Abstract
Background
The impact of serrated polyps on the advanced colorectal neoplasia (CRN) risk in inflammatory bowel disease (IBD) patients is unknown. Serrated polyps are histologically categorized as hyperplastic polyps (HPs), sessile serrated lesions (SSLs), and traditional serrated adenomas (TSAs).
Aims
We aimed (1) to characterize the serrated polyps in IBD patients, (2) to identify factors associated with the presence of serrated polyps in IBD, and (3) to assess the CRN risk in IBD patients with serrated polyps.
Methods
We established a retrospective cohort of IBD patients with and without colonic serrated polyps. Cox-regression analysis with time-dependent variables was used to compare advanced CRN risk in IBD patients with and without serrated polyps.
Results
Of the 621 enrolled IBD patients, 198 had a serrated polyp (92 HPs, 88 SSLs without dysplasia, 13 SSLs with dysplasia, and 5 TSAs). Independent factors associated with serrated polyps were ulcerative colitis (UC) (odds ratio (OR) 1.77, 95% confidence interval (CI) 1.19–2.62, p = 0.005), male gender (OR 1.63, 95% CI 1.11–2.40, p = 0.013), and older age (per year increase, OR 1.06, 95%CI 1.05–1.08, p < 0.001). TSAs and SSLs with dysplasia were risk factors for subsequent advanced CRN (HR 13.51, 95% CI 3.11–58.68, p < 0.001), while HPs (HR 1.98, 95% CI 0.46–8.60, p = 0.36) and SSLs without dysplasia (HR 0.87, 95% CI 0.11–6.88, p-0.89) did not impact the subsequent advanced CRN risk.
Conclusions
UC, male gender and older age were associated with the presence of serrated polyps. The majority of serrated polyps (91%) were HPs and SSL without dysplasia and did not affect the CRC risk. However TSAs and SSLs with dysplasia, representing a small subgroup of serrated polyps (9%), were associated with subsequent advanced CRN.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10620-022-07485-w.
Keywords: Serrated polyp, Colorectal cancer, Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis
Introduction
Colorectal cancer (CRC) is one of the most detrimental complications of colonic inflammatory bowel disease (IBD), with an incidence that is estimated 1.5–twofold higher compared to the general population [1]. Therefore, IBD patients undergo regular surveillance colonoscopies to remove precancerous lesions and prevent CRC. According to international guidelines, the interval between surveillance colonoscopies depends on the individual risk profile [2–5]. One of the most important risk factors for CRC is the presence of colitis-associated low-grade dysplasia (LGD) or high-grade dysplasia (HGD) [6]. However, it is unknown if the presence of colorectal serrated polyps in IBD impacts the colorectal neoplasia (CRN) risk and how it should impact surveillance guidelines.
In the general population, approximately 25% of sporadic CRCs arise via serrated precursor lesions [7]. Serrated polyps include hyperplastic polyps (HPs), sessile serrated lesions (SSLs) and traditional serrated adenomas (TSAs). They develop through a distinct molecular pathway and have a distinct endoscopic appearance compared to conventional adenomas. Before 2010 serrated polyps were poorly recognized by endoscopists and most serrated polyps were regarded as harmless HPs [7]. Since then, several studies reported that patients with serrated polyps have a 2- to 4- fold increased risk of synchronous and metachronous advanced CRN (including HGD and CRC). [8, 9]
To date, only few small retrospective studies described the risk of CRN in IBD patients with serrated polyps [10–15]. These studies did not show an increased CRN risk in patients with HPs, while one study including 78 patients reported that IBD patients with serrated polyps with dysplasia had a higher risk of advanced CRN [10]. However, these studies were published before the renewed World Health Organisation (WHO) criteria from 2019 were adopted for subclassification of serrated polyps [16]. Moreover, since most studies included almost only HPs, the CRN risk of other types of serrated polyps in IBD patients remains unclear.
To fill this knowledge gap, we established a large retrospective IBD cohort undergoing endoscopic surveillance. We aimed (1) to characterize serrated polyps in IBD patients, (2) to identify factors associated with the presence of serrated polyps in IBD, and (3) to assess the CRN risk in IBD patients with serrated polyps.
Materials and Methods
Study Design and Outcomes
We adopted a retrospective cohort study design to characterize IBD patients with serrated polyps, and to assess the (advanced) CRN risk in patients with and without serrated polyps. Primary endpoints were CRN defined as either LGD, HGD or CRC; and advanced CRN defined as HGD and CRC.
Study Population
We established a cohort of IBD patients undergoing standardized CRC surveillance according to the British Society of Gastroenterology guidelines [2] at the Radboud University Medical Center, Nijmegen, The Netherlands. Patients were identified using an electronic search in the local endoscopy and histopathology databases. This search included key terms for ‘surveillance’ and/or ‘colonoscopy’ in combination with key terms for IBD (‘ulcerative colitis’, ‘Crohn’s disease’, ‘inflammatory bowel disease’). Subsequently, IBD diagnoses were verified in the individual patients’ medical charts.
