Abstract
Some lipopolysaccharide (LPS) preparations from S- or R-form members of the family Enterobacteriaceae and oral black-pigmented bacteria (Porphyromonas gingivalis and Prevotella intermedia) are known to activate LPS-refractory C3H/HeJ macrophages. When contaminating proteins are removed from R-form LPS of Enterobacteriaceae by repurification, however, this ability is lost. In the present study, we investigated the capacity of LPS from P. gingivalis, P. intermedia, Salmonella minnesota, and Salmonella abortusequi to induce production of tumor necrosis factor (TNF) in gamma interferon-primed C3H/HeJ macrophages before and after repurification. P. abortusequi S-LPS was fractionated by centrifugal partition chromatography into two LPS forms: SL-LPS, having homologous long O-polysaccharide chains, and SS-LPS having short oligosaccharide chains. Prior to repurification, all LPS forms except SL-LPS induced TNF production in both C3H/HeJ and C3H/HeN macrophages. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that repurification removed contaminating protein from the preparations, and repurified SS-LPS and S. minnesota Ra-LPS no longer stimulated TNF production in C3H/HeJ macrophages, although C3H/HeN macrophages remained responsive. In contrast, repurified oral bacterial LPS retained the capacity to induce TNF production in C3H/HeJ macrophages. Oral bacterial LPS preparations also were not antagonized by excess inactive, repurified SL-LPS; Ra-LPS; Rhodobacter sphaeroides lipid A, a competitive LPS antagonist, or paclitaxel, an LPS agonist, and they were comparatively resistant to polymyxin B treatment. Nevertheless, oral bacterial LPS was less toxic to d-galactosamine-treated C3H/HeN mice than was LPS from Salmonella. These findings indicate that the active molecule(s) and mode of action of LPS from P. gingivalis and P. intermedia are quite different from those of LPS from Salmonella.
C3H/HeJ is a unique mutant mouse strain derived from C3H/He mice. As a result of a genetic defect, they lack the ability to respond to endotoxin or lipopolysaccharide (LPS) derived from the cell walls of gram-negative bacteria or the lipid A component thereof (reviewed in reference 25). A recent study demonstrated that the mutation of C3H/HeJ mice is located in the Tlr4 gene (30). While C3H/HeJ cells are profoundly refractory to some highly purified LPS, however, the cells remain responsive to other endotoxins (e.g., Boivin) in which the endotoxin protein or lipid A-associated protein (35) is known to be bioactive. In addition, LPS from Brucella abortus (21, 33), Pseudomonas aeruginosa (29), Porphyromonas (Bacteroides) gingivalis (5, 7), and Bacteroides fragilis (12) are also known to activate C3H/HeJ cells. LPS isolated from rough (R-form) mutant members of the family Enterobacteriaceae had also been thought capable of stimulating C3H/HeJ cells, but Manthey and Vogel (19) clearly demonstrated that the effect disappeared when protein associated with the LPS was removed by repurification.
P. gingivalis and Prevotella intermedia are the dominant gram-negative bacteria in the periodontal pockets of patients with periodontitis, and they are considered to be the major pathogens associated with periodontal diseases (38, 41). LPS of P. gingivalis and P. intermedia has been suggested as a possible virulence factor, acting by stimulation of host cells to induce production of proinflammatory mediators (28). Their LPS possess unique chemical and biological properties different from those of LPS of Enterobacteriaceae (15–17, 27, 28). The low endotoxic activity of P. gingivalis LPS has been suggested to be due to the unique chemical structure of its lipid A (15, 27).
LPS from wild type (S-form) organisms of Enterobacteriaceae is a glycolipid complex composed of three distinct structural elements: an O-antigenic repeating polysaccharide, a core oligosaccharide, and a lipophilic component designated lipid A. Wild-type strains synthesize LPS with long polysaccharide chains, the so-called S-form LPP. In R-form strains, biosynthesis of the O polysaccharide and, in some cases, the core oligosaccharide is defective. Consequently, R-form strains synthesize LPS, generally termed R-chemotype or R-form LPS, with shorter saccharide chains. As it happens, during cell wall biosynthesis, S-form bacteria also produce incomplete, R-form LPP. We previously showed that the native S-form LPS from Salmonella abortusequi contains both S-form (SL-LPS) and R-form (SS-LPS) LPS which were separable by centrifugal partition chromatography (CPC) and that their respective endotoxicities, as assessed by macrophage activation, were quite different (34). In the present study, we set out to clarify whether SL- and SS-LPS are capable of inducing C3H/HeJ macrophages to produce tumor necrosis factor (TNF), and if so, whether the active principle can be removed by repurification by the method of Manthey and Vogel (19). We also determined whether LPS isolated from the oral bacteria P. gingivalis and Prevotella intermedia retain the capacity to induce TNF production in C3H/HeJ macrophages after repurification.
MATERIALS AND METHODS
Mice.
C3H/HeN and C3H/HeJ mice were bred and maintained in the Animal Faculty of the Jichi Medical School under standard care. Female mice were used at 8 to 12 weeks of age. In individual experiments, age-matched mice were used.
LPS.
LPS from P. gingivalis 381 and P. intermedia ATCC 25611 were prepared by using a hot phenol-water extraction procedure (40). LPS of Rhodobacter sphaeroides ATCC 17023 (RsDPLA) was prepared as previously described (32). Ra-chemotype LPS (Ra-LPS) from Salmonella minnesota R595 was kindly provided by K. Hisatsune, Josai University, Sakado, Japan. Ra-LPS from S. minnesota R60 was obtained from List Biological Laboratories, Inc., Campbell, Calif. S-form LPS from Escherichia coli O111:B4, S. abortusequi, and wild-type S. minnesota were purchased from Sigma Chemical Co., St. Louis, Mo.
