Abstract
Lyme disease, a chronic multisystemic disorder that can affect the skin, heart, joints, and nervous system is caused by Borrelia burgdorferi sensu lato. Lyme disease spirochetes were previously shown to bind glycosaminoglycans (GAGs). In the current study, the GAG-binding properties of eight Lyme disease strains were determined. Binding by two high-passage HB19 derivatives to Vero cells could not be inhibited by enzymatic removal of GAGs or by the addition of exogenous GAG. The other six strains, which included a different high-passage HB19 derivative (HB19 clone 1), were shown to recognize both heparan sulfate and dermatan sulfate in cell-binding assays, but the relative efficiency of binding to these two GAGs varied among the strains. Strains N40, CA20-2A, and PBi bound predominantly to heparan sulfate, PBo bound both heparan sulfate and dermatan sulfate roughly equally, and VS461 and HB19 clone 1 recognized primarily dermatan sulfate. Cell binding by strain HB19 clone 1 was inhibited better by exogenous dermatan sulfate than by heparin, whereas heparin was the better inhibitor of binding by strain N40. The GAG-binding preference of a Lyme disease strain was reflected in its cell-type-specific binding. Strains that recognized predominantly heparan sulfate bound efficiently to both C6 glioma cells and EA-Hy926 cells, whereas strains that recognized predominantly dermatan sulfate bound well only to the glial cells. The effect of lyase treatment of these cells on bacterial binding was consistent with the model that cell-type-specific binding was a reflection of the GAG-binding preference. We conclude that the GAG-binding preference varies with the strain of Lyme disease spirochete and that this variation influences cell-type-specific binding in vitro.
Lyme borreliosis, caused by the spirochete Borrelia burgdorferi sensu lato, is a chronic, multisystemic illness found in Europe, Asia, and North America (30). The bacterium is introduced into the human host by the bite of the Ixodes tick, and local infection of the skin results in the characteristic rash, erythema migrans. After this local infection, the spirochete can disseminate via the blood to other sites, including the joints, heart, nervous system, and skin. The bacterium can survive for long periods of time in some of these tissues, giving rise to chronic manifestations of Lyme disease, such as arthritis, neuroborreliosis, and acrodermatitis.
The molecular mechanisms that promote the infection of specific tissues by the spirochete or its survival in the mammalian hosts are not well understood. The adherence of bacteria to host tissues is the first step in the establishment of many infections (10), and B. burgdorferi is known to attach to a wide variety of cell types in vitro, such as lymphocytes (7), platelets (11), epithelial cells (32), endothelial cells (6, 31), and neuroglia (13). B. burgdorferi recognizes several classes of host cell components, including integrins (3, 4), glycolipids (12), and proteoglycans (15, 17, 22, 24).
Proteoglycans are composed of a protein core covalently linked to glycosaminoglycans (GAGs). GAGs are long, linear, highly sulfated heteropolymers of hexosamine residues alternating with another sugar, usually a uronic acid (20, 29). Based on the identity of the hexosamine, the common GAG chains are divided into two groups, glucosaminoglycans and galactosaminoglycans. Each group can be further subdivided into different classes on the basis of epimerization of the glycan chain and the extent and location of the sulfate groups. Thus, heparan sulfate (and the more highly sulfated model analog, heparin) is a glucosaminoglycan, while chondroitin-4-sulfate (also known as chondroitin sulfate A), chondroitin-6-sulfate (chondroitin sulfate C), and dermatan sulfate (chondroitin sulfate B) comprise the galactosaminoglycans. The different classes of GAGs are often distinguished from each other experimentally on the basis of their sensitivity to different lyases (20).
Several isolates of B. burgdorferi recognize heparan sulfate and dermatan sulfate for cell binding (17, 22, 24). GAGs are ubiquitously expressed by mammalian cells and thus could promote bacterial attachment to many tissues colonized by B. burgdorferi. Consistent with this hypothesis, GAGs were shown to promote binding of B. burgdorferi N40 clone D10/E9 to diverse cell types in vitro, including endothelial, glial, and neuronal cells (24). The particular class of GAG that was critical for bacterial attachment, however, varied with cell type:heparan sulfate mediated attachment to endothelial cells, while both heparan sulfate and dermatan sulfate participated in attachment to glial cells (24). In the current study, we examined the recognition of GAGs by several strains of Lyme disease spirochete. The results indicate that GAG-binding preferences vary among strains and that these differences in GAG binding result in differences in cell-type-specific binding.
MATERIALS AND METHODS
Bacterial strains and cell lines.
The strains used in this study are described in Table 1. All strains of Borrelia spp. were cultured in MKP base medium (MKP-S) (26) supplemented with 7% human serum, as described previously (3, 24). The cell lines used in this study are described in Table 2. Vero (monkey kidney epithelial) cells were cultured in RPMI 1640 supplemented with 10% NuSerum (Collaborative Research). C6 (rat) glioma cells were cultured in RPMI 1640 supplemented with 8% fetal bovine serum (FBS), and EA-Hy926 cells were cultured in Dulbecco modified Eagle medium (DMEM; high glucose) supplemented with 1% hypoxanthine-aminopterin-thymidine (Gibco-BRL, Bethesda, Md.) and 10% FBS. Each of the above cell lines was grown at 37°C in an atmosphere containing 5% CO2. 293 (human kidney) cells were cultured in a 1:1 mix of DMEM (low glucose; Gibco-BRL) and Ham’s F12 medium (Gibco-BRL) supplemented with 10% FBS and grown at 37°C in an atmosphere containing 7% CO2.
