Abstract
Background
Patients with cirrhosis often develop portal hypertension-associated splenomegaly and hypersplenism, potentially causing severe cytopenia.
AIMS
Systematic assessment on the impact of transjugular intrahepatic portosystemic shunt (TIPS) implantation on platelet count (PLT), hemoglobin (Hb), and white blood cell count (WBC).
Methods
Patients with cirrhosis undergoing covered TIPS implantation were retrospectively included. Patients with malignancies or hematologic disorders were excluded. Hematology lab work was recorded at baseline (pre-TIPS) and at regular intervals after TIPS.
Results
One hundred ninety-two patients (male: 72.4%, age: 56 ± 10 years; MELD: 12.1 ± 3.6) underwent TIPS implantation. Higher-grade (≥ G2) thrombocytopenia (PLT < 100 G/L) was present in 54 (28.7%), ≥ G2 anemia (Hb < 10 g/dL) in 57 (29.7%), and ≥ G2 leukopenia (WBC < 2 G/L) in 3 (1.6%) patients pre-TIPS, respectively. Resolution of ≥ G2 thrombocytopenia, anemia, and leukopenia occurred in 24/55 (43.6%), 23/57 (40.4%), and 2/3 (66.7%), respectively. Similar results were also observed in the subgroup of patients without ‘bleeding’ TIPS-indication, with improvements of G ≥ 2 thrombocytopenia and of G ≥ 2 anemia in 19.8% and 10.2% of patients after TIPS, respectively.
Conclusions
Thrombocytopenia, anemia, and leukopenia frequently improved after TIPS. Therefore, moderate- to higher-grade thrombocytopenia should not be regarded as a contraindication against TIPS, but rather be considered in case of severe thrombocytopenia—particularly prior to surgery or interventions.
Graphical Abstract
Supplementary Material
The online version of this article (10.1007/s10620-022-07443-6) contains supplementary material, which is available to authorized users.
Keywords: Hypersplenism, Thrombocytopenia, Anemia, TIPS, Portal hypertension, Cirrhosis
Introduction
The development of portal hypertension (PH) in advanced chronic liver disease (ACLD) accounts for severe complications in patients with cirrhosis [1], such as development of ascites or variceal bleeding. PH-induced splanchnic venous congestion may also cause splenomegaly [2–4]. Splenomegaly has a prevalence of 50%–100% in cirrhosis depending on the underlying etiology [2] and is often complicated by hypersplenism-associated cytopenia [5]. Hypersplenism mostly induces thrombocytopenia; however, anemia and leukopenia may also occur [6, 7]. Patients with cirrhosis and PH may also present with non-hypersplenism associated causes for cytopenia [6, 8–10], such as iron deficiency due to gastrointestinal blood loss or malnutrition [6, 11, 12], or deprived levels of thrombopoietin (TPO) due to hepatic insufficiency [13]. Since platelet count (PLT) indirectly correlates with severity of PH, i.e., hepatic venous pressure gradient (HVPG), PLT is widely recognized as a valuable marker for noninvasive assessment of PH [14–16].
Both refractory ascites and severe/recurrent variceal bleeding can be effectively treated by placing a transjugular intrahepatic portosystemic shunt (TIPS) [1, 14, 17–19]. TPO agonists may be used to increase the PLT count before invasive procedures [20]. Moreover, splenectomy and other interventions such as partial splenic artery embolization or liver transplantation have shown to improve thrombocytopenia in distinct patient collectives [5, 13, 21, 22]. However, the effect of TIPS on cytopenia has not yet been fully characterized. Prior studies have yielded controversial results regarding improvement of thrombocytopenia after TIPS implantation [23–25], and data on the course of anemia or leukopenia after TIPS are scarce.
Methods
Study Design and Patient Cohort
This retrospective cohort study was conducted at two tertiary care centers in Vienna, Austria. We assessed whether TIPS implantation improves hypersplenism-associated cytopeniacytopenia (thrombocytopenia, anemia, leukopenia) in consecutive patients undergoing TIPS implantation between 1999 and 2017 fulfilling the following inclusion criteria: (i) diagnosis of cirrhosis (ii) indication for TIPS due to refractory ascites or variceal hemorrhage (iii) age > 18 years, (iv) implantation of a polytetrafluoroethylene (PTFE)-covered TIPS and (v) available hematology lab reports prior to TIPS (baseline) and at least at one follow-up visit between 3 and 24 months after TIPS placement. Patients with hepatocellular carcinoma, other malignancies, or primary hematologic disorders were excluded from analysis.
Data Acquisition
Clinical and epidemiological data were extracted from medical histories, and laboratory data from the respective digital patient management systems. TIPS intervention data were collected from the respective intervention reports of each study center, while spleen diameter was assessed by local radiologists specifically for this study (see below).
Parameters
We recorded hemoglobin (Hb) values, platelet count, and white blood cell count (WBC) at the following time points: at baseline (0–10 days prior to TIPS implantation), and at approx. 3 months (M3; ± 3 weeks), 6 months (M6; ± 6 weeks), 12 months (M12; ± 10 weeks), and 24 months (M24; ± 20 weeks) after TIPS implantation, where available.