Reagents.
Polymyxin B and paclitaxel (Taxol) were obtained from Sigma Chemical Co. Murine recombinant gamma interferon (IFN-γ) was provided by Shionogi Pharmaceutical Co., Osaka, Japan.
Fractionation of wild-type LPS into LPS with and without O-polysaccharides.
LPS isolated from wild-type S. abortusequi is actually a mixture of two LPS forms: SL-LPS, having homologous long O-polysaccharide chains, and SS-LPS, which, like R-form LPS, lacks most O-saccharide chains. The two LPS preparations were isolated from each other by CPC as previously described (34). Briefly, the triethylamine (TEA) salt of LPS from S. abortusequi (10 mg) was applied to a Sanki LLB-M CPC apparatus (Sanki Engineering, Kyoto, Japan) being used with a solvent system consisting of 1-butanol–tetrahydrofuran–methanol–water (10/7/1/20, vol/vol) at 25°C and 1,900 rpm. The fractions were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by silver staining. Fractions rich in SL- and SS-LPS were respectively pooled and used for experiments as SL-LPS and SS-LPS. Dry-weight recoveries for the pooled fractions were 45 and 17%, respectively.
Repurification of LPS using a modified phenol-water extraction procedure.
Ra-LPS, P. gingivalis LPS, P. intermedia LPS, SL-LPS, and SS-LPS were repurified by detergent-modified phenol-water extraction as described by Manthey and Vogel (19). Briefly, LPS was suspended in H2O (5 mg of LPS/ml) containing 0.2% TEA and 0.5% sodium deoxycholate, and the sample (1 vol) was extracted with an equal volume of a 9:1 (wt/wt) phenol-water solution. The phenol phase was then re-extracted with 1 volume of H2O containing 0.2% TEA and 0.5% sodium deoxycholate, and the aqueous phase was re-extracted with 1 volume of 9:1 (wt/wt) phenol-water. The aqueous phase was then adjusted to 75% ethanol and 30 mM sodium acetate, and the LPS was allowed to precipitate at −20°C for 1 h. Recovery of Salmonella LPS in the aqueous phase was determined by measuring 3-keto-3-deoxyocturonic acid.
Analysis of LPS and protein by SDS-PAGE.
As described by Manthey and Vogel (19), diluted samples were boiled for 5 min with 0.33 volume of 4× loading buffer. LPS was then resolved by SDS–13% PAGE and visualized by silver staining in accordance with the manufacturer’s (Bio-Rad Laboratories, Richmond, Calif.) instructions. The resolved proteins were blotted onto nitrocellulose transfer membranes and stained in colloidal gold solution for 1 h (Bio-Rad Laboratories). The molecular weights of the endotoxin proteins were determined by comparison with known protein standards (Low Molecular Weight Range Molecular Weight Markers; Bio-Rad Laboratories).
Macrophage isolation and culture.
Murine peritoneal macrophages were isolated by peritoneal lavage 4 days after intraperitoneal (i.p.) injection of 1.5 ml of 3% Brewer thioglycolate broth (Difco Laboratories, Detroit, Mich.). The cells were washed with serum-free RPMI 1640 medium (ICN Biomedicals, Costa Mesa, Calif.) containing 4 mM l-glutamine, 100-U/ml penicillin, and 100-μg/ml streptomycin and plated in 96-well plates (Nunc, Roskilde, Denmark) at a concentration of 2 × 105 cells/well. After the cells had been incubated for 2 h at 37°C under an atmosphere of 95% air–5% CO2, they were washed with serum-free RPMI 1640 medium to remove nonadherent cells. The remaining cells were incubated for an additional 3.5 h in the presence of various doses of LPS in 200 μl of RPMI 1640 medium also containing (i) 2% heat-inactivated fetal bovine serum (JRH Biosciences, Lenexa, Kans.) in the case of C3H/HeN macrophages or (ii) 2% heat-inactivated fetal bovine serum plus 20-U/ml murine recombinant IFN-γ in the case of C3H/HeJ macrophages. After the incubation period, the culture supernatants were collected for TNF assay. Contaminating endotoxin concentrations in culture media and serum was quantified by using a modified Limulus amebocyte lysate test (Endospeci Test Kit; Seikagaku Corporation, Tokyo, Japan), and found to be 4.8 and 2.4 pg/ml, respectively.
TNF bioassay.
Culture supernatants were assayed for TNF bioactivity in a standard cytotoxicity assay using actinomycin D-treated L929 cells as described previously (13).
Determination of LD50 for mice.
Groups of C3H/HeN mice (four to eight per group) were simultaneously injected i.p. with GalNac (18 mg/mouse) and LPS (0.1 ng to 10 μg/mouse). Mortality was scored 72 h after injection, and the 50% lethal dose (LD50) was calculated by the method of Reed and Muench.
RESULTS
SDS-PAGE following CPC fractionation and repurification of S. abortusequi LPS.
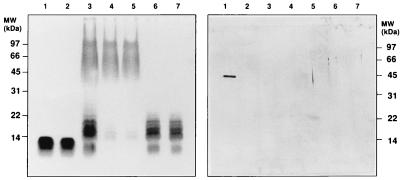
As expected, SDS-PAGE of Westphal-type LPS (40) prepared from wild-type S. abortusequi resolved two distinct bands revealing the presence of two LPS molecules (Fig. 1, left panel, lane 3): a relatively broad band with a higher molecular weight (SL-LPS) and a relatively narrow band with a lower molecular weight (SS-LPS). SL-LPS and SS-LPS were subsequently separated by CPC (Fig. 1, left panel, lanes 4 and 6). S. minnesota Ra-LPS was extracted in phenol-chloroform-petroleum ether (9) and had a molecular weight comparable to that of SS-LPS (Fig. 1, left panel, lane 1). Repurification of the isolated Ra-LPS, SL-LPS, and SS-LPS using a modified phenol-water extraction procedure had no further effect on the gels (Fig. 1, left panel, lanes 2, 5, and 7).
