Abstract
Background
T1 rectal cancer (RC) patients are increasingly being treated by local resection alone but uniform surveillance strategies thereafter are lacking. To determine whether different local resection techniques influence the risk of recurrence and cancer-related mortality, a meta-analysis was performed.
Methods
A systematic search was conducted for T1RC patients treated with local surgical resection. The primary outcome was the risk of RC recurrence and RC-related mortality. Pooled estimates were calculated using mixed-effect logistic regression. We also systematically searched and evaluated endoscopically treated T1RC patients in a similar manner.
Results
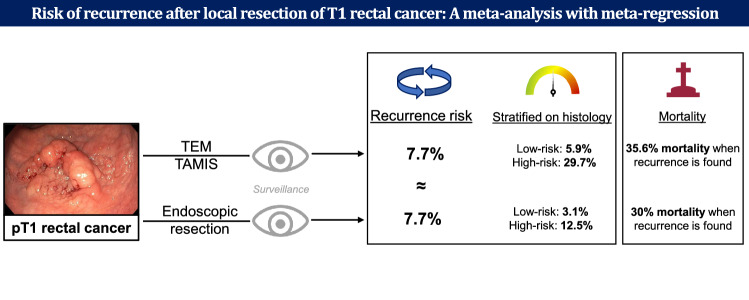
In 2585 unique T1RC patients (86 studies) undergoing local surgical resection, the overall pooled cumulative incidence of recurrence was 9.1% (302 events, 95% CI 7.3–11.4%; I2 = 68.3%). In meta-regression, the recurrence risk was associated with histological risk status (p < 0.005; low-risk 6.6%, 95% CI 4.4–9.7% vs. high-risk 28.2%, 95% CI 19–39.7%) and local surgical resection technique (p < 0.005; TEM/TAMIS 7.7%, 95% CI 5.3–11.0% vs. other local surgical excisions 10.8%, 95% CI 6.7–16.8%). In 641 unique T1RC patients treated with flexible endoscopic excision (16 studies), the risk of recurrence (7.7%, 95% CI 5.2–11.2%), cancer-related mortality (2.3%, 95% CI 1.1–4.9), and cancer-related mortality among patients with recurrence (30.0%, 95% CI 14.7–49.4%) were comparable to outcomes after TEM/TAMIS (risk of recurrence 7.7%, 95% CI 5.3–11.0%, cancer-related mortality 2.8%, 95% CI 1.2–6.2% and among patients with recurrence 35.6%, 95% CI 21.9–51.2%).
Conclusions
Patients with T1 rectal cancer may have a significantly lower recurrence risk after TEM/TAMIS compared to other local surgical resection techniques. After TEM/TAMIS and endoscopic resection the recurrence risk, cancer-related mortality and cancer-related mortality among patients with recurrence were comparable. Recurrence was mainly dependent on histological risk status.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00464-022-09396-3.
Keywords: T1 rectal cancer, Local surgical resection, Therapeutic endoscopy, Follow-up, Recurrence
The introduction of population-based screening has resulted in an increased number of early invasive, or T1, rectal cancers (T1RC) [1]. Over the last years, a shift can be observed from major surgery towards local, organ-preserving endoscopic or surgical resection techniques as primary treatment for these tumors.
The decision whether to perform additional total mesorectal excision (TME) after local resection mainly depends on the oncological risk (which is based on histological high-risk features for lymph node metastasis (LNM) [2]), operative risk and patient preferences. Considering the limited accuracy of the histological risk stratification models, and the significant morbidity and decrease in quality of life that are associated with TME, there has been an increased tendency towards close-surveillance strategies after local resection of T1RC [3–6].
Surveillance after local resection of T1RC is currently quite heterogeneous [7], and needs to be optimized to improve the efficacy of surveillance. To determine the optimal surveillance strategy, it is important to determine the risk, type and prognosis of cancer recurrences that could occur. This meta-analysis aims to estimate the cumulative incidence of RC recurrence and RC-related mortality for patients with local surgically resected T1RC and to compare this with results of endoscopically treated T1RC patients.
Materials and methods
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement [8]. Information regarding the search strategy, data extraction, definitions and classifications, and risk of bias assessment can be found in the Supplementary methods. Approval of the institutional review board (IRB) and written consent was not needed.
Selection criteria for local surgical resection
A systematic literature search was conducted in PubMed, Embase, Web of Science and Cochrane Library from inception until May 19, 2021. Inclusion criteria were: 1. histologically confirmed pT1RCs 2. local surgical resection alone, 3. the proportion of recurrences after local surgical resection of T1RCs was reported 4. original peer-reviewed articles. Exclusion criteria were: 1. prior or additional therapy (e.g., endoscopic resection, oncological surgery, chemotherapy or radiotherapy), 2. hereditary predisposition for CRC, 3. inflammatory bowel disease, 4. studies with < 5 patients with T1RC undergoing local surgical resection, 5. studies without original patient data (e.g., reviews or meta-analyses), 6. conference abstracts, 7. animal studies and 8. non-English or non-German articles. In case of overlapping cohorts, the cohort with the largest number of patients, or covering the largest period of time was selected.
T1RCs were defined as rectal tumors with histologic tumor invasion through the muscularis mucosae and into, but not beyond, the submucosa. Local surgical resection was defined as any type of local resection that was used to excise a rectal tumor without lymph node dissection, and that did not use flexible endoscopy (i.e., no endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (eFTR), endoscopic mucosal resection (EMR), or snare polypectomy). High-risk criteria for LNM defined by The Japanese Society for Cancer of the Colon and Rectum (JSCCR) include: positive resection margins, deep submucosal invasion, grade 3 differentiation, lymphovascular invasion and high-grade tumor budding [2].
Selection criteria for local endoscopic resection
Data of endoscopically treated T1RC patients were extracted from our previous meta-analysis on recurrences after local endoscopic resection of T1 colorectal cancer[9]. This search was updated until May 19, 2021 and additional data regarding primary outcomes or main study characteristics for the subgroup of T1RC patients were requested from the corresponding authors. The in- and exclusion criteria of the current analysis were similar to those of the previous analysis [9], except for treatment and location (Supplementary Table 1).
