Abstract
Lentivirus and adeno-associated viruses are invaluable tools for biotechnology applications due to their genetic material delivery abilities both in vitro and in vivo. However, their large-scale productions with Good Manufacturing Practices yield low efficiency when adherent and serum dependent HEK293 (Human Embryonic Kidney) cells are used as the host. To increase production efficiency, HEK293 cells are adapted to grow in suspension using commercially available and chemically defined serum-free mediums. Suspended cells can be transiently transfected for viral vector production; however, significant improvements are still needed to increase yield and thereby cost effectiveness. Here, we evaluated four most preferred commercially available mediums that are IVY, FreeStyle293, LV-MAX, and BalanCD HEK293 for the transient transfection feasibility of lentiviral (LV) and adeno-associated virus serotype 2 (AAV2) production in FlorabioHEK293 suspension cells. The highest transfection efficiency was over 90% and obtained by using polyethyleneimine (PEI) 25 K and by media adaptation in IVY without using any transfection enhancer. For the first time the feasibility of HEK293 cells, which were adapted to grow in suspension culture by Florabio and IVY media, were tested for virus production. This study demonstrates the best transfection medium for scalable and optimized production of Lentivirus and Adeno-Associated Virus in suspended HEK293 cell culture.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10616-022-00551-1.
Keywords: Transfection media , Virus production , LV , AAV2 , Suspension cell culture
Introduction
Virus production techniques, originally developed for vaccine manufacturing, are becoming increasingly important in other fields of the biopharmaceutical industry (Yvonne Genzel and Udo Reichl 2007). The scope of therapeutic strategies has been expanded to include recombinant protein expression, gene therapy and even cancer treatment (Bucher et al. 2021; Goradel et al. 2022). To achieve desired therapeutic effects, viruses should be in high quantities to reach required MOI number for cell infection and should be stable in long term storage. Thus, effective upstream production must be combined with optimized cell culture conditions. Consequently, the design of bioproduction for viruses is strongly dependent on the virus-host cell interactions, in vitro synthesis, and release of virus particles. Complex multi-step processes are required for virus production, however low virus titers and virus particle temperature sensitivity limits the production scale. To address all requirements of commercial products, each step of virus production must be scalable, reproducible and must produce high virus titers. In order to reach high virus titers, cell culture methods with most appropriate virus-host cell pairing, and the best serum-free cell culture mediums should be established (Grein et al. 2017).
Although transient gene transfection is a rapid method for research purposes, the quantity of recombinant product remains mainly in milligrams per Liter using transient transfection of HEK293 cells (Wright 2009). In a few studies, the higher level of recombinant protein production in kg-ton quantities has been shown in bioreactors using CHO cells (De Jesus and Wurm 2011). Enhanced product quantities can be achieved via well-controlled transfection efficiency. Factors affecting the efficiency of transfection can be counted as the type of host cell, suitable transfection agent and culture conditions (Valkama et al. 2017; Legmann 2020). Development of virus production methods for Lentivirus (LV) and Adeno-associated Virus (AAV) are in progress to address the challenges such as large-scale production, efficient vector delivery, and cellular toxicity (Bulcha et al. 2021).
Host cell is an important factor for functional assembly of viral products. The viability of transiently transfected cells, level of gene and its corresponding protein expression and post-translational modifications, which are necessary for functional viral proteins, are major considerations in host cell selection. Different types of mammalian cell lines such as COS (CV-1 in Origin with SV40 genes), HEK293 (Human Embryonic Kidney), CHO (Chinese Hamster Ovary) (Rajendra et al. 2017), and BHK (Baby Hamster Kidney) cells (Hesse et al. 2003) have been investigated (Baldi et al. 2012) as the “cell factory” to produce successful viral vaccines and recombinant proteins. Suspension mammalian cell lines are preferred for large-scale production due to better control over culture conditions and reduced culture duration (Haldankar et al. 2006). Serum-free suspension HEK293 cell culture is widely preferred for virus production, because of its steady growth in various commercial media and well-characterized cellular characteristics (Chahal et al. 2014).
Most efficient transient transfection systems such as PEI and lipofectamine are developed for adherent cells, such as the COS-7 cell line. Versatile nucleic acid carriers (e.g., PEI, liposomes, calcium phosphate) are required for efficient transfection to suspension cells as well (Karolewski et al. 2004; Tang et al. 2015). It has been reported that stirred tank bioreactors (20 to 100 L) can be utilized using either calcium phosphate co-precipitation (Kumar et al. 2019) or polyethylenimine (PEI)-mediated gene delivery methods (Longo et al. 2013). For industrial production, optimized protocols are widely used with PEI 25 K (Linear, 25 kDa), which is very cost-effective (Reed et al. 2006). Gene delivery methods with PEI 25 K have been proven in several types of host cells and laboratory animals (Longo et al. 2013). However, there is still room for improvement of PEI protocol to decrease the amount of DNA and enable production in larger volumes (Raymond et al. 2011).
Culture conditions providing sufficient production volumes, pH buffering capacity and optimum ventilation are considered factors to achieve maximum transfection efficiency (Griffiths and Racher 1994; Longo et al. 2013). In biotechnological production, utilization of serum-free mediums is a pre-requisite because ingredients of animal-derived serum may cause impairment in human health. Additionally, animal-derived serum is expensive for the scale-up production and has serious scientific and ethical concerns regarding its harvest and production (Brunner et al. 2010; Miki and Takagi 2015). Taking serum, the vital composition of ingredients and growth factors, out of the culture media comes with the challenge of formulating the essential chemical compositions for proper cell health. During the development of serum-free mediums, important supplements such as insulin, transferrin, selenium, and glucose, the vital ingredients for cellular proliferation, should be adjusted (Ghasemi et al. 2019). However, generating one optimal culture media for all cell types and virus particles may be impossible since each virus and host cell combination has different requirements depending on cell growth characteristics and virus infection kinetics. Therefore, several serum-free transfection mediums are designed for the growth of commonly used cell lines in the market. Some serum-free cell culture mediums are specifically designed for virus production such as Gibco™ CTS™ LV-MAX™ Lentiviral Production System (Bundy et al. 2020). Several research groups have been studied on the best cell culture media to improve stability and efficient production of virus particles of interest (Croyle et al. 2001; Kissmann et al. 2008; Cervera et al. 2015).
In this study, the efficiency of four different commercially available serum-free cell culture media, BalanCD HEK293, Freestyle293, LV-MAX and IVY were evaluated for virus production in suspension HEK293 cells. The cultivation media were analyzed for transfection and transduction efficiencies to determine the best medium for efficient production of LV and AAV2 particles. The efficiency of viral particle productions at 24 h, 48 h, and 72 h by using PEI 25 K as the nucleic acid carrier was investigated.
Materials and methods
Cell cultures
FlorabioHEK293 and FlorabioMRC5 cell lines adapted to suspension culture by Florabio company (Florabio, İzmir, Turkey) were cultured in serum-free Adaptation Medium (ADP, Florabio, İzmir, Turkey) supplemented with 4 mM L-glutamine. Cells were grown in 20 mL culture volume by shaking orbitary at 140 rpm in a 5% CO2 incubator at 37 °C (Thermo Scientific™ BBD 6220 CO2 incubator equipped with Edmund Bühler, KS-15 shaker). Both transfected and transduced cells were grown in different commercial transfection mediums; IVY (Florabio, Turkey), BalanCD HEK293 (Irvine Scientific, 91165), LV-MAX (Gibco, A35684), Freestyle293 (Gibco, 12338002) supplemented with 4 mM L-glutamine. For media adaptation of the suspended cells, FlorabioHEK293 cells were grown in IVY at least three passages until achieving 98% cell viability at each cultivation day. For the transfection efficiency and titer analysis, the same experimental procedures were followed for both 4 mL and 20 mL culture production.
Plasmid constructs and purification
AAV2 construction plasmids; an AAV2 vector expressing tdTomato under CBA promoter, an pHGTI-adeno1, (PF1236, PlasmidFactory, Genbank accession no. M73260, coordinates 9840–13258, 21442–28139, and 30819–35810) to provide adenoviral helper function and 7M8 capsid vector (Addgene, Cat No: 64839) were obtained from Dr. Cavit Agca at Sabanci University as a kind gift. For LV construction, pLenti-CMV-GFP-puro, pmD2.G (plasmid #12259) and psPAX2 (plasmid #12260) were obtained from Addgene. Plasmids were amplified in E. coli and purified using the Genopure Plasmid Midi Kit (Roche Molecular Systems, Inc.). The concentration of plasmid DNAs were analyzed by using NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific Inc.).
