Abstract
Background
Hydrogen and methane breath tests (HMBT) are widely used clinical investigations but lack standardization. To address this, the North American Consensus (NAC) group published evidence-based recommendations for HMBT.
Aims
To evaluate results obtained using NAC recommendations for HMBT, compared to retrospective data that utilized guidelines previously recommended.
Methods
HMBT data from 725 patients referred for small intestinal bacterial overgrowth (SIBO) and/or carbohydrate malabsorption (CM) testing were analyzed. Data were compared regarding dose of substrate for SIBO testing (16 vs. 10 g lactulose, and 50 vs. 75 g glucose) and the effect of post-ingestion sampling period for malabsorption testing. The effect of different recommended cut-off values for SIBO were examined.
Results
Substrate dose did not affect methane production. 10 g lactulose significantly reduced positive SIBO results compared to 16 g lactulose (42 vs. 53%, p = 0.04). 75 g glucose significantly increased positive results compared to 50 g glucose (36 vs. 22%, p = 0.04). Provoked symptoms were significantly more prevalent in patients testing positive by both North American Consensus and Ledochowski cut-off values.
34.5% of patients tested positive for CM at 180-min compared to 28% at 120-min (not significant, p = 0.19).
Conclusions and Inferences
10 g lactulose substrate produces fewer positive SIBO results than 16 g lactulose, while 75 g glucose dose produces more positive SIBO results than 50 g. Performing CM breath tests for 180 min increases number of positive results when compared to 120 min. SIBO cut-off timings require further investigation, but our findings broadly support the NAC recommendations for SIBO and CM testing.
Keywords: Breath tests, Microbiome, Lactulose, Small intestine, Fermentation
Introduction
Unexplained gastrointestinal (GI) symptoms are extremely common in the general population with up to 47% of females fulfilling the symptom-based Rome IV criteria for one or functional gastrointestinal disorder [1], contributing to poor quality of life for patients and economic burden to society [2–4]. Despite this high prevalence, diagnostic and treatment pathways remain sub-optimal and the majority of prescribed treatments are based on the alleviation of symptoms rather than addressing their underlying cause [5, 6].
One proposed cause of persistent GI symptoms is small intestinal bacterial overgrowth (SIBO). The current gold standard investigation for SIBO is microbial culture of a jejunal aspirate, obtained at enteroscopy [7]. This investigation is invasive, expensive, time consuming, and not widely performed in clinical practice [8, 9]. In addition, the investigation poses risks of both false positives, through contamination by oral bacteria or saliva, and false negatives, through irregular distribution of bacteria through the bowel causing aspiration of a non-representative sample or through aspiration of cultivation-resistant species [7, 10]. Consequentially, the use of hydrogen and methane breath tests for the assessment of SIBO are becoming increasingly popular in clinical practice [11].
HMBT is based on the observation that hydrogen and methane gases expelled in human breath is derived from intestinal bacteria as a product of fermentation of undigested carbohydrates. The pattern of excreted breath hydrogen can therefore give an indication of bacterial fermentation in response to oral carbohydrate load [12]. HMBT is a tertiary investigation used in patients that have often had symptoms for many years and undergone many other, clinical investigations [13] and offers a simple and non-invasive alternative to small bowel aspirate. However, HMBT are subject to significant criticism [2, 14], for instance, the sensitivity and specificity of these tests is difficult to assess due to methodological issues, differences in substrate (and dose) used, restrictions applied before and during the test, and interpretation of the results [10, 15]. A second use of HMBT is to assess carbohydrate mal-absorption (CM) most commonly lactose and fructose substrates [16].
The 2017 North American Consensus for hydrogen and methane breath testing has sought to address some of these issues [17]. The consensus report, based on evidence and expert opinion, proposed a series of 26 statements with regards to the performance of HMBT, including indications for testing, preparation, performance, interpretation of results, and remaining gaps in knowledge.
Following publication of The North American Consensus, we adapted our practice in line with these recommendations and the aim of this study was to objectively assess the impact of these changes on patients results and symptoms during the investigation before and after this change.