Data acquisition
Data extraction and risk of bias assessment were independently performed by 3 authors (ND, HD, PO). In case of disagreement without consensus after discussion, a fourth assessor (JB) was decisive. Relevant study-level parameters and individual patient-level data of recurrence cases were extracted. The risk of bias was assessed using a modified Newcastle–Ottawa Scale [10]. An additional random data check was performed by the decisive assessor to ensure the data quality.
Study outcomes
The primary outcome was the cumulative incidence of RC recurrence (locoregional or distant) and RC-related mortality during follow-up. Locoregional recurrence was defined as endoluminal cancer at the primary resection site or pelvic LNM. Distant recurrence was defined as any metastasis outside the pelvic area. Secondary outcomes were the cumulative incidence of locoregional RC recurrence only, any locoregional RC recurrence and any distant recurrence.
Statistical analysis
All analyses were performed in R v4.1.0 [11] using the package metafor v3.0.1 [12]. Cumulative incidences of all study outcomes were modeled on the logit scale using mixed-effects logistic regression (13). Thereafter, results were transformed back to proportions and presented as point estimates with 95%-confidence intervals (95% CI). The risk of publication bias was examined using a funnel plot with the square root of the study size on the y-axis [14].
Statistical heterogeneity was quantified using I2 statistic and tau-squared (τ2). Univariable meta-regression and subgroup analyses were performed to explore possible sources of heterogeneity with predefined potential predictors: study characteristics (e.g., publication year, study design), individual items from the risk of bias assessment, follow-up characteristics (e.g., duration and intensity), and clinical characteristics (e.g., resection technique, histology). Only studies with subgroups of ≥ 5 patients, for whom the exact number of events could be determined, were included in meta-regression and subgroup analyses. Meta-regression was only performed when at least 10 studies could be analysed [15]. p values < 0.05 were considered statistically significant.
Results
Study characteristics for local surgical resection
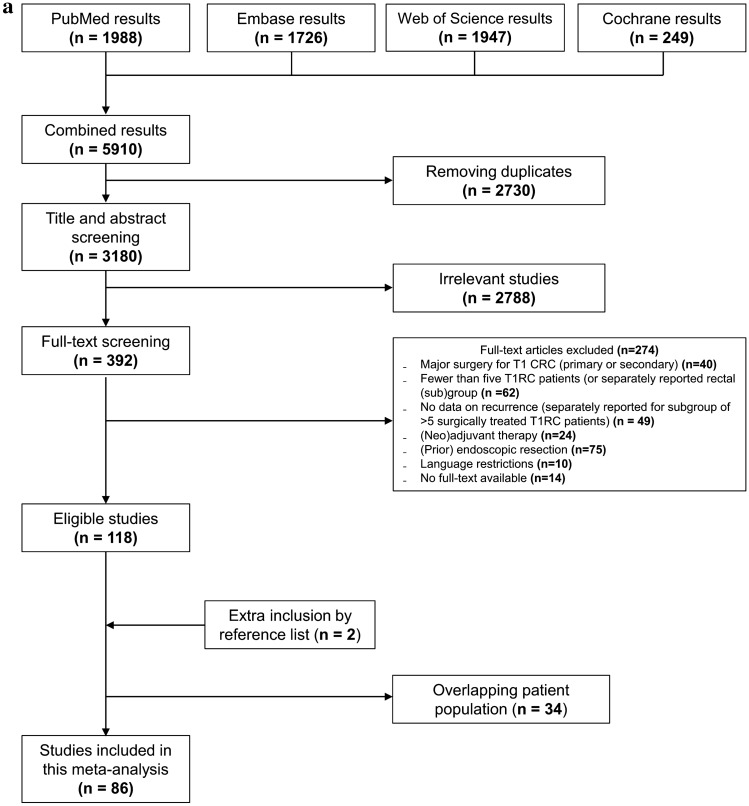
Our search identified 5910 articles, of which 86 reported unique patient cohorts and were included (Fig. 1a) [16–101]. These studies consisted of 2585 patients undergoing local surgical resection for T1RC, with data on the cumulative incidence of recurrence. Eighty-five studies also reported separate incidences of locoregional and distant recurrences.
Fig. 1.
Flow diagram of the selection process for studies on local surgical resection (a) and endoscopic resection (b). (C)RC (colo)rectal cancer
The extracted data and risk of bias assessment of the included studies are shown in Supplementary analyses. Most studies were performed in Europe (46 studies, n = 1506 patients), followed by North America (20 studies, n = 608), Asia (17 studies, n = 438), South America (2 studies, n = 15) and Australia (one study, n = 618). No obvious asymmetry was observed in the funnel plot (Supplementary Fig. 1).
In 41 studies the transanal endoscopic microsurgery (TEM) technique was investigated and transanal minimally invasive surgery (TAMIS) in 4. The majority of patients in the remaining 41 studies underwent other local surgical resection techniques with direct visualization; these were grouped as “local excision” (LE; e.g., Park method or using the Ferguson anoscope). Fifty-five studies reported data on the resection plane; almost all patients in these studies underwent a full-thickness resection (99.2%). The mean and minimum follow-up could be determined in 15 (range, 18.2–72.5) and 51 studies (range 1–60), respectively. Complete data on follow-up schemes (i.e., which follow-up modalities and intervals per modality) was reported in 42 studies; schemes were classified as “not strict” in 6 (14.3%), “strict” in 13 (31.0%), and “very strict” in 23 studies (54.8%). The definitions used for these groups are shown in the Supplementary methods. A flow diagram of the study process is shown in Fig. 2.
Fig. 2.
Flow diagram of the study process. TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery
Pooled estimates of all included studies
Overall, 302 out of 2585 patients experienced recurrence after local surgical resection. The pooled cumulative incidence of any RC recurrence was 9.1% (95% CI 7.3–11.4%; I2 = 68.3%; Fig. 3).
Fig. 3.