TABLE 1.
Bacterial strains used in this study
| Straina | No. of passagesb | Source or reference |
|---|---|---|
| B. burgdorferi sensu stricto | ||
| N40 clone D10/E9 | 8 | 5, 21 |
| HB19-W | High | A. Barbour (28) |
| HB19-R1 | High | A. Barbour (28) |
| HB19 clone 1 | High | 21 |
| CA20-2A | 6 | P. Rosa (5, 27) |
| B. garinii PBi | 8 | V. Preac-Mursic (5, 34) |
| B. afzelii PBo | 8 | V. Preac-Mursic (5, 34) |
| B. afzelii VS461 | 13 | V. Preac-Mursic (5, 25) |
The Lyme disease spirochetes B. burgdorferi sensu stricto, B. garinii, and B. afzelii are members of B. burgdorferi sensu lato (33).
High, greater than 50 passages.
TABLE 2.
Cell lines used in this study
| Cell line | Cell type | Predominant GAG recognized by B. burgdorferi N40a | Source or reference |
|---|---|---|---|
| Vero | Kidney fibroblast-like | Heparan sulfate | ATCC (CCL 81) |
| 293 | Kidney epithelial | Dermatan sulfate | ATCC (CRL 1573) |
| C6 | Glial | Dermatan and heparan sulfate | ATCC (CCL 107) |
| EA-Hy926 | Endothelial | Heparan sulfate | 8 |
Identified by effect of specific lyase digestion of monolayers on binding by B. burgdorferi N40 (24).
Labeling of B. burgdorferi.
Radiolabeled B. burgdorferi strains were prepared by growth in modified MKP medium supplemented with 50 to 100 μCi of [35S]methionine and [35S]cysteine per ml. Briefly, 100 ml of methionine-free RPMI 1640 with l-glutamine was supplemented with 0.6 g of HEPES, 0.07 g of sodium citrate, 0.3 g of dextrose, 0.08 g of sodium pyruvate, 0.04 g of N-acetylglucosamine, and 0.2 g of sodium bicarbonate and adjusted to pH 7.6. Then, 20 ml of 7% gelatin, 7 ml of pooled human sera, 6.1 ml of 20% bovine serum albumin (BSA), and 3.75 ml of 8% neopeptone, all of which had been dialyzed against phosphate-buffered saline (PBS), were added to the medium. This methionine-free medium was supplemented with 35S-labeled protein labeling mix (NEG-072; NEN Dupont, Wilmington, Del.) to a final concentration of 50 to 100 μCi/ml and then sterilized by filtration through a 0.22-μm (pore size) filter. B. burgdorferi cultures were concentrated by centrifugation and added to the labeling medium at a concentration of approximately 5 × 108 bacteria per ml. After 6 to 8 h of growth at 33°C, the culture was diluted 1:10 in MKP medium and cultured overnight at 33°C. The bacteria were washed, concentrated by centrifugation for 15 min at 15,000 × g, washed again, and stored as aliquots at −80°C in MKP-S containing 20% glycerol, as described earlier (3).
GAG-mediated attachment of labeled spirochetes to mammalian cells. (i) Bacterial binding assays.
One to two days prior to each assay, the mammalian cells to be tested were lifted with 0.05% trypsin–0.53 mM EDTA (Gibco-BRL) and plated in Nunc 96-well breakapart microtiter plates coated with Yersinia pseudotuberculosis invasin protein, which promotes cell attachment by binding a subset of β1-chain integrins (18). Just prior to the addition of bacteria, confluent cell monolayers were washed twice in PBS (150 mM NaCl, 16.9 mM K2HPO4, 4.8 mM KH2PO4; pH 7.4). Frozen aliquots of radiolabeled bacteria were thawed, suspended at 1 × 108 to 2 × 108/ml in MKP-S, and incubated for 2 h at room temperature to allow for physiologic recovery of the bacteria. Dark-field microscopy indicated that in all cases, more than 90% of the spirochetes showed intact morphology and vigorous motility. The bacteria were diluted 1:3 into 10 mM HEPES–10 mM glucose–50 mM NaCl (pH 7.0) and added to quadruplicate wells at approximately 106 bacteria/well. To promote host cell-bacterium contact, the microtiter plates were centrifuged at 190 × g for 5 min at 20°C and then rocked at 20°C for 1 h. Unbound bacteria were removed by washing the monolayers three times in PBS supplemented with 0.2% BSA. After the integrity of the monolayers was confirmed microscopically, the plates were air dried, and bound bacteria in each well were quantitated by liquid scintillation. For each assay, the bacterial binding to identically treated wells without mammalian cells was determined, and this value was always less than 2%.
(ii) Inhibition with exogenous GAGs or an inhibitor of proteoglycan synthesis.
To test the effect of exogenous GAGs on bacterial attachment, radiolabeled bacteria were incubated for 30 min at room temperature in MKP-S supplemented with various concentrations of GAGs and diluted 1:3 into 10 mM HEPES–10 mM glucose–50 mM NaCl (pH 7.0) prior to the addition of bacteria to monolayers. Heparin, chondroitin-4-sulfate, chondroitin-6-sulfate, and dermatan sulfate were purchased from Sigma Chemical Co. (St. Louis, Mo.). To inhibit the addition of heparan sulfate and chondroitin sulfate GAGs to the protein core of proteoglycans, mammalian cells were cultured overnight in medium supplemented with 5 mM p-nitrophenyl-β-d-xyloside (Sigma) or, as a control, 5 mM p-nitrophenyl-α-d-galactoside (19, 24).