Materials and Methods
Study Participants
Study participants were outpatients referred to The Functional Gut Clinic for hydrogen and methane breath testing for investigation of unexplained GI symptoms including but not limited to bloating, abdominal pain, and change to bowel habit, in the absence of organic pathology to explain these symptoms. Patients attending for small intestinal bacterial overgrowth (SIBO) testing and/or CM testing between 2014 and 2017 were included. Patients were included following referral by gastroenterologist or GI surgeon for HMBT if they were aged over 18, and had adhered to the preparations required for the test, including a strict pre-study low fermentable diet and 12 h fast, cessation of antibiotics 4 weeks prior to the test, cessation of probiotics, laxatives/stool softeners, stool bulking agents and motility agents for at least one week prior to testing, and no tests which require cleansing of the bowel e.g. colonoscopy or barium enema for at least one week prior to the test. Proton Pump Inhibitor (PPI) use was not restricted. Patients with high baseline hydrogen values (> 20 ppm), suggestive of failure to adhere to the pre-study diet, were excluded from analysis.
Hydrogen and Methane Breath Test Protocol
725 patients were included in the study. All patients were asked to adhere to a low fermentable diet in the 24 h prior to HMBT and a 12 h fast. Patients provided a baseline end-expiratory breath sample before ingesting the sugar substrate dissolved in 200 ml of water. Subsequent end-expiratory breath samples were taken at regular intervals for 2–3 h following ingestion.
Samples were collected in foil bags and analyzed using desktop Bedfont GastroCH4ECK® Gastrolyzer® (Bedfont Scientific, Kent, UK). Patients were also given a symptom sheet to record symptoms of bloating, abdominal pain, and nausea on a visual analogue scale (VAS) of 0–10 with each breath sample (0 = no symptoms, 10 = worse possible symptoms). All patients were asked to avoid eating, drinking, smoking, exercising and sleeping during the breath test.
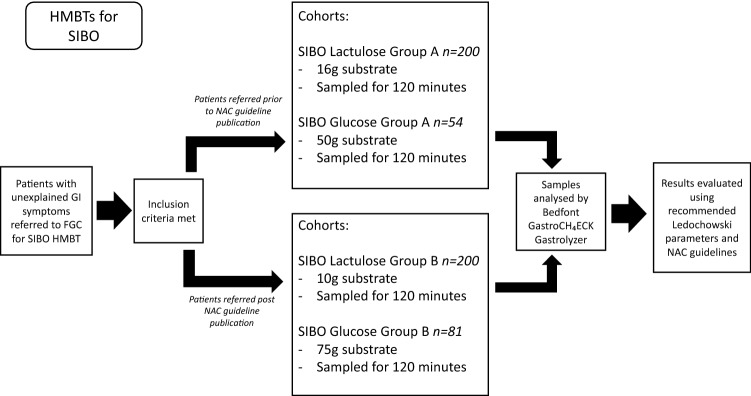
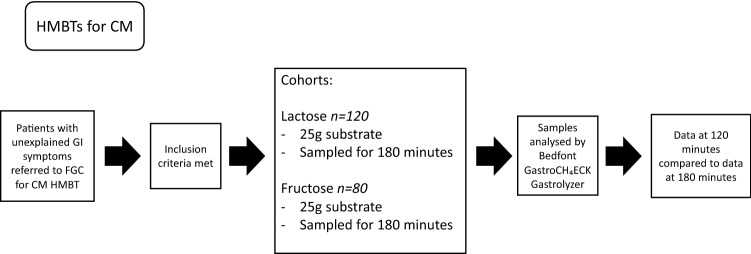
Sugar substrate quantities and duration of test were changed as part of an update in breath test protocol in June 2017 following the publication of the North American Consensus document [17]. Prior to June 2017, 50 g glucose or 16 g lactulose was administered for a SIBO test, which was changed to 75 g glucose and 10 g lactulose post-June 2017 (Fig. 1). Prior to June 2017, the duration of lactose and fructose malabsorption tests was 120 min, this was extended to 180 min (Fig. 2). Patients were grouped into cohorts based on their sugar substrate and dose.
Fig. 1.
Flowchart to demonstrate the methods process for analysis of SIBO glucose and lactulose breath test data, from subject selection, analysis of samples, and interpretation of results
Fig. 2.
Flowchart to demonstrate the methods process for analysis of lactose and fructose carbohydrate malabsorption breath test data, from subject selection, analysis of samples, and interpretation of results
Cohorts
SIBO Test Cohorts
SIBO Lactulose A – 16 g Lactulose group. Patients performing SIBO Lactulose breath test prior to June 2017 ingested 16 g lactulose (in 200 ml water). Samples were taken at 20-min intervals for 120 min.