FIG. 1.
SDS-PAGE and Western blot analysis of P. minnesota Ra-LPS and S. abortusequi LPS. S. abortusequi S-form LPS was fractionated into SL-LPS and SS-LPS by CPC. Some preparations were repurified by a modified phenol-water extraction procedure. The LPS were submitted to SDS–13% PAGE, and the gels were visualized by silver staining (left panel). Proteins were blotted onto nitrocellulose and stained with colloidal gold (right panel). Lanes: 1, Ra-LPS (0.8 μg); 2, repurified Ra-LPS (0.8 μg); 3, S. abortusequi LPS (2.5 μg); 4, SL-LPS (1.5 μg); 5, repurified SL-LPS (1.5 μg); 6, SS-LPS (0.8 μg); 7, repurified SS-LPS (0.8 μg). MW, molecular mass.
Another set of SDS-PAGE blots were stained with colloidal gold in order to detect protein. As shown in the right panel of Fig. 1, Ra-LPS possessed a major protein component with a molecular mass of 41 kDa. After repurification, however, that protein had disappeared. Other LPS species showed no trace of proteins including the 41-kDa protein before or after repurification.
LPS-induced TNF production in C3H/HeJ macrophages.
C3H/HeJ macrophages are known to be refractory to S-form LPS extracted by the phenol-water procedure (40), although if cultured in the presence of IFN-γ (1, 3), they may respond to LPP. Indeed, we have at times observed that phenol-water-extracted S-form LPS stimulates TNF production in IFN-γ-primed, C3H/HeJ macrophages. Therefore, six preparations of commercially available phenol-water-extracted S-form LPS from S. abortusequi were tested for the capacity to induce TNF production in IFN-γ-primed C3H/HeJ macrophages. At a concentration of 1 μg/ml, four of the LPS preparations elicited marginal levels of TNF synthesis (<100 U/ml), whereas two of the preparations were significantly more efficacious and elicited synthesis of >500-U/ml TNF.
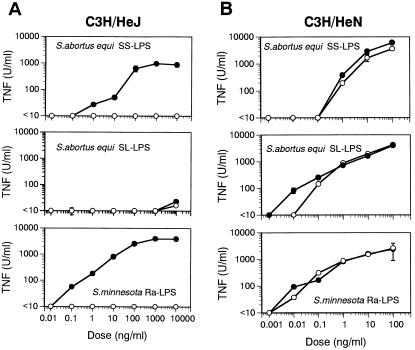
Activation of C3H/HeJ macrophages by SS-LPS and SL-LPS before and after repurification.
One of the two aforementioned S. abortusequi LPS capable of activating IFN-γ-primed C3H/HeJ macrophages was fractionated into SS- and SL-LPS, and samples of the respective fractions, as well as samples of Ra-LPS, were then repurified by phenol-water extraction. Their capacities to induce TNF production in IFN-γ-primed C3H/HeJ or unprimed C3H/HeN macrophages were then assessed. Prior to repurification, SS-LPS and Ra-LPS each stimulated IFN-γ-primed C3H/HeJ macrophages, although that capacity was lost when the LPS preparations were repurified (Fig. 2A); SL-LPS lacked the ability to induce TNF production both before or after repurification. In contrast, all three LPS preparations induced TNF production in C3H/HeN macrophages, regardless of repurification (Fig. 2B).
FIG. 2.
TNF production induced by SS-LPS and SL-LPS before and after repurification in C3H/HeN and C3H/HeJ macrophages. C3H/HeJ macrophages were pretreated with IFN-γ (20 U/ml) for 2 h. C3H/HeJ (A) and C3H/HeN (B) macrophages were then incubated with the indicated doses of SS-LPS, SL-LPS, or Ra-LPS for 4 h in triplicate wells. TNF activity in the supernatants was determined by cytotoxicity assay using L929 cells. TNF secretion by unstimulated cells was below detectable levels in all experiments. Filled circles indicate LPS without repurification, and open circles indicate repurified LPS (assuming 100% recovery of LPS after repurification). Each point represents the mean ± the standard error. The data are from one of two independent experiments with similar results.
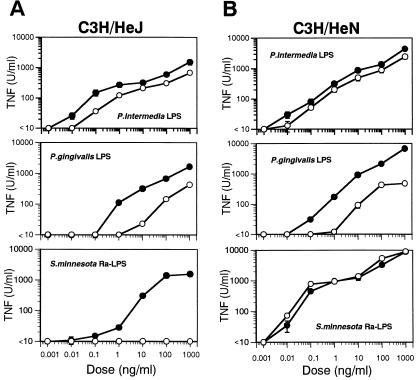
Effect of repurification on SDS-PAGE and induction of TNF by LPS from P. intermedia and P. gingivalis.
LPS from some strains of oral bacteria are known to activate C3H/HeJ and C3H/HeN cells (5, 7). Prior to repurification, LPS obtained from P. intermedia and P. gingivalis by phenol-water extraction as well as Ra-LPS induced TNF production in both C3H/HeJ and C3H/HeN macrophages (Fig. 3A and B). Moreover, all but Ra-LPS remained active even after repurification, although TNF production induced by repurified P. gingivalis LPS decreased slightly (Fig. 3B). With respect to C3H/HeJ macrophages, the capacity to elicit TNF production was retained by repurified LPS from P. intermedia and P. gingivalis but was entirely absent from repurified Ra-LPS (Fig. 3A). SDS-PAGE carried out before and after repurification revealed no remarkable changes in gels visualized with silver stain (data not shown). On the other hand, gels stained with colloidal gold confirmed that the protein component in Ra-LPS was lost during repurification (data not shown).