Next, we identified all serrated polyps in the established cohort (including histopathology slides for histopathological review). To this end, we performed a search in the local histopathology database from January 1996 to December 2017 using different terms for serrated polyps (metaplastic polyp, hyperplastic polyp, sessile serrated lesion, traditional serrated adenoma).
To assess whether the CRN risk of IBD patients with a serrated polyp with dysplasia was comparable to the risk in patients with colitis-associated LGD, we selected from the study cohort a control group of IBD patients who developed LGD but no serrated polyps. Only patients with colitis-associated LGD, defined as LGD located in a colonic area with (prior) inflammation, and at least one follow-up colonoscopy were included in this group.
Inclusion and Exclusion Criteria
All patients with a diagnosis of IBD (ulcerative colitis (UC), Crohn’s disease (CD) and IBD-unclassified (IBD-U)) who received at least 1 complete surveillance colonoscopy were eligible for inclusion. Exclusion criteria were defined as follows: HGD and/or CRC diagnosis before IBD development, serrated polyps before IBD diagnosis, serrated polyps that could not be confirmed after histopathology review, serrated polyps before 1996 (histopathology slides are available since 1996), patients with hereditary CRC syndromes such as Lynch syndrome and familial adenomatous polyposis, patients with an IBD duration of less than 8 years or patients with proctitis only (since these patients are not eligible for surveillance [2, 17]), objection against the use of data, and insufficient clinical data.
Data Collection
The following baseline characteristics were extracted from the patients’ medical charts: gender, age, family history of CRC, diagnosis of concomitant primary sclerosing cholangitis (PSC), and smoking status. Regarding IBD, we collected information on IBD type, age at IBD diagnosis, and disease extent. Extensive disease was defined as inflammation extending proximal of the splenic flexure in UC or an estimated colonic involvement of > 50% in CD. Non-extended disease included left-sided UC or segmental CD with 30–50% colonic involvement. To study the association of inflammation and serrated polyps, a mean inflammation score was calculated by averaging the inflammation scores of all colonoscopies (no inflammation = 0, non-extensive inflammation = 1, extensive inflammation = 2), reflecting the average extent of inflammation at colonoscopies [18, 19]. Date and outcomes of all IBD colonoscopies were extracted including the development of serrated polyps and CRN. Synchronous CRN was defined as neoplasia that was detected during the same colonoscopy as the serrated polyp. Metachronous CRN was defined as neoplasia that was detected at any follow-up colonoscopy or at subsequent colectomy. Furthermore, we extracted the date and type of colectomy. A colectomy was defined as either a subtotal (i.e., a colectomy only leaving the rectum in situ) or a total colectomy. Follow-up data were collected until August 1, 2019.
Histopathology Review
Histopathology slides from all serrated polyps and lesions with CRN were reviewed by an expert IBD pathologist (I.N., S.V., R.P.). This was performed in a blinded fashion. In case of doubt, slides were discussed and re-assessed by the other pathologists in order to obtain consensus. Slides with serrated polyps were reviewed according to the renewed WHO criteria [16]. Serrated polyps were classified into HP, SSL without dysplasia, SSL with dysplasia, or TSA (always with dysplasia) [16]. HPs are polyps with a normal architecture with crypts that are evenly spaced, but the superficial epithelium shows serration up to two-thirds of the crypts. According to the updated WHO criteria, the presence of a single unequivocally distorted crypt is considered diagnostic for an SSL. TSAs are the least common serrated polyps. The cells of these villous polyps contain prominent eosinophilic cytoplasm and have narrow slits. [7]
Grade of CRN was classified into LGD, HGD, or CRC [20]. Revised results were used for further analyses.
IBD Surveillance Strategy
In the 1990’s, surveillance colonoscopies in IBD patients were performed using standard-definition white light endoscopy with targeted biopsies of abnormalities, in combination with random biopsies. Between 2005 and 2010, high-definition white light endoscopy was adopted as the mainstay endoscopic technique. Following updates in IBD surveillance guidelines in 2008, chromoendoscopy was gradually implemented in some patients. Chromoendoscopy involves pan-colonic dye-spraying using either 0.3% indigo carmine or 0.1% methylene blue, along with targeted biopsies of abnormal areas. The interval between surveillance colonoscopies is based on the guidelines of the British Society of Gastroenterology. [2]
Statistics
Descriptive statistics were used to describe the baseline characteristics. Continuous outcomes are reported as means including standard deviation (SD) if normally distributed, and as medians with interquartile range (IQR) if non-normally distributed. Logistic regression analysis was used to compare baseline factors between patients with and without serrated polyps. Factors with a p-value < 0.1 in univariable analysis were included in multivariable logistic regression analysis.