SIBO Lactulose B – 10 g Lactulose group. Patients performing SIBO Lactulose breath test post June 2017 ingested 10 g lactulose (in 200 ml water). Samples were taken at 20-min intervals for 120 min.
SIBO Glucose A – 50 g Glucose group. Patients performing SIBO Glucose breath test prior to June 2017 ingested 50 g glucose (in 200 ml water). Samples were taken at 20-min intervals for 120 min.
SIBO Glucose B – 75 g Glucose group. Patients performing SIBO Glucose breath test post June 2017 ingested 75 g glucose (in 200 ml water). Samples were taken at 20-min intervals for 120 min.
Carbohydrate Malabsorption Test Cohorts
Lactose A – Patients performing lactose breath testing prior to June 2017 ingested 25g lactose (in 200ml water). Diagnosis of SIBO had previously been excluded with glucose or lactulose breath test. Samples were taken at 20-minute intervals for 120 minutes.
Lactose B – Patients performing lactose breath testing post June 2017 ingested 25g lactose (in 200ml water). Diagnosis of SIBO had previously been excluded with glucose or lactulose breath test. Samples were taken at 20-minute intervals for 180 minutes.
Fructose A – Patients performing fructose breath testing prior to June 2017 ingested 25g fructose (in 200ml water). Diagnosis of SIBO had previously been excluded with glucose or lactulose breath test. Samples were taken at 20-minute intervals for 120 minutes.
Fructose B - Patients performing fructose breath testing post June 2017 ingested 25g fructose (in 200ml water). Diagnosis of SIBO had previously been excluded with glucose or lactulose breath test. Samples were taken at 20-minute intervals for 180 minutes.
Analysis of Results
Breath samples were analyzed by Bedfont GastroCH4ECK® Gastrolyzer® (Bedfont Scientific, Kent UK), which recommends the use of a rise of ≥ 10 ppm hydrogen from baseline within 60 min of ingestion to inform a positive result based on parameters outlined by Ledochowski 2008 [18]. Therefore, these parameters primarily were used to diagnose SIBO in this study, however a rise of > 20 ppm hydrogen within 90 min of substrate ingestion was also noted for comparison to NAC recommended diagnostic criteria. The presence of ≥ 10 ppm methane at any point during the test was considered positive for presence of methane.
A positive result for lactose or fructose malabsorption was determined following a rise of > 20 ppm hydrogen or methane from baseline at any point post-ingestion of substrate [17].
Categorical variables were expressed as percentages. Unpaired t-tests, chi-square and Fisher’s exact tests were used to compare groups. P < 0.05 was adopted as the criterion for statistical significance and all analyses were performed using proprietary software (GraphPad Prism, Version 7, La Jolla, California, USA).
Results
Lactulose Breath Tests
Of 200 patients administered 16 g lactulose as part of a hydrogen and methane breath test (Cohort A), 106 patients (53%) demonstrated a rise of ≥ 10 ppm hydrogen within the first 60 min post-ingestion, suggestive of a positive result for SIBO. Of 200 patients administered 10 g lactulose as substrate for hydrogen and methane breath test (Cohort B), as recommended by the North American Consensus, 84 patients (42%) tested positive for SIBO (Fig. 3). Significantly more patients tested positive with 16 g lactulose compared to 10 g (OR: 0.6422, 95% CI 0.44–0.96, p = 0.04).
Fig. 3.
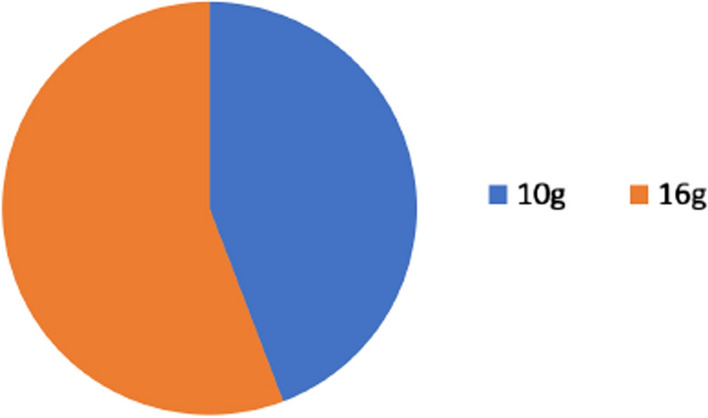
Higher dose (16 g) of lactulose substrate in hydrogen and methane breath test produced more positive SIBO results than lower dose (10 g)
No difference in the presence of methane was seen between dose groups, as both 10 g and 16 g lactulose dose groups saw a prevalence of 15.5% (31/200 patients).
