major impact in shaping this difference. Although previous works found such an association exclusively in older, peri- and postmenopausal women [26], our data detected a significant difference already in the group 40–60 years old. We are unable to state whether this is because of a different region and population (genetic background, lifestyle factors, environmental influences, proportion of postmenopausal women in the age group 40–60 years of age, etc.) or because of a secular trend.
Arterial wall properties are the result of complex biological interactions. Thus, the value of pulse wave velocity needs to be carefully addressed, and this is why multivariable analysis, accounting for traditional cardiovascular risk factors, is so important in interpreting our results. There is still some controversy regarding whether systolic or mean blood pressure should be used in such models, however. Unsurprisingly, in both correlation analysis and simple linear regression, pulse wave velocity was significantly associated with both systolic and mean blood pressures (Supplementary Table S1). From a biological perspective, while systemic vascular resistance is mainly determined by the peripheral arteries and it is translated into the mean arterial pressure, pressure fluctuations in the circulation depend on arterial compliance, mainly determined by aorta and large arteries [27, 28]. It is therefore usual to appreciate arterial compliance changes while assessing pulse pressure and systolic blood pressure, but not necessarily mean arterial pressure [29]. Thus, although several studies had detected a mathematical relationship between pulse wave velocity and mean blood pressure, we felt that biologically it made more sense to correct pulse wave velocity (which is a measure of arterial stiffness) by systolic rather than by mean blood pressure (Table 5 and Supplementary Table S3). As this issue is still controversial, sensitivity analyses correcting for mean blood pressure were performed (Supplementary Tables S2 and S4), mainly confirming the same results and therefore supporting the robustness of our analysis.
This study has strengths and limitations, partly related to the recruitment strategy, which was based on voluntary participation. Both general practitioners and regional authorities were informed about the project, and participation was offered free of charge to local residents. The voluntary participation might be seen either as a limitation (volunteering bias) or a strength (high population coverage) of this study. Indeed, the bias effect due to the voluntary, non-randomized inclusion of the subjects in this long-term cohort project may have been substantially tempered by the high proportion of participants, when compared to the whole population. The similar sex distribution in participants and the whole population further supports this assumption. The failure to assess the participants’ menopausal status and the cross-sectional design of the current analysis are relevant limitations of this analysis.
The methods used to measure brachial blood pressure and central hemodynamic parameters can be seen both as a strength and as a limitation. The standardized measurement of peripheral and central hemodynamic factors with an operator-independent, non-invasive device is a notable strength of this study [10–13]. Moreover, patients were allowed to sit for 5 min and at least two blood pressure readings were taken, as recommended at the time of study performance [30]. However, only the second blood pressure value was retained (and not the mean of the last two readings as recommended by the newer guidelines [6]). Furthermore, for pulse wave velocity we tolerated a beat-to-beat variation up to 1.0 m/s, while some authorities recommend to repeat a third measurement when the first two differ by more than 0.5 m/s [21]. Although most studies on pulse wave velocity used tonometric devices, we used an oscillometric device, which is user-friendly and easy to integrate into routine clinical care. While this represents a potential strength, Arteriograph® comes with its own limitations. First, it is unknown whether the cutoff value of 10 m/s traditionally used for tonometric devices can be applied also to the Arteriograph®. Second, it is virtually impossible to determine the exact location of the artery reflection wave [31] and its dependency from heart rate might have influenced the magnitude of the significant association we detected between heart rate and pulse wave velocity. However, it is important to note that this device has been validated both with respect to oscillometric devices [32] and invasive measures [12].
Conclusions
The results of this study, performed in a countryside region of Southern Switzerland, confirm that traditional cardiovascular risk factors are important modulators of arterial stiffness also in a rural population. Furthermore, they show that aortic pulse wave velocity increases with age and in subjects with one or more traditional cardiovascular risk factors and that, with advancing age, it is higher in women than men. Further parameters including emerging individual risk factors and population-based risk factors, as well as their association with cardiovascular events, scheduled to be scrutinized in the ongoing long-term cohort SWICOS study, will allow a better understanding of the underlying pathophysiological mechanisms.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Dr. Lava is the current recipient of research grants from Fonds de perfectionnement, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland; Fondation SICPA, Prilly, Switzerland; Fondazione Dr. Ettore Balli, Bellinzona, Switzerland; Fondazione per il bambino malato della Svizzera italiana, Bellinzona, Switzerland and Frieda Locher-Hofmann Stiftung, Zürich, Switzerland. Authors would like to particularly thank all study participants for their involvement in the study.
Funding
This study is supported by an unrestricted grant from the SHK Stiftung für Herz und Kreislaufkrankheiten, Hergiswil, Switzerland.
Author Contributions
PE initiated the study. The SWICOS steering committee (FM, AWS, GP, PS, AG, PE) contributed to the design and development of the study protocol. Conceptualization of this analysis: LP, DR, SAGL. Methodology: LP, DR. Formal analysis: DR, MGB, SAGL. Investigation: LP, FM. Resources: FM, PE, AWS, RSB, PMS, AG. Data curation: LP, DR, MGB, SAGL. Preparation of the original draft: MGB, SAGL. Review and editing: MGB, SAGL. Visualization: SAGL. Supervision: GP, PS, AG, MGB, SAGL. Funding acquisition: FM, PE, AWS, GP, PS, AG. All authors have read and agreed to the submitted version of the manuscript.