Forest plot with cumulative incidences of any RC recurrence after local surgical resection. To visualize incidence estimates of studies with 0 events, a continuity correction of + 0.5 was applied. Values of the pooled estimates, I2 and τ2 are calculated using a model without continuity correction
Meta-regression
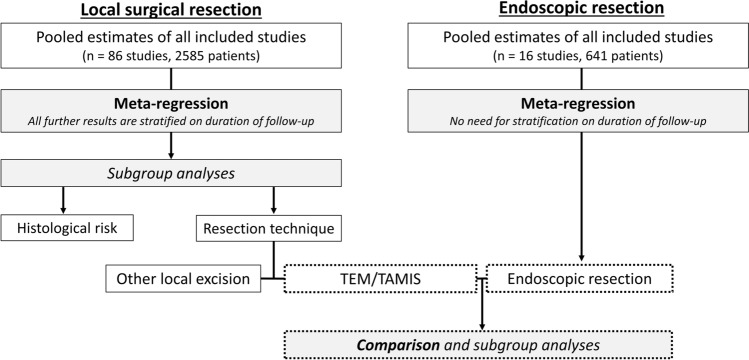
In meta-regression, histological risk status (low-risk vs. high-risk) and, local surgical resection technique (TEM/TAMIS vs. other local surgical excisions) were associated with the risk of recurrence (Supplementary Tables 2 and 3). Therefore, subgroup analyses were performed for histological risk status and local surgical resection technique. Further analyses were stratified according to the duration of follow-up because risk of recurrence increased with longer mean follow-up duration (Supplementary Table 2). Results for studies with ≥ 2-year follow-up are shown below; results for all studies, for studies with a ≥ 5 years follow-up and detailed information regarding the meta-regression results are shown in Supplementary results.
Pooled estimates of studies with ≥ 2 years follow-up
The pooled cumulative incidence of any RC recurrence was 9.2% (194/1713 events; 95% CI 7.1–11.9%; I2 = 60.8%; Supplementary Fig. 2). Pooled incidences of all secondary outcomes are shown in Supplementary analyses. The pooled incidence of RC-related mortality was 1.9% (31/898 events, 27 studies; 95% CI 0.9–4.2%; I2 = 69.3%; Supplementary Fig. 3). The RC-related mortality rate among patients with recurrence was 28.7% (31/108). All of these patients died of disease progression.
Subgroup analyses in studies with ≥ 2 years follow-up
Low-risk versus high-risk
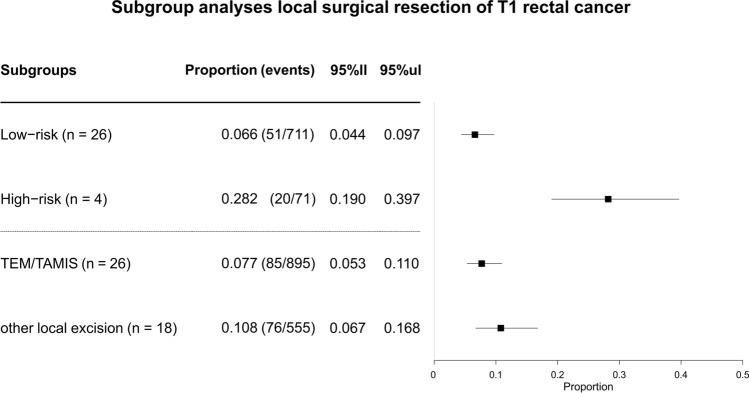
Twenty-six studies reported a subgroup of ≥ 5 patients with low-risk T1RC and sufficient data on recurrence, and 4 studies did so for high-risk T1RCs. The definitions of low- and high-risk T1RCs were diverse. Most studies used 3 risk criteria: differentiation grade was used the most and tumor budding the least (Supplementary Fig. 4). The cumulative incidence of any RC recurrence was 6.6% for low-risk T1RCs (51/711 events; 95% CI 4.4–9.7%; I2 = 22.4%) and 28.2% for high-risk T1RCs (20/71 events; 95% CI 19–39.7%; I2 = 0.0%) (Fig. 4).
Fig. 4.
Forest plot with cumulative incidences of any RC recurrence after local surgical resection with subgroups based on histological risk status and local surgical resection technique. 95%ll 95% confidence interval lower limit, 95%ul 95% confidence interval upper limit, TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery
TEM/TAMIS versus local excision
The cumulative incidence of RC recurrence was 7.7% after TEM/TAMIS (85/895 events; 95% CI 5.3–11.0%; I2 = 47.7%) and 10.8% after local surgical excision techniques with direct visualization (76/555 events; 95% CI 6.7–16.8%; I2 = 65.3%) (Fig. 4). This difference was mainly due to an increased incidence of endoluminal local-site recurrences; 4.7% for the TEM/TAMIS (50/859 events; 95% CI 2.9–7.6%; I2 = 44.2%) and 7.2% for local excision (38/480 events; 95% CI 4.2–12%; I2 = 29.9%). This subgroup analyses confirmed that TEM/TAMIS is superior to other local surgical excision techniques with regard to recurrence. Outcomes of the TEM/TAMIS technique will therefore be compared to the endoscopic data. Secondary outcomes for all subgroup analyses are detailed in Supplementary results and Supplementary analyses.