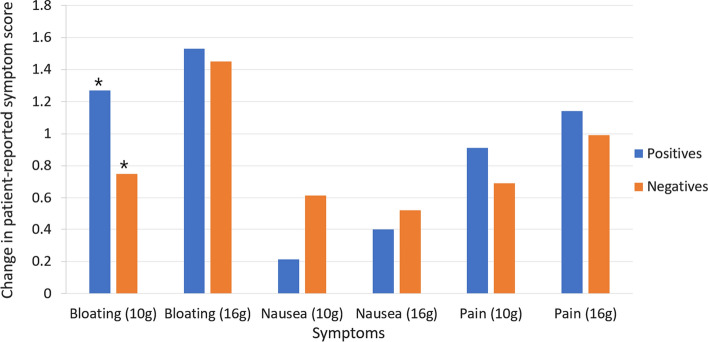
Between lactulose dose groups, there was no significant difference in number of patients experiencing an increase in bloating, pain, or nausea during the test. However, the average increase in patient perception of bloating in Cohort B was 1, while Cohort A substrate was 1.5 (p = 0.04). A significant difference in symptoms of pain and nausea severity between dose groups was not seen.
A difference in bloating severity was seen between patients testing positive for SIBO and those testing negative for SIBO in Cohort B (10 g lactulose), but not in Cohort A (16 g lactulose) (Fig. 4). Patients testing positive for SIBO following 10 g lactulose had an average increase of 1.27 in patient perception of bloating during the test, while those with a negative result in the same dose group had an average increase of 0.75 in bloating (p = 0.047).
Fig. 4.
Mean increase in patient-reported symptoms during lactulose breath test, in both 10 g (post-consensus) and 16 g (pre-consensus) substrate groups. Blue indicates patients testing positive for SIBO or methane while orange indicates patients testing negative for both SIBO and methane. * = p < 0.05
In this study, patients were considered positive for SIBO following a rise of ≥ 10 ppm hydrogen within the first 60 min post-ingestion of lactulose (Ledochowski parameters). In cohort A, patients administered 16 g lactulose, 106 patients tested positive by Ledochowski parameters, while 108 patients tested positive by NAC parameters of a rise of ≥ 20 ppm hydrogen from baseline within 90 min post-ingestion. Analysis of results using the two different guidelines lead to contradicting interpretations of results in 30 patients (X2(1, N = 200) 97.63, p < 0.001). Of patients testing positive by one guideline only (by NAC guidelines or by Ledochowski guidelines) 53.3% of patients had a rise in reported symptoms concurrent with rise in gas production. Of patients testing positive by both NAC and Ledochowski guidelines, 68.5% had concurrent rise in patient reported symptoms (p = 0.13). Following 16 g lactulose, significantly more patients with positive results by both criteria reported an increase in symptoms during the test than patients with a negative result by both criteria (68.5% compared to 50% p = 0.014). No significant difference in number of patients reporting increased symptoms was seen between patients with a negative result and those with a positive result by one guideline only (50% compared to 53%, p = 0.756).
In cohort B, following 10 g lactulose 84 out of 200 patients tested positive by Ledochowski criteria, however when following NAC criteria 113 patients tested positive for SIBO. Contradictory interpretation of results between the two guidelines was seen in 49 patients (X2 (1, N = 200) 97.63, p < 0.001). Of patients testing positive by one guideline only (by NAC guidelines or by Ledochowski guidelines) 42.8% of patients had a rise in reported symptoms concurrent with rise in gas production. Of patients testing positive by both NAC and Ledochowski guidelines, 74.3% had concurrent rise in patient reported symptoms (p < 0.001). Following 10 g lactulose, significantly more patients with positive results for SIBO by both NAC and Ledochowski criteria reported an increase in symptoms during the test when compared to patients with a negative result by both criteria (74.3% compared to 41.6%, p < 0.001). No significant difference in number of patients reporting increased symptoms was seen between patients with a negative result and those with a positive result by one guideline only (41.6% compared to 42.9%, p = 0.886).
Glucose Breath Tests
Of 81 patients administered 75 g glucose as part of a hydrogen and methane breath test (Cohort B), as recommended by the North American Consensus, 29 patients (36%) demonstrated a rise of ≥ 10 ppm hydrogen within the first 60 min post-ingestion, indicative of a positive result for SIBO. Of 54 patients administered 50 g glucose as substrate for hydrogen and methane breath test (Cohort A), 12 patients (22%) tested positive for SIBO. This difference was significant (OR: 0.5093, 95% CI 0.27–0.97, p = 0.04)).