FIG. 3.
Effect of repurification of TNF production induced by P. intermedia LPS, P. gingivalis LPS, and Ra-LPS. C3H/HeJ macrophages were pretreated with IFN-γ (20 U/ml) for 2 h. C3H/HeJ (A) and C3H/HeN (B) macrophages were incubated for 4 h with the indicated doses of P. intermedia LPS, P. gingivalis LPS, or Ra-LPS. TNF activity in the supernatants was then determined by cytotoxicity assay using L929 cells. TNF secretion by unstimulated cells was below detectable levels in all experiments. Filled circles indicate LPS without repurification, and open circles indicate repurified LPS. The doses of repurified LPS are based on the intensity of silver staining in SDS-PAGE. The yields of LPS during repurification were more than 80%. The data are from one of two independent experiments with similar results.
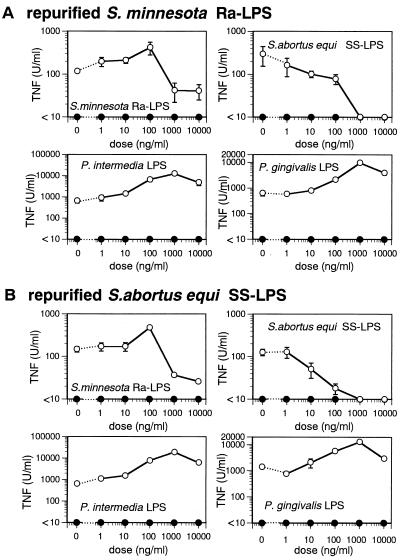
Antagonistic effect of repurified Ra-LPS and SS-LPS on LPS-induced TNF production.
Repurified Ra-LPS and SS-LPS were incapable of eliciting TNF production in IFN-γ-primed C3H/HeJ macrophages, even at a concentration of 10 μg/ml. We were interested to know, therefore, whether these repurified, inactive LPS would interfere with TNF production elicited by other active LPS in IFN-γ-primed C3H/HeJ macrophages. We observed that repurified, inactive Ra-LPS (Fig. 4A) and SS-LPS (Fig. 4B) each dose dependently inhibited TNF production elicited by their nonrepurified counterparts. In contrast, the inactive LPS failed to inhibit TNF production induced by P. intermedia LPS and P. gingivalis LPP.
FIG. 4.
Inhibitory effect of repurified Ra-LPS and SS-LPS on LPS-induced TNF production in IFN-γ-primed C3H/HeJ macrophages. C3H/HeJ macrophages were pretreated with IFN-γ (20 U/ml) for 2 h and then cultured for an additional 2 h in the presence or absence of the indicated doses of repurified Ra-LPS (A) or SS-LPS (B). TNF production was then elicited by addition of 10-ng/ml Ra-LPS, SS-LPS, or P. intermedia LPS or 100-ng/ml P. gingivalis LPS (open circles) and incubation for 4 h. TNF activity in the supernatants was determined by cytotoxicity assay using L929 cells. As a negative control (closed circles), some cultures were exposed only to repurified Ra-LPS (A) or SS-LPS (B). TNF secretion by unstimulated cells was below detectable levels in all experiments. Each point represents the mean ± the standard error of triplicate cultures. The data are from one of two independent experiments with similar results.
Taken together, the data presented so far strongly suggest that the active molecules present in LPS from oral bacteria differ substantially from those present in Ra-LPS and SS-LPP.
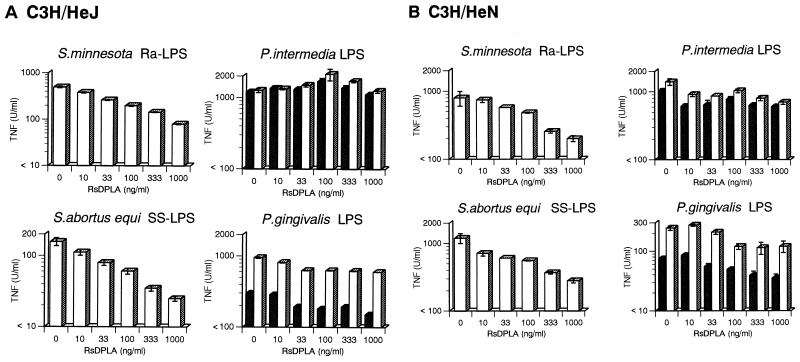
Effect of RsDPLA and paclitaxel on TNF production induced by P. intermedia LPS, P. gingivalis LPS, Ra-LPS, and SS-LPP.
RsDPLA, an LPS from R. sphaeroides, is known to be a specific LPS antagonist and has been shown to inhibit a variety of LPS-evoked responses in macrophages (18). For instance, LPS-induced TNF production in macrophages is specifically blocked by RsDPLA, as is the binding of 125I-labelled LPS (13). Conversely, paclitaxel is an LPS-like agonist (6, 20): it stimulates TNF production in murine macrophages but has little effect on LPS-refractory C3H/HeJ macrophages. The present experiments were carried out to determine the effects of RsDPLA and paclitaxel on TNF production elicited by P. intermedia LPS, P. gingivalis LPS, Ra-LPS, and SS-LPS in C3H/HeN and IFN-γ-primed C3H/HeJ macrophages. RsDPLA and paclitaxel were each added to separate cultures 1 h before LPP.