TEM/TAMIS versus endoscopic resection
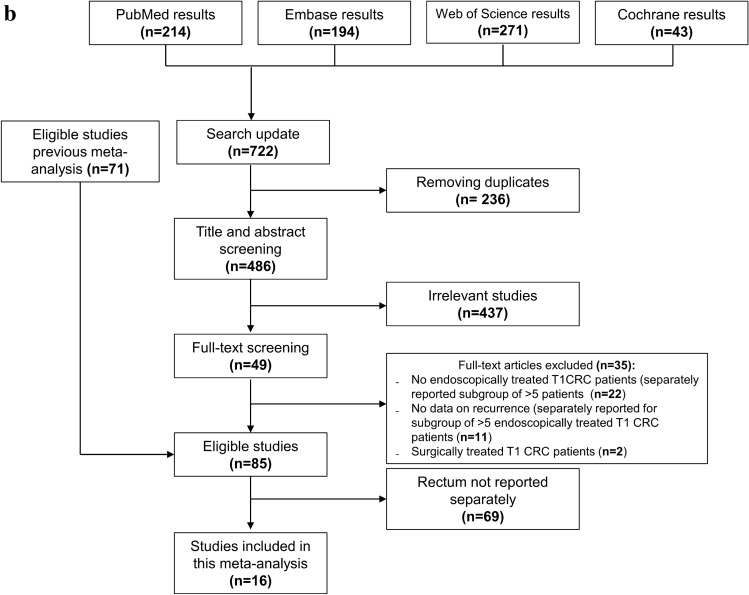
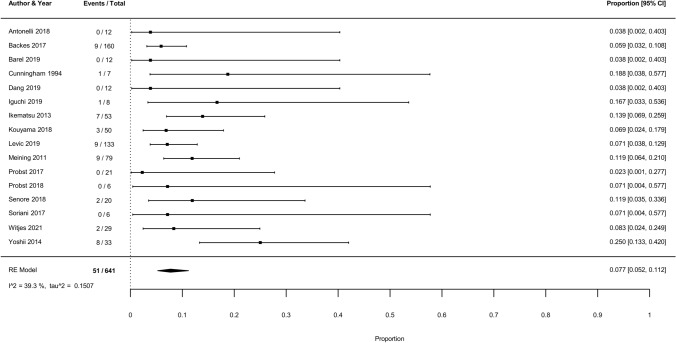
The previous meta-analysis and search update yielded 16 eligible studies with 641 patients and 51 recurrences (Fig. 1B, Supplementary analyses) [26, 102–116]. The studied endoscopic resection techniques included ESD, eFTR, EMR, and snaring polypectomy. “Very strict” follow-up schemes were reported in 50.0% (13/26) of the TEM/TAMIS studies and in 37% (10/27) of the endoscopic studies (Supplementary Fig. 5). The pooled incidence of RC recurrence was comparable between endoscopically treated (7.7%; 95% CI 5.2–11.2%; I2 = 39.3%; Fig. 5) and TEM/TAMIS-treated patients with ≥ 2 years follow-up (7.7%). Also after correcting for the proportion of low- and high-risk T1RCs, meta-regression showed no statistical difference between TEM/TAMIS and endoscopic resection (p = 0.244).RC-related mortality was also comparable between endoscopically treated (2.3%; 95% CI 1.1–4.9%; I2 = 18.4%) and TEM/TAMIS-treated patients (2.8%; 95% CI 1.2–6.2%; I2 = 48.9%) and among the recurrence cases (30.0% versus 35.6%, respectively). The timing of the recurrences after endoscopic resection is shown in Supplementary Fig. 6. The overall pooled incidence of RC recurrence after TEM/TAMIS and endoscopic resections combined was 7.7% (136/1536 events, 95% CI 5.9–10.0%; I2 = 46.2%).
Fig. 5.
Forest plot with cumulative incidences of any RC recurrence after endoscopic resection. To visualize incidence estimates of studies with 0 events, a continuity correction of + 0.5 was applied. Values of the pooled estimates, I2 and τ2 are calculated using a model without continuity correction
The risk of recurrence for low-risk T1RC was 5.9% after TEM/TAMIS (27/406 events, 95% CI 3.4–10.0%; I2 = 24.1%) and 3.1% after endoscopic resection (4/128 events, 95% CI 1.2–8.0%; I2 = 0.0%). Twenty-four of these low-risk recurrences were endoluminal (2 with synchronous locoregional LNM; 9 also presented with distant metastasis at the time of the local recurrence or later), the other 7 were distant metastasis. For 29 of the 31 low-risk T1RC recurrences it was stated that the local resection was complete. For the other 2 recurrence cases this was not stated explicitly. For high-risk T1RCs the risk of recurrence was 29.7% after TEM/TAMIS (11/37 events, 95% CI 17.3–46.1%; I2 = 0.0%) and 12.5% after endoscopic resection (25/200 events, 95% CI 8.6–17.8%; I2 = 0.0%). In 29 of the 43 studies on local endoscopic resections for T1RC, 4–5 JSCCR risk criteria were used; for studies on TEM/TAMIS this was 5 of the 33 studies (Supplementary Fig. 4). Other secondary outcomes are shown in Supplementary results.
Discussion
This meta-analysis is the first to meticulously analyze the long-term outcomes of T1RC patients treated by local surgical resection, and to relate these outcomes to those of endoscopically treated T1RC patients. The overall recurrence risk after local surgical resection of T1RC was found to be around 9%.
Meta-regression analysis demonstrated that the risk of recurrence was significantly affected by several factors, including resection technique. In line with previous studies [117], our subgroup analyses confirmed that TEM/TAMIS (7.7%) is superior to other local surgical excision techniques using direct visualization (10.8%) with regard to recurrence. Although TEM/TAMIS were introduced later, it is unlikely that this biased the results because meta-regression showed no association between publication year and risk of recurrence. Instead, the difference could mainly be attributed to an increased risk of endoluminal local-site recurrences. This suggests that the oncological superiority of TEM/TAMIS is most likely explained by the camera-assisted visualization, and the use of a pneumorectum, which allows for improved visualization of tumor margins and increases the chance of achieving a complete resection. Tumor height may also have influenced the outcome of local surgical resections. Unfortunately data on tumor height was scarcely reported and could not always be extracted for the correct subgroup, therefore it was not possible to further stratify our results.
Another factor that significantly influenced the recurrence risk was histological risk status. This was in accordance with findings of our previous meta-analysis [9]. In subgroup analyses the difference between low- and high-risk tumors was confirmed for both TEM/TAMIS-treated (5.9% recurrence risk for low-risk T1RC vs. 29.7% for high-risk T1RC) and endoscopically treated patients (3.1% recurrence risk for low-risk T1RC vs. 12.5% for high-risk T1RC). There appears to be a difference in the risk of recurrence for high-risk T1RC treated by TEM/TAMIS or endoscopic resection (TEM/TAMIS: 11/37 events in 2 studies, endoscopic resection: 25/200 events in 8 studies). Due to the limited number of studies included in this subgroup analysis, it was not possible to draw any valid conclusions on these findings.