The presence of methane was not significantly different between dose groups. Out of 54 patients administered 50 g glucose, 1 patient tested positive for methane, and out of 81 patients administered 75 g glucose, 7 tested positive for methane (p > 0.05).
Between glucose dose groups, there was no significant difference in number of patients reporting an increase in symptoms during the test (p > 0.05). Increase in symptoms during the test was reported by significantly more patients with a positive result (61.9%) than patients with a negative result (43.9%), p = 0.048.
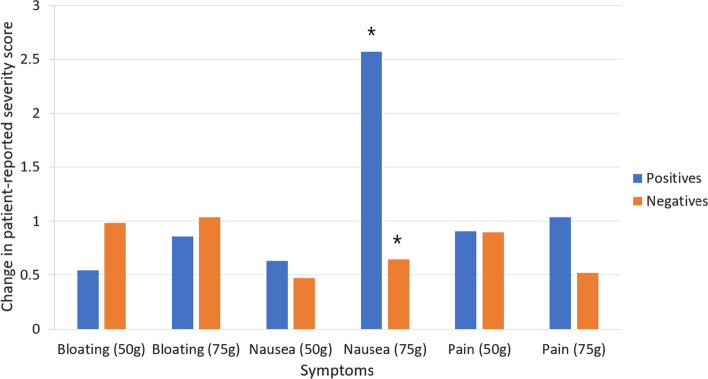
During the length of the test, the average increase in severity of abdominal pain and bloating was not significantly different between glucose dose groups. Average change in nausea severity during the test was significantly higher (2.57) in patients in Cohort B (75 g) when compared to Cohort A (50 g) dose group (average increase in nausea 0.64) (Fig. 5), however this effect was only seen in patients testing positive for SIBO (p = 0.007). No significant difference in change in nausea severity was seen between dose groups in those testing negative for SIBO.
Fig. 5.
Mean increase in patient-reported symptoms during glucose breath test in both 50 g (pre-consensus) and 75 g (post-consensus) substrate groups. Blue indicates patients testing positive for SIBO or methane, while orange indicates patients testing negative for SIBO and methane. * = p < 0.05
Carbohydrate Malabsorption Breath Tests
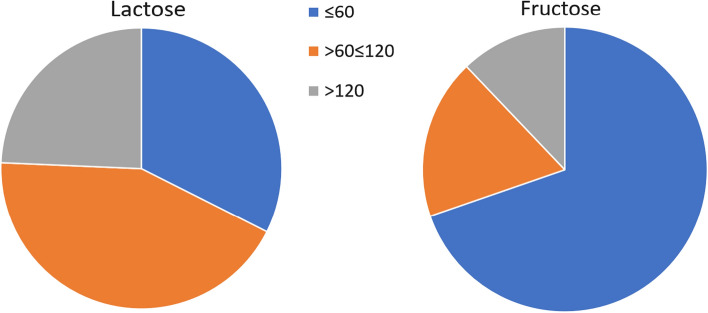
Of 120 patients tested for lactose malabsorption, 22.5% tested positive for malabsorption at 120 min. When extended to 180 min, as recommended by the North American Consensus, 30% of patients tested positive for lactose malabsorption (p > 0.05). Of the patients testing positive for lactose malabsorption, 34.3% demonstrated a significant rise in gases (> 20 ppm) within 60 min of ingestion.
Of 80 patients taking a hydrogen and methane breath test for fructose malabsorption, 36.3% of patients tested positive for malabsorption at 120 min. When extended to 180 min, as recommended by the North American Consensus, 41.3% of patients tested positive for fructose malabsorption (p > 0.05). Of the patients testing positive for fructose malabsorption, 69.8% demonstrated a significant rise of gases (> 20 ppm) within 60 min of ingestion.
Time at which a rise of > 20 ppm hydrogen was demonstrated in lactose or fructose testing is demonstrated in Fig. 6.
Fig. 6.
Time of significant rise in hydrogen gas (> 20 ppm) in patients testing positive for carbohydrate malabsorption
Discussion
The sensitivity and specificity of HMBTs in assessing SIBO and carbohydrate malabsorption is variably reported due to methodological issues and large differences in substrate dose used, restrictions followed prior to the test, and duration of sampling [10, 12]. The North American Consensus 2017 attempted to standardize the process of breath testing and reduce heterogeneity in practice between centers [17]. This study assessed the effects of changing clinical practice in line with North American Consensus guidelines and reviewed its impact on patient diagnosis.