Size measurements of PEI: DNA complex
PEI: DNA complexes were prepared in different mediums following the same method as explained under ‘Transfection’ section. Briefly, DNA and PEI, both dissolved in culture medium, were mixed and incubated at room temperature for 30 min. Samples were diluted with distilled water before the analysis. Size of the PEI: DNA complexes in different culture mediums was measured using dynamic light scattering (DLS) by using a Zetasizer Nano ZS (Malvern Panalytical).
Size measurement of produced viruses (both LV and AAV) was also performed by using Zetasizer Nano ZS (Malvern Panalytical). The refractive indexes of LV and AAV2 viruses were used as reported in the literature (Mueller et al. 2012; Pang et al. 2016). First, viruses were collected from cell supernatants of transfected HEK293 cells and then filtered with a 0.45 μm filter. Samples were diluted with distilled water before DLS measurement.
Transient transfection
The day before transfection, HEK 293 cells were seeded on 6-well plates at a density of 1 × 106 cells/ml in transfection mediums. At the day of transfection, cell density of 2 × 106 was checked using the Countess II cell counter (Thermo Sci.). For LV production; DNA plasmids pLenti-CMV-GFP-puro (20 µg), pmD2.G (4 µg), and psPAX2 (16 µg) were mixed in a 1.5 ml microcentrifuge tube. For AAV production, DNA plasmids AAV helper (23.52 µg), AAV2-tdTomato (8.24 µg) and 7m8 capsid (8.24 µg) were mixed in a separate microcentrifuge tube. Plasmids were then mixed with PEI 25 K (Linear- 25 kDa, Polysciences Catalog # 23966) at a ratio of 1 µg of total DNA to 4 µl of 1 mg/ml PEI 25 K (1:4) for 30 min at room temperature for LV production and the same protocol was performed with 1:7 ratio for AAV2 production. PEI: DNA complexes were added dropwise into the cell culture. All experiments were repeated as three independent biological replicates. After small scale production in 4 ml with 8 µg of total plasmid, scale-up to 20 ml was also tested with 40 µg of total plasmid for transfection in IVY. To increase transfection efficiency, a commercial transfection enhancer was also tested. NATE™ (InvivoGen, lyec-nate) nucleic acid transfection enhancer was added dropwise to the culture before transfection according to manufacturer’s instructions.
At 24 h, 48 and 72 h of post-transfection, cell culture supernatants were collected, combined, and stored at + 4 °C until virus harvesting or stored at − 20 °C until further biochemical analysis. The collected mediums were replaced with fresh medium at all time points. The combined supernatants from 3 time points were filtered through Amicon Ultra 15 100 K and 50 K filters (Merck Millipore) at 4000×g for 15 min. Concentrated viruses were stored at − 80 °C. Additionally, 500 µl of supernatant was collected at post-transfection days for biochemical analysis to measure glucose consumption and lactate accumulation using Radiometer ABL90 Analyzer.
Reporter assays
Tandem dimeric (pseudo-monomeric) derivative of DsRed (tdTomato) expressions for AAV2 transfection and GFP expression for LV transfections were analyzed at 24 h, 48 and 72 h post-transfection using both fluorescent microscopy imaging and flow cytometry. 40 fluorescence microscopy images were taken for each transfection day by using Axio Observer Fluorescent Microscope (Zeiss) and each image was analyzed with ImageJ (NIH) to measure the total number of cells in bright-field and fluorescence. Based on the ImageJ toolbox (Schneider et al. 2012), images taken from the samples for each channel were converted to 16-bit grayscale. All cells were highlighted using the “threshold” tool and then counted with the “analyze particles” tool. The automatically calculated particle ratio between brightfield and fluorescent images gives tdTomato/GFP transfection efficiency as a percentage of total cell number. Experiments were conducted as three independent biological repeats with two technical replicates, whereas eight different locations from each sample were visualized under the microscope at each transfection day. For flow cytometry analyzes, the cells were centrifuged at 1000 rpm for 5 min after transfection and then washed with 1X PBS. Cold 70% ethanol fixation was applied for each sample. The percentage of cells expressing tdTomato/GFP was determined by using BD LSRFortessa (BD Biosciences). At least 10.000 cells were analyzed per sample and the gate was set against non-modified cells.
Functional titer
HEK293 suspension cells were seeded on 6-well plates at a density of 1 × 106 cells per well for 4 h prior to transduction. Serial dilutions of AAV2 or LV stocks were freshly prepared in selected mediums for transduction. Virus dilutions (10− 1 to 10− 5) were added into the culture medium including protamine sulfate (8 µg/ml final concentration). The plate was centrifuged at 2400 rpm at 32 °C for 2 h before returning it to the cell culture incubator. After 48 h, cells were harvested and analyzed by BD LSRFortessa Flow Cytometer. For detection of tdTomato- and GFP-transduced cells, PE and FITC channels were used, respectively. AAV and LV virus titers were calculated by using the following formula:
Genome containing titer
tdTomato and GFP gene expressions during AAV (express tdTomato) and LV (express GFP) transduction were measured as an indication of genome containing titer. Briefly, total RNA extraction from transduced cell pellets was applied using GENEzol Reagent (Geneaid) following the product manufacturer’s guidance. The concentration of total RNA was measured by using Nanodrop 2000 spectrophotometer (Thermo Scientific). For reverse transcription of RNAs, 1 µg of RNA per dilution was treated with DNase I at 37 °C for 30 min. The samples were then incubated with 25 mM EDTA at 65 °C for 10 min. RNA was reverse transcribed by using RevertAid First Strand cDNA Transcription System (Thermo Scientific, USA). The reaction was prepared by combining RevertAid Reverse Transcriptase, RiboLock RNase Inhibitor, 5X Reaction Buffer, dNTP Mix, and Random Hexamer Primer in 20 µl of total reaction volume at 25 °C for 10 min, 37 °C for 1 h. The reaction continued at 72 °C for 10 min. Real Time Quantitative-PCR was carried out to quantify gene expression levels following transduction using LightCycler480 (Roche) in a total reaction volume of 10 µL by using SYBR Green I. Forward and reverse primers are as follows: for GFP: 5′- TGACCCTGAAGTTCATCTGC-3′ and 5′-GTCTTGTAGTTGCCGTCGTC-3′; tdTomato: 5′-GGCACCAATITTCCCCCTGA-3′ and 5′-CGACCAGATAATGCCCTCCA-3′. Threshold cycles (Ct) were used to determine vector genome copies per ml using standard curve of viral vector dilutions (Aurnhammer et al. 2012).
Statistical analysis
Two-tailed Student’s t-test and one-way ANOVA were carried out pairwise to analyze the statistical significance of data. The two-way ANOVA test followed by Bonferroni’s post hoc analyses were applied where appropriate. Values of p < 0.05 were considered as significant.