As shown in Fig. 5, TNF production elicited by LPS from P. intermedia and P. gingivalis was not suppressed by RsDPLA, whereas RsDPLA dose dependently inhibited the TNF production elicited by Ra-LPS and SS-LPP. Paclitaxel had no effect on LPS-induced TNF production in IFN-γ-primed C3H/HeJ macrophages, although higher doses of dimethyl sulfoxide, the vehicle used to dissolve paclitaxel, appeared to suppress TNF production somewhat (Fig. 6, open circles).
FIG. 5.
Antagonistic effect of RsDPLA on TNF production induced by Ra-LPS, SS-LPS, P. intermedia LPS, and P. gingivalis LPP. INF-γ-primed C3H/HeJ (A) and unprimed C3H/HeN (B) macrophages and were incubated with the indicated doses of RsDPLA for 1 h, and then Ra-LPS (10 ng/ml), SS-LPS (10 ng/ml), P. intermedia LPS (10 ng/ml), and P. gingivalis LPS (100 ng/ml) were added to respective cultures, which were then incubated for 4 h. TNF activity in the supernatants was then determined by cytotoxicity assay. Open bars, LPS without repurification; dotted bars, repurified LPP. Bars depict means ± the standard errors of triplicate cultures. The data are from one of two independent experiments with similar results.
FIG. 6.
Effect of paclitaxel on LPS-induced TNF production in IFN-γ-primed C3H/HeJ macrophages. C3H/HeJ macrophages were pretreated for 2 h with IFN-γ (20 U/ml) and then incubated with the indicated doses of paclitaxel for 1 h (filled circles). As a control, some cultures received equivalent amounts of the vehicle (dimethyl sulfoxide; open circles). The macrophages were cultured in the presence or absence of unrepurified Ra-LPS (10 ng/ml), SS-LPS (10 ng/ml), P. intermedia LPS (10 ng/ml), or P. gingivalis LPS (100 ng/ml) for 4 h, and then TNF activity in the supernatants was determined by cytotoxicity assay. Each point represents the mean ± the standard error. The data are from one of two independent experiments with similar results.
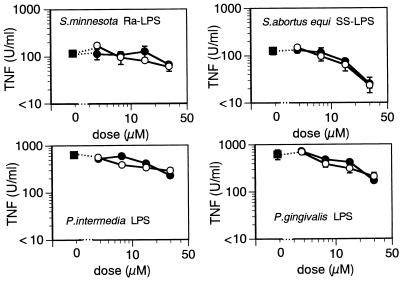
Sensitivity of P. intermedia LPS, P. gingivalis LPS, Ra-LPS, and SS-LPS to polymyxin B.
Polymyxin B neutralizes many of the biological activities of LPS by binding to lipid A (22, 23). We tested the sensitivity of repurified preparations of P. intermedia LPS, P. gingivalis LPS, and nonrepurified Ra-LPS and SS-LPS to polymyxin B. High or low concentrations of these preparations were incubated with various doses of polymyxin B; the mixtures were then added to C3H/HeN or C3H/HeJ macrophage cultures, and evoked TNF production was assessed (Fig. 7).
FIG. 7.
Effect of polymyxin B on TNF production induced by P. intermedia LPS, P. gingivalis LPS, Ra-LPS, or SS-LPP. High (1,000 and/or 100 ng/ml) or low (10 ng/ml) concentrations of repurified P. intermedia and P. gingivalis LPS and unrepurified Ra-LPS and SS-LPS were incubated for 30 min with the indicated doses of polymyxin B. The polymyxin B-LPS mixture was added to INF-γ (20 U/ml)-primed C3H/HeJ (A) or unprimed C3H/HeN (B) macrophages, which were then incubated for 4 h, and then TNF activity in the supernatants was determined by cytotoxicity assay. The bars depict the means ± the standard errors of triplicate cultures. The data are from one of two independent experiments with similar results.
In C3H/HeN macrophage cultures, increasing doses of polymyxin B suppressed the TNF production elicited by both high (100 ng/ml) and low (10 ng/ml) doses of Ra-LPS and SS-LPS (Fig. 7B, left panels), as well as by low doses (10 ng/ml) of P. intermedia LPS and P. gingivalis LPS (Fig. 7B, right panels). On the other hand, polymyxin B did not attenuate the TNF production induced by high doses of P. intermedia LPS and P. gingivalis LPS (100 and 1,000 ng/ml, respectively; Fig. 7B, right panels). Similar results were obtained with IFN-γ-primed C3H/HeJ macrophages, although the magnitude of the polymyxin B-evoked inhibition was somewhat smaller (Fig. 7A). Thus, P. intermedia LPS and P. gingivalis LPS were apparently substantially less sensitive to polymyxin B than were Ra-LPS and SS-LPP.
Lethal toxicity of LPS from oral bacteria in GalNac-loaded C3H/HeN mice.
Since P. gingivalis LPS always appeared less efficacious than P. intermedia LPS, we examined the toxicity of repurified preparations of these LPS in GalN-loaded C3H/HeN mice (8). Consistent with its greater ability to induce TNF production, P. intermedia proved to be more toxic than P. gingivalis; indeed, P. gingivalis LPS was nontoxic (Table 1). Overall, we found the following toxicity order: Ra-LPS > P. intermedia LPS >> P. gingivalis LPP. Groups of C3H/HeN mice (four to eight per group) were simultaneously injected i.p. with GalN (18 mg/mouse) and various doses (0.01 ng to 10 μg/mouse) of repurified LPP. Mortality was scored 72 h after the challenge. The LD50s calculated by the method of Reed and Muench were as follows: S. minnesota Ra-LPS, 1.15 ng; P. intermedia LPS, 14.5 ng; P. gingivalis LPS, >10,000 ng.