We found significantly fewer positive results for SIBO were reported with a lactulose dose of 10 g as recommended by NAC, when compared with a higher dose of 16 g. As lactulose has been shown to decrease small bowel transit time [19] it is possible that a larger lactulose dose accelerates transit resulting in false positive results for SIBO as normal colonic fermentation was falsely attributed to the small intestine. The higher proportion of patients testing positive for SIBO following 16 g lactulose dose could be therefore due to false positives, and by reducing the dose to 10 g the risk of a false positive result may be reduced. The presence of methane was not affected by substrate dose, suggesting that presence of methane is independent of lactulose dose, and in fact a single fasting baseline breath measurement for methane may be sufficient to obtain a clinically useful assessment of methanogenesis.
Analysis of symptoms during the study suggests that 10 g dose of lactulose invokes a lower severity of symptoms during the test thus making the test more comfortable for patients, as increase in severity of bloating experienced by patients following 10 g lactulose was significantly less than with 16 g. When looking at patients with positive results only, there was no significant difference in symptoms in patients between substrate groups, suggesting that patients testing positive for SIBO are no more likely to experience symptoms whether they were administered 10 g or 16 g.
The results also demonstrated a significant difference in bloating severity between positive and negative patients administered 10 g lactulose, with patients with positive results recording a higher increase in severity. The same effect is not seen with the 16 g group, suggesting the increased measure of lactulose could provoke SIBO-like symptoms in those testing negative. Therefore, a 10 g lactulose substrate as part of a SIBO breath test is the preferred dose, as recommended by the NAC, due to reduction in likelihood of false positives and severity of symptoms in patients. In addition, lactulose is readily available in oral solution sachets of 10 g promoting ease of use of tests with this substrate dose.
Significantly more patients were diagnosed with SIBO when 75 g of glucose was used as test substrate compared to 50 g. As glucose is absorbed in the proximal small bowel, it is not fermented by colonic bacteria therefore these are likely to be true positives. It has been suggested a dose of 50 g glucose provides an underestimation of SIBO, as availability of substrate for bacterial fermentation in the distal small bowel is reduced [15]. Therefore, increasing glucose dose in this study is thought to improve sensitivity of SIBO diagnosis. The presence of methane was not affected by substrate dose, suggesting that presence of methane is independent of glucose dose. The number of patients reporting an increase in symptoms during the test did not increase with the glucose dose, suggesting that 75 g glucose provides improved diagnostic sensitivity without excessively amplifying patient symptoms. Although an increase in average severity of nausea was seen with the higher glucose dose this was only seen in patients with a positive result, suggesting that this was a symptom of underlying bacterial overgrowth. This significant increase in nausea in SIBO-positive patients only may suggest nausea to be a more important symptom in assessing SIBO, the presence of which during a SIBO breath test may improve diagnostic sensitivity. Symptoms experienced by patients with negative results were not different between dose groups suggesting that, unlike with lactulose, a larger substrate dose does not induce overgrowth-like symptoms in those testing negative for SIBO. Therefore, an increased dose of 75 g glucose is recommended for a SIBO breath test, in agreement with the North American Consensus, to improve diagnostic sensitivity without provoking significant symptoms. In addition, glucose is readily available as 75 g dose, as this is commonly used in oral glucose tolerance testing for diabetics [20].
Extending the duration of a CM test to 180 min demonstrated that around 20% of positive results for CM occurred after 120 min. These patients would have been given a falsely negative diagnosis for CM with a shorter test. Therefore, this data supports the North American Consensus position in extending malabsorption tests to 180 min, to avoid false negative studies. The data also demonstrates a high proportion of positive results occurring within 60 min of ingestion. At this point, a rise in gas in response to carbohydrate could be caused by fermentation within the small intestine and could be falsely attributed to CM when SIBO is the true cause [14]. Therefore, the data supports the need for a SIBO breath test with lactulose or glucose substrate prior to carbohydrate malabsorption to avoid false positive results.