Kaplan Meier curve and log-rank analysis were used to compare cumulative incidences of CRN between HPs, SSLs with dysplasia, SSLs without dysplasia, and TSAs. All serrated polyps containing dysplasia (thus, SSLs with dysplasia and TSAs) were pooled in analysis. Time to event was calculated from the date of the first serrated polyp until (advanced) CRN or censoring. The first serrated polyp was regarded as the index polyp. Only CRN identified after index serrated polyp detection was regarded as an event. Patients who had an index lesion in their (sub) total colectomy specimen were excluded. Patients who had synchronous HGD or CRC were excluded from all log-rank analyses. In addition, patients who had synchronous LGD were excluded as well in a sensitivity analysis. Patients were censored at last follow-up colonoscopy, or if performed, at the moment of (sub) total colectomy given the impact on the subsequent CRC risk. [21].
Finally, we compared the risk of metachronous CRN between IBD patients with serrated polyps and IBD patients with colitis-associated LGD (without serrated polyps).
Cox regression analysis was used to compare the risk of advanced CRN between IBD patients with and without serrated polyps. Here, time to event was calculated from the moment of IBD diagnosis until advanced CRN or censoring. To limit the risk of immortal time bias, serrated polyps were included in the Cox regression models as a time-changing covariate. We subsequently corrected for the confounders gender, concomitant diagnosis of PSC, history of smoking, IBD type, family history of CRC and the mean inflammation score.
A 2-tailed p-value of < 0.05 was considered to be statistically significant. Data analysis was performed using the SPSS statistical software (version 22, IBM, Chicago ILL).
Ethical Considerations
The study was approved by the medical ethical committee at the Radboud university medical center, Nijmegen (2017–3645).
Results
Patient Selection
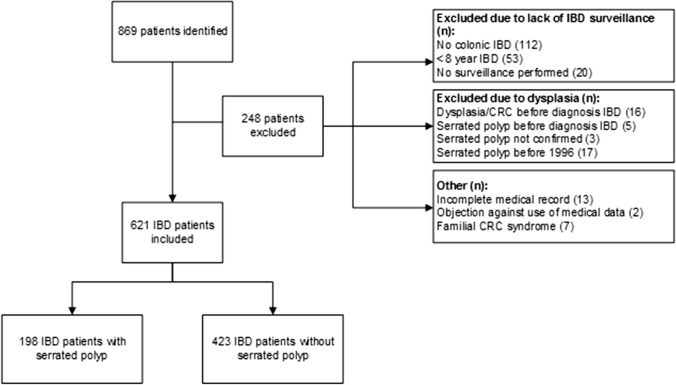
A total of 621 patients were eligible for inclusion, including 198 patients who had at least one serrated polyp after revision (Fig. 1). A total of 47 patients had LGD without a serrated polyp. Baseline characteristics of patients with and without serrated polyps are shown in Table 1. The index serrated polyp was an HP in 91 patients, an SSL in 102 patients, and a TSA in five patients. More detailed baseline characteristics per subgroup of serrated polyp are provided in Supplementary Table 1. Although we observed that the absolute mean inflammation score was higher in patients with SSL with dysplasia (0.67) and TSA (0.73) than in patients with HP (0.58) and SSL without dysplasia (0.50), this difference did not reach statistical significance (p = 0.25).
Fig. 1.
Flowchart of inclusion of patients. IBD = inflammatory bowel disease, CRC = colorectal cancer
Table 1.
Baseline characteristics of included patients with low-grade dysplasia
| Characteristic | IBD patients with serrated polyp (n = 198) | IBD patients without serrated polyp (n = 423) |
|---|---|---|
| Male, n (%) | 102 (51.5%) | 177 (41.8) |
| Disease | ||
| Ulcerative colitis, n (%) | 126 (63.6) | 206 (49.2) |
| Crohn's disease, n (%) | 65 (32.8) | 208 (48.7) |
| IBD-unclassified, n (%) | 7 (3.5) | 9 (2.1) |
| Age at IBD diagnosis in years (± SD) | 37.0 (14.5) | 26.9 (10.4) |
| Follow-up after IBD diagnosis in years, mean (± SD) | 22.2 (13.6) | 21.3 (10.6) |
| Years between consecutive colonoscopies, mean (± SD) | 3.0 (1.7) | 3.3 (2.0) |
| Mean inflammation score (± SD) | 0.56 (0.47) | 0.50 (0.49) |
| Family history of CRC (%) | 28 (14) | 60 (14) |
| Serrated polyps | – | |
| Hyperplastic polyp | 91 | |
| Sessile serrated lesion with dysplasia | 13 | |
| Sessile serrated lesion without dysplasia | 89 | |
| Traditional serrated adenoma | 5 | |
| Age at serrated polyp, mean (± SD) | 54.8 (12.0) | – |
| Follow-up after index serrated polyp in years, median (IQR) | 3.0 (0–5.8) | – |
IBD = inflammatory bowel disease, CRC = colorectal cancer, SD = standard deviation, IQR = interquartile range
The mean follow-up after IBD diagnosis was 22.2 (± 13.6) and 21.3 (± 10.6) years in patients with and without a serrated polyp, respectively. The median follow-up time after the index serrated polyp was 3.0 years. During follow-up 14/92 patients (15%) with an HP developed an SSL and 25/101 patients (25%) with an SSL developed a second SSL.