When comparing TEM/TAMIS to endoscopic resections, we observed that overall recurrence rates (7.7% and 7.7%, respectively), RC-related mortality rates (2.8% and 2.3%, respectively) and mortality rates among recurrences (35.6% and 30.0%, respectively) were quite similar. A randomized non-inferiority trial is pending to confirm these results [118]. Despite the similarities in oncological outcomes, we found that risk stratification and follow-up varied considerably between local surgically and endoscopically treated T1RC patients. Firstly, the number of JSCCR criteria used for risk stratification were quite different, which makes it difficult to compare recurrence risks stratified by histology. Two-third of studies on endoscopic resections used > 3 criteria to define high-risk tumors, but among studies on TEM/TAMIS only ~ 15% used > 3 criteria. This has most probably caused an overestimation of the recurrence risk in the group of TEM/TAMIS-treated low-risk T1RC, as some of these patients would have been classified as high-risk if more JSCCR criteria had been used. However, it was impossible to draw any valid conclusions on the clinical relevance of each high-risk criterion from these results, as the available data did not allow us to study the criteria individually. More universal histological assessment of T1RC by a dedicated pathologist is therefore warranted. Secondly, the reported follow-up schemes of TEM/TAMIS-treated T1RC patients were often much stricter than the schemes of endoscopically treated patients (Supplementary Fig. 6), but compliance to these schemes were rarely reported. Considering the comparable outcomes of TEM/TAMIS and endoscopic resection, it appears that at a certain point further intensifying the follow-up, using current follow-up modalities, might not necessarily lead to increased detection of recurrences or improved prognosis of T1RC patients. However, the optimal surveillance intensity in terms of clinical outcomes remains to be elucidated.
The risk of recurrence after local resection seems higher for T1 cancers in the rectum compared to T1 cancers throughout the colon. Here, we found a risk of recurrence for rectal T1 cancers of 7.7% (after endoscopic resection or TEM/TAMIS), which is higher than the 3.3% for endoscopically treated T1 cancers at sites throughout the colorectum [9]. A similar difference was seen in the subgroup of low-risk (endoscopically treated T1 colorectal cancer: 0.7%; endoscopically or TEM/TAMIS-treated low-risk T1 rectal cancer: 3.1–5.9%) and high-risk cancers (endoscopically treated T1 colorectal cancer: 7.0%; endoscopically or TEM/TAMIS-treated low-risk T1 rectal cancer: 12.5–29.7%). These results suggest rectal T1 cancers are associated with worse outcomes compared to colonic T1 cancers, independent of histological risk status. Plausible contributing factors include differences in anatomic structures and tumor biology [119].
The most important limitation of this meta-analysis relates to the quality of the included studies. The selection of studies for this meta-analysis was performed as thoroughly as possible, to prevent the exclusion of important studies. However, several studies did not specifically study T1RC patients treated by local resection alone. Therefore, data on patient, treatment, tumor size, tumor height, histological, follow-up and individual recurrence characteristics could not always be fully extracted. This resulted in a smaller number of studies in various subgroup analyses and for some studies in not receiving the maximum assessment scores on risk of bias. Secondly, there was some statistical heterogeneity, which could be expected a priori considering the heterogeneity in the resection techniques and follow-up. Therefore, we performed extensive meta-regression and subgroup analyses, which yielded lower heterogeneity estimates. Lastly, the definition of the rectum was left to the discretion of the authors of included studies, to avoid exclusion of many relevant articles that did not clearly state a definition. However, due to the technical limitations of transanal local excisions proximal to the rectum, it is not likely that cancers outside the rectum were included in this meta-analysis.
Clinical implications
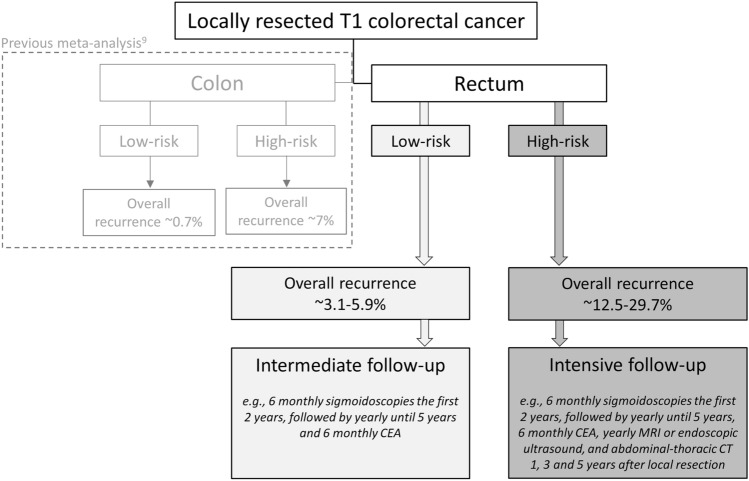
Based on our study findings, we propose the following surveillance recommendations and key points for future research. Firstly, T1RC patients should be offered a different follow-up than T1 colon cancer patients and the surveillance should be stratified for histological risk status. There is no need to stratify surveillance for local resection technique when the T1RC is removed endoscopically or by TEM/TAMIS. All T1RCs that are removed locally should be offered surveillance (provided that possible findings will have clinical consequences) because even for low-risk T1RCs the recurrence risk is 3.1–5.9%. Patients with locally resected low-risk T1RC should be offered surveillance rather than completion TME because we think that in these patients the potential drawbacks from oncological surgery are greater than the possible benefits. We propose a 5-year moderately intensive follow-up scheme that should focus on the local-endoluminal site where most recurrences seem to develop (e.g., 6 monthly (recto)sigmoidoscopies the first 2 years, and then yearly until 5 years; and 6 monthly CEA). Patients with high-risk T1RC should be offered completion TME surgery because of the relatively high-risk of recurrence, as is recommended in current guidelines [2, 120]. If oncological surgery is not feasible we propose a 5-year intensive follow-up scheme focusing on the detection of endoluminal, locoregional lymph node and distant recurrences (e.g., 6 monthly (recto)sigmoidoscopies the first 2 years, and then yearly until 5 years; 6 monthly CEA; yearly MRI or endoscopic ultrasound, and abdominal-thoracic computed tomography at 1, 3 and 5 years). This follow-up scheme only seems beneficial for those patients in whom salvage surgery or treatment of metastases seems feasible in the future. An overview of our main study findings and surveillance recommendations is shown in Fig. 6. Further prospective studies are necessary to study the optimal method, the optimal timing, cost-effectiveness of surveillance and the impact of surveillance on the prognosis.