In the North American Consensus document, the parameters used for a positive SIBO result for both lactulose and glucose were set at a rise in hydrogen of ≥ 20 ppm above baseline within 90 min of ingestion. The Association of Gastrointestinal Physiologists (AGIP) recommend the use of lactulose over glucose as a first line assessment as it provides a full bowel assessment [21], however there are concerns that a 90-min cut-off may increase the incidence of false positive results due to lactulose arriving in the proximal colon within 90 min. Conversely, a more conservative measure of a rise of ≥ 10 ppm hydrogen within 60 min, as used in this study and in accordance with Ledochowski guidelines, may increase false negative results. In this study, a significant number of patients had contradicting diagnoses when interpreted with the two contrasting guidelines. When considering patient-reported symptoms during 10 g lactulose breath test, a correlation with gas production was seen in significantly more patients testing positive by both guidelines than those testing negative or those testing positive by one guideline only. A reproduction in typical symptoms following ingestion of substrate in line with an increase in gas production is considered support for a positive SIBO diagnosis, as patient symptoms are demonstrated to correlate with increased fermentation [22–24]. As a significantly higher rate of symptom correlation is demonstrated in patients testing positive by NAC and Ledochowski parameters combined, these results suggest that a positive SIBO result considered by a rise of > 10 ppm hydrogen within 60 min, and a rise of > 20 ppm within 90 min of ingestion, may be most clinically relevant. This combined approach may reduce both false positives and false negatives in response to lactulose tests, and allows for use of clinical judgement and review of patient-reported symptoms in borderline cases.
The use of different cut-off values in interpretation of SIBO breath tests will become increasingly validated as more data emerge regarding treatment outcomes following SIBO diagnosis, or further study of breath testing with co-existing imaging using tagged substrate to establish optimum cut off values. In the meantime, clinical judgement by an experienced clinician and/or within a multi-disciplinary team can reduce the risk of false positive results and reduce the consequential risk of inappropriate antibiotic use in the instance of borderline positive results. Glucose HMBT could also be undertaken following borderline lactulose HMBT to provide more confidence in a SIBO diagnosis.
This study is limited by the absence of a suitable gold standard investigation for the diagnosis of SIBO to confirm or deny SIBO in those with a positive breath test. Culture of jejunal aspirate is considered the gold standard, however this is highly invasive and not considered appropriate in clinical practice. In addition, the techniques are not standardized, and risk of both false positives, through contamination by oral flora, or false negatives, through difficulty aspirating a sufficient and representative sample, is high and can lead to large over- or under-estimation of SIBO [10, 12, 25, 26]. In the absence of a suitable gold standard investigation to serve as a reliable comparator, the most appropriate criteria for interpretation of SIBO breath testing cannot be reliably determined.
A further limitation of this study is the exclusion of hydrogen sulfide analysis in breath samples. There are several species of bacteria that can reside in the colon and small intestine and sequester H2 produced through fermentation to produce H2S gas [27, 28]. Many IBS patients may have SIBO caused by sulfate reducing bacteria as opposed to hydrogen and methane producing species, and thus measuring hydrogen and methane alone could underestimate the true prevalence of SIBO [29]. At present, the assessment of breath HsS as a biomarker for intestinal sulfate-reducing bacteria is not routinely utilized in the assessment of SIBO [30].
The findings of this study broadly support the parameters outlined in the NAC document for hydrogen and methane breath testing. The findings support the recommended approach for breath testing, including substrate dose and study length. However, the conclusions of this study question the recommended interpretation of SIBO breath testing, as combining NAC-recommended diagnostic parameters with Ledochowski parameters may optimize sensitivity and specificity. This study also highlights the importance of measuring patient-reported abdominal symptoms during HMBT to support a positive diagnosis and to offer clarity in cases of borderline positive results. The North American Consensus represents a positive first step in standardizing breath test diagnosis, which aims to improve accuracy and reliability of breath testing across all centers.