Alterations in blood cell counts were graded as follows:
Thrombocytopenia:
-
o
No thrombocytopenia (grade, G0) at PLT ≥ 150 G/L;
-
o
G1: PLT 100–149 G/L;
-
o
G2: PLT 50–99 G/L;
-
o
G3: PLT < 50 G/L,
-
•
Anemia:
-
o
No Anemia (G0): Hb at or above the lower limit of normal (LLN: male: ≥ 13.5 g/dL, female: ≥ 12.5 g/dL);
-
o
G1: Hb 10.0 g/dL—LLN;
-
o
G2: Hb 7.5—10,0 g/dL;
-
o
G3: Hb < 7.5 g/dL.
-
•
Leukopenia:
-
o
No Leukopenia WBC ≥ 4.00 G/L;
-
o
G1: 2.00–3.99 G/L;
-
o
G2: 1.00–1.99 G/L;
-
o
G3: < 1.00 G/L.
MELD was calculated as previously described [26].
TIPS-Procedure
TIPS stent graft implantations were performed according to standard operating procedures as previously described [17–19, 27, 28]. Portosystemic pressure gradient (PPG) was measured by radiologists intra-procedurally by subtracting the pressure within the right atrium from the directly measured portal vein pressure prior to TIPS dilatation. Afterward, patients were surveilled during routine clinical visits with routine blood draws (including complete blood counts) every 3–6 months after TIPS insertion.
Measurement and Dynamics of Splenomegaly
When available, spleen size was assessed by an experienced radiologist at the largest cranial-caudal diameter at level of the hilus in coronary reconstruction in cross-sectional imaging at the following time points: at baseline (within 3 months prior to TIPS), within 12 months (short-term follow-up), and later than 12 months (long-term follow-up) after TIPS implantation. A spleen diameter of > 11 cm was regarded as splenomegaly. Changes in spleen diameter by ± > 2.0 cm or by > 10% were considered relevant increases/decreases.
Sub-analyses
We also evaluated the course of blood cell counts after TIPS implantation according to etiology of liver disease (alcoholic liver disease, ALD vs. viral etiology vs. other etiologies) and in a sub-group of patients undergoing TIPS without a bleeding-related indications.
Statistics
All statistical analyses were performed using Microsoft Excel (Microsoft Office 2016, Microsoft Corporation/Impressa Systems, Santa Rosa, California, USA), GraphPad Prism 9.0.0 (Prism 9, GraphPad Software, LLC, San Diego, California, USA) and SPSS Statistics V.26 (IBM SPSS Statistics for Windows, Version 26.0.0.0., IBM Corp., Armonk, NY, USA). Categorical variables are presented as absolute numbers (percentage) of patients with a certain characteristic. Metric variables were tested for normal distribution using the Kolmogorov–Smirnov-test. Normally distributed variables are presented as mean ± standard deviation (SD) and skewed variables as median (interquartile range, IQR) unless otherwise specified. Changes in blood counts over time were assessed by paired t-test or the Wilcoxon matched-pairs signed rank test, wherever applicable. Spearman’s rank correlation coefficient was calculated when assessing correlations between two metric variables. In case of missing values, patients were excluded for the respective analyses.
We further calculated multivariable regression models to evaluate independent predictors for improvement of moderate-to-high grade cytopenia. We entered age, etiology of liver disease (ALD vs. non-ALD etiology), MELD/MELD-Na, in addition to any variable with a p value ≤ 0.100 in univariate analysis into a step-wise backwards binomial logistic regression analysis. To assess risk factors for mortality, we computed multivariable Cox regression analyses, where patients were censored at day of liver transplant or last follow-up, and death was regarded an event. Two-sided p values were calculated, and significance level was set at p < 0.05.
Ethics
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the human research committees of the Medical University of Vienna (1760/2014) and the City of Vienna (MA15, 14-264-VK). The need for written consent was waived.
Results
Study population (Fig. 1, Table 1, Table2)
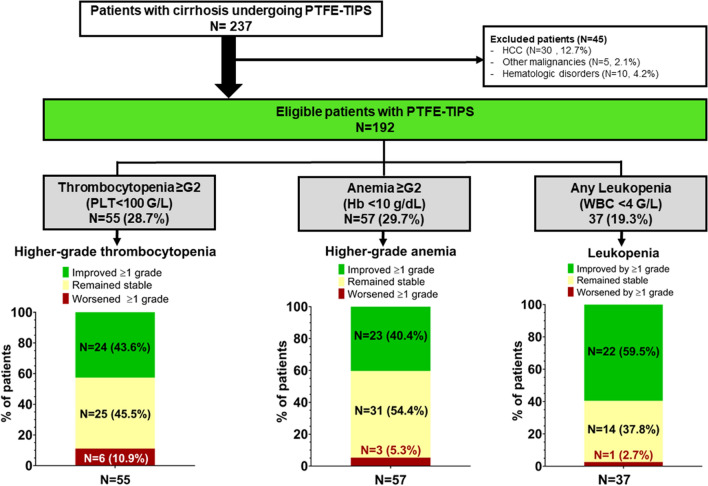
Fig. 1.
Patient Flowchart. Abbreviations: PTFE, polytetrafluoroethylene; TIPS, transjugular intrahepatic portosystemic shunt; HCC, hepatocellular carcinoma; LLN, lower limit of normal; WBC, white blood cell count. Lower Limit of normal (LLN) of hemoglobin: males: 13.5 g/dL, females: 12.5 g/dL
Table 1.