Results
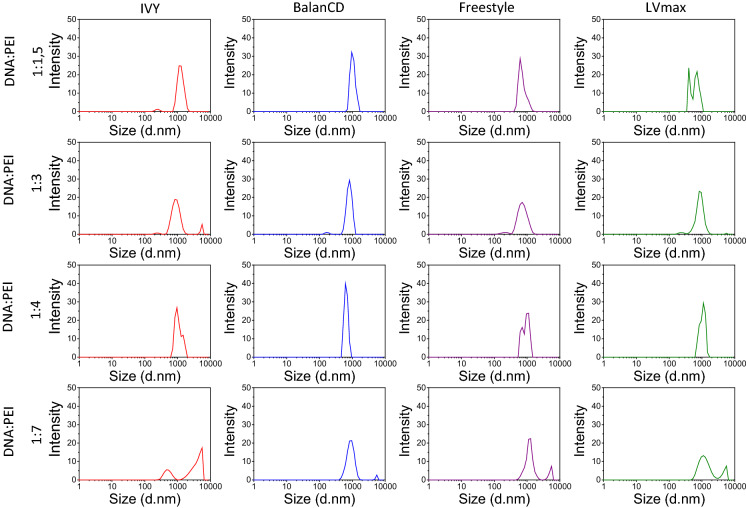
To reveal scalable and optimized transfection conditions for LV and AAV2 production in HEK293 suspension cultures, transfection efficiencies of four commercial transfection mediums were evaluated. Initially, DNA:PEI ratios were tested for proper complex formation by measuring the size of various DNA: PEI complexes with Malvern Nanosizer ZS. Different DNA:PEI ratios (1:1.5, 1:3, 1:4 and 1:7) were analyzed to achieve the highest transfection efficiency using minimum amount of DNA. The hydrodynamic sizes of DNA: PEI complexes of AAV2 plasmids at 1:4 ratio in FreeStyle293, IVY, LV-MAX, and BalanCD mediums were 1032 ± 143 nm, 984 ± 162 nm, 1045 ± 194 nm, and 648 ± 83 nm, respectively. At 1:7 ratio, hydrodynamic sizes of polyplexes in FreeStyle, IVY, LV-MAX, and BalanCD mediums became 1189 ± 302 nm, 510 ± 126 nm, 1228 ± 465 nm, and 897 ± 220 nm, respectively. PEI complexes of AAV2 plasmids also showed second peaks in micrometers range (between 4000 and 5500 nm), which might be a result of aggregation of complexes (Fig. 1). We selected the DNA:PEI ratio of 1:7 for AAV2 transfections, because the transfection efficiencies are increased with polyplexes size. The hydrodynamic sizes of polyplexes of LV plasmids at 1:4 ratio in FreeStyle293, IVY, LV-MAX, and BalanCD mediums were 755 ± 113 nm, 674 ± 98 nm, 931 ± 237 nm, and 875 ± 205 nm, respectively. On the other hand, at 1:7 ratio, sizes of polyplexes in FreeStyle293, IVY, LV-MAX, and BalanCD mediums were increased to 1048 ± 131 nm, 1156 ± 196 nm, 1309 ± 166 nm, and 957 ± 109 nm, respectively. The elevated PEI concentration significantly increased the DNA: PEI complex size, however the size ranges of complexes became narrower with increasing PEI amount from 1:1,5 to 1:4 DNA:PEI ratios. On the other hand, LV plasmids at a DNA:PEI ratio of 1:7 showed smaller sized polyplexes that are observed as second peaks at 717 ± 70 nm, 913 ± 106 nm, and 557 ± 42 nm in FreeStyle293, LV-MAX, and BalanCD mediums, respectively. Polyplexes of LV plasmids at 1:7 ratio might be dissociated due to the arising of second peaks, which had lower hydrodynamic sizes than polyplexes prepared at 1:4 ratio. Therefore, 1:4 DNA: PEI ratio was suggested to be the optimal ratio for LV production (Fig. 2).
Fig. 1.
DLS analysis of DNA: PEI complex with different ratios for AAV2 plasmids. Results were averaged from 3 measurements
Fig. 2.
DLS analysis of DNA: PEI complex with different ratios for LV plasmids. Results were averaged from 3 measurements
IVY media showed highest transfection efficiency for AAV2 plasmids
For studying transfection efficiency for AAV2 production, 1:7 DNA: PEI ratio was applied on HEK293 suspension cell culture in a 6-well plate. FlorabioHEK293 cells were inoculated with 1 × 106 cells/ml in subjected transfection mediums. Next day, cells reached to 2 × 106 cells/ml were transfected considering cell viability above the 90%. According to microscopy images (Fig. 3), an increased number of tdTomato-positive cells were observed at post-transfection days.
Fig. 3.
AAV2 production with PEI 25 K-mediated transfection in IVY. Fluorescent images of FlorabioHEK293 cells were captured 24, 48 and 72 h post transfection at 10X magnification with the Axio Observer (Zeiss) cell imaging system. Representative merged images of Brightfield and tdTomato are presented from three independent experiments. Scale bar: 100 μm
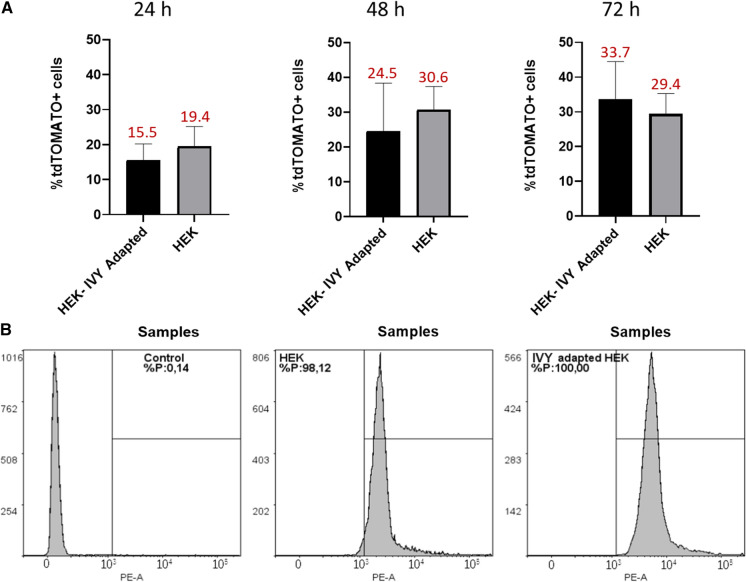
At each transfection day, IVY media showed highest transfection efficiency for AAV2 production based on tdTomato + signal compared to other subjected media indicating that transfection efficiency of IVY medium was significantly high compared with other media (p < 0.05) (Fig. 4A). At 72 h, the number of tdTomato-positive cells was higher than the other days in all subjected media composition. Flow cytometry analysis supported the microscopic data that represent highest transfection efficiency of IVY with 97% of tdTomato-positive cells (Fig. 4B). Like IVY, Freestyle293 (74%) and LV-MAX (60%) media also have a remarkable percentage of tdTomato-positive cells, however, any prominent percentage of transfection efficiency could not be obtained with BalanCD media. The reason for the reduced level of transfection efficiency in BalanCD could be low cell viability within this composition compared to other media (Fig. S1). On the other hand, low cell viability in LVmax might be due to the production of viruses and PEI/DNA complexes.
Fig. 4.
Transfection efficiency of AAV2 vector system based on tdTomato signals in different commercial mediums. A Transfected cells % was calculated from fluorescent microscopy analysis whereas non-transfected FlorabioHEK293 served as negative control. B Percentage of + tdTomato cells at 72 h post-transfection (% of total population) was given using Flow Cytometry (BD LSRFortessa Flow Cytometer). FACS graphs of FlorabioHEK293 suspension cells were taken at 72 h post transfection after cold 70% ethanol fixation. Data is analyzed using FlowLogic software. Results represent the mean ± SD. Significant differences in tdTomato protein expressions between mediums were assessed with one way ANOVA; *p value < 0.05, **p value < 0.005, ***p value < 0.0005. Each experiment was replicated at least three times
To evaluate the transfection efficiency of IVY in higher culture volume, the transient transfection process was scaled up to 20 mL culture volume by using shaking flasks. The effect of media adaptation on transfection efficiency was also evaluated in 20 mL culture conditions.
In both flow cytometry and microscopy analysis, the number of tdTomato-positive cells were not significantly high in media-adapted FlorabioHEK293 cells for the initial time points of culture compared to non-adapted cells (Fig. 5A, B). Notably, a significant effect of media adaptation was observed at 72 h post-transfection in microscopy analysis, showing the highest transfection rate of viral plasmids (Fig. 5A).
Fig. 5.