Declarations
Conflicts of interests
Charlotte K Pitcher, Jordan J Haworth BSc, Sam Treadway MSc and Anthony R Hobson PhD are employees and/or shareholders of Functional Gut Diagnostics Ltd and/or The Functional Gut Clinic. Adam D Farmer declares that he has no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lam CY, Palsson OS, Whitehead WE, Sperber AD, Tornblom H, Simren M, et al. Rome IV Functional Gastrointestinal Disorders and Health Impairment in Subjects With Hypermobility Spectrum Disorders or Hypermobile Ehlers-Danlos Syndrome. Clin Gastroenterol Hepatol. 2020;19:227. doi: 10.1016/j.cgh.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, Vanner S , et al. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simrén M, Svedlund J, Posserud I, Björnsson ES, Abrahamsson H. Health-related quality of life in patients attending a gastroenterology outpatient clinic: Functional disorders versus organic diseases. Clin Gastroenterol Hepatol. 2006;4:187–195. doi: 10.1016/S1542-3565(05)00981-X. [DOI] [PubMed] [Google Scholar]
- 4.Tack J, Stanghellini V, Mearin F, Yiannakou Y, Layer P, Coffin B, et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterology. 2019 doi: 10.1186/s12876-019-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou S, Liu X, Wang X, Xi F, Luo X, Yao L, et al. Pharmacological and non-pharmacological treatments for irritable bowel syndrome: Protocol for a systematic review and network meta-analysis. Medicine (Baltimore). 2019;98(30):e16446. doi: 10.1097/MD.0000000000016446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99. doi: 10.3390/jcm6110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16(24):2978–2990. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: Pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013;4:223–231. doi: 10.1177/2040622313496126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimentel M, Saad RJ, Long MD, Rao SS. ACG clinical guideline : Small intestinal bacterial overgrowth. Am J Gastroenterol. 2020;4:1–14. doi: 10.14309/ajg.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 10.Erdogan A, Rao SSC, Gulley D, Jacobs C, Lee YY, Badger C. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;27(4):481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- 11.Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol [Internet]. 2014;20:2482–2491. doi: 10.3748/wjg.v20.i10.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Stefano M, Quigley EMM. The diagnosis of small intestinal bacterial overgrowth: Two steps forward, one step backwards? Neurogastroenterol Motil. 2018;30:e13494. doi: 10.1111/nmo.13494. [DOI] [PubMed] [Google Scholar]
- 13.Black CJ, Ford AC. Rational investigations in irritable bowel syndrome. Vol. 11. Frontline Gastroenterology. BMJ Publishing Group. 2019;11:140–7. doi: 10.1136/flgastro-2019-101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simrén M, Stotzer P-O. Use and abuse of hydrogen breath tests. Gut. 2006;55(3):297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdogan A, Rao SSC, Gulley D, Jacobs C, Lee YY, Badger C. Possible underestimation of SIBO in IBS patients: is lack of Glucose Breath Test standardization responsible? Neurogastroenterol Motil. 2015;27(8):1192–1193. doi: 10.1111/nmo.12603. [DOI] [PubMed] [Google Scholar]
- 16.Goebel-Stengel M, Stengel A, Schmidtmann M, van der Voort I, Kobelt P, Mönnikes H. Unclear abdominal discomfort: Pivotal Role of carbohydrate malabsorption. J Neurogastroenterol Motil. 2014;20(2):228. doi: 10.5056/jnm.2014.20.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezaie A, Buresi M, Lembo A, Lin H, McCallum R, Rao S, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American consensus. Am J Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledochowski M, Ledochowski E, Eisenmann A. Hydrogen breath tests. 1st ed. 2008;1–66
- 19.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Association of Gastrointestinal Physiologists. Association of Gastrointestinal Physiologists ( AGIP ) Proposed Standardised Testing Protocol for Hydrogen / Methane Breath Testing ( HMBT ) to Assess Small Intestinal Bacterial Overgrowth ( SIBO ) and Carbohydrate Malabsorption Introduction Objective of. 2019.
- 22.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd SJ, Lomer MCE, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–17. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 24.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 25.Kerckhoffs APM, Visser MR, Samsom M, van der Rest ME, de Vogel J, Harmsen W, et al. Critical evaluation of diagnosing bacterial overgrowth in the proximal small intestine. J Clin Gastroenterol. 2008;42:1095–1102. doi: 10.1097/MCG.0b013e31818474d7. [DOI] [PubMed] [Google Scholar]
- 26.Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3:112–122. [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson GR, Macfarlane GT, Cummings JH. Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut. 1993;34:437–439. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci U S A. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banik GD, De A, Som S, Jana S, Daschakraborty SB, Chaudhuri S, et al. Hydrogen sulphide in exhaled breath: a potential biomarker for small intestinal bacterial overgrowth in IBS. J Breath Res. 2016;10:026010. doi: 10.1088/1752-7155/10/2/026010. [DOI] [PubMed] [Google Scholar]
- 30.Birg A, Hu S, Lin HC. Reevaluating our understanding of lactulose breath tests by incorporating hydrogen sulfide measurements. JGH Open. 2019;3:228–233. doi: 10.1002/jgh3.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]