Patient characteristics
| Patients receiving PTFE-TIPS | 192 (100%) |
|---|---|
| Male (n, %) | 139, 72.4% |
| Age (years; µ ± SD) | 56.0 ± 10.0 |
|
Etiology (n, %) Alcoholic liver disease Viral Alcoholic and viral liver disease Autoimmune and/or cholestatic Nonalcoholic steatohepatitis Cryptogenic Other |
133, 69.3% 17, 8.9% 16, 8.3% 6, 3.2% 1, 0.5% 16, 8.3% 3, 1.6% |
|
Main indication for TIPS (n, %) Refractory/recurrent ascites Variceal bleeding: |
109, 56.8% 83, 43.2% |
|
Child–Pugh Scorea (median, IQR) Child A (n, %) Child B (n, %) Child C (n, %) |
9 (8–10) 11, 5.7% 122, 63.5% 57, 29.7% |
|
MELD (points; µ ± SD) MELD-Na (points; µ ± SD) |
12.1 ± 3.6 15.9 ± 4.6 |
|
PPG (mmHg; µ ± SD) Pre-TIPS Post-TIPS Reduction by TIPS |
19.9 ± 5.6 7.6 ± 3.5 − 12.4 ± 4.9 |
aData for CTP assessment was not available in N = 2 patients.
bCross-sectional imaging-based pre-TIPS spleen diameter assessment was available in 67 patients.
Table 2.
Prevalence of cytopenias in patients undergoing TIPS implantation
| Nr. of patients | 192 (100%) |
|---|---|
|
Platelet count (G/L; median, IQR) Mild thrombocytopenia (G1: 100–149 G/L; n, %) Moderate/severe thrombocytopenia (G2/G3: ≤ 100 G/L; n, %) |
131, 94–198 54, 28.1% 55, 28.7% |
|
Hemoglobin (g/dL; µ ± SD) Mild anemia (G1: Hb: 10-m ≤ 13.5/f: ≤ 12.5 g/dL; n, %) Moderate/severe anemia (G2/G3: Hb < 10 g/dL; n, %) |
10.8 ± 1.9 114, 59.4% 57, 29.7% |
|
White blood cell count (G/L; median, IQR) Mild leukopenia (G1: 2–4 G/L; n, %) Moderate/severe leukopenia (G2/G3: < 2 G/L; n, %) |
5.90, 4.37–7.65 34, 17.7% 3, 1.6% |
|
Bicytopenia (n, %) Pancytopenia (n, %) |
75, 39.1% 32, 16.7% |
237 patients met the inclusion criteria. 45 patients were excluded due to primary hematologic disorders and malignancies. The final study population comprised 192 patients undergoing PTFE-TIPS implantation (n = 109, 56.8% for ascites; n = 83, 43.2% for variceal bleeding). Mean MELD was 12.1 ± 3.6, and most patients had Child–Pugh class B (63.5%) or class C (29.7%) cirrhosis. Among 67 patients with cross-sectional imaging readily available, almost 90% presented with splenomegaly > 11 cm prior to TIPS implantation.
In total, thrombocytopenia was prevalent in 56.8% of patients and anemia in 89.1%, while less than 20% presented with leukopenia. Moderate-to-severe (G2/G3) thrombocytopenia was present in 28.7%, G2/G3 anemia in 29.7%, and G2 leukopenia in three patients (1.6%). No patient was diagnosed with G3 leukopenia at baseline.
Effect of TIPS on Thrombocytopenia
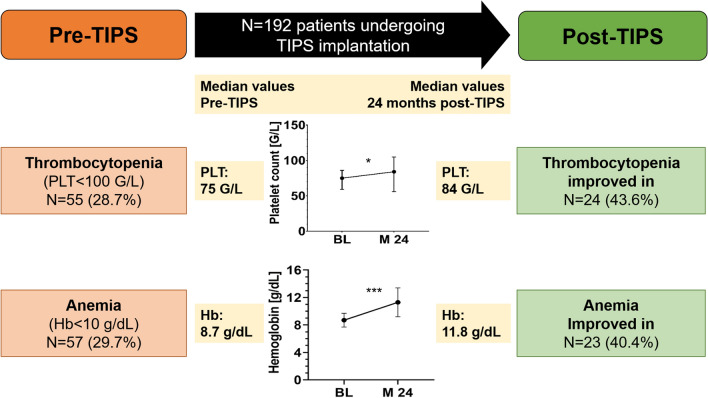
Median platelet count at baseline was 131 G/L (see online source Table-S1). Mild thrombocytopenia (G1) was present in 54 (28.1%) patients, while 46 patients (24.0%) were diagnosed with moderate (G2) and 9 patients (4.7%) with severe (G3) thrombocytopenia (Table 2). Improvement of PLT was more pronounced, the lower the pre-TIPS PLT: While only 16.7% of patients with G1 thrombocytopenia improved to G0, 43.6% of patients with G2/G3 thrombocytopenia improved by ≥ 1 stage (Fig. 1); conversely, 48.1% of patients with G1 thrombocytopenia worsened by ≥ 1 grade, but only 10.9% with G2/3 thrombocytopenia (vs. G1: p = 0.002).
In patients with G1 thrombocytopenia pre-TIPS, PLT decreased after TIPS implantation at M3, but increased again to values similar to the pre-TIPS level at M24 (Fig. 2a and b). Importantly, after TIPS implantation, PLT levels remained stable in patients with moderate (G2) thrombocytopenia at baseline, but severe (G3) thrombocytopenia improved significantly and sustainably to G2 (see also online sources Table ST1, Figure SF1).