Determination of the transfection efficiencies of AAV2 vector system in IVY in 20 mL culture media-adapted and non-adapted FlorabioHEK293 suspension cells. A Transfected cells % was calculated from fluorescent microscopy analysis whereas non-transfected FlorabioHEK293 served as negative control. B Percentage of + tdTomato cells 72 h post-transfection (% of total population) was given using FACS (BD LSRFortessa Flow Cytometer). FACS graphs of FlorabioHEK293 suspension cells were taken at 72 h post transfection after ethanol fixation. Data is analyzed using FlowLogic software. Results represent the mean ± SD. Each experiment was replicated at least three times
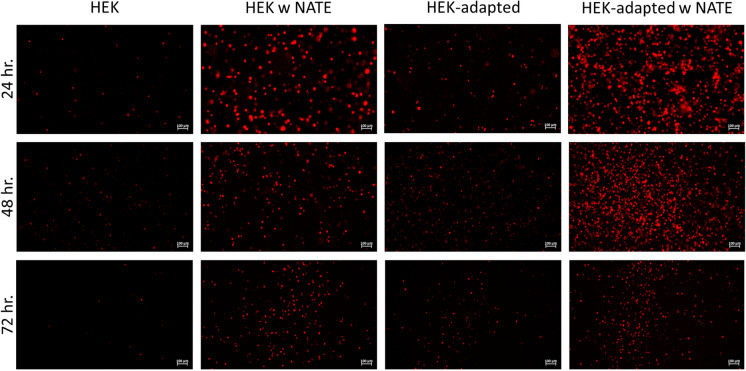
Next, the effect of a commercial transfection enhancer on the transfection efficiency was tested. NATE™ (Invivogen) boosts transfection by inhibiting nucleic acid sensing pathways of host cells, thereby allowing plasmid transcription according to the manufacturer. In Fig. 6, the effect of media adaptation and transfection enhancer on transfection efficiency for AAV generation in HEK293 suspension cell culture was given. At 24 and 48 h of post-transfection, transfection efficiency of media-adapted cells is highest in the presence of enhancers. However, media adaptation led to no impact without transfection enhancer at 72 h of post-transfection. Additionally, it was observed that transfection enhancer and media adaptation have the same effect on tdTomato-positive cell signal at 48 h of post-transfection. Thus, media adaptation may be used for increasing transfection efficiency due to cost-efficiency and sufficient optimization for HEK293 cells transfected in IVY. On the other hand, media adaptation may not be a sufficient optimization approach for other types of cell lines. To test this, we used another suspension cell line, FlorabioMRC5. First, we investigated transfection performance of BalanCD, IVY and serum-free DMEM in AAV2 production by using FlorabioMRC5 cells (Fig. S2). At 48 h of post-transfection, IVY medium showed significantly higher transfection efficiency than BalanCD medium (p < 0.05). The day after, IVY still showed the highest transfection efficiency compared to BalanCD (p < 0.05) and serum-free DMEM (p < 0.5). Similar to the data obtained in HEK293 cells, IVY media was adequate for AAV2 production in FlorabioMRC5 cells. Then, the effect of media adaptation and enhancer on transfection efficiency in IVY were tested (Fig. S3). Only media-adapted MRC5 cells showed higher fluorescence signals than those of only enhancer or adapted-enhancer supplemented cells at 48 h post-transfection. These results indicated that media adaptation could be a sufficient improvement for increasing transfection efficiency in different cell lines.
Fig. 6.
The effect of enhancer and media adaptation on transfection efficiency for AAV2 production with PEI 25 K mediated transfection. Fluorescent images of FlorabioHEK293 suspension cells were captured 24-, 48 h and 72 h post transfection at 10× magnification with the Axio Observer cell imaging system. Representative images are presented from three independent experiments. Scale bar: 100 μm
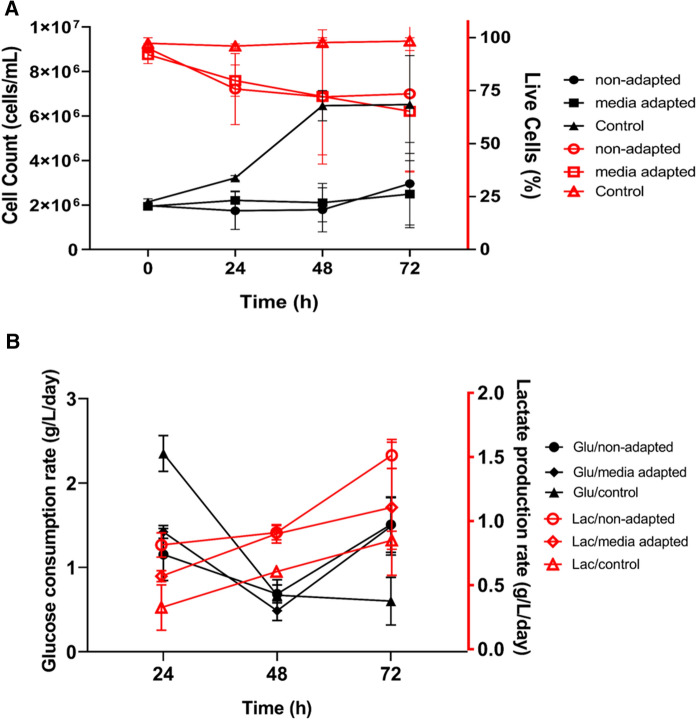
In 20 mL cell cultures, growth rate was measured by counting cell number and analyzing metabolic activities of cells, including glucose consumption and lactate production Fig. 7. According to cell count data, growth rate of transfected HEK293 cells (media-adapted and non-adapted) was slow with an increased cell number to 3 × 106 from 2 × 106 in 72 h of post-transfection. Non-transfected HEK293 control cells, on the other hand, exhibit an exponential growth curve with elevated cell number up to 7 × 106 at 72 h. Accordingly, live cell percentage was kept above 95% in control cells at all time points. However, the percentage of live cells in non-adapted cells decreased prominently to 75% within 24 h in line with lower cell number at 24 h of post-transfection. Percentage of live cells of media-adapted culture decreased linearly. At 72 h of post-transfection, the lowest live-cell ratio of media-adapted cells was observed (Fig. 7a). At this time point, total cell number was similar in adapted and non-adapted cell cultures.
Fig. 7.
Monitoring state of cellular physiology after transfection for AAV2 production. Cell viability and total cell concentration (A), and glucose and lactate concentrations (B) during progress of cultivation and post transfection of the FlorabioHEK293 cells. Cell density (top/filled symbols), percentage of viability (top/open symbols), glucose (bottom/filled symbols) and lactate (bottom/open symbols) concentration of non-adapted HEK293 cells are indicated as a circle symbol, media adapted cells are square symbol, and non-transfected control cells are arrow symbol
Glucose concentration in the medium was decreased, while lactate concentration was increased during cultivation time. Glucose content of both media-adapted and non-adapted cell cultures were decreased to 4 g/L from 4.8 g/L within 24 h. At 48 h of post transfection, glucose consumption rate was decreased compared to first 24 h (Fig. 7b). Glucose content linearly decreased to 3.6 g/L from 2.3 g/L in non-transfected control cells during cultivation, while lactate production linearly increased. In addition, lactate content of media-adapted cells slightly increased to 0.63 g/L from 0.57 g/L within the last 24 h. Statistical analysis indicated a significant effect of time (p < 0.0001) and media adaptation - time interaction (p = 0.0002) on glucose concentration. Bonferroni’s post hoc analyses showed significant differences between media adapted HEK293 cells and control cells as well as between non-adapted cells and control cells at every time point.
Freestyle media showed highest transfection efficiency for LV plasmids
Transfection efficiency for LV production was analyzed with a similar approach applied for AAV2. Microscopic analysis of GFP signals in post-transfected cells indicated increasing transfection efficiencies up to 72 h of transfection in all media evaluated (Fig. 8 and Fig. S6).
Fig. 8.
LV production with PEI 25 K-mediated transfection in FreeStyle293 Media. Fluorescent images of FlorabioHEK293 cells were captured 24, 48 and 72 h post transfection at 10× magnification with the Axio Observer (Zeiss) cell imaging system. Representative merged images of Brightfield and GFP are presented from three independent experiments. Scale bar: 100 μm
According to image analysis, Freestyle medium exhibited the highest transfection efficiency with 16.38% transfected cells at 72 h. IVY represented the second highest transfection efficiency rate at all time points and showed the highest efficiency at 48 h with 6.90% transfected cells (Fig. 9A). In addition, flow cytometry data showed that 95% and 43.5% of HEK293 suspension cells expressed GFP, when transfected in FreeStyle293 and IVY mediums, respectively (Fig. 9B). These commercial mediums outperformed BalanCD and LV-MAX with the transfection efficiencies of ~ 34.5 and ~ 30% of the number of cells expressing GFP, respectively.
Fig. 9.
Transfection efficiency of LV vector systems based on GFP signals in different commercial mediums. A Transfected cells % was calculated from fluorescent microscopy analysis whereas non-transfected HEK293 served as negative control. B Percentage of + GFP cells 72 h post-transfection (% of total population) from FACS (BD LSRFortessa Flow Cytometer) results. FACS graphs of FlorabioHEK293 suspension cells were taken at 72-h post transfection after ethanol fixation. Data is analyzed using FlowLogic software. Results represent the mean ± SD. Significant differences in GFP protein expressions between mediums were assessed with one way ANOVA; *p value < 0.05, **p value < 0.005, ***p value < 0.0005. Each experiment was replicated at least three times
Next, media adaptation was evaluated in 20 mL culture to increase transfection efficiency of the lentivirus vector system in IVY. At 24 and 48 h post transfection, FlorabioHEK293 cells, adapted to IVY, showed increased transfection efficiency compared to non-adapted cells (Fig. 10A). The difference was more significant for the first 24 h with a p value of < 0.005. The difference continued to decrease during following days and when reached to 72 h there was no significant difference between adapted and non-adapted culture (Fig. 10A). However, flow cytometry analysis at 72 h showed a significant difference in the number of GFP-positive cells between media-adapted and non-adapted HEK293 cells, where non-adapted cells exhibit 27.42% GFP-positive signal, that was as high as 65.60% for adapted cells (Fig. 10B).