(iii) Inhibition by enzymatic removal of specific classes of GAG.
The effect of enzymatic removal of different classes of GAGs on bacterial attachment was determined as previously described (22). Briefly, monolayers were incubated with 0.5 U of heparinase I, heparitinase (heparinase III), or chondroitinase ABC (all from Sigma) per ml for 2 h at 37°C in RPMI 1640 supplemented with 1% BSA, 10−2 trypsin inhibitory units of aprotinin per ml, and 150 μg of phenylmethylsulfonyl fluoride per ml. The conditions for lyase treatment were previously shown to (i) specifically release 35S-labeled GAG from the monolayer surface (22) and (ii) result in maximal inhibition of binding to 293 and C6 cells, i.e., a level of binding indistinguishable from the binding to empty wells or to cells treated with an inhibitor of proteoglycan synthesis (e.g., see Fig. 5). In addition, multiple B. burgdorferi strains were tested in parallel, and each lyase inhibited binding by at least one strain, indicating that all lyases were enzymatically active in each experiment. Each strain was assayed on at least three separate occasions.
FIG. 5.
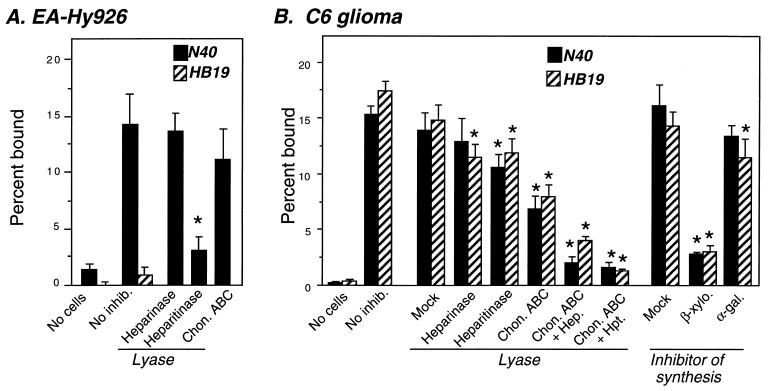
Different GAG binding preferences of Lyme disease spirochetes lead to differences in cell-type-specific binding. (A) Empty wells, untreated, or lyase-treated EA-Hy926 cells were incubated with radiolabeled strain N40 (solid bars) or HB19 clone 1 (hatched bars), and the percent bound bacteria was determined (see Materials and Methods). Each bar represents the average ± the SD of four determinations. (B) Empty wells (No cells) and untreated (No inhib.), mock-, or lyase-treated C6 glioma cells or C6 glioma cells grown in the presence of the GAG synthesis inhibitor p-nitrophenyl-β-d-xyloside or the control sugar p-nitrophenyl-α-d-galactoside were incubated with radiolabeled strain N40 (solid bars) or HB19 clone 1 (hatched bars), and the percent bound bacteria was determined. Significant (P < 0.05) differences in binding to mock- versus lyase- or β-d-xyloside-treated monolayers were determined by t-test analysis and are indicated by asterisks.
(iv) Statistical analysis.
The statistical significance of differences in bacterial binding after mock versus lyase or xyloside treatment of monolayers or in the presence versus the absence of exogenous GAG was determined by two-tailed t-test analysis with Microsoft Excel version 4.0.
RESULTS
Lyme disease spirochetes vary in their dependence on GAGs for attachment to Vero cells.
B. burgdorferi N40 clone D10/E9 (herein simply referred to as N40), a low-passage infectious strain, was previously shown to bind Vero cell GAGs much more efficiently than HB19 clone 1, a high-passage noninfectious strain (22). To test whether other Lyme disease strains varied in their ability to recognize GAGs, we examined GAG binding by a collection of seven other Lyme disease strains, which included other high-passage derivatives of HB19, as well as genetically diverse Lyme disease spirochetes (Tables 1 and 3). We initially tested GAG-binding by using the fibroblast-like Vero cells, a cell line we have previously used extensively for this purpose (22).
TABLE 3.
Heparin and dermatan sulfate inhibit Vero cell binding by a subset of Lyme disease spirochetes
| Inhibitor | IC50 (μg/ml)a for strain:
|
||||||
|---|---|---|---|---|---|---|---|
| N40 | CA20-2A | PBi | VS461 | HB19-W | HB19-R1 | HB19 clone 1 | |
| Heparin | 8.0 | 1.9 | 3.0 | 0.4 | >500 | >500 | NAb |
| Dermatan sulfate | 32 | 32 | 50 | 35 | >500 | >500 | NA |
| Chondroitin-6-sulfate | >500 | >500 | >500 | >500 | >500 | >500 | NA |
| Chondroitin-4-sulfate | >500 | >500 | >500 | >500 | >500 | >500 | NA |
Attachment of B. burgdorferi sensu lato strains to Vero cells was determined in the presence of various concentrations of GAG (see Materials and Methods). IC50 indicates the estimated concentration of GAG at which bacterial binding is 50% of the level of binding in the absence of inhibitor. The percentages of spirochetes bound in the absence of inhibitors were as follows: N40, 21.4 ± 1.3%; CA20-2A, 7.1 ± 0.8%; PBi, 11.4 ± 0.8%; VS461, 17.3 ± 2.0%; HB19-W, 36.1 ± 0.9%; HB19-OspR1, 32.3 ± 1.4%.