Fig. 2.
Improvement of thrombocytopenia, anemia and leukopenia over time. a, c, e: absolute and b, c, f: relative changes in a, b: platelet count (PLT); c, d: hemoglobin and e, f: white blood cell count (WBC) over time, stratified by severity of the underlying cytopenia. Denotations: *: p < 0.05, **: p < 0.01; ***: p < 0.001
Effect of TIPS on Anemia
Mean baseline hemoglobin was 10.8 ± 1.9 g/dL (Table-S1). In total, 114 patients (59.4%) were diagnosed with mild (G1) anemia, 52 patients (27.1%) with moderate (G2) and 5 patients (2.6%) with severe (G3) anemia prior to TIPS (Fig. 2c). In patients with mild (G1) anemia, despite a slight drop at M3 by < 1 g/dL that recovered at M6, Hb remained mostly stable (Fig. 2c and d). Among patients with G2/3 anemia, Hb levels significantly and permanently improved by ≥ 1 grade in 40.4% of patients (Fig. 1, 2c and d). The proportion of patients with G2/3 anemia improved from approx. 30% at baseline to 24% at 12 months.
Changes in White Blood Cell Count (WBC) After TIPS Implantation
Leukopenia (WBC < 4G/L) was uncommon in patients undergoing PTFE-TIPS implantation, as only 17.7% were diagnosed with G1 (WBC: 2–3.99G/L), 1.5% (3 patients) with G2 (WBC: 1-2G/L) and no patient with G3 leukopenia (WBC: < 1G/L) at baseline. Approximately 60% of patients with leukopenia improved by ≥ 1 grade after TIPS, while G1 leukopenia worsened to G2/3 in one patient (Fig. 1). Overall, WBC increased significantly after TIPS implantation, and normalized in most patients in under a year (Table ST1, Fig. 2e–f).
Course of Splenomegaly After TIPS and Correlation to Blood Counts
Pre-TIPS cross-sectional images were reviewed in 133 patients. The median pre-TIPS spleen diameter was 14.5 cm (IQR: 12.5–16.25), as 115/133 (86.5%) showed pre-TIPS splenomegaly. 85 patients underwent a follow-up cross-sectional imaging scan that covered data on spleen diameter: 38 patients had a second “short-term” imaging scan within 12 months (median 4.0 months, IQR 1.3–7.3 months) with a “short-term” post-TIPS spleen diameter of 14.0 cm (p = 0.347), and 55 patients had a “long-term” (> 12 months after TIPS; median 43.7 months, IQR 19.0–70.7 months) follow-up imaging showing a median “long-term” post-TIPS spleen diameter of also 14.0 cm (p = 0.251).
Although greater pre-TIPS spleen diameter correlated with the presence of thrombocytopenia (splenomegaly > 11 cm: 66.1% vs. no splenomegaly (≤ 11 cm): 16.7%; p = 0.001, see Table-ST3) as well as with improvement of platelet count after TIPS (median spleen diameter in patients with post-TIPS increase in PLT by > 20%: 15.5 cm (IQR: 13.2–18.8 cm); stable PLT: 14.6 cm (IQR: 13.0–16.5 cm); decrease in PLT by > 20%: 14.0 cm (IQR: 12.0–15.2 cm); p = 0.0093), these effects were not observed for Hb and WBC.
Predictive Factors for Improvement of Thrombocytopenia and Anemia After TIPS
In a sub-analysis (Table ST2), we evaluated potential predictive indicators for improvement of advanced (G2/G3) thrombocytopenia and anemia after TIPS implantation. Variceal bleeding as indication for TIPS did not impact on the likelihood of post-TIPS improvement of thrombocytopenia, and neither did PPG nor liver function tests (p > 0.05 in univariate analysis). Only severity of thrombocytopenia (G3 vs. G2; p = 0.007) predicted improvement of thrombocytopenia in univariate analysis. In the multivariable model, G3 thrombocytopenia emerged as strong predictor (OR = 32.000, p = 0.003) for improving thrombocytopenia with TIPS; similarly, in a different model using PLT as a parameter instead of thrombocytopenia grade, higher PLT emerged as an independent negative predictor for improvement of PLT (OR: 0.683, p = 0.028), indicating higher response rates with lower platelet values.
Pre-TIPS variceal bleeding (p = 0.001), low baseline Hb (p = 0.026) and lower MELD-Na were associated with improvement of anemia in univariate analysis (Table-S2B). In the multivariable model only variceal bleeding as TIPS-indication (OR 5.585, p = 0.022) and low baseline hemoglobin count (per g/dL: OR 0.352, p = 0.020) remained independent predictors for improvement of anemia.
Sub-analysis of Patients Receiving TIPS for Ascites
Similar results on improvements of thrombocytopenia and anemia were seen in the subgroup of patients receiving PTFE-TIPS for ascites who do not hold the potential bias related to significant bleeding-induced cytopenia (see online sources Table-ST4, Figures-SF2).
Sub-analysis According to Etiology
When stratifying patients according to their etiology of liver disease, advanced thrombocytopenia was numerically more prevalent in viral hepatitis than in ALD or other etiologies (52.9% vs. 26.8% vs. 23.1%, respectively, p = 0.063). However, there was no difference with regards to G2/3 anemia at baseline (P = 0.724). The course of G2/3 thrombocytopenia after TIPS implantation did not differ between etiologies, and G2/3 anemia improved significantly after TIPS regardless of etiology.