Fig. 10.
Determination of the LV-GFP transfection efficiencies of IVY in 20 mL culture adapted and non-adapted HEK293 suspension cells. A Transfected cells % was calculated from fluorescent microscopy analysis whereas non-transfected HEK293 served as negative control. Results represent the mean ± SD. B Percentage of + GFP cells 72 h post-transfection of IVY in 20 mL culture adapted and non-adapted HEK293 suspension cells (% of total population) from FACS (BD LSRFortessa Flow Cytometer) results. FACS graphs of HEK293 suspension cells were taken at 72 h post transfection after ethanol fixation. Data is analyzed using FlowLogic software. Significant differences in GFP protein expressions between mediums were assessed with one way ANOVA; *p value < 0.05, **p value < 0.005, ***p value < 0.0005. Each experiment was repeated independently at least three times
The LV transfection was initiated at a cell concentration of 2 × 106 as shown in Fig. 11a. Subsequently, the growth rate of both HEK293 cells (media-adapted and non-adapted) were decreased in line with previous results obtained in AAV2 production. In medium, glucose concentration was decreased to 4.1 g/L from 4.9 g/L, while lactate concentration was increased to 0.65 g/L from 0.5 g/L between 24 and 48 h of post transfection. Glucose consumption rate decreased over time, while lactate production rate increased during cultivation. (Fig. 11b). Statistical analyses indicated a significant effect for the media adaptation (p = 0.02),on glucose concentration in the transfection mediums. On the other hand, Bonferroni’s post hoc analyses showed significant differences between media-adapted (p = 0.0001) and non-adapted (p < 0.0001) HEK293 cells compared to control cells at 72 h of post transfection.
Fig. 11.
Monitoring state of cellular physiology after transfection for LV production. Total number and living cells (A), and glucose and lactate concentrations (B) at indicated time points. Total cell number (top/filled symbols), percentage of viability (top/open symbols), glucose (bottom/filled symbols) and lactate (bottom/open symbols) concentration of non-adapted FlorabioHEK293 cells are shown by circle, media-adapted cells are shown by square, and non-transfected control cells are shown by arrow
Transduction efficiency of AAV2 and LV products in IVY and FreeStyle293 media
AAV2 and LV are efficient gene delivery tools, which can infect both dividing and non-dividing cells, as well as cell types that are difficult to transfect. Suspension cell lines are known to be difficult to transduce because of reduced attachment of the viruses (O’Doherty et al. 2000). Hence, spinoculation was used for providing viral and cellular attachment via centrifugation. In this method, viral particles and target cells were centrifuged together in a 6-well plate (Remley et al. 2021) and then viral vector titer is evaluated by qPCR. The qPCR method is utilized to measure the vector genome copies per ml, which refers to the genome containing titer (Gallaher and Berk 2013). In addition to this method, we performed infectivity assays to determine the functional titer (TU/ml) (Ho and Too 2019). This assay shows the efficiency of viral particles that are capable of transducing gene expression in target cells. To test the transduction efficiency of subjected commercial mediums, viral products, generated in the same commercial mediums were used for infection. Furthermore, DLS was used to demonstrate the existence of LV and AAV2 virions after transfections (Perry and Rayat 2021) (Fig. S4).
In Fig. 12A, the LV transduction efficiency assessed by GFP-positive cells was determined as 50.1% in FreeStyle media, which was the highest ratio due to its transfection efficiency. Besides, the 50% lentiviral transduction efficiency in FreeStyle media followed with 30% efficiency by those in IVY media, however there is no significant difference between the same dilutions of each of these two media. AAV transduction efficiency in IVY media, determined by tdTomato-positive cells, was found to be 32%, while tdTomato-positive signals in FreeStyle media remained at 20.6% (Fig. 12B). In genome containing titer analysis, a higher AAV2 vector genome per ml was observed to 1.6 × 109 vg/ml in IVY media, followed by 1.4 × 107, 7 × 106, 5.6 × 106 for FreeStyle293, LvMax, BalanCD media, respectively (Fig. 12C). On the other hand, a higher LV vector genome per ml was observed to 8.8 × 109 vg/ml in FreeStyle293 media, followed by 7.8 × 109, 3.3 × 109, and 5.7 × 106 for LvMax, IVY, BalanCD media respectively (Fig. 12D). All data about transfection efficiency (%GFP + or %tdTomato+), genome containing titer (Vg/ml) and functional titer (TU/ml) were summarized in Table 1.
Fig. 12.
The examination of genome containing titer and functional titers of LV and AAV2 in FreeStyle293 and IVY mediums. A Representative images of the flow cytometric analysis of the FlorabioHEK293 suspension cells transduced with the GFP-expressing lentivirus. B Representative images of the flow cytometric analysis of the FlorabioHEK293 suspension cells transduced with the tdTomato-expressing AAV2. C Summary graph of RT-PCR measurements of the FlorabioHEK293 suspension cells transduced with the tdTomato-expressing AAV2 and D RT-PCR measurements of the FlorabioHEK293 suspension cells transduced with the GFP-expressing lentivirus. The HEK293T cells were suspended in 1X PBS 48 h after the transduction for the flow cytometric analysis, while 5 × 105 cells were denatured with GENEzol Reagent for RT-PCR analysis. FS is for FreeStyle293; LVX is for Lvmax; BCD is for BalanCD; IVY is for IVY media. All summary graphs show mean ± SD. Percentage of + GFP or + tdTomato cells 48 h post-infection (% of total population) was given using FACS (BD LSRFortessa Flow Cytometer) and data was analyzed using FlowJo_v10.8.1.
Table 1.
Production Yields of LV and AAV2 in the selected transfection mediums
| Transfection mediums | Vector type | Cell culture volume (ml) | Transfection efficiency (%) | Genome containing titer (Vg/ml ) | Functional titer (TU/ml) |
|---|---|---|---|---|---|
| IVY | pLenti-GFP-puro | 4 | 44 | 3.3 × 109 | 4.5 × 106 |
|
AAV2 CBA-tdTomato |
4 | 98 | 1.6 × 109 | 6.1 × 106 | |
| FreeStyle293 | pLenti-GFP-puro | 4 | 95 | 8.8 × 109 | 6.05 × 106 |
|
AAV2 CBA-tdTomato |
4 | 75 | 1.4 × 107 | 3.3 × 106 | |
| LVmax | pLenti-GFP-puro | 4 | 30 | 7.8 × 109 | 1.2 × 106 |
|
AAV2 CBA-tdTomato |
4 | 61 | 7.1 × 106 | 1.4 × 106 | |
| BalanCD | pLenti-GFP-puro | 4 | 35 | 5.9 × 107 | 4 × 105 |
|
AAV2 CBA-tdTomato |
4 | 5 | 5.6 × 106 | 3.4 × 105 |
Consequently, the data obtained by flow cytometry and real-time PCR (Fig. 12) indicated that IVY and FreeStyle medium showed the highest genome containing titer and functional viral titer than the other two commercial mediums, namely Lvmax and BalanCD (Fig. S5), which was consistent with their transfection efficiencies.
Discussion
The AAV and LV viral vector systems are widely used for various research and preclinical translational studies, such as gene therapy, vaccine manufacturing and drug development (Naso et al. 2017; Liu et al. 2021; Ku et al. 2021). The size of AAV particles is smaller than LV particles, resulting in better dissemination in specific tissues like muscle, kidney and heart which are posing the principal barrier and limiting vector spreading (Wang et al. 2005; Rubin et al. 2019). Instead, LV vectors can integrate into the host genome providing stable expression. Development of robust, scalable, and high-yield viral production processes are crucial due to diverse application areas of AAV and LV particles in medicine. To generate maximum amounts of recombinant virus as efficient gene delivery tools with AAV and LV vector systems, suspension cell cultures are commonly preferred and recommended (Subedi et al. 2015; Zhao et al. 2020). However, there is a need for an optimized medium, which could be used for both efficient transfection and transduction to produce viruses in suspension cells. So far, only a few studies reported the importance of the effect of transfection media on transfection efficiency of viral vectors (De Los Milagros Bassani Molinas et al. 2014; Chahal et al. 2014). Therefore, in this study, we compared the performance of AAV and LV production as well as the infection feasibility of IVY medium and 3 well-known transfection mediums: FreeStyle293, BalanCD, and LV-MAX in FlorabioHEK293 suspension cell culture. Moreover, an efficient viral vector delivery and transduction protocol was produced in suspension culture.