NA, not applicable (this strain does not bind Vero cells [22]).
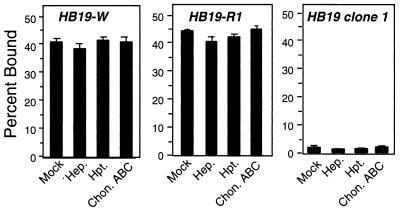
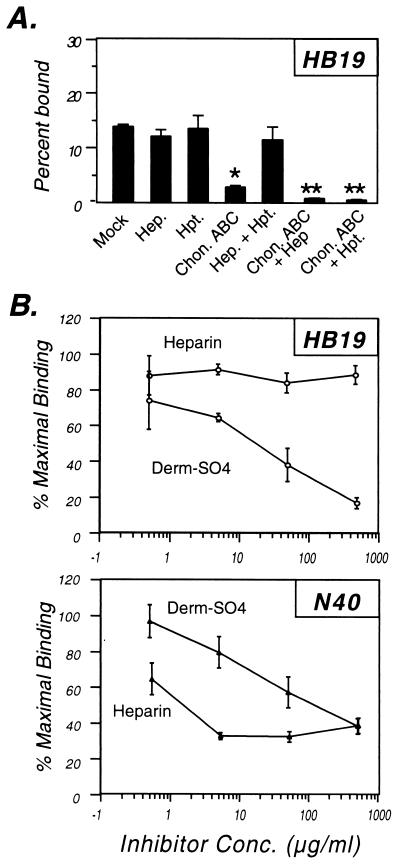
Binding by a different high-passage clone of HB19 (herein referred to as HB19-W) and HB19-R1 (a derivative of HB19-W that does not express the major outer surface proteins OspA or OspB [28]) bound very well to Vero cells, in contrast to HB19 clone 1 (Fig. 1). Exogenous GAGs did not inhibit binding by HB19-W and HB19-R1 (Table 3), suggesting that these strains may bind to Vero cells in a GAG-independent manner. Consistent with this hypothesis, the enzymatic removal of different classes of GAG with specific lyases had no effect on cell binding by these strains, in spite of the fact that parallel digestions of Vero cells significantly diminished binding by other Lyme disease strains (Fig. 1; see also Fig. 2 and 3). Combining lyase treatment with two other treatments to inhibit GAG-mediated attachment (i.e., the inhibition of proteoglycan synthesis with β-xyloside and the addition of the GAG-binding protein platelet factor 4) also had no effect on binding by HB19-W and HB19-R1 (data not shown). The binding phenotypes of the three HB19 derivatives suggest that different derivatives of the same strain differ significantly in their cell-binding activities and that HB19-W and HB19-R1 bind Vero cells predominantly via a GAG-independent mechanism.
FIG. 1.
High-passage derivatives of strain HB19 display no GAG-dependent binding to Vero cells but differ in their cell attachment activity. Prior to the addition of radiolabeled bacteria, Vero cells were mock treated or were treated with the indicated lyase (see Materials and Methods). Hep., heparinase digestion; Hpt., heparitinase digestion; Chon. ABC, chondroitinase ABC digestion. To ensure that all lyases were enzymatically active in each experiment, multiple strains were tested in parallel, and each lyase inhibited binding by at least one strain (see Fig. 2 and Materials and Methods). Each bar represents the average ± the standard deviation (SD) of four determinations.
FIG. 2.
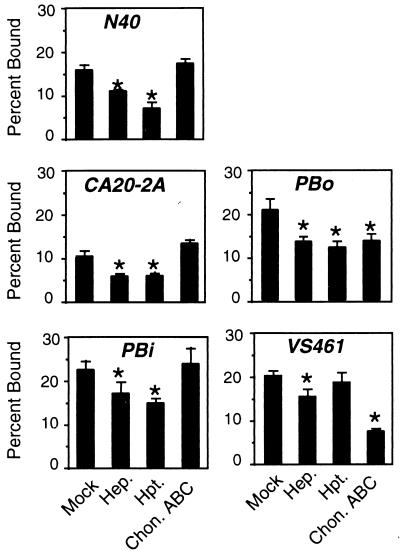
The species of GAGs that promote attachment of Lyme disease spirochetes to Vero cells varies with bacterial strain. Prior to the addition of radiolabeled bacteria, Vero cells were mock treated or were treated with the indicated lyase. Hep., heparinase digestion; Hpt., heparitinase digestion; Chon. ABC, chondroitinase ABC digestion. Controls to ensure that all lyases were enzymatically active in each experiment were performed as described in the Fig. 1 legend and in Materials and Methods. Each bar represents the average ± the SD of four determinations. Significant (P < 0.05) differences in binding to mock- versus lyase-treated monolayers were determined by t-test analysis and are indicated by asterisks. Each strain was assayed on at least three separate occasions, and a representative experiment is shown.
FIG. 3.
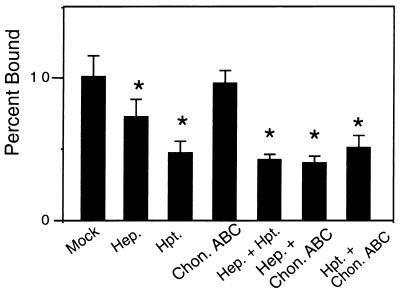
A component of N40 attachment to Vero cells is independent of GAGs. Vero cells were mock treated or were treated with the indicated lyases prior to the addition of radiolabeled strain N40. Hep., heparinase digestion; Hpt., heparitinase digestion; Chon. ABC, chondroitinase ABC digestion. Each bar represents the average ± the SD of four determinations. Significant (P < 0.05) differences in binding to mock- versus lyase-treated monolayers were determined by t-test analysis and are indicated by asterisks.