Discussion
To our knowledge, our study is first to investigate the effects of TIPS on all three hematopoietic cell lines in patients who received PTFE-covered stents. Thrombocytopenia and anemia were prevalent in most patients prior to TIPS, and higher-grade (i.e., G2/3) thrombocytopenia and anemia significantly improved consistently after the procedure. While mild and moderate leukopenia also tended to improve after TIPS, the respective patient numbers were low and thus, no strong conclusion can be drawn in this regard.
In 1999, Gschwantler et al. [23] reported an improvement of PLT after TIPS implantation in comparison to a control group of patients who did not undergo TIPS. In their longitudinal prospective study, median PLT of the 55 included patients who underwent TIPS implantation increased by approximately 20% over the first year after TIPS, while PLT of the 110 controls significantly decreased. Moreover, PLT of patients with < 100G/L at baseline increased by > 25%, but none normalized. Massoud et al. [24] also reported significant increases in PLT after TIPS, averaging + 22% in a total of 74 patients undergoing TIPS implantation and reporting higher increases (+ 36%) in patients with moderate thrombocytopenia and highest increases (+ 55%) in patients with severe thrombocytopenia at baseline. We observed similar effects of PTFE-TIPS on PLT in our study with a comparable increase in PLT of + 19.8% in patients with G2/G3 thrombocytopenia (up to + 75.6% at M12 in patients with G3 thrombocytopenia). Besides the grade of thrombocytopenia at baseline, Massoud et al. [24] did not identify any meaningful predictor for improvement of thrombocytopenia after TIPS.
In contrast, Barney et al. [25] did not report a significant increase in PLT levels after TIPS but a slight improvement in Hb [25]. Although their findings are indeed in line with the results from our general study population, by stratifying patients according to cytopenia severity, we found that PLT tended to decrease in patients with PLT ≥ 100 G/L, but increased in patients with lower baseline values, especially in the relevant sub-groups of patients with G3 thrombocytopenia and anemia at baseline. Unfortunately, the study by Barney et al. [25] lacked sub-analyses and included patients with HCC, and thus, the beneficial effect of TIPS on hematology may have been masked by their pooled analysis. Ultimately, the improvement of higher-grade thrombocytopenia after TIPS implantation may suggest that severe thrombocytopenia should actually not be regarded as a contraindication for TIPS implantation (as previously proposed) [1]. In contrast, Barney et al. [25] did not report a significant increase in PLT levels after TIPS but a slight improvement in Hb [25]. Although their findings are indeed in line with the results from our general study population, by stratifying patients according to cytopenia severity, we found that PLT tended to decrease in patients with PLT ≥ 100 G/L, but increased in patients with lower baseline values, especially in the relevant sub-groups of patients with G3 thrombocytopenia and anemia at baseline. Unfortunately, the study by Barney et al. [25] lacked sub-analyses and included patients with HCC, and thus, the beneficial effect of TIPS on hematology may have been masked by their pooled analysis. Ultimately, the improvement of higher-grade thrombocytopenia after TIPS implantation may suggest that severe thrombocytopenia should actually not be regarded as a contraindication for TIPS implantation (as previously proposed).
Studies in liver-transplanted patients reported a significant reduction in spleen volume in patients with pre-transplant splenomegaly, although normalization of spleen volume was rare [29–31]. A recent study by Liu et al. [32] investigated the effects of TIPS implantation on thrombocytopenia and splenomegaly (spleen volume) in 104 patients mostly (mostly (95%) with variceal bleeding as TIPS-indication. The authors found an impressive correlation between improvement of platelet count and a decrease in splenic volume after TIPS [32] which, however, was likely affected by the underlying acute bleeding events.
Regrettably, we did not routinely measure spleen elastography nor assessed erythropoietin or thrombopoietin prior to TIPS implantation. Therefore, our retrospective study design did not allow to decipher the underlying pathophysiology responsible for the improvement of cytopenias after TIPS, such as a decrease in splenic congestion or amelioration of hyporegenerative anemia/thrombocytopenia. However, at our study centers, only patients who are decompensated with a history of recent or recurrent variceal bleeding and/or intractable/refractory ascites are referred to TIPS implantation. In case of clinical re-compensation (e.g., diuretic control of ascites [33] and/or a single variceal bleeding episode with an uncomplicated clinical course under secondary prophylaxis in a low-risk setting) in patients who present with low benefit/risk ratio (e.g., HVPG ≤ 12 mmHg), we consider the TIPS intervention to no longer be indicated. As a result, all patients included into this study presented with CSPH at time of TIPS placement, which is why we highly suspect PH and associated splenomegaly to be the major drivers in the pathogenesis of hypersplenism and cytopenia in our patient collective.