Characteristics of Serrated Polyps in IBD
Table 2 illustrates the location of the detected serrated polyps. Index HPs were located in the distal colon in 90% (53% rectum and 37% left-sided colon), and rarely in the transverse colon (6.0%) or ascending colon (4%). SSLs without dysplasia and SSLs with dysplasia were located in the proximal (transverse or ascending) colon in 32 and 38%, respectively. In addition, 60% of TSAs were located in the proximal colon. The mean size of the serrated polyp as reported in the pathology report was 5.3 mm in lesions with dysplasia versus 3.5 mm in serrated polyps without dysplasia (p = 0.06). The mean size of TSAs was 7.3 mm. 162/198 (82%) lesions were visible while 18/198 (9%) resulted from random biopsies (the method was not specified in the report in 16 patients).
Table 2.
Location of the serrated polyps
| Location | HPs n = 91 | SSLs without dysplasia n = 89 | SSLs with dysplasia n = 13 | TSAs n = 5 | ||||
|---|---|---|---|---|---|---|---|---|
| Not specified | 8 | 7 | 0 | 0 | ||||
| Location specified | n = 83 | n = 82 | n = 13 | n = 5 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Rectum | 44 | 53 | 28 | 34 | 3 | 23 | 1 | 20 |
| Left colon | 31 | 37 | 28 | 34 | 5 | 38 | 1 | 20 |
| Transverse colon | 5 | 6 | 8 | 10 | 2 | 15 | 1 | 20 |
| Ascending colon | 3 | 4 | 18 | 22 | 3 | 23 | 2 | 40 |
HP = hyperplastic polyp, SSL = sessile serrated lesion, TSA = traditional serrated adenoma, n = number
Factors Associated with the Presence of Serrated Polyps in IBD
A diagnosis of UC, male gender, and age (per year increase) were associated with the presence of serrated polyps in IBD in the univariable logistic regression analysis (Table 3). There was no difference in mean inflammation score, smoking history, family history of CRC or PSC diagnosis. In line, multivariable logistic regression analysis identified UC (odds ratio (OR) 1.77, 95% confidence interval (CI) 1.19–2.62, p = 0.005), male gender (OR 1.63, 95% CI 1.11–2.40, p = 0.013), and age (1.06, 95%CI 1.05–1.08, p < 0.001) as independent factors associated with the presence of serrated polyps. Excluding HPs from this analysis, only UC (OR 2.31, 95% CI 1.35–3.95, p = 0.002) and older age (OR 1.08, 95% CI 1.06–1.10, p < 0.001) were associated with the presence of serrated polyps, as shown in Supplementary Table 1. Male gender was included in the multivariable analysis but did not reach statistical significance (p = 0.051).
Table 3.
Factors associated with the presence of serrated polyps
| Baseline | OR uni-variable | 95% CI | P-value | OR multi-variable (final model) | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Ulcerative colitis | 1.95 | 1.37–2.78 | < 0.001 | 1.77 | 1.19–2.62 | 0.005 |
| Male | 1.51 | 1.07–2.12 | 0.02 | 1.63 | 1.11–2.40 | 0.013 |
| PSC | 1.68 | 0.72–3.90 | 0.23 | – | – | – |
| Age at IBD diagnosis (per year increase) | 1.07 | 1.05–1.08 | < 0.001 | 1.06 | 1.05–1.08 | < 0.001 |
| Mean inflammation score | 1.25 | 0.87–1.81 | 0.23 | – | – | – |
| Smoking | 1.01 | 0.72–1.43 | 0.94 | – | – | – |
| Family history of CRC | 0.99 | 0.61–1.62 | 0.99 |
IBD = Inflammatory bowel disease; PSC = Primary sclerosing cholangitis; CI = Confidence interval, OR = Odds ratio, CRC = colorectal cancer
In bold factors with a p-value < 0.05
Neoplasia Risk
Risk of Metachronous and Synchronous (Advanced) CRN in IBD Patients with Serrated Polyps
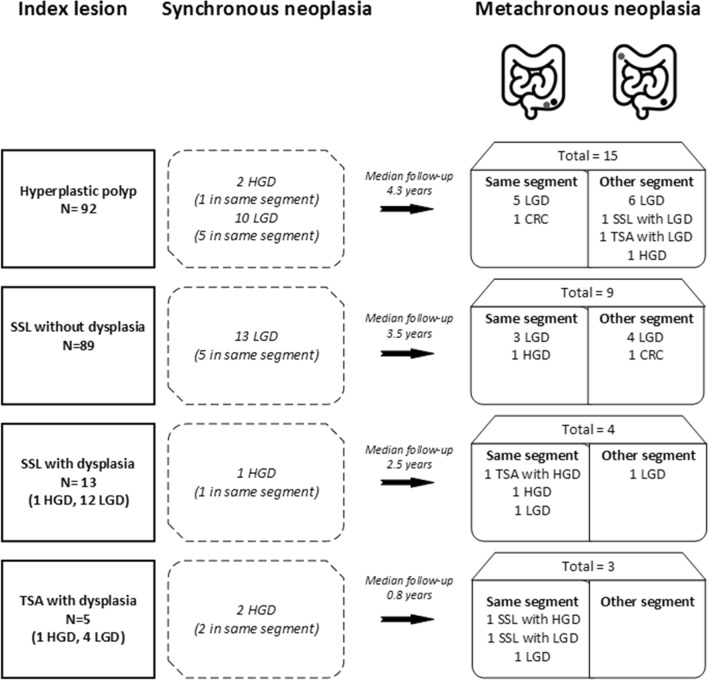