Vero cell binding by the other strains (N40, CA20-2A, PBi, and VS461) was blocked by exogenous GAG, and in each case, the 50% inhibitory concentration (IC50) for the heparin (a heparan sulfate analog) was lower than for the dermatan sulfate (Table 3). The more potent inhibitory activity of heparin compared to dermatan sulfate in this assay could simply reflect the greater negative charge of heparin. In order to determine the preference of GAG recognition among these strains, Vero cell attachment by a number of spirochete strains was measured after enzymatic cleavage of specific classes of GAGs by using various lyases. Heparinase was used for the removal of heparin-related GAGs, heparitinase for heparan sulfate-related GAGs, and chondroitinase ABC for dermatan and chondroitin sulfates. In addition to examining N40, CA20-2A, PBi, and VS461, the GAG-binding preference of B. afzelii PBo was assessed.
As previously shown, heparin/heparan sulfate GAGs primarily mediated attachment of strain N40 to Vero cells, because attachment was inhibited by treatment with heparinase or heparitinase, while chondroitinase ABC had no effect (reference 22 and Fig. 2). The inhibition of binding by heparinase or heparitinase digestion was not complete, likely reflecting the expression of a GAG-independent binding pathway by this strain (4, 14). Consistent with this hypothesis, combination lyase digestion had no greater inhibitory effect than heparinase or heparitinase digestion alone (Fig. 3). The mechanism of Vero cell attachment by strains CA20-2A and PBi resembled that of strain N40, in that binding by these strains was also inhibited better by heparinase and heparitinase than by chondroitinase ABC (Fig. 2).
Analysis of the last two strains, PBo and VS461, indicated alternate GAG-binding preferences: attachment of both strains was inhibited by chondroitinase ABC digestion, indicating that dermatan or chondroitin sulfates were required for maximal binding (Fig. 2). Heparinase and heparitinase digestion of Vero cells also resulted in a significant decrease in binding by strain PBo, indicating a mixed GAG binding preference for this strain. VS461 binding was inhibited only slightly by heparinase, suggesting that dermatan or chondroitin sulfates are likely to play the more important role than heparan sulfate in host cell recognition by this strain.
High-passage B. burgdorferi clone 1 recognizes dermatan sulfate better than heparin.
The high-passage strain B. burgdorferi HB19 clone 1 was previously demonstrated to express very little heparin-binding activity and to attach inefficiently to Vero cells (Fig. 1 and reference 22). The finding that different Lyme disease strains express variant GAG-binding preferences raised the possibility that HB19 clone 1 may bind GAGs but not the specific GAGs that are expressed by Vero cells. Indeed, as previously shown (4), this strain efficiently bound 293 cells (14.5% of inoculum bound) (Fig. 4A). Dermatan or chondroitin sulfate primarily mediated bacterial attachment to 293 cells, because digestion with chondroitinase ABC diminished bacterial binding, whereas digestion with heparinase or heparitinase alone had little effect. Nevertheless, HB19 clone 1 apparently has the ability to (weakly) bind heparin or heparan sulfate, because heparinase or heparitinase when used in combination with chondroitinase ABC had significant inhibitory effects on HB19 clone 1 attachment (Fig. 4A).
FIG. 4.
Strains N40 and HB19 clone 1 demonstrate differences in their preferences for dermatan sulfate or heparin. (A) 293 cells were mock treated or were treated with the indicated lyase(s) prior to the addition of radiolabeled bacteria, and the percent bound bacteria was determined (see Materials and Methods). Hep., heparinase digestion; Hpt., heparitinase digestion; Chon. ABC, chondroitinase ABC digestion. Each bar represents the average ± the SD of four determinations. Significant (P < 0.05) differences in binding to mock- versus chondroitinase-treated monolayers were determined by two-tailed t-test analysis and are indicated by single asterisks. Double asterisks indicate significant differences in binding to chondroitinase versus combination lyase digestions. (B) Attachment of strain N40 and HB19 clone 1 was determined in the presence of various concentrations of heparin or dermatan sulfate (see Materials and Methods). Binding is expressed relative to the binding in the absence of inhibitor. In the experiment shown, the binding of N40 in the absence of inhibitor was 28.1% of the inoculum, and binding of HB19 clone 1 was 16.4%.
That HB19 clone 1 preferentially binds dermatan sulfate was further demonstrated by inhibition of 293 cell attachment with exogenous GAGs. Dermatan sulfate was a much better inhibitor of HB19 clone 1 attachment than was heparin (Fig. 4B; Table 4). In contrast, N40 attachment to 293 cells was inhibited better by heparin than by dermatan sulfate.
TABLE 4.
Different GAG inhibition profiles expressed by two B. burgdorferi strains
| Inhibitor | IC50 (μg/ml)a for strain:
|
|
|---|---|---|
| N40 | HB19 clone 1 | |
| Heparin | 1.3 | >500 |
| Dermatan sulfate | 60 | 18 |
| Chondroitin-6-sulfate | >500 | >500 |
| Chondroitin-4-sulfate | >500 | >500 |
Attachment of B. burgdorferi N40 and HB19 to 293 cells was determined in the presence of various concentrations of GAG (see Materials and Methods). IC50 indicates the estimated concentration of GAG at which bacterial binding is 50% of the level of binding in the absence of inhibitor. The percentages of the spirochetes bound in the absence of inhibitors were as follows: N40, 28.3 ± 2.8%; HB19 clone 1, 18.1 ± 2.7%.