Hepatic decompensation, development of acute-on-chronic liver failure and mortality have all been linked to systemic inflammation (e.g., caused by bacterial translocation, ongoing viral infection, or alcohol intoxication) in patients with CLD [33–39]. Thus, it is to be expected that patients in need for TIPS implantation would present with elevated inflammation markers prior to TIPS, and that decompression of portal pressure would improve systemic inflammation and reduce the risk of further complications such as ACLF and mortality [40]. These effects have indeed been demonstrated both by prescription of non-cardioselective beta-blockers [36, 41] and by TIPS implantation in patients with increased risk of PH-associated complications and death [28, 42–45]. While decompression of PH by TIPS might ameliorate inflammation in some patients (by limiting bacterial translocation) and reduce splanchnic venous vascular shear stress [42], some patients do experience a further increase in splenic stiffness (which is considered a marker for portal hypertension and inflammation) after TIPS that is associated with poor post-procedural outcome and increased mortality [46]. Additionally, mechanical hemolysis of erythrocytes and platelets may impede hematologic improvement or actually worsen blood cell counts in patients undergoing TIPS, as was demonstrated by Ansari-Gilani et al. [47] who reported improvement in platelet counts only in patients with G2-3 thrombocytopenia and patent TIPS according to Doppler sonography. Mechanical destruction of blood cells could be the cause for the decline in blood cell counts in our patients without apparent hematologic hypersplenism (i.e., no or G1 cytopenias).
Since pre-TIPS blood counts can be considerably influenced by bleedings events, either directly due to blood loss/iron deficiency or indirectly by infection/inflammation-associated acute phase reactions [48], we also performed sub-analyses in patients who only presented with ascites as the sole indication for TIPS. The results were similar to those of the entire study cohort, with continuous improvement of higher-grade anemia and normalization of leukopenia after TIPS [48].
In 2011, Bureau et al. [49] recommended TIPS implantation in patients with a baseline PLT count of > 75 G/L and a bilirubin level of < 50 µmol/mL, since according to their data, lower PLT values were associated with increased post-TIPS mortality. We were not able to verify this finding in our cohort; in our multivariable model (data not shown), surprisingly, neither thrombocytopenia nor any of the MELD components (INR, bilirubin, creatinine) emerged as predictors for mortality after TIPS, but pre-TIPS PPG, ascites as indication for TIPS and low WBC count (p < 0.010). Contrariwise, cytopenia improvement did not appear to influence post-TIPS mortality, either. We hypothesize that (baseline) leukopenia is associated with more severe portal hypertension-associated hypersplenism than thrombocytopenia, considering that only patients with radiologically evident splenomegaly had WBC < 4 G/L. On the other hand, leukopenia was very rare in our cohort, as leukopenia may be masked by chronic liver injury-associated inflammation (which in itself may be associated with increased mortality) [36, 50].
Strengths of our study include the large sample size compared to previous studies and the detailed stratification of patients according to severity of thrombocytopenia and anemia. In addition, our study is the first to systematically focus on all three types of hematopoietic cell lines and their longitudinal course after TIPS implantation.
Nonetheless, our study has some limitations: First, due to its retrospective nature, some blood count results were missing. Second, we may have introduced a selection bias by not including patients with a follow-up of < 3 months (our first follow-up time point); moreover, selection bias may have aggravated over time due to loss of follow-up and mortality. Third, the stratified analysis is prone to a phenomenon termed regression toward the mean. Fourth, we had to merge G2/G3 thrombocytopenia/anemia and all grades of leukopenia for several analyses, which precluded us to evaluate the effects of TIPS in detail within the distinct groups. Finally, the study lacks a matched control group of patients without TIPS.
In conclusion, thrombocytopenia with PLT < 100 G/L, anemia with Hb < 10 g/dL, and leukopenia improved sustainably after covered TIPS. This underlines the role of advanced PH and PH-associated hypersplenism on high-grade cytopenia in cirrhosis. Therefore, we recommend that higher-grade anemia and thrombocytopenia not be regarded as absolute contraindications for TIPS implantation, but to be considered in the individual benefit/risk ratio assessment when screening for TIPS, especially in patients who are scheduled to undergo major interventions or surgery (e.g., liver transplantation) who might actually profit from pre-surgical TIPS.
Supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- PH
Portal hypertension
- ACLD
Advanced chronic liver disease
- TPO
Thrombopoietin
- PLT
Platelet count
- HVPG
Hepatic venous pressure gradient
- TIPS
Transjugular intrahepatic portosystemic shunt
- PTFE
Polytetrafluoroethylene
- Hb
Hemoglobin
- M
Month
- G
Grade
- LLN
Lower limit of normal
- MELD
Model for end-stage liver disease
- PG
Portal-systemic pressure gradient
- SD
Standard deviation
- IQR
Interquartile ratio
- SEM
Standard error of the mean
- WBC
White blood cell count
- ALD
Alcohol-related liver disease
- INR
International normalized ratio
- FU
Follow-up
- PS
Child’s Pugh’s Score
- CRP
C-reactive protein
- HE
Hepatic encephalopathy
- OHE
Overt hepatic encephalopathy
- OLT
Orthotopic liver transplantation
Author's contribution
Conceptualization was contributed by TB, KL, MM, TR. Data acquisition and analysis were contributed by TB, KL, CV, MM, TR. Writing—original draft preparation, was contributed by TB, KL, MM, TR. Resources were contributed by MS, FW, DB, BS, BS, LH, MJ, TG, FK, MM, TR. All authors read and approved the final manuscript.
Funding
Open access funding provided by Medical University of Vienna. The authors received no financial support for the research, authorship, and/or publication of this article.