DISCUSSION
In an earlier study (34), we used CPC to show that S-form S. abortusequi LPS could be fractionated into SL-LPS having long, S-form O-polysaccharide chains and SS-LPS having short, R-form oligosaccharide chains. SL-LPS and SS-LPS also differed in the ability to induce TNF production in murine macrophage-like J774.1 cells: SS-LPS induced TNF production in serum-free culture medium, whereas SL-LPS required the presence of serum in the culture medium (34).
LPS-refractory C3H/HeJ and C57BL/10ScCr mice, as well as the cells isolated from them, are known to be generally unresponsive to LPS from Enterobacteriaceae (e.g., E. coli and Salmonella), although they are sometimes responsive to R-form LPS (reviewed in reference 25). In addition, macrophages from Mycobacterium bovis BCG-infected C3H/HeJ mice (26) and uninfected C3H/HeJ macrophages cultured in the presence of either IFN-γ (1, 3) or the calcium ionophore A23187 (1, 26) are responsive to LPP. IFN-γ enhances LPS-induced TNF production by augmenting the transcription rate and stability of TNF mRNA (11). Priming of C3H/HeJ macrophages with IFN-γ also appears to facilitate the “decision” of macrophages to respond to LPS.
Manthey and Vogal (19) showed that the ability of LPS to activate LPS-refractory mice and their cells disappeared when the LPS was repurified and contaminating protein was removed. In the present study, although repurification did not affect TNF induction in LPS-responsive C3H/HeN macrophages, it completely blocked the ability of SS-LPS and Ra-LPS to activate IFN-γ-primed, LPS-refractory C3H/HeJ macrophages (Fig. 2A). Combined with the effect of repurification on colloidal gold staining of SDS-PAGE gels (Fig. 1, right panel), these results strongly suggest that a protein associated with SS-LPS and Ra-LPS is necessary for activation of IFN-γ-primed C3H/HeJ macrophages; most likely, the active molecule is a lipid A-associated protein, as described by Manthey and Vogel (19).
LPS from both P. gingivalis and P. fragilis, as well as lipid A-associated protein, are known to activate cultured cells isolated from LPS-refractory C3H/HeJ and C57BL/10ScCr mice (5, 7, 39), and evidence suggests that activation of LPS-refractory C3H/HeJ mice by P. gingivalis LPS is specifically mediated by the lipid A portion of LPS (37). We nonetheless observed that LPS from P. gingivalis and P. intermedia were capable of inducing TNF production in C3H/HeJ and C3H/HeN macrophages even after repurification and, presumably, removal of lipid A-associated protein. The responses were somewhat weakened, however, especially in the case of P. gingivalis LPS (Fig. 3). LPS derived from P. gingivalis possesses chemical constituents different from those derived from Enterobacteriaceae, including the core saccharide region of the LPS (16, 17), as well as the lipid A portion (16, 27). The chemical structure of the lipid A of P. gingivalis is characterized by the absence of ester-linked phosphate at the 4′ position of glucosamine disaccharide and the presence of fatty acids possessing considerable lengths of acyl chains (16, 27). Thus, in contrast to activation by Ra-LPS and SS-LPS, activation of C3H/HeJ macrophages by LPS from P. intermedia and P. gingivalis is apparently elicited by the LPS with unique structures themselves and not by lipid A-associated protein. In other words, the chemical configuration and/or the mode of action of the active entity in P. intermedia and P. gingivalis LPS is quite different from that of the active entity in Ra-LPS and SS-LPP. Furthermore, the fact that Ra-LPS was more potent against C3H/HeN than C3H/HeJ macrophages (Fig. 2) is consistent with the idea that an impurity activates C3H/HeJ macrophages. Conversely, the equipotency of P. intermedia and P. gingivalis LPS against C3H/HeJ and C3H/HeN macrophages is consistent with the idea that the LPS is the stimulant for both cells. These results also support our conclusion that the active molecular part is the lipid A portion of P. intermedia and P. gingivalis LPS and that its mode of action against macrophages is apparently different from those of Ra-LPS.
Unique aspects of the effect of P. intermedia and P. gingivalis LPS on C3H/HeJ macrophages were also made manifest by competitive binding experiments using repurified Ra-LPS, repurified SS-LPS, and RsDPLA. Repurified Ra-LPS and SS-LPS did not antagonize the actions of LPS from P. intermedia and P. gingivalis, although they competed with their nonrepurified counterparts (Fig. 4). Moreover, RsDPLA, which competitively antagonizes LPS receptor binding (10, 13, 14, 18, 31, 36), dose dependently inhibited TNF production elicited by Ra-LPS and SS-LPS, but it did not antagonize the actions of P. intermedia or P. gingivalis LPS (Fig. 5), suggesting that macrophage receptors for P. intermedia and P. gingivalis LPS are different from those for RsDPLA, Ra-LPS, and SS-LPP.
The chemical structure of paclitaxel, which is clinically used as an anticancer drug, is very much unlike that of LPP. Interestingly, paclitaxel induced RsDPLA-sensitive TNF production in C3H/HeN macrophages, yet 3H-labelled paclitaxel binding to macrophages was not inhibited by LPS or RsDPLA (13), which suggests that the receptor for paclitaxel is different from that for LPS but is located very close to the LPS receptor. The findings of the present study are consistent with that notion, since paclitaxel had little or no effect on the actions of Ra-LPS, SS-LPS, P. intermedia LPS, and P. gingivalis LPS (Fig. 6).