B. burgdorferi strains that express different GAG binding preferences display different binding preferences for cultured glial and endothelial cells.
It was previously shown that the binding of B. burgdorferi N40 to cultured endothelial cells was mediated primarily by heparan sulfate, whereas binding to glial or neural cells was mediated by both heparan and dermatan sulfates (Table 2 and reference 24). Thus, N40 bound efficiently to the endothelial cell line EA-Hy926 in a heparitinase-inhibitable manner (Fig. 5A) and to C6 glioma cells in a heparitinase- and chondroitinase-inhibitable manner (Fig. 5B).
We postulated that preferential recognition of dermatan sulfate by strains such as HB19 clone 1 could result in the preferential attachment to glial cells compared to endothelial cells. Indeed, HB19 clone bound well to C6 cells but not to EA-Hy926 cells (Fig. 5). GAG binding was responsible for HB19 clone 1 attachment to C6 glioma cells because attachment was almost completely inhibited by β-xyloside, an inhibitor of GAG synthesis, or by digestion with a combination of heparitinase and chondroitinase ABC (Fig. 5B). Chondroitinase ABC digestion had a somewhat greater effect on bacterial attachment than did heparitinase digestion. The critical GAG chain removal from the glial cells by chondroitinase ABC is likely to be dermatan sulfate, because exogenous dermatan sulfate inhibited HB19 clone 1 attachment to these cells, whereas chondroitin-4-sulfate or chondroitin-6-sulfate did not (data not shown). These results suggest that both N40 and HB19 clone 1 can bind glial cells primarily by recognizing dermatan sulfate, but HB19 clone 1, by virtue of its relative inability to recognize heparan sulfate, is not able to bind to EA-Hy926 cells.
If the selective binding to glial cells by HB19 clone 1 and the “promiscuous” binding to both glial and endothelial cells by N40 are reflections of their GAG-binding preferences, then the GAG-binding preferences of the other strains characterized in this study should also correlate with selective or promiscuous binding. Assessment of the binding of strains PBi, PBo, and VS461 to C6 and EA-Hy926 cells confirmed this prediction. On the basis of lyase digestion, VS461 bound Vero cells primarily via dermatan sulfate (Fig. 2), and this strain, like HB19 clone 1, bound to C6 glioma cells but not EA-Hy926 cells (Table 5). Strain PBi, like N40, recognized primarily heparan sulfate on Vero cells and bound well to both C6 and EA-Hy926 cells. Strain PBo displayed a mixed GAG-binding preference on Vero cells, requiring both dermatan sulfate and heparan sulfate for maximal binding—this strain also expressed an intermediate selectivity of cell attachment, binding to glial cells efficiently and to EA-Hy926 cells poorly but above the background levels. Assessment of cell binding after lyase digestion of C6 and EA-Hy926 cells revealed that, as predicted, chondroitin or dermatan sulfates played a more important role than heparan sulfate in C6 glioma attachment by all of the strains (Table 5). Similarly, for the two strains proficient at binding EA-Hy926 cells (PBi and PBo), endothelial cell attachment depended upon heparan sulfate.
TABLE 5.
GAG-binding preference and differential binding to glial and endothelial cells
| Straina | Vero cell GAG recognizedb | C6 glioma
|
EA-Hy926
|
||||
|---|---|---|---|---|---|---|---|
| % Boundc | % Inhibitiond
|
% Boundc | % Inhibitione
|
||||
| Heparinase | Chondroitinase ABC | Heparinase | Chondroitinase ABC | ||||
| N40 | Hep-SO4 | 18.2 ± 1.9 | 30.1* | 47.2* | 13.6 ± 1.6 | 80.7* | 18.1 |
| PBi | Hep-SO4 | 40.4 ± 2.7 | 37.7* | 42.1* | 16.3 ± 3.2 | 58.8* | 0.0 |
| PBo | Derm-SO4, Hep-SO4 | 18.8 ± 2.4 | 39.3* | 49.3* | 4.0 ± 0.2 | 51.9* | 4.1 |
| VS461 | Derm-SO4 > Hep-SO4 | 16.0 ± 2.5 | 42.1* | 50.2* | 2.3 ± 1.7 | NA | NA |
| HB19 clone 1 | None | 14.9 ± 1.3 | 22.7* | 46.5* | 0.4 ± 0.4 | NA | NA |
Similar results for N40 and HB19 were presented in Fig. 5; these results are included in this table for ease of comparison with other strains.
The putative class(es) of GAG that is an important mediator of bacterial attachment to Vero cells was inferred by the effect (or lack of effect) of heparinase or chondroitinase ABC digestion on bacterial binding (see Fig. 1 and 2). (For simplicity of presentation, the putative GAG that mediates bacterial attachment rather than the inhibitory lyase is given). Hep-SO4, binding was significantly (P < 0.05) inhibited by digestion of the monolayer with heparinase or heparitinase; Derm-SO4, binding was significantly inhibited by chondroitinase ABC; Derm-SO4 > Hep-SO4, both classes of lyase significantly inhibited binding, and chondroitinase ABC had a greater inhibitory effect than heparinase; None, binding was not significantly higher than to empty wells.