Declarations
Conflict of interest
Mattias Mandorfer served as a speaker and/or consultant and/or advisory board member for W.L. Gore and Associates. Thomas Reiberger received grant support and speaking honoraria from W.L. Gore and Associates. Maria Schoder served as a speaker and/or consultant and/or advisory board member for W.L. Gore and Associates.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and its later amendments. It was approved by the Ethics Committees of the Medical University of Vienna (1760/2014) and of the City of Vienna (MA15, 14–264-VK). This research study was conducted retrospectively from data obtained for clinical purposes, and all the procedures being performed were part of the routine care. Due to the retrospective nature of the study, the need for informed patient consent was waived by the local Ethics Committees.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Association for the Study of the Liver (EASL). EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. [DOI] [PubMed]
- 2.Gibson PR, Gibson RN, Ditchfield MR, Donlan JD. Splenomegaly—an insensitive sign of portal hypertension. Aust N Z J Med. 1990;20:771–774. doi: 10.1111/j.1445-5994.1990.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 3.Von Herbay A, Frieling T, Häussinger D. Color doppler sonographic evaluation of spontaneous portosystemic shunts and inversion of portal venous flow in patients with cirrhosis. J Clin Ultrasound. 2000;28:332–339. doi: 10.1002/1097-0096(200009)28:7<332::AID-JCU3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141–155. doi: 10.1586/egh.12.83. [DOI] [PubMed] [Google Scholar]
- 5.Lv Y, Gong X, Xie X, Wang B, Yang Y, Li Y. Clinical study on the relationship between hematocytopenia and splenomegaly caused by cirrhotic portal hypertension. Cell Biochem Biophys. 2014;70:355–360. doi: 10.1007/s12013-014-9920-9. [DOI] [PubMed] [Google Scholar]
- 6.Scheiner B, Semmler G, Maurer F, Schwabl P, Bucsics TA, Paternostro R, et al. Prevalence of and risk factors for anaemia in patients with advanced chronic liver disease. Liver Int. 2020;40:194–204. doi: 10.1111/liv.14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Y, Yee Lau W, Wu H, Han XY, Gong X, Liu N, et al. Causes of peripheral cytopenia in hepatitic cirrhosis and portal hypertensive splenomegaly. Exp Biol Med. 2017;242:744–749. doi: 10.1177/1535370217693113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778–793. doi: 10.1111/liv.13317. [DOI] [PubMed] [Google Scholar]
- 10.Mandorfer M, Peck-Radosavljevic M, Ferenci P, Reiberger T. Dynamics of platelet count after sustained virologic response do not mirror those of hepatic venous pressure gradient. Liver Int. 2020;40:988–989. doi: 10.1111/liv.14273. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama S, Hirayama C, Yamamoto S, Koda M, Udagawa A, Kadowaki Y, et al. Red blood cell status in alcoholic and non-alcoholic liver disease. J Lab Clin Med. 2001;138:332–337. doi: 10.1067/mlc.2001.119106. [DOI] [PubMed] [Google Scholar]
- 12.Pfisterer N, Riedl F, Pachofszky T, Gschwantler M, König K, Schuster B, et al. Outcomes after placement of a SX-ELLA oesophageal stent for refractory variceal bleeding—a national multicentre study. Liver Int. 2019;39:290–298. doi: 10.1111/liv.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi H, Beppu T, Shirabe K, Maehara Y, Baba H. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol. 2014;20:2595–2605. doi: 10.3748/wjg.v20.i10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Franchis R, On behalf of the Baveno VI Faculty Expanding consensus in portal hypertension: report of the baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102–111.e1. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol. 2019;25:308–329. doi: 10.3748/wjg.v25.i3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiberger T, Püspök A, Schoder M, Baumann-Durchschein F, Bucsics T, Datz C, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III) Wien Klin Wochenschr. 2017;129:135–158. doi: 10.1007/s00508-017-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucsics T, Hoffman S, Grünberger J, Schoder M, Matzek W, Stadlmann A, et al. ePTFE-TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int. 2018;38:1036–1044. doi: 10.1111/liv.13615. [DOI] [PubMed] [Google Scholar]
- 19.Bucsics T, Schoder M, Diermayr M, Feldner-Busztin M, Goeschl N, Bauer D et al. Transjugular intrahepatic portosystemic shunts (TIPS) for the prevention of variceal re-bleeding – A two decades experience. PLoS One. 2018;9:1–15. [DOI] [PMC free article] [PubMed]
- 20.Peck-Radosavljevic M, Simon K, Iacobellis A, Hassanein T, Kayali Z, Tran A, et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L-PLUS 2) Hepatology. 2019;70:1336–1348. doi: 10.1002/hep.30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu K, Meng X, Qian J, Huang M, Li Z, Guan S, et al. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig Liver Dis. 2009;41:411–416. doi: 10.1016/j.dld.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Pandey UC, Jussa ZM, Qureshi IM, Al-Dhohayan A, Shareef ZA. Partial splenic embolization for the treatment of hypersplenism in tropical splenomegaly. Trop Doct. 1997;27:114–115. doi: 10.1177/004947559702700228. [DOI] [PubMed] [Google Scholar]
- 23.Gschwantler M, Vavrik J, Gebauer A, Kriwanek S, Schrutka-Kölbl C, Fleischer J, et al. Course of platelet counts in cirrhotic patients after implantation of a transjugular intrahepatic portosystemic shunt - a prospective, controlled study. J Hepatol. 1999;30:254–259. doi: 10.1016/S0168-8278(99)80071-3. [DOI] [PubMed] [Google Scholar]
- 24.Massoud OI, Zein NN. The effect of transjugular intrahepatic portosystemic shunt on platelet counts in patients with liver cirrhosis. Gastroenterol Hepatol. 2017;13:286–291. [PMC free article] [PubMed] [Google Scholar]
- 25.Barney EJ, Little EC, Gerkin RD, Ramos AX, Kahn J, Wong M, et al. Coated transjugular intrahepatic portosystemic shunt does not improve thrombocytopenia in patients with liver cirrhosis. Dig Dis Sci. 2012;57:2430–2437. doi: 10.1007/s10620-012-2162-z. [DOI] [PubMed] [Google Scholar]
- 26.UNOS. OPTN/UNOS policy note. 29072019 2016:1–23. https://optn.transplant.hrsa.gov/media/1575/policynotice_20151101.pdf Accessed 13.12.2020.