Polymyxin B destroys the biological activity of LPS and lipid A isolated from Enterobacteriaceae (e.g., E. coli and Salmonella spp. [24]). We found that pretreating Ra-LPS or SS-LPS with polymyxin B completely blocked their ability to elicit TNF production in either C3H/HeN or C3H/HeJ macrophages. By contrast, LPS from P. intermedia and P. gingivalis were relatively resistant to polymyxin B (Fig. 7). The polysaccharides and fatty acids of P. gingivalis LPS are certainly unlike those of LPS from Enterobacteriaceae (reviewed in reference 5), and this likely underlies their resistance to polymyxin B. In addition, the observation that when LPS was present at higher concentrations it was more resistant to polymyxin B than when it was present at lower concentrations suggests that both polymyxin B-sensitive and polymyxin B-resistant endotoxic molecules are present in P. gingivalis LPS. If so, when a low dose of LPS containing a lesser amount of a polymyxin B-resistant molecule is treated with polymyxin B, the quantity of active endotoxic molecules remaining might not be sufficient to elicit a peak response. On the other hand, at the higher concentration, the number of active molecules may be sufficient to elicit robust responses even in the presence of polymyxin B. This hypothesis, however, remains to be tested.
TNF production induced in C3H/HeN macrophages by oral bacterial LPS was similar to that elicited by Ra-LPS (Fig. 3B). TNF is thought to be one of the major causative factors in endotoxic shock and death (23). However, the lethal toxicities in GalNac-sensitized mice of the LPS examined in this study were not consistent with that picture (see Results). Ra-LPS was toxic, but P. intermedia LPS was substantially less so and P. gingivalis LPS was nontoxic. Given their efficacy with respect to induction of TNF synthesis, we do not know the reason why injection of P. intermedia LPS or P. gingivalis LPS did not cause endotoxic shock. However, shock is a complex phenomenon entailing activation of complement, coagulation, fibrinolytic, and kinin pathways and resulting in release of vasoactive peptides and an array of cytokine mediators, including TNF, interleukin-1 (IL-1), IL-6, IL-8, and nitric oxide from macrophages and other cell types (4, 23). The released mediators, in turn, trigger the characteristic biological effects. Endotoxic shock and death would, therefore, result from the integrated action of all of these mediators rather than that of TNF alone (2).
REFERENCES
- 1.Akagawa K S, Kamoshita K, Onodera S, Tokunaga T. Restoration of lipopolysaccharide-mediated cytotoxic macrophage induction in C3H/HeJ mice by interferon-γ or a calcium ionophore. Jpn J Cancer Res. 1987;78:279–287. [PubMed] [Google Scholar]
- 2.Amura C R, Chen L C, Hirohashi N, Lei M G, Morrison D C. Two functionally independent pathways for lipopolysaccharide-dependent activation of mouse peritoneal macrophages. J Immunol. 1997;159:5079–5083. [PubMed] [Google Scholar]
- 3.Beutler B, Tkacenko V, Milsark I, Krochin N, Cerami A. Effect of γ-interferon on cachectin expression by mononuclear phagocytes: reversal of the Lpsd (endotoxin resistance) phenotype. J Exp Med. 1986;164:1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone R C. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramanti T E, Wong G G, Weintraub S T, Holt S C. Chemical characterization and biologic properties of lipopolysaccharide from Bacteroides gingivalis strains W50, W83, and ATCC 33277. Oral Microbiol Immunol. 1989;4:183–192. doi: 10.1111/j.1399-302x.1989.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 6.Ding A H, Porteu F, Sanchez E, Nathan C F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara T, Ogawa T, Sobue S, Hamada S. Chemical, immunobiological and antigenic characterizations of lipopolysaccharides from Bacteroides gingivalis strains. J Gen Microbiol. 1990;136:319–326. doi: 10.1099/00221287-136-2-319. [DOI] [PubMed] [Google Scholar]
- 8.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;75:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanos G, Lüderitz O, Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 10.Golenbock D T, Hampton R Y, Qureshi N, Takayama K, Raetz C R H. Lipid A-like molecules that antagonize the effects of endotoxinson human monocytes. J Biol Chem. 1991;266:19490–19489. [PubMed] [Google Scholar]
- 11.Hayes M P, Freemann S L, Donnelly R P. IFN-γ priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and mRNA stability. Cytokine. 1995;7:427–435. doi: 10.1006/cyto.1995.0058. [DOI] [PubMed] [Google Scholar]
- 12.Joiner K A, McAdam K P W, Kasper D L. Lipopolysaccharides from Bacteroides fragilis are mitogenic for spleen cells from endotoxin responder and nonresponder mice. Infect Immun. 1982;36:1139–1145. doi: 10.1128/iai.36.3.1139-1145.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirikae F, Kirikae T, Qureshi N, Takayama K, Morrison D C, Nakano M. CD14 is not involved in Rhodobacter sphaeroides diphosphoryl lipid A inhibition of tumor necrosis factor alpha and nitric oxide induction by taxol in murine macrophages. Infect Immun. 1995;63:486–497. doi: 10.1128/iai.63.2.486-497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirikae T, Shade F U, Zahringer U, Kirikae F, Brade H, Kusumoto S, Kusama T, Rietschel E T. The significance of the hydrophilic backbone and the hydrophobic fatty acid regions of lipid A for macrophage binding and the cytokine induction. FEMS Immunol Med Microbiol. 1994;8:13–16. doi: 10.1111/j.1574-695X.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumada H, Haishima Y, Umemoto T, Tanamoto K. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol. 1995;177:2098–2106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumada H, Kondo S, Umemoto T, Hisatsune K. Chemical structure of the 2-keto-3-deoxyoctonate region of lipopolysaccharide isolated from Porphyromonas (Bacteroides) gingivalis. FEMS Microbiol Lett. 1993;108:75–79. doi: 10.1111/j.1574-6968.1993.tb06076.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumada H, Watanabe K, Umemoto T, Haishima Y, Kondo S, Hisatsune K. Occurrence of O-phosporylated 2-keto-3-deoxyoctonate in the lipopolysaccharide of Bacteroides gingivalis. FEMS Microbiol Lett. 1988;51:77–80. [Google Scholar]