To date, several approaches were reported to improve protocols for scalable AAV (serotype 2) manufacturing due to their importance in clinical research, such as phase 1 clinical trial for retinal neovascularization (Ali et al. 1996). Therefore, we studied with an AAV2 vector in this research. For AAV2 production, PEI-facilitated transfection of pHelper, 7M8 capsid and pAAV2-CMA-tdTomato plasmids showed the highest transfection efficiency (98%), generating the highest volumetric productivity (1.6 × 109 vg/ml) using IVY. Transfection with FreeStyle293 media resulted in a transfection efficiency of 75% and the second highest AAV productivity of 1.4 × 107 vg/ml. Consistent with the literature, our comparison among IVY, FreeStyle293, BalanCD and LVmax mediums showed that IVY had higher transfection and transduction performance. For instance, in a previous study, PEI-mediated transfection of three different plasmids into suspension HEK293 culture, AAV serotypes (2–9) productivity of 2 × 108 vg/ml was observed (Blessing et al. 2019). Similar to our data, Grieger et al. reported that PEI-facilitated three plasmid transfection revealed volumetric productivity of 1 × 109 − 3.3 × 1010 vg/ml of different AAV serotypes (Grieger et al. 2016). On the other hand, Zhao et al. reported an AAV productivity of 3 × 1011 vg/ml of HEK293T suspension culture with PEI transfection using FreeStyle293 medium (Zhao et al. 2020). In this study, 52 different conditions were used as a design-of experiment approach to optimize cell lines, harvest times, cell densities and plasmid concentrations for different AAV serotypes (Zhao et al. 2020). Due to various data in several studies led to a shift of attention to the cellular dynamics of the virus production process. To understand dynamics of recombinant virus production, a mechanistic model for synthesis of AAV viral vectors by triple plasmid transfection was developed by Nguyen et al. 2021. This model suggested that viral DNA replication and capsid synthesis should be sufficiently coordinated, and Rep protein is important for capsid synthesis at later stages of virus assembly. Despite several efforts, limitations still exist for obtaining high titer of virus, sustained biological activity and robust production process (Guan et al. 2022). In this context, transfection enhancers and cell adaptation to media were offered for viral vector delivery to increase transfection efficiency (Graham et al. 1977; Xie et al. 2013; Cervera et al. 2015; Ho and Too 2019).
Some well-known transfection enhancers are used as a general practice in literature such as sodium butyrate for lentivirus production (Olsen and Sechelski 2008). Also, trichostatin A, valproic acid, sodium butyrate, dimethyl sulfoxide (DMSO), lithium acetate, caffeine, hydroxyurea, or nocodazole are used to increase virus-like particle production in HEK293 suspension cell culture in previous studies (Cervera et al. 2015). In this study, NATE (a commercial transfection enhancer for cell lines which are difficult to transfect) was used for AAV transfection and suspension cells were adapted to IVY for several passages. Although NATE together with media-adaptation had the higher efficiency for the first two days of post-transfection, there was not much difference at 72 h. In our data, the collective effect of NATE and media adaptation on AAV transfection efficiency was insignificant for HEK293 cell lines compared to the effect of media adaptation alone. These results proposed that media adaptation could be sufficient for improving virus production in HEK293 cells, however different cell lines might exhibit different responses. In scale-up (20 mL) transfection, we showed that there is a significant effect of medium adaptation on transfection efficiency compared to non-adapted cells. Thus, the media adaptation could be a cost-effective alternative to transfection enhancers in HEK293 cells.
In this study, both of fluorescence microscopy and flow cytometry methods were used to monitor transfection efficiency. Fluorescence microscopy data was given for laboratories which have not access to flow cytometry instrument and for checking transfection efficiency at different time points. However, flow cytometry is more reliable than fluorescence microscopy in terms of fluorescence intensity measurement based on the sensitivity range and data collection method. Flow cytometry measures higher values of percentage transfection compared to microscopy, as also can be seen in our data (Marjanovič et al. 2014). Although cultivation was continued for 72 h to obtain highest amount of viral yield both for LV and AAV production, transfection efficiency at 24 h post-transfection showed the best medium correlating maximum virus yield.
For LV production, PEI-facilitated transfection of pLenti-CMV-GFP-puro, pmD2.G and psPAX2 plasmids showed the highest transfection efficiency with 95%, generating the highest volumetric productivity (8.8 × 109 vg/ml) and yielding 6.1 × 106 TU/ml functional titer in FreeStyle293 medium. Furthermore, the transfection with IVY resulted in a transfection efficiency of 44% and the second highest LV productivity of 3.3 × 109 vg/ml and that of functional titer was 4.5 × 106 TU/ml. Consistent with the previous reports, our comparison among IVY, FreeStyle293, BalanCD, LVmax mediums showed that FreeStyle293 medium and IVY had high transduction performance. Ferreira et al. 2021 reported that most of lentivirus production systems with transient transfection yield lentivirus within functional titer of 106–108 transduction units per ml (TU/ml) (Ferreira et al. 2021). These lentivirus production systems commonly used cationic transfection agents; PEI and calcium phosphate, to form a complex with negatively charged nucleic acids for both suspension and adherent cell cultures. The quality of the genome containing particles compared to the functional particles of the products belonging to FreeStyle293 and IVY was 1.4 × 103 vp/TU and 7 × 102 vp/TU, respectively. These results are comparable to the characteristics of lentivirus achieved in previous studies (Merten et al. 2010; Ausubel et al. 2012). The lower value of vp/TU indicates the higher vector quality; therefore, a very high ratio may be used as a marker for defective particles. In our study, the lowest value of vp/TU was obtained with IVY and BalanCD, however, BalanCD medium had the lowest genome containing particles and functional titer of 5.9 × 107 vg/ml and 4 × 105 TU/ml. Consequently, IVY provides highest vector quality for lentivirus products, however, FreeStyle293 medium was found the most efficient one.
Viral titers revealed that IVY and FreeStyle293 media surpassed the efficiency of other media for AAV2 and LV production, respectively. The main differences between AAV2 and LV production were vector plasmids and the applied DNA: PEI ratio. The sizes of DNA: PEI complex in this study were compatible with the literature (Xie et al. 2013). However, a slight decrease in size of polyplexes at DNA: PEI ratio of 1:7 as second peaks which may be due to dissociation of polyplexes. When the amount of PEI increased, the size of polyplexes were also increased until a saturated DNA:PEI ratio, and after the threshold, sizes of polyplexes started to decrease. Moreover, the amount of free PEI polymers are increased with the increased DNA:PEI ratio, which could increase cytotoxicity of PEI:DNA complex solutions (Perevyazko et al. 2012). Polyplexes, prepared by PEI showed size-dependent transfection efficiency, in which, by increasing size of polyplexes from nanoscale to microscale, transfection efficiencies were also increased (Pezzoli et al. 2017). Similarly, our AAV2 transfection results demonstrated that micrometer-sized polyplexes increased the transfection efficiency which supported this statement. Thus, different transfection success during AAV production in different commercial media may depend on their differential production processes, including DNA uptake from the medium, endosomal escape, nuclear entry, plasmid degradation, capsid protein synthesis, transgene rescue and host polymerase-dependent replication, etc. (Nguyen et al. 2021).
Since, high amount of plasmid DNA and transfection reagents are required for large-scale viral production, transient transfection is not cost-effective method. It also cause additional cost-increasing factors, such as batch-to-batch variability and short cultivation time to harvest. Thus, previous studies described stable inducible cell lines and stable constitutive packaging cell lines for lentivirus production (Ferreira et al. 2021). The virus titer of IVY and FreeStyle293 in our study was comparable to lentivirus yield achieved in studies of stable cell lines without large-scale production (Sanber et al. 2015; Piovan et al. 2017; Tomás et al. 2018). When viewed from this aspect, transient transfection protocol which is suggested in this study is promising for virus production in all laboratories.