Figure 2 illustrates the risk of synchronous and metachronous neoplasia. Here, only the first lesion with metachronous neoplasia is reported. Synchronous CRN was present in 13.0, 14.6, 7.7, and 40% of patients with an HP, SSL without dysplasia, SSL with dysplasia, and TSA with dysplasia, respectively. At follow-up, 6/18 patients (33.3%) with serrated polyps with dysplasia (TSA and SSL) developed metachronous CRN in the same colonic segment, while only 10/180 (5.5%) of patients with serrated polyps without dysplasia developed CRN in the same segment (p < 0.001). Although only two patients had CRC as first metachronous lesion, an additional four patients developed CRC during further follow-up (index lesion included SSL with LGD (n = 3), HP (n = 2) and TSA with LGD (n = 1)).
Fig. 2.
Serrated polyps and risk of synchronous and metachronous neoplasia. HP = hyperplastic polyp, SSL = sessile serrated lesion, TSA = traditional serrated adenoma, LGD = low-grade dysplasia, HGD = high-grade dysplasia
Figure 3 illustrates the cumulative incidence of CRN after the index polyp. The 2-year cumulative incidence of CRN was 7.4, 6.8, 44, and 50% after the detection of an HP, an SSL without dysplasia, an SSL with dysplasia, and a TSA, respectively. Patients with a TSA or SSL with dysplasia had an increased incidence of metachronous CRN compared to patients with HPs and an SSL without dysplasia (log-rank test, p < 0.001). This difference remained if patients with synchronous colitis-associated LGD were excluded from the analysis (sensitivity analysis, p < 0.001). Patients with an SSL without dysplasia had a similar CRN risk as patients with HPs (p = 0.78). Of note, 6/72 patients with one SSL without dysplasia developed metachronous CRN (8.3%; 5 LGD and 1 advanced CRN) versus 2/17 patients who had an SSL without dysplasia more than once (11.8%; 2 LGD; p = 0.65). Patients with a serrated polyp without dysplasia with synchronous LGD had a higher cumulative advanced CRN risk than those without synchronous LGD (p < 0.001).
Fig. 3.
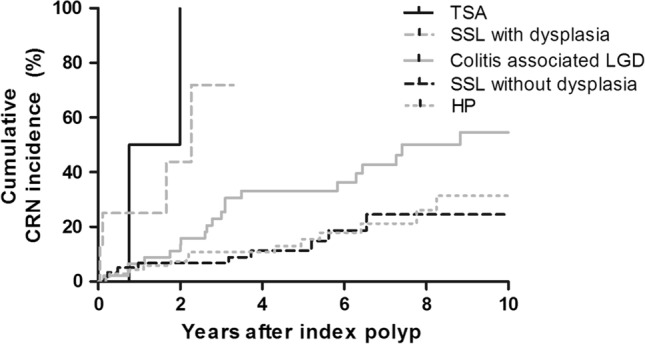
Kaplan–Meier plot illustrating the risk of metachronous colorectal neoplasia in IBD patients with serrated polyps or colitis-associated LGD. Patients with synchronous advanced colorectal neoplasia at index colonoscopy were excluded from this analysis. HP = hyperplastic polyp, SSL = sessile serrated lesion, TSA = traditional serrated adenoma, LGD = low-grade dysplasia, CRN = colorectal neoplasia
Cumulative Risk of Metachronous CRN in IBD Patients with Serrated Polyps Versus Colitis-Associated LGD
We compared the metachronous CRN risk between IBD patients with serrated polyps and IBD patients with colitis-associated LGD without serrated polyps (n = 47). The mean size of the colitis-associated LGD was 4.2 mm. Baseline characteristics of this control cohort are described in Supplementary Table 2. Patients with LGD were younger at IBD diagnosis, and had a lower mean inflammation score than patients with serrated polyps. Patients with a TSA or an SSL with dysplasia had a higher cumulative incidence of metachronous CRN compared with patients with colitis-associated LGD (p < 0.001, Fig. 3). After correction for the mean inflammation score, we still observed a higher risk (HR 2.86, 95% confidence interval 1.03–7.99, p = 0.04). Adjusting for other covariates including gender, concomitant diagnosis of PSC, (history of) smoking, IBD type, family history of CRC and mean inflammation score yielded similar results.