- 18.Lynn W A, Golenbock D T. Lipopolysaccharide antagonists. Immunol Today. 1992;13:271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 19.Manthey C L, Vogel S N. Elimination of trace endotoxin protein from rough chemotype LPS. J Endotoxin Res. 1994;1:84–91. [Google Scholar]
- 20.Manthey C L, Vogel S N. Taxol: a promising endotoxin research tool. J Endotoxin Res. 1994;1:189–198. [Google Scholar]
- 21.Moreno E, Berman D T. Brucella abortus lipopolysaccharide is mitogenic for spleen cells of endotoxin resistant C3H/HeJ mice. J Immunol. 1979;123:2915–2919. [PubMed] [Google Scholar]
- 22.Morrison D C, Betz S J, Jacobs D M. Isolation of a lipid A bound polypeptide responsible for ‘LPS-initiated’ mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976;144:840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison D C, Danner R L, Dinarello C A, Munford R S, Natanson C, Pollack M, Spitzer J J, Ulevitch R J, Vogel S N, McSweegan E. Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future direction. J Endotoxin Res. 1994;1:71–83. [Google Scholar]
- 24.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacteria LPS. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 25.Nakano M, Shinomiya H. The Lps mutational defect in C3H/HeJ mice. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. 1. Molecular biochemistry and cellular biology. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 311–328. [Google Scholar]
- 26.Nakano M, Yanasu H, Terada Y, Shinomiya H, Saito S. LPS-induced protein phosphorylation and monokine production in calcium ionophare-stimulated or BCG-infected C3H/HeJ macrophages. In: Levin J, Alving C R, Munford R S, Stütz P L, editors. Bacterial endotoxin: recognition and effector mechanisms. Amsterdam, The Netherlands: Excerpta Medica; 1993. pp. 293–304. [Google Scholar]
- 27.Ogawa T. Chemical structure of lipid A from Porphyromonas (Bacteroides) gingivalis lipopolysaccharide. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa T. Immunobiological properties of chemically defined lipid A from lipopolysaccharide of Porphyromonas (Bacteroides) gingivalis. Eur J Biochem. 1994;219:737–742. doi: 10.1111/j.1432-1033.1994.tb18552.x. [DOI] [PubMed] [Google Scholar]
- 29.Pier G B, Markham R B, Eardley D. Correlation of the biological responses of C3H/HeJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981;127:184–191. [PubMed] [Google Scholar]
- 30.Poltrak A, Xiaolong H, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castafnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi N, Takayama K, Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991;59:441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi N, Takayama K, Meyers K C, Kirkland T N, Bush C A, Chen L, Wang R, Cotter R J. Chemical reduction of 3-oxo and unsaturated groups in fatty acids of diphosporyl lipid A from lipopolysaccharide of Rhodopseudomonas sphaeroides. Comparison of biological properties before and after reduction. J Biol Chem. 1991;266:6532–6538. [PubMed] [Google Scholar]
- 33.Spellman J M, Reed N D. Immune and mitogenic responses by BALB/c, C3H/HeJ, and nude mice to Brucella abortus bacteria and lipopolysaccharide. Infect Immun. 1979;24:371–378. doi: 10.1128/iai.24.2.371-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suda Y, Kirikae T, Shiyama T, Yasukochi T, Kirikae F, Nakano M, Rietschel E T, Kusumoto S. Macrophage activation in response to S-form lipopolysaccharides (LPS) separated by centrifugal partition chromatography from wild-type LPS: effects of the O-polysaccharide portion of LPP. Biochem Biophys Res Commun. 1995;210:678–685. doi: 10.1006/bbrc.1995.1713. [DOI] [PubMed] [Google Scholar]
- 35.Sultzer B M, Goodman G W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976;144:821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takayama K, Qureshi N, Beutler B, Kirkland T N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989;57:1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanamoto K, Azumi S, Haishima Y, Kumada H, Umemoto T. The lipid A moiety of Porphyromonas gingivalis lipopolysaccharide specifically mediates the activation of C3H/HeJ mice. J Immunol. 1997;158:4430–4436. [PubMed] [Google Scholar]
- 38.Tanner A. Microbial succession in the development of periodontal disease. In: Hamada S, Holt S C, McGhee J R, editors. Periodontal disease: pathogens and host immune responses. Tokyo, Japan: Quintessence; 1991. pp. 13–25. [Google Scholar]
- 39.Wannemuehler M J, Michalek S M, Jirillo M, Williamson S I, Hirasawa M, McGhee J R. LPS regulation of the immune responses: Bacteroides endotoxin induces mitogenic, polyclonal, and antibody responses in classical LPS responsive but not C3H/HeJ mice. J Immunol. 1984;133:299–305. [PubMed] [Google Scholar]
- 40.Westphal O, Lüderitz O. Chemische Erforschung von Lipopolysacchariden Gram negativer Bakterien. Angew Chem. 1954;66:407–417. [Google Scholar]
- 41.Zambon J J, Grossi S, Dunford R, Haraszthy V I, Preus H, Genco R J. Epidemiology of subgingival bacterial pathogens in periodontal disease. In: Genco R J, Hamada S, Lehner T, McGhee J, Mergenhagen S, editors. Molecular pathogenesis of periodontal disease. Washington, D.C: American Society for Microbiology; 1994. pp. 3–12. [Google Scholar]