LV production generally causes inherent cytotoxicity and low stability results in low titers compared to other viral systems. In this regard, the best medium both for packaging and infection is of high importance to improve the virus titer and thus, reduce the costs. It seems that the serum-free IVY medium was sufficient for optimal cell growth and efficient processes of viral systems, such as transfection and transduction in FlorabioHEK293 suspension cell culture.
Conclusion
For an efficient virus production protocol, there are four key considerations: (1) type of transfection reagent, (2) cell density, (3) DNA and transfection reagent ratio, (4) cell culture media (Sung and Kim 2019). The focus of the current study was to evaluate the effects of cell culture medium to the production of viral particles in a suspension cell culture. Our study confirmed that the certain serum-free transfection mediums (IVY and FreeStyle293) showed the highest transfection efficiencies in HEK293 suspension cells compared to others (BalanCD and LV-MAX). As a simple and cost-effective choice, IVY media reveals great success for LV and AAV2 viral vector systems compared to other serum-free mediums, while Freestyle 293 media came into prominence for LV production. Moreover, our improved protocol can be used for viral production and transduction to overcome difficulties of suspension cell cultures that require laborious handling. Our protocol suggests that same media can be used for both transfection and transduction with no need for Opti-MEM or transfection enhancers (ex. sodium butyrate, MgCl2 etc.) for transfection. Transduction efficiencies can be increased with spinoculation where the cells don’t need to be resuspended or splitted after centrifuge. Thus, a cost-effective, laborless and efficient protocol for suspended HEK293 cells were constructed. Consequently, our improved protocol with IVY media provides scalable, simple, and successful viral vector applications in HEK293 suspension cells.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This project is financially supported by Sabanci University Nanotechnology Research and Application Center (SUNUM).
Author contributions
G.C.T. and A.A.Y. conducted the experiments, performed the statistical analysis, wrote, and edited the original draft. C.E., A.C., O.K., and S.C. conceived and participated in the study design. O.K. and S.C. reviewed the manuscript. All authors contributed to and have approved the final version of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gizem Çelebi Torabfam and Abuzer Alp Yetişgin have contributed equally.
Contributor Information
Gizem Celebi Torabfam, Email: gizemcelebi@sabanciuniv.edu.
Abuzer Alp Yetisgin, Email: yalp@sabanciuniv.edu.
Ozlem Kutlu, Email: ozlemkutlu@sabanciuniv.edu.
Sibel Cetinel, Email: cetinel@sabanciuniv.edu.
References
- Ali RR, Reichel MB, Thrasher AJ, et al. Gene Transfer into the Mouse Retina Mediated by an Adeno-Associated Viral Vector. Hum Mol Genet. 1996;5:591–594. doi: 10.1093/HMG/5.5.591. [DOI] [PubMed] [Google Scholar]
- Alton EWFW, Beekman JM, Boyd AC, et al. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax. 2017;72:137–147. doi: 10.1136/THORAXJNL-2016-208406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurnhammer C, Haase M, Muether N, et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods. 2012;23:18–28. doi: 10.1089/HGTB.2011.034. [DOI] [PubMed] [Google Scholar]
- Ausubel LJ, Hall C, Sharma A, et al. Production of CGMP-grade lentiviral vectors. Bioprocess Int. 2012;10:32–43. [PMC free article] [PubMed] [Google Scholar]
- Baldi L, Hacker DL, Meerschman C, Wurm FM. Large-scale transfection of mammalian cells. Methods Mol Biol. 2012;801:13–26. doi: 10.1007/978-1-61779-352-3_2. [DOI] [PubMed] [Google Scholar]
- Blessing D, Vachey G, Pythoud C, et al. Scalable Production of AAV Vectors in Orbitally Shaken HEK293 Cells. Mol Ther Methods Clin Dev. 2019;13:14–26. doi: 10.1016/J.OMTM.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Frank J, Appl H, et al. Serum-free cell culture: the serum-free media interactive online database. Altex. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- Bucher K, Rodríguez-Bocanegra E, Dauletbekov D, Fischer MD. Immune responses to retinal gene therapy using adeno-associated viral vectors - Implications for treatment success and safety. Prog Retin Eye Res. 2021 doi: 10.1016/J.PRETEYERES.2020.100915. [DOI] [PubMed] [Google Scholar]
- Bulcha JT, Wang Y, Ma H, et al. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021 doi: 10.1038/S41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy M, Thompson K, Liu C, et al. Optimization of the Gibco™ CTS™ LV-MAX™ Lentiviral Production System in Stirred Tank Bioreactors. Cytotherapy. 2020;22:S206. doi: 10.1016/J.JCYT.2020.04.087. [DOI] [Google Scholar]
- Cervera L, Fuenmayor J, González-Domínguez I, et al. Selection and optimization of transfection enhancer additives for increased virus-like particle production in HEK293 suspension cell cultures. Appl Microbiol Biotechnol. 2015;99:9935–9949. doi: 10.1007/S00253-015-6842-4. [DOI] [PubMed] [Google Scholar]
- Chahal PS, Schulze E, Tran R, et al. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J Virol Methods. 2014;196:163–173. doi: 10.1016/J.JVIROMET.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle MA, Cheng X, Wilson JM. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther. 2001;8:1281–1290. doi: 10.1038/sj.gt.3301527. [DOI] [PubMed] [Google Scholar]
- De Jesus M, Wurm FM. Manufacturing recombinant proteins in kg-ton quantities using animal cells in bioreactors. Eur J Pharm Biopharm. 2011;78:184–188. doi: 10.1016/J.EJPB.2011.01.005. [DOI] [PubMed] [Google Scholar]
- De Molinas LMilagrosB, Beer M, Hesse C, et al. Optimizing the transient transfection process of HEK-293 suspension cells for protein production by nucleotide ratio monitoring. Cytotechnology. 2014;66:493–514. doi: 10.1007/s10616-013-9601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MV, Cabral ET, Coroadinha AS. Progress and Perspectives in the Development of Lentiviral Vector Producer Cells. Biotechnol J. 2021 doi: 10.1002/BIOT.202000017. [DOI] [PubMed] [Google Scholar]
- Gallaher SD, Berk AJ. A rapid Q-PCR titration protocol for adenovirus and helper-dependent adenovirus vectors that produces biologically relevant results. J Virol Methods. 2013;192:28–38. doi: 10.1016/j.jviromet.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel Y, Reichl U. Vaccine Production Program Vaccine Production Program. In: Pörtner R, editor. Animal Cell Biotechnology. 2. Humana Press; 2007. pp. 457–473. [Google Scholar]
- Ghasemi N, Bandehpour M, Ranjbari J. Optimization of Key Factors in Serum Free Medium for Production of Human Recombinant GM-CSF Using Response Surface Methodology. Iran J Pharm Res IJPR. 2019;18:146. doi: 10.22037/IJPR.2020.112322.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goradel NH, Alizadeh A, Hosseinzadeh S, et al. Oncolytic virotherapy as promising immunotherapy against cancer: mechanisms of resistance to oncolytic viruses. Future Oncol. 2022;18:245–259. doi: 10.2217/FON-2021-0802. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grein TA, Weidner T, Czermak P. Concepts for the Production of Viruses and Viral Vectors in Cell Cultures. In: Gowder SJT, editor. New Insights into Cell Culture Technology. London: IntechOpen; 2017. [Google Scholar]
- Grieger JC, Soltys SM, Samulski RJ. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol Ther. 2016;24:287–297. doi: 10.1038/MT.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JB, Racher AJ. Cultural and physiological factors affecting expression of recombinant proteins. Cytotechnology. 1994;15:3–9. doi: 10.1007/BF00762374. [DOI] [PubMed] [Google Scholar]
- Guan J-S, Chen K, Si Y, et al. Process Improvement of Adeno-Associated Virus Production. Front Chem Eng. 2022;0:1. doi: 10.3389/FCENG.2022.830421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldankar R, Li D, Saremi Z, et al. Serum-free suspension large-scale transient transfection of CHO cells in WAVE bioreactors. Mol Biotechnol. 2006;34:191–199. doi: 10.1385/MB:34:2:191. [DOI] [PubMed] [Google Scholar]