Fig. 6.
Overview of the main study findings and surveillance recommendations. CEA carcinoembryonic antigen, MRI magnetic resonance imaging, CT computed tomography
Conclusion
Patients with T1 rectal cancer may have a significantly lower recurrence risk after TEM/TAMIS compared to other local surgical resections. After TEM/TAMIS and endoscopic resection the recurrence risk, cancer-related mortality and cancer-related mortality among patients with recurrence were comparable. Recurrence was mainly dependent on histological risk status. Based on our findings we propose a more uniform histology-based surveillance strategy for T1 rectal cancer patients treated by local resection alone.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary methods and results (DOCX 51 kb)
Supplementary figure 1. Funnel plot for the cumulative incidence of any rectal cancer recurrence. The points correspond to the incidences of individual studies, and the vertical line in the funnel plot indicates the summary estimate. To visualize studies with 0 events, a continuity correction of +0.5 was applied (TIF 147486 kb)
Supplementary figure 2. Forest plot with cumulative incidences of any RC recurrence after local surgical resection in patients with ≥ 2 years follow-up. To visualize incidence estimates of studies with 0 events, a continuity correction of +0.5 was applied. Values of the pooled estimates, I2 and τ2 are calculated using a model without continuity correction (TIF 147486 kb)
Supplementary figure 3. Forest plot with cumulative incidences of rectal cancer-related mortality after local surgical resection in patients with ≥ 2 years follow-up. To visualize incidence estimates of studies with 0 events, a continuity correction of +0.5 was applied.Values of the pooled estimates, I2 and τ2 are calculated using a model without continuity correction (TIF 147486 kb)
Supplementary figure 4. Number of JSCCR criteria used for histological risk stratification for studies on TEM/TAMIS and endoscopic resection. TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery, JSCCR Japanese Society for Cancer of the Colon and Rectum (TIF 147486 kb)
Supplementary figure 5. Strictness of follow-up for studies on endoscopic resection and TEM/TAMIS. TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery (TIF 147486 kb)
Supplementary figure 6. Time to any rectal cancer recurrence after endoscopic resection (TIF 147486 kb)
Supplementary figure 7. Time to any rectal cancer recurrence after local surgical resection (TIF 147486 kb)
Supplementary figure 8. Treatment of recurrence after local surgical resection. a. local endoluminal recurrence, b. locoregional recurrence, c. local/locoregional + distant recurrence, d. distant recurrence. TME total mesorectal excision, CRT chemoradiotherapy (TIF 2367 kb)
Supplementary figure 9. Time between rectal cancer recurrence and rectal cancer-related mortality (TIF 831 kb)
Supplementary table 1. In- and exclusion criteria for the endoscopic sub-section. CRC colorectal cancer, RC rectal cancer (DOCX 14 kb)
Supplementary table 2. Meta-regression with study characteristics and risk of bias. Potential predictors of statistical inter-study heterogeneity for the outcome "any rectal cancer recurrence". (DOCX 21 kb)
Supplementary table 3. Meta-regression with clinical characteristics. Potential predictors of statistical inter-study heterogeneity for the outcome "any rectal cancer recurrence". LE local excision, TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery, AV anal verge, DL dentate line, LVI lymphovascular invasion (DOCX 20 kb)
Supplementary file: Prisma checklist (DOCX 31 kb)
Funding
None.
Data availability
Data file, analytic methods and study materials will be made available in the supplementary data. This meta-analysis was not pre-registered.
Declarations
Disclosures
Jeanin van Hooft is a consultant of Boston Scientific, Cook Medical, Olympus and Medtronic; and received a research grant from Cook Medical and Abbvie. Jurjen Boonstra is a consultant of Boston Scientific. These disclosures do not directly relate to the content of this work. Nik Dekkers, Hao Dang, Jolein van der Kraan, Saskia le Cessie, Philip Oldenburg, Jan Schoones, Alexandra Langers, Monique van Leerdam, Yara Backes, Katarina Levic, Alexander Meining, Giorgio Saracco, Fabian Holman, Koen Peeters, Leon Moons, Pascal Doornebosch and James Hardwick disclose no conflicts.
Institutional review board
Approval of the institutional review board and written consent was not needed.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nik Dekkers and Hao Dang—Shared first authorship.