By contrast, patients with an HP (p = 0.027) or an SSL without dysplasia (p = 0.017) had a lower cumulative incidence of metachronous CRN compared with patients with colitis-associated LGD.
We did not observe a higher cumulative CRN risk in serrated polyps that developed within an area of (prior) inflammation (p = 0.19), and similarly not after excluding HPs from the analysis (p = 0.60).
Risk of Advanced CRN in IBD Patients with Versus Without Serrated Polyps
In the Cox-regression analysis with the presence of serrated polyps as a time-changing factor, serrated polyps with dysplasia (TSA and SSL) were associated with an increased subsequent advanced CRN risk (HR 13.51, 95% CI 3.11–58.68, p < 0.001) compared to IBD patients without a serrated polyp. This association remained after correcting for the confounders gender, concomitant diagnosis of PSC, (history of) smoking, IBD type, family history of CRC and mean inflammation score (adjusted HR 6.02, 95% CI 1.06–34.32). Specifically assessing patients with SSLs with dysplasia resulted in an HR of 7.96 (95% CI 1.45–43.62, p = 0.017). In contrast, neither HPs (HR 1.98, 95% CI 0.46–8.60, p = 0.36) nor SSLs without dysplasia (HR 0.87, 95% CI 0.11–6.88, p-0.89) were associated with an increased risk of advanced CRN compared to IBD patients without a serrated polyp.
Discussion
In this cohort study including 621 patients with colonic IBD undergoing CRC surveillance, we observed that TSAs and SSLs with dysplasia were associated with an increased risk of advanced CRN (HR 13.51) compared with IBD patients without a serrated polyp. By contrast, HPs and SSLs without dysplasia were not associated with an increased advanced CRN risk. UC, male gender, and older age were associated with the presence of serrated polyps.
We found an increased CRN and advanced CRN risk in patients with serrated polyps with dysplasia. This is in line with previous literature, although previous studies were performed before the latest classification system for serrated polyps was adopted [16]. One previous study (n = 78 patients) reported a shorter advanced CRN-free survival time in IBD patients with an index SSL with dysplasia compared to serrated polyps without dysplasia (p = 0.002) [10]. A recent study reported that 4/30 IBD patients with a TSA developed advanced CRN [14], while another study including 25 IBD patients with an SSL reported a high rate of synchronous CRN (36%) and metachronous CRN (8 of 13 patients who had follow-up), although this study did not distinguish between SSLs with and without dysplasia [11]. In contrast, a retrospective cohort including 115 patients (of whom 112 with an HP) reported a low metachronous CRN risk in IBD patients with HPs [13]. In line, we observed that IBD patients with serrated polyps without dysplasia had no increased risk of advanced CRN, and a lower risk of metachronous CRN than patients with colitis-associated LGD.
We observed a higher CRN risk in patients with serrated polyps with dysplasia compared to patients with colitis-associated LGD. However, this result may be biased by the higher mean inflammation score in patients with serrated polyps. Similarly, several studies conducted in the general population demonstrated that the CRC risk of serrated polyps is comparable or increased in comparison with conventional adenomas [9, 22, 23]. One may speculate that this finding is the result of easily missed and/or incompletely removed serrated polyps given their subtle and flat endoscopic appearance.
In our cohort, HPs were rarely located in the proximal (transverse or ascending) colon (9.6%), while SSLs were located more frequently in the proximal colon (32% SSL without dysplasia; 38% SSL with dysplasia). Likewise, a previous study in IBD patients reported that serrated polyps with dysplasia were proximally located in 20% [10]. In contrast, in the general population 70–80% of all SSLs are detected in the proximal colon [7, 11], although SSLs with dysplasia might be located more throughout the colon [24]. We observed that serrated polyps with dysplasia (including TSAs and SSLs) were generally larger than serrated polyps without dysplasia, although this did not reach statistical significance (mean size 5.3 vs 3.5 mm, p = 0.06). In line, previous studies reported that TSAs and SSLs are generally larger than HPs. [25–27].