Binding of radiolabeled bacteria to confluent monolayers was quantitated as described in Materials and Methods. Each datum point represents the mean ± the SD of four replicate samples. Less than 2% of bacteria bound to identically treated wells without mammalian cells (not shown).
Binding to cells was measured after treatment of monolayers with the indicated lyase. For all strains, binding was inhibited by both chondroitinase ABC and heparinase digestion. Significant (P < 0.05) differences in binding to mock- versus lyase-treated monolayers were determined by t-test analysis and are indicated by asterisks.
Binding to cells was measured after treatment of monolayers with the indicated lyase. For the three strains that bound significantly above the background levels (N40, PBi, and PBo), binding to EA-Hy926 cells was significantly inhibited by heparinase digestion but not by chondroitinase ABC digestion. Significant (P < 0.05) differences in binding to mock- versus lyase-treated monolayers were determined by t-test analysis and are indicated by asterisks.
DISCUSSION
Initiation and maintenance of infection of a host by various pathogens often involves interactions between microbial and eukaryotic surface components (10). B. burgdorferi has been shown to recognize GAGs on the surface of cultured mammalian cells (17, 22). GAGs are ubiquitously expressed on the surface of mammalian cells and in extracellular matrix, and we undertook this study to investigate whether the preference for different species of GAG varies among Lyme disease spirochetes and to examine the potential contribution of GAG recognition to cell-type-specific attachment.
Diverse Lyme disease spirochetes were tested for GAG recognition. Lyme disease spirochetes express multiple pathways for attachment to cells and matrix (3, 12, 15), and two high-passage derivatives of HB19 (HB19-W and HB19-R1) bound well to Vero cells in a GAG-independent manner. Another high-passage derivative of HB19 (HB19 clone 1) did not bind Vero cells at all. Thus, none of the high-passage HB19 derivatives could be demonstrated to recognize Vero cell GAGs. Isaacs previously used HeLa cells to show that low-passage HB19 expressed a GAG-binding activity that was apparently lost or modified upon in vitro culture (17).
Binding of each of the other five strains to Vero cells was diminished by exogenous GAGs and/or by enzymatic digestion of GAGs from the cell surface. The removal of different classes of GAGs from the surface of Vero cells resulted in strain-specific effects on spirochetal binding and indicated several different GAG-binding preferences among the strains, including the following: (i) N40, CA20-2A, and PBi recognized predominantly heparin/heparan sulfate on the surface of Vero cells; (ii) PBo bound to Vero cells by using both heparan sulfate and dermatan sulfate; and (iii) VS461 attached to Vero cells primarily via a chondroitinase ABC-sensitive GAG chain(s), presumably dermatan sulfate. The ability of strain VS461 to recognize dermatan sulfate better than does strain N40 is consistent with the previous observation that VS461, but not N40, efficiently bound to CHO-pgsD cells, which express galactosaminoglycans but not glucosaminoglycans (9, 22).
HB19 clone 1 provided the clearest evidence of an alternate GAG-binding preference because it did not recognize Vero cell GAGs yet still bound well to dermatan sulfate GAGs expressed by 293 cells. Binding to 293 cells was inhibited better by exogenous dermatan sulfate than by heparin. It was previously shown that charge is a critical determinant for recognition of GAGs by B. burgdorferi (17, 22, 23), but the finding that HB19 clone 1 recognizes dermatan sulfate better than the more highly charged GAG heparin indicates that the structure of the glycan backbone of the GAG is likely to play an additional role in bacterial binding.
It is important to note that although different Lyme disease strains clearly vary in their relative preferences for heparan sulfate or dermatan sulfate, these preferences are not absolute, and nearly all of the strains retain some ability to recognize both GAGs. With the exception of HB19 clone 1, cell attachment was inhibited by either exogenous heparin or dermatan sulfate. In addition, for the strains tested (N40, HB19 clone 1, VS461, and PBo), enzymatic removal of both glucosaminoglycans and galactosaminoglycans diminished cell attachment to a greater extent than removal of either class alone (Fig. 3 and 4 and data not shown). The ability to bind more than one class of GAG is typical of GAG-binding receptors (20).
The variation in GAG-binding preference could be explained by hypothesizing that the Lyme disease spirochete expresses a single GAG-binding receptor but that strain variations in this receptor result in different GAG-binding preferences. An alternative model is that some strains (such as N40) express multiple GAG receptors, each specific for a particular class of GAGs, while other strains (such as HB19 clone 1) express only a receptor for dermatan sulfate. Complicating the analysis of GAG binding still further are the observations that (i) an uncloned version of B. burgdorferi N40 expresses two proteins that bind decorin, a collagen-associated chondroitin or dermatan sulfate proteoglycan (15, 16), and (ii) B. burgdorferi B31 binds aggrecan, a cartilage chondroitin sulfate proteoglycan (18a). Neither heparin nor aggrecan efficiently blocked binding of uncloned N40 to decorin (15), and dermatan sulfate did not efficiently inhibit B31 attachment to aggrecan (18a), findings consistent with the bacterial expression of multiple proteoglycan- and GAG-binding pathways. Ultimately, an understanding of the relationships between these binding activities will require further characterization of all of the bacterial molecules involved. The recent cloning of decorin-binding proteins should facilitate this analysis (15).