Disclosures
Dr. Franco Muggli and Prof. Paul Erne are advisors to TensioMed GmbH (Switzerland). Prof. Erne served as advisor to Inovise Medical (USA), the manufacturer of the Audicor device. Ms Lucrezia Pusterla has nothing to disclose. Dr. Dragana Radovanovic has nothing to disclose. Prof. Andreas W. Schoenenberger has nothing to disclose. Dr. Renate Schoenenberger-Berzins has nothing to disclose. Prof. Gianfranco Parati has nothing to disclose. Prof. Paolo Suter has nothing to disclose. Dr. Sebastiano A.G. Lava has nothing to disclose. Prof. Augusto Gallino has nothing to disclose. Prof. Mario G. Bianchetti has nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments. The SWICOS study is registered at ClinicalTrials.gov (NCT02282748).
Study Approval Statement
This study protocol was reviewed and approved by the Ethics Committee of Nordwest-und Zentralschweiz, protocol code EKNZ 2014-209.
Consent to Participate Statement
Written informed consent was obtained from participants. In subjects unable to provide informed consent, it was obtained from a proxy.
Data Availability
The data presented in this study are available upon reasonable request from the corresponding author.
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed]
- 2.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2013;34(10):719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 3.Csige I, Ujvárosy D, Szabó Z, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. 2018;2018:3407306. doi: 10.1155/2018/3407306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi: 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 6.Williams B, Mancia G, Spiering W, et al. 2018 practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36(12):2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 7.Conklin DJ, Schick S, Blaha MJ, et al. Cardiovascular injury induced by tobacco products: assessment of risk factors and biomarkers of harm. A Tobacco Centers of Regulatory Science compilation. Am J Physiol Heart Circ Physiol. 2019;316(4):H801–H827. doi: 10.1152/ajpheart.00591.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce GL. Mechanisms and subclinical consequences of aortic stiffness. Hypertension. 2017;70(5):848–853. doi: 10.1161/HYPERTENSIONAHA.117.08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horváth IG, Németh A, Lenkey Z, et al. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens. 2010;28(10):2068–2075. doi: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 13.Marčun Varda N, Močnik M. Pulse wave velocity, central haemodynamic parameters, and markers of kidney function in children. Kidney Blood Press Res. 2022;47(1):43–49. doi: 10.1159/000519340. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Lombard J, Rundek T, et al. Relationship of neighborhood greenness to heart disease in 249 405 US medicare beneficiaries. J Am Heart Assoc. 2019;8(6):e010258. doi: 10.1161/JAHA.118.010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han SJ, Lee SH. Nontraditional risk factors for obesity in modern society. J Obes Metab Syndr. 2021;30(2):93–103. doi: 10.7570/jomes21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenenberger AW, Muggli F, Parati G, et al. Protocol of the Swiss Longitudinal Cohort Study (SWICOS) in rural Switzerland. BMJ Open. 2016;6(11):e013280. doi: 10.1136/bmjopen-2016-013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepori L, Radovanovic D, Schoenenberger AW, et al. Age-dependency of cardiometabolic risk and protective factors in females living in a countryside area of Switzerland. Praxis (Bern 1994) 2021;110(5):252–256. doi: 10.1024/1661-8157/a003622. [DOI] [PubMed] [Google Scholar]
- 19.Schoenenberger AW, Radovanovic D, Muggli F, et al. Prevalence of ideal cardiovascular health in a community-based population—results from the Swiss Longitudinal Cohort Study (SWICOS) Swiss Med Wkly. 2021;151:w30040. doi: 10.4414/smw.2021.w30040. [DOI] [PubMed] [Google Scholar]
- 20.Adler C, Ellert U, Neuhauser HK. Disagreement of the two oscillometric blood pressure measurement devices, Datascope Accutorr Plus and Omron HEM-705CP II, and bidirectional conversion of blood pressure values. Blood Press Monit. 2014;19(2):109–117. doi: 10.1097/MBP.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 21.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 22.Ogola BO, Zimmerman MA, Clark GL, et al. New insights into arterial stiffening: does sex matter? Am J Physiol Heart Circ Physiol. 2018;315(5):H1073–H1087. doi: 10.1152/ajpheart.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74(11):2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 24.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 25.Reference Values for Arterial Stiffness' Collaboration Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31(19):2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staessen JA, van der Heijden-Spek JJ, Safar ME, et al. Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens. 2001;15(8):511–518. doi: 10.1038/sj.jhh.1001226. [DOI] [PubMed] [Google Scholar]
- 27.Nichols WW, Edwards DG. Arterial elastance and wave reflection augmentation of systolic blood pressure: deleterious effects and implications for therapy. J Cardiovasc Pharmacol Ther. 2001;6(1):5–21. doi: 10.1177/107424840100600102. [DOI] [PubMed] [Google Scholar]
- 28.McVeigh GE, Bratteli CW, Morgan DJ, et al. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension. 1999;33(6):1392–1398. doi: 10.1161/01.hyp.33.6.1392. [DOI] [PubMed] [Google Scholar]
- 29.Gangadharan N, Venkatachalapathi A, Jebaraj B, et al. Electrical modelling of tissue experiments confirms precise locations of resistance and compliance in systemic arterial tree-they are mutually exclusive. Clin Exp Pharmacol Physiol. 2022;49(2):242–253. doi: 10.1111/1440-1681.13606. [DOI] [PubMed] [Google Scholar]
- 30.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31(10):1925–1938. 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed]
- 31.Westerhof BE, van den Wijngaard JP, Murgo JP, Westerhof N. Location of a reflection site is elusive: consequences for the calculation of aortic pulse wave velocity. Hypertension. 2008;52(3):478–483. doi: 10.1161/HYPERTENSIONAHA.108.116525. [DOI] [PubMed] [Google Scholar]
- 32.Baulmann J, Schillings U, Rickert S. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. 2008;26(3):523–528. doi: 10.1097/HJH.0b013e3282f314f7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.