Serrated polyps were detected more often in UC than in CD, which is in line with previous studies [10, 11]. However, we found no association between colonic inflammation and the development of serrated polyps. Previous studies reported that molecular alterations of serrated polyps are similar between IBD and non-IBD patients and the impact of inflammation on serrated polyps remains unclear [10, 28]. Furthermore, serrated polyps in IBD patients were found more often with increasing age, similar to the general population [7]. In addition, we found male gender to be associated with the presence of serrated polyps. Although several other studies reported a higher risk in men as well, it is assumed that in general men and woman have an equivalent risk. [7]
Our findings may impact current surveillance guidelines. Given the increased advanced CRN risk in IBD patients with serrated polyps with dysplasia, we propose a surveillance strategy for these IBD patients similar to that of IBD patients with colitis-associated LGD following current European and American guidelines [2, 4, 17]. This would result in initially yearly surveillance colonoscopy following removal of a serrated polyp with dysplasia. The high risk of metachronous CRN in the same colonic segment that harbored the serrated polyp with dysplasia may suggest incomplete resection of these lesions, or segmental inflammatory changes increasing this CRN risk. Previous studies that reported a higher risk of incomplete resection of serrated polyps compared to conventional adenomas in a non-IBD population [29, 30]. This indicates that this colonic segment should be monitored closely with optimal endoscopic visualization. Since we did not identify serrated polyps without dysplasia as a risk factor for advanced CRN in IBD, we argue that these lesions should not impact surveillance intervals and do not result in yearly surveillance colonoscopies.
Our study has several strengths, including the rigorous collection of data, the histopathology review by expert pathologists according to the renewed [2019] WHO criteria, and the control group of IBD patients without serrated polyps. The setting of an IBD population undergoing CRC surveillance makes our cohort representative for clinical practice. However, there are also some limitations. It is known that serrated polyps may be difficult to detect, especially in the past before high-definition colonoscopes were used. Moreover, inter-observer variability exists between pathologists in clinical practice7, which may have resulted in a lower rate of serrated polyps. Second, although the mean interval between surveillance colonoscopies was similar between patients with and without serrated polyps, the individual differences in surveillance intervals and visualisation techniques may have resulted in different adenoma detection rates. Third, although we established the largest cohort of patients with serrated polyps to date, the number of index serrated polyps with dysplasia was relatively small. This might have resulted in a lack of power to detect other significant differences such as the size of the dysplastic and non-dysplastic serrated polyps. However, despite the small number of index lesions with dysplasia we still observed a significant higher cumulative risk of CRN.
In conclusion, in a relatively large cohort of IBD patients undergoing surveillance, we observed that only serrated polyps with dysplasia (including TSAs and SSLs with dysplasia) were associated with an increased risk of advanced CRN while HPs and SSLs without dysplasia (91% of the cohort) were not. These findings suggest that the detection of serrated polyps with dysplasia may warrant a similar surveillance strategy to that of IBD patients with colitis-associated LGD, while serrated polyps without dysplasia do not require this intensified surveillance.
Supplementary Information
Below is the link to the electronic supplementary material.
Author's Contribution
No additional writing assistance was used for this manuscript. MJ, SV, IN, RP, YH, LD, and FH all contributed to the design of the study. MJ collected data and analyzed the data. SV, IN, and RP reviewed histopathology. MJ drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors have approved the final version of this manuscript.
Funding
This research received no specific grant form any funding agency in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lauranne A.A.P. Derikx and Frank Hoentjen: Shared last authorship.
Contributor Information
Michiel E. de Jong, Email: Michiel.E.deJong@radboudumc.nl
Iris D. Nagtegaal, Email: Iris.Nagtegaal@Radboudumc.nl
Shoko Vos, Email: Shoko.Vos@Radboudumc.nl.
Rachel S. van der Post, Email: Chella.vanderPost@Radboudumc.nl
Yasmijn van Herwaarden, Email: Yasmijn.vanHerwaarden@Radboudumc.nl.
Lauranne A. A. P. Derikx, Email: Lauranne.Derikx@Radboudumc.nl
Frank Hoentjen, Email: Frank.Hoentjen@Radboudumc.nl, Email: Hoentjen@ualberta.ca.