- Hesse F., Ebel M., Konisch N., Sterlinski R., Kessler W., Wagner R. Comparison of a Production Process in a Membrane-Aerated Stirred Tank and up to 1000-L Airlift Bioreactors Using BHK-21 Cells and Chemically Defined Protein-Free Medium. Biotechnology Progress. 2003;19(3):833–843. doi: 10.1021/bp0257630. [DOI] [PubMed] [Google Scholar]
- Ho YK, Too HP. Development of a laboratory scalable process for enhancing lentivirus production by transient transfection of HEK293 adherent cultures. Gene Ther. 2019;27:482–494. doi: 10.1038/s41434-020-0152-x. [DOI] [PubMed] [Google Scholar]
- Karolewski BA, Watson DJ, Parente MK, Wolfe JH. Comparison of Transfection Conditions for a Lentivirus Vector Produced in Large Volumes. Hum Gene Ther. 2004;14:1287–1296. doi: 10.1089/104303403322319372. [DOI] [PubMed] [Google Scholar]
- Kissmann J, Ausar SF, Rudolph A, et al. Stabilization of measles virus for vaccine formulation. Hum Vaccin. 2008;4:350–359. doi: 10.4161/HV.4.5.5863. [DOI] [PubMed] [Google Scholar]
- Ku MW, Bourgine M, Authié P, et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29:236–249e6. doi: 10.1016/J.CHOM.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Nagarajan A, Uchil PD. Calcium Phosphate-Mediated Transfection of Adherent Cells or Cells Growing in Suspension: Variations on the Basic Method. Cold Spring Harb Protoc. 2019;2019:705–708. doi: 10.1101/PDB.PROT095455. [DOI] [PubMed] [Google Scholar]
- Legmann R. Transient transfection at large scale for clinical AAV9 vector manufacturing. Cytotherapy. 2020;22:S151. doi: 10.1016/J.JCYT.2020.03.312. [DOI] [Google Scholar]
- Liu D, Zhu M, Zhang Y, Diao Y. Crossing the blood-brain barrier with AAV vectors. Metab Brain Dis. 2021;36:45–52. doi: 10.1007/S11011-020-00630-2. [DOI] [PubMed] [Google Scholar]
- Longo PA, Kavran JM, Kim MS, Leahy DJ. Transient Mammalian Cell Transfection with Polyethylenimine (PEI) Methods Enzymol. 2013;529:227. doi: 10.1016/B978-0-12-418687-3.00018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjanovič I, Kandušer M, Miklavčič D, et al. Comparison of Flow Cytometry, Fluorescence Microscopy and Spectrofluorometry for Analysis of Gene Electrotransfer Efficiency. J Membr Biol. 2014;247:1259–1267. doi: 10.1007/s00232-014-9714-4. [DOI] [PubMed] [Google Scholar]
- Merten OW, Charrier S, Laroudie N, et al. Large-Scale Manufacture and Characterization of a Lentiviral Vector Produced for Clinical Ex Vivo. Gene Ther Appl. 2010;22:343–356. doi: 10.1089/HUM.2010.060. [DOI] [PubMed] [Google Scholar]
- Miki H, Takagi M. Design of serum-free medium for suspension culture of CHO cells on the basis of general commercial media. Cytotechnology. 2015;67:689. doi: 10.1007/S10616-014-9778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C, Ratner D, Zhong L, et al. Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol. 2012 doi: 10.1002/9780471729259.mc14d01s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/S40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TNT, Sha S, Hong MS, et al. Mechanistic model for production of recombinant adeno-associated virus via triple transfection of HEK293 cells. Mol Ther Methods Clin Dev. 2021;21:642–655. doi: 10.1016/J.OMTM.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty U, Swiggard WJ, Malim MH. Human Immunodeficiency Virus Type 1 Spinoculation Enhances Infection through Virus Binding. J Virol. 2000;74:10074. doi: 10.1128/JVI.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JC, Sechelski J. Use of Sodium Butyrate to Enhance Production of Retroviral Vectors Expressing CFTR cDNA. Hum Gene Ther. 2008;6:1195–1202. doi: 10.1089/HUM.1995.6.9-1195. [DOI] [PubMed] [Google Scholar]
- Pang Y, Song H, Cheng W. Using optical trap to measure the refractive index of a single animal virus in culture fluid with high precision. Biomed Opt Express. 2016 doi: 10.1364/boe.7.001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perevyazko IY, Vollrath A, Pietsch C, et al. Nanoprecipitation of poly(methyl methacrylate)-based nanoparticles: effect of the molar mass and polymer behavior. J Polym Sci Part A Polym Chem. 2012;50:2906–2913. doi: 10.1002/POLA.26071. [DOI] [Google Scholar]
- Perry C, Rayat ACME. Lentiviral Vector Bioprocessing. Viruses. 2021;13:268. doi: 10.3390/V13020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzoli D, Giupponi E, Mantovani D, Candiani G. Size matters for in vitro gene delivery: investigating the relationships among complexation protocol, transfection medium, size and sedimentation. Sci Rep. 2017 doi: 10.1038/SREP44134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovan C, Marin V, Scavullo C, et al. Vectofusin-1 promotes RD114-TR-pseudotyped lentiviral vector transduction of human HSPCs and T lymphocytes. Mol Ther Methods Clin Dev. 2017;5:22–30. doi: 10.1016/J.OMTM.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendra Y, Balasubramanian S, Hacker DL. Large-Scale Transient Transfection of Chinese Hamster Ovary Cells in Suspension. Methods Mol Biol. 2017;1603:45–55. doi: 10.1007/978-1-4939-6972-2_3. [DOI] [PubMed] [Google Scholar]
- Raymond C, Tom R, Perret S, et al. A simplified polyethylenimine-mediated transfection process for large-scale and high-throughput applications. Methods. 2011;55:44–51. doi: 10.1016/J.YMETH.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Reed SE, Staley EM, Mayginnes JP, et al. Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods. 2006;138:85–98. doi: 10.1016/J.JVIROMET.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Remley VA, Jin J, Sarkar S, et al. High efficiency closed-system gene transfer using automated spinoculation. J Transl Med. 2021;19:1–15. doi: 10.1186/S12967-021-03126-4/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JD, Nguyen TV, Allen KL, et al. Comparison of Gene Delivery to the Kidney by Adenovirus, Adeno-Associated Virus, and Lentiviral Vectors After Intravenous and Direct Kidney Injections. Hum Gene Ther. 2019;30:1559. doi: 10.1089/HUM.2019.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanber KS, Knight SB, Stephen SL, et al. Construction of stable packaging cell lines for clinical lentiviral vector production. Sci Rep. 2015 doi: 10.1038/SREP09021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012. 2012;97:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Johnson RW, Moniz HA, et al. High yield expression of recombinant human proteins with the transient transfection of HEK293 cells in suspension. J Vis Exp. 2015 doi: 10.3791/53568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res 2019. 2019;231 23:1–7. doi: 10.1186/S40824-019-0156-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Garson K, Li L, Vanderhyden BC. Optimization of lentiviral vector production using polyethylenimine-mediated transfection. Oncol Lett. 2015;9:55. doi: 10.3892/OL.2014.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás HA, Rodrigues AF, Carrondo MJT, Coroadinha AS. LentiPro26: novel stable cell lines for constitutive lentiviral vector production. Sci Rep. 2018 doi: 10.1038/S41598-018-23593-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkama AJ, Leinonen HM, Lipponen EM, et al. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther 2018. 2017;251 25:39–46. doi: 10.1038/gt.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkama AJ, Oruetxebarria I, Lipponen EM, et al. Development of Large-Scale Downstream Processing for Lentiviral Vectors. Mol Ther Methods Clin Dev. 2020 doi: 10.1016/j.omtm.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chai L, Liang S, et al. Fetal calf serum exerts an inhibitory effect on replication of duck hepatitis A virus genotype 1 in duck embryo fibroblast cells. Viruses. 2020;12:1–14. doi: 10.3390/v12010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/NBT1073. [DOI] [PubMed] [Google Scholar]
- Wright JF. Transient Transfection Methods for Clinical Adeno-Associated Viral Vector Production. Hum Gene Ther. 2009;20:698. doi: 10.1089/HUM.2009.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Xinyong G, Xianjin C, Yayu W. PEI/DNA formation affects transient gene expression in suspension Chinese hamster ovary cells via a one-step transfection process. Cytotechnology. 2013;65:263–271. doi: 10.1007/s10616-012-9483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Lee KJ, Daris M, et al. Creation of a High-Yield AAV Vector Production Platform in Suspension Cells Using a Design-of-Experiment Approach. Mol Ther Methods Clin Dev. 2020;18:312–320. doi: 10.1016/J.OMTM.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.