- 27.Peck-Radosavljevic M, Angermayr B, Datz C, Ferlitsch A, Ferlitsch M, Fuhrmann V, et al. Austrian consensus on the definition and treatment of portal hypertension and its complications (Billroth II) Wien Klin Wochenschr. 2013;125:200–219. doi: 10.1007/s00508-013-0337-z. [DOI] [PubMed] [Google Scholar]
- 28.Bucsics T, Schoder M, Goeschl N, Schwabl P, Mandorfer M, Diermayr M, et al. Re-bleeding rates and survival after early transjugular intrahepatic portosystemic shunt (TIPS) in clinical practice. Dig Liver Dis. 2017;49:1360–1367. doi: 10.1016/j.dld.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Chezmar JL, Redvanly RD, Nelson RC, Henderson JM. Persistence of portosystemic collaterals and splenomegaly on CT after orthotopic liver transplantation. Am J Roentgenol. 1992;159:317–320. doi: 10.2214/ajr.159.2.1632346. [DOI] [PubMed] [Google Scholar]
- 30.Se HK, Lee JM, Jin YC, Suh KS, Yi NJ, Joon KH et al. Changes of portosystemic collaterals and splenic volume on CT after liver transplantation and factors influencing those changes. Am J Roentgenol. 2008;191:W8–W16. [DOI] [PubMed]
- 31.Chikamori F, Nishida S, Selvaggi G, Tryphonopoulos P, Moon JI, Levi DM, et al. Effect of liver transplantation on spleen size, collateral veins, and platelet counts. World J Surg. 2010;34:320–326. doi: 10.1007/s00268-009-0314-x. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Ma J, Yang C, Ye T, Meng J, Shi Q, et al. Impact of TIPS on splenic volume and thrombocytopenia. Am J Roentgenol. 2021:1–6. [DOI] [PubMed]
- 33.Monteiro S, Grandt J, Uschner FE, Kimer N, Madsen JL, Schierwagen R, et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021;70:379–387. doi: 10.1136/gutjnl-2019-320170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardi M, Moreau R, Angeli P, Schnabl B. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2021;74:819–828. doi: 10.1016/j.jhep.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Jachs M, Hartl L, Schaufler D, Desbalmes C, Simbrunner B, Eigenbauer E et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta-blocker therapy translates into improved clinical outcomes. Gut. 2021;70:1758–1767. [DOI] [PubMed]
- 37.Ferstl P, Trebicka J. Acute decompensation and acute-on-chronic liver failure. Clin Liver Dis. 2021;25:419–430. doi: 10.1016/j.cld.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Arroyo V, Angeli P, Moreau R, Jalan R, Clària J, Trebicka J, et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74:670–685. doi: 10.1016/j.jhep.2020.11.048. [DOI] [PubMed] [Google Scholar]
- 39.Mandorfer M, Simbrunner B. Prevention of first decompensation in advanced chronic liver disease. Clin Liver Dis. 2021;25:291–310. doi: 10.1016/j.cld.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Trebicka J. Emergency TIPS in a Child-Pugh B patient: when does the window of opportunity open and close? J Hepatol. 2017;66:442–450. doi: 10.1016/j.jhep.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Queck A, Carnevale R, Uschner FE, Schierwagen R, Klein S, Jansen C, et al. Role of portal venous platelet activation in patients with decompensated cirrhosis and TIPS. Gut. 2019;69:20–21. doi: 10.1136/gutjnl-2019-319044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 44.Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 45.Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082–1091. 10.1016/j.jhep.2020.04.024. [DOI] [PubMed]
- 46.Jansen C, Möller P, Meyer C, Kolbe CC, Bogs C, Pohlmann A, et al. Increase in liver stiffness after transjugular intrahepatic portosystemic shunt is associated with inflammation and predicts mortality. Hepatology. 2018;67:1472–1484. doi: 10.1002/hep.29612. [DOI] [PubMed] [Google Scholar]
- 47.Ansari-Gilani K, Tonekaboni BS, Nakamoto DA, Esfeh JM. Utility of Doppler ultrasonography for predicting improvement of platelet count after transjugular intrahepatic portosystemic shunt. Gastroenterol Rep. 2017;5:305–308. doi: 10.1093/gastro/gow031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simbrunner B, Beer A, Wöran K, Schmitz F, Primas C, Wewalka M, et al. Portal hypertensive gastropathy is associated with iron deficiency anemia. Wien Klin Wochenschr. 2020;132:1–11. doi: 10.1007/s00508-019-01593-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bureau C, Métivier S, D’Amico M, Péron JM, Otal P, Pagan JCG, et al. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPS. J Hepatol. 2011;54:901–907. doi: 10.1016/j.jhep.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 50.Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2020;74:819–828. doi: 10.1016/j.jhep.2020.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.