References
- 1.Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet (London, England) 2020;395:123–131. doi: 10.1016/s0140-6736(19)32545-0. [DOI] [PubMed] [Google Scholar]
- 2.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 3.Farraye FA, Odze RD, Eaden J, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–774. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Farraye FA, Odze RD, Eaden J, et al. AGA Medical Position Statement on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Shergill AK, Lightdale JR, Bruining DH, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81(1101–1121):e1101–1113. doi: 10.1016/j.gie.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 6.De Jong ME, Van Tilburg SB, Nissen LHC, et al. Long-term Risk of Advanced Neoplasia After Colonic Low-grade Dysplasia in Patients With Inflammatory Bowel Disease: A Nationwide Cohort Study. Journal of Crohn's and Colitis. 2019;13:1485–1491. doi: 10.1093/ecco-jcc/jjz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crockett SD, Nagtegaal I. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 8.He X, Hang D, Wu K, et al. Long-term Risk of Colorectal Cancer After Removal of Conventional Adenomas and Serrated Polyps. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erichsen R, Baron JA, Hamilton-Dutoit SJ, et al. Increased Risk of Colorectal Cancer Development Among Patients With Serrated Polyps. Gastroenterology. 2016;150:895–902.e895. doi: 10.1053/j.gastro.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Ko HM, Harpaz N, McBride RB, et al. Serrated colorectal polyps in inflammatory bowel disease. Modern Pathology. 2015;28:1584–1593. doi: 10.1038/modpathol.2015.111. [DOI] [PubMed] [Google Scholar]
- 11.Jackson WE, Achkar JP, Macaron C, et al. The Significance of Sessile Serrated Polyps in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2213–2220. doi: 10.1097/mib.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 12.Iacucci M, Hassan C, Fort Gasia M, et al. Serrated adenoma prevalence in inflammatory bowel disease surveillance colonoscopy, and characteristics revealed by chromoendoscopy and virtual chromoendoscopy. Canadian journal of gastroenterology & hepatology. 2014;28:589–594. doi: 10.1155/2014/386540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Gibson JA, Schulte S, et al. Clinical, pathologic, and outcome study of hyperplastic and sessile serrated polyps in inflammatory bowel disease. Human pathology. 2015;46:1548–1556. doi: 10.1016/j.humpath.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Miller GC, Liu C, Bettington ML, et al. Traditional serrated adenoma-like lesions in patients with inflammatory bowel disease. Human pathology. 2020;97:19–28. doi: 10.1016/j.humpath.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Choi WT, Wen KW, Rabinovitch PS, et al. DNA content analysis of colorectal serrated lesions detects an aneuploid subset of inflammatory bowel disease-associated serrated epithelial change and traditional serrated adenomas. Histopathology. 2018;73:464–472. doi: 10.1111/his.13652. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organisation . Classification of Tumours of the Digestive Tract. Lyon: IARC Press; 2019. [Google Scholar]
- 17.Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. Journal of Crohn's and Colitis. 2015;9:945–965. doi: 10.1093/ecco-jcc/jjv141. [DOI] [PubMed] [Google Scholar]
- 18.de Jong ME, Gillis VELM, Derikx LAAP, et al. No Increased Risk of Colorectal Neoplasia in Patients With Inflammatory Bowel Disease and Postinflammatory Polyps. Inflamm Bowel Dis. 2019 doi: 10.1093/ibd/izz261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoud R, Shah SC, ten Hove JR, et al. No Association Between Pseudopolyps and Colorectal Neoplasia in Patients With Inflammatory Bowel Diseases. Gastroenterology. 2019;156:1333–1344.e1333. doi: 10.1053/j.gastro.2018.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Human pathology. 1983;14:931–968. doi: 10.1016/S0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 21.Derikx L, Nissen LHC, Smits LJT, et al. Risk of Neoplasia After Colectomy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:798–806.e720. doi: 10.1016/j.cgh.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Lu F-I, van Niekerk DW, Owen D, et al. Longitudinal Outcome Study of Sessile Serrated Adenomas of the Colorectum: An Increased Risk for Subsequent Right-sided Colorectal Carcinoma. The American journal of surgical pathology. 2010;34:927–934. doi: 10.1097/PAS.0b013e3181e4f256. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Emilsson L, Bozorg SR, et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study. The Lancet Gastroenterology & Hepatology. 2020;5:537–547. doi: 10.1016/S2468-1253(20)30009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouwens MWE, van Herwaarden YJ, Winkens B, et al. Endoscopic characterization of sessile serrated adenomas/polyps with and without dysplasia. Endoscopy. 2014;46:225–235. doi: 10.1055/s-0034-1364936. [DOI] [PubMed] [Google Scholar]
- 25.Turner KO, Genta RM, Sonnenberg A. Lesions of All Types Exist in Colon Polyps of All Sizes. The American journal of gastroenterology. 2018;113:303–306. doi: 10.1038/ajg.2017.439. [DOI] [PubMed] [Google Scholar]
- 26.Torlakovic E, Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. The American journal of surgical pathology. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Bettington ML, Walker NI, Rosty C, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Modern pathology. 2015;28:414–427. doi: 10.1038/modpathol.2014.122. [DOI] [PubMed] [Google Scholar]
- 28.Odze RD, Brien T, Brown CA, et al. Molecular alterations in chronic ulcerative colitis-associated and sporadic hyperplastic polyps: a comparative analysis. The American journal of gastroenterology. 2002;97:1235–1242. doi: 10.1111/j.1572-0241.2002.05696.x. [DOI] [PubMed] [Google Scholar]
- 29.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80.e71. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen IB, Bretthauer M, Kalager M, et al. Incomplete endoscopic resection of colorectal polyps: a prospective quality assurance study. Endoscopy. 2021;53:383–391. doi: 10.1055/a-1243-0379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.