It has been suggested that different strains of Lyme disease spirochete are associated with different clinical manifestations of the illness (1, 2, 33). The results presented here demonstrate that the differences in GAG-binding preferences among strains can result in differences in cell-type-specific binding in vitro. The relationship between attachment of in vitro-cultured bacteria to mammalian cells and infection of particular tissues remains to be defined, and no determinants of tissue tropism have yet been identified for this pathogen. Determining whether attachment to specific cell types in vitro is related to the colonization of particular tissues during infection will require further study.
ACKNOWLEDGMENTS
We thank Louis Rosenfeld, Biswajit Lahiri, Trudy Morrison, Ira Schwarz, and Jenifer Coburn for helpful discussion and careful review of the manuscript and Barbara Johnson for communication of unpublished results. Alan Barbour, Patti Rosa, Vera Preac-Mursic, Bettina Wilske, and Tom Schwan provided strains, and Cora-Jean Edgell provided the endothelial cell line used in this study.
This work was supported by NIH grant R01-AI 37601 to J.M.L. J.M.L. was a Pew Scholar in the Biomedical Sciences and is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Anthonissen F M, De Kesel M, Hoet P P, Bigaignon G H. Evidence for the involvement of different genospecies of Borrelia in the clinical outcome of Lyme disease in Belgium. Res Microbiol. 1994;145:327–331. doi: 10.1016/0923-2508(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 2.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J-C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 3.Coburn J, Leong J, Erban J. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc Natl Acad Sci USA. 1993;90:7058–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn J, Magoun L, Bodary S C, Leong J M. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn J L, Barthold S W, Leong J M. Diverse Lyme disease spirochetes bind integrin αIIbβ3 on human platelets. Infect Immun. 1994;62:5559–5567. doi: 10.1128/iai.62.12.5559-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comstock L E, Thomas D D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989;57:1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorward D W, Fischer E R, Brooks D M. Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease. Clin Infect Dis. 1997;25(Suppl. 1):S2–S8. doi: 10.1086/516169. [DOI] [PubMed] [Google Scholar]
- 8.Edgell C J, McDonald C C, Graham J B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esko J D. Genetic analysis of proteoglycan structure, function and metabolism. Curr Opin Cell Biol. 1991;3:805–816. doi: 10.1016/0955-0674(91)90054-3. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 11.Galbe J L, Guy E, Zapatero J M, Peerschke E I, Benach J L. Vascular clearance of Borrelia burgdorferi in rats. Microb Pathogen. 1993;14:187–201. doi: 10.1006/mpat.1993.1019. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Monco J C, Fernandez-Villar B, Rogers R C, Szczepanski A, Wheeler C M, Benach J L. Borrelia burgdorferi and other related spirochetes bind to galactocerebroside. Neurology. 1992;42:1341–1348. doi: 10.1212/wnl.42.7.1341. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Monco J C, Fernandez-Villar B, Benach J L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989;160:497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Monco J C, Fernandez-Villar B, Rogers R C, Szczepanski A, Wheeler C M, Benach J L. Borrelia burgdorferi and other related spirochetes bind to galactocerebroside. Neurology. 1992;42:1341–1348. doi: 10.1212/wnl.42.7.1341. [DOI] [PubMed] [Google Scholar]
- 15.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson M S, Cassatt D R, Guo B P, Patel N K, McCarthy M P, Dorward D W, Hook M. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs R. Borrelia burgdorferi bind to epithelial cell proteoglycan. J Clin Invest. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promoted bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 18a.Johnson, B. Personal communication.
- 19.Kato Y, Kimata K, Ito K, Karasawa K, Suzuki S. Effect of β-d-xyloside and cycloheximide on the synthesis of two types of proteochondroitin sulfate in chick embryo cartilage. J Biol Chem. 1978;253:2784–2789. [PubMed] [Google Scholar]
- 20.Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 21.Leong J M, Moitoso de Vargas L, Isberg R R. Binding of cultured mammalian cells to immobilized bacteria. Infect Immun. 1992;60:683–686. doi: 10.1128/iai.60.2.683-686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong J M, Morrissey P E, Ortega-Barria E, Pereira M E A, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong J M, Robbins D, Rosenfeld L, Lahiri B, Parveen N. Structural requirements for glycosaminoglycan recognition by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1998;66:6045–6048. doi: 10.1128/iai.66.12.6045-6048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leong J M, Wang H, Magoun L, Field J A, Morrissey P E, Robbins D, Tatro J B, Coburn J, Parveen N. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect Immun. 1998;66:994–999. doi: 10.1128/iai.66.3.994-999.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marconi R, Konkel M, Garon C. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect Immun. 1993;61:2611–2617. doi: 10.1128/iai.61.6.2611-2617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks: culture conditions and antibiotic susceptibility. Zentbl Bakteriol Hyg. 1986;263:112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 27.Rosa P, Schwan T, Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992;6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 28.Sadziene A, Rosa P, Thompson P, Hogan D, Barbour A. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992;176:799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silbert J E, Bernfield M, Kokenyesi R. Proteoglycans: a special class of glycoproteins. In: Montreuil J, Vliegenthart J F G, Schachter J, editors. Glycoproteins II. Oxford, United Kingdom: Elsevier; 1997. [Google Scholar]
- 30.Steere A C. Lyme disease. New Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 31.Szczepanski A, Furie M B, Benach J L, Lane B P, Fleit H B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990;85:1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas D D, Comstock L E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989;57:1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dam A, Kulper H, Vos K, Widjojokusumo A, de Jongh B, Spanjaard L, Ramselaar A, Kramer M, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 34.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]