References
- 1.Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lezoche E, Paganini AM, Fabiani B, et al. Quality-of-life impairment after endoluminal locoregional resection and laparoscopic total mesorectal excision. Surg Endosc. 2014;28(1):227–234. doi: 10.1007/s00464-013-3166-2. [DOI] [PubMed] [Google Scholar]
- 4.Bennis M, Parc Y, Lefevre JH, et al. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg. 2012;255(3):504–510. doi: 10.1097/SLA.0b013e31824485c4. [DOI] [PubMed] [Google Scholar]
- 5.van Groningen JT, van Hagen P, Tollenaar R, et al. Evaluation of a Completion Total Mesorectal Excision in Patients After Local Excision of Rectal Cancer: A Word of Caution. J Natl Compr Canc Netw. 2018;16(7):822–828. doi: 10.6004/jnccn.2018.7026. [DOI] [PubMed] [Google Scholar]
- 6.Bosch SL, Teerenstra S, de Wilt JHW, et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45(10):827–841. doi: 10.1055/s-0033-1344238. [DOI] [PubMed] [Google Scholar]
- 7.Gijsbers K, de Graaf W, Moons LMG, et al. High practice variation in risk stratification, baseline oncological staging, and follow-up strategies for T1 colorectal cancers in the Netherlands. Endosc Int Open. 2020;8(9):E1117–E1122. doi: 10.1055/a-1192-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang H, Dekkers N, le Cessie S, et al. Risk and Time Pattern of Recurrences After Local Endoscopic Resection of T1 Colorectal Cancer: A Meta-analysis. Clin Gastroenterol Hepatol. 2020;20(2):e298–e314. doi: 10.1016/j.cgh.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Wells GA SB, O’Connell D et al (2020) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 6 Apr 2020
- 11.R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed: 3 June 2021
- 12.Viechtbauer W (2020) metafor: meta-analysis package for R. https://CRAN.R-project.org/package=metafor. Accessed 3 June 2021
- 13.Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 14.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2. Chichester (UK): John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert MR, Atallah SB, deBeche-Adams TC, et al. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56(3):301–307. doi: 10.1097/DCR.0b013e31827ca313. [DOI] [PubMed] [Google Scholar]
- 17.Amann M, Burghardt J, Stratz C, et al. Transanal endoscopic microsurgery in treatment of small rectal T1 high-risk, T2 and T3 carcinomas combined with radiochemotherapy. Eur Surg. 2015;47(5):226–237. [Google Scholar]
- 18.Ambacher T, Kasperk R, Schumpelick V. Einfluß der transanalen Excision auf die Rezidivrate beim Stadium-I-Rectumcarcinom im Vergleich zu radikal resezierenden Verfahren. Chirurg. 1999;70(12):1469–1474. doi: 10.1007/s001040050088. [DOI] [PubMed] [Google Scholar]
- 19.Araki Y, Isomoto H, Shirouzu K. Video-assisted gasless transanal endoscopic microsurgery: a review of 217 cases of rectal tumors over the past 10 years. Dig Surg. 2003;20(1):48–52. doi: 10.1159/000068866. [DOI] [PubMed] [Google Scholar]
- 20.Araujo SEA, Seid VE, de Araujo HL, et al. Transanal endoscopic microsurgery: a Brazilian initial experience in private practice. Hepatogastroenterology. 2012;59(118):1822–1827. doi: 10.5754/hge12021. [DOI] [PubMed] [Google Scholar]
- 21.Bacić D, Durut I, Bukvić N, et al. Transanal endoscopic microsurgery (TEM)–alternative or a method of choice in treating tumors of the rectum with appropriately selected patients? Coll Antropol. 2014;38(4):1127–1130. [PubMed] [Google Scholar]
- 22.Balani A, Turoldo A, Braini A, et al. Local excision for rectal cancer. J Surg Oncol. 2000;74(2):158–162. doi: 10.1002/1096-9098(200006)74:2<158::AID-JSO15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Balyasnikova S, Read J, Tait D, et al. The results of local excision with or without postoperative adjuvant chemoradiotherapy for early rectal cancer among patients choosing to avoid radical surgery. Colorectal Dis. 2017;19(2):139–147. doi: 10.1111/codi.13477. [DOI] [PubMed] [Google Scholar]
- 24.Bamba Y, Itabashi M, Hirosawa T, et al. Follow-up and recurrence of T1 colorectal cancer. Int Surg. 2006;91(1):12–16. [PubMed] [Google Scholar]
- 25.Baral J. Transanal endoscopic microsurgical submucosa dissection in the treatment of rectal adenomas and T1 rectal cancer. Coloproctology. 2018;40(5):364–372. doi: 10.1007/s00053-018-0291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barel F, Cariou M, Saliou P, et al. Histopathological factors help to predict lymph node metastases more efficiently than extra-nodal recurrences in submucosa invading pT1 colorectal cancer. Sci Rep. 2019;9(1):8342. doi: 10.1038/s41598-019-44894-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleday R, Breen E, Jessup JM, et al. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum. 1997;40(4):388–392. doi: 10.1007/BF02258381. [DOI] [PubMed] [Google Scholar]
- 28.Budhoo H. Transanal excision of early rectal carcinoma-review of a personal series. Colorectal Dis. 2000;2(2):73–76. doi: 10.1046/j.1463-1318.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 29.Casalots A, Serra-Aracil X, Mora-Lopez L et al (2021) T1 rectal adenocarcinoma: a different way to measure tumoral invasion based on the healthy residual submucosa with its prognosis and therapeutic implications. J Gastrointest Surg 25(10):2660–2667 [DOI] [PubMed]
- 30.Chorost MI, Petrelli NJ, McKenna M, et al. Local excision of rectal carcinoma. Am Surg. 2001;67(8):774–779. [PubMed] [Google Scholar]
- 31.Coco C, Magistrelli P, Netri G, et al. Combined modality therapy in low risk (T2N0) rectal cancer. Rays. 1995;20(2):156–164. [PubMed] [Google Scholar]
- 32.Dafnis G, Påhlman L, Raab Y, et al. Transanal endoscopic microsurgery: clinical and functional results. Colorectal Dis. 2004;6(5):336–342. doi: 10.1111/j.1463-1318.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- 33.Demartines N, von Flüe MO, Harder FH. Transanal endoscopic microsurgical excision of rectal tumors: indications and results. World J Surg. 2001;25(7):870–875. doi: 10.1007/s00268-001-0043-2. [DOI] [PubMed] [Google Scholar]
- 34.D'Hondt M, Yoshihara E, Dedrye L et al (2017) Transanal endoscopic operation for benign rectal lesions and T1 carcinoma. JSLA 21(1):e2016.00093 [DOI] [PMC free article] [PubMed]
- 35.Doornebosch PG, Ferenschild FT, de Wilt JH, et al. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum. 2010;53(9):1234–1239. doi: 10.1007/DCR.0b013e3181e73f33. [DOI] [PubMed] [Google Scholar]
- 36.Elmessiry MM, Van Koughnett JA, Maya A, et al. Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis. 2014;16(9):703–709. doi: 10.1111/codi.12657. [DOI] [PubMed] [Google Scholar]
- 37.Endreseth BH, Myrvold HE, Romundstad P et al ( 2005) Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum 48(7):1380–1388 [DOI] [PubMed]
- 38.Fegiz G, Indinnimeo M, Gozzo P, et al. Low rectal cancer–what is the choice? Dis Colon Rectum. 1994;37(2 Suppl):S35–41. doi: 10.1007/BF02048429. [DOI] [PubMed] [Google Scholar]
- 39.Floyd ND, Saclarides TJ. Transanal endoscopic microsurgical resection of pT1 rectal tumors. Dis Colon Rectum. 2006;49(2):164–168. doi: 10.1007/s10350-005-0269-4. [DOI] [PubMed] [Google Scholar]
- 40.Ganai S, Kanumuri P, Rao RS, et al. Local recurrence after transanal endoscopic microsurgery for rectal polyps and early cancers. Ann Surg Oncol. 2006;13(4):547–556. doi: 10.1245/ASO.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Gavilanes Calvo C, Manuel Palazuelos JC, Alonso Martín J, et al. Transanal endoscopic operations for rectal tumours. Cir Esp. 2014;92(1):38–43. doi: 10.1016/j.ciresp.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez QH, Heslin MJ, Shore G et al (2003) Results of long-term follow-up for transanal excision for rectal cancer. Am Surg 69(8):675–678; discussion 678 [PubMed]
- 43.Greenberg JA, Shibata D, Herndon JE II et al (2008) Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum 51(8):1185–1191; discussion 91–94. [DOI] [PubMed]
- 44.Guerrieri M, Gesuita R, Ghiselli R, et al. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol. 2014;20(28):9556–9563. doi: 10.3748/wjg.v20.i28.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horn A, Halvorsen JF, Morild I. Transanal extirpation for early rectal cancer. Dis Colon Rectum. 1989;32(9):769–772. doi: 10.1007/BF02562126. [DOI] [PubMed] [Google Scholar]
- 46.Hoth JJ, Waters GS, Pennell TC. Results of local excision of benign and malignant rectal lesions. Am Surg. 2000;66(12):1099–1103. [PubMed] [Google Scholar]
- 47.Huang YJ, Huang YM, Wang WL, et al. Surgical outcomes of robotic transanal minimally invasive surgery for selected rectal neoplasms: A single-hospital experience. Asian J Surg. 2020;43(1):290–296. doi: 10.1016/j.asjsur.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Huh JW, Park YA, Lee KY, et al. Recurrences after local excision for early rectal adenocarcinoma. Yonsei Med J. 2009;50(5):704–708. doi: 10.3349/ymj.2009.50.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong WK, Park JW, Choi HS, et al. Transanal endoscopic microsurgery for rectal tumors: experience at Korea's National Cancer Center. Surg Endosc. 2009;23(11):2575–2579. doi: 10.1007/s00464-009-0466-7. [DOI] [PubMed] [Google Scholar]
- 50.Jones HJS, Hompes R, Mortensen N, et al. Modern management of T1 rectal cancer by transanal endoscopic microsurgery: a 10-year single-centre experience. Colorectal Dis. 2018;20(7):586–592. doi: 10.1111/codi.14029. [DOI] [PubMed] [Google Scholar]
- 51.Junginger T, Goenner U, Hitzler M, et al. Influence of Local Recurrence and Distant Metastasis on Prognosis After Local Excision of Rectal Carcinoma. Anticancer Res. 2016;36(2):763–768. [PubMed] [Google Scholar]
- 52.Kanehira E, Tanida T, Kamei A, et al. A single surgeon's experience with transanal endoscopic microsurgery over 20 years with 153 early cancer cases. Minim Invasive Ther Allied Technol. 2014;23(1):5–9. doi: 10.3109/13645706.2013.868814. [DOI] [PubMed] [Google Scholar]
- 53.Keller DS, Tahilramani RN, Flores-Gonzalez JR, et al. Transanal Minimally Invasive Surgery: Review of Indications and Outcomes from 75 Consecutive Patients. J Am Coll Surg. 2016;222(5):814–822. doi: 10.1016/j.jamcollsurg.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Kimura CMS, Kawaguti FS, Nahas CSR, et al. Long-term outcomes of endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumors. J Gastroenterol Hepatol. 2021;36(6):1634–1641. doi: 10.1111/jgh.15309. [DOI] [PubMed] [Google Scholar]
- 55.Kosciñski T, Malinger S, Drews M. Local excision of rectal carcinoma not-exceeding the muscularis layer. Colorectal Dis. 2003;5(2):159–163. doi: 10.1046/j.1463-1318.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 56.Kwakye G, Curran T, Uegami S, et al. Locally Excised T1 Rectal Cancers: Need for Specialized Surveillance Protocols. Dis Colon Rectum. 2019;62(9):1055–1062. doi: 10.1097/DCR.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 57.Lamont JP, McCarty TM, Digan RD et al (2000) Should locally excised T1 rectal cancer receive adjuvant chemoradiation? Am J Surg 180(6):402–405; discussion 5–6. [DOI] [PubMed]
- 58.Langer C, Liersch T, Süss M, et al. Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis. 2003;18(3):222–229. doi: 10.1007/s00384-002-0441-4. [DOI] [PubMed] [Google Scholar]
- 59.Lebedyev A, Tulchinsky H, Rabau M, et al. Long-term results of local excision for T1 rectal carcinoma: the experience of two colorectal units. Tech Coloproctol. 2009;13(3):231–236. doi: 10.1007/s10151-009-0521-3. [DOI] [PubMed] [Google Scholar]
- 60.Lee S, Woo CG, Lee HJ, et al. Effectiveness of adjuvant radiotherapy after local excision of rectal cancer with deep submucosal invasion: a single-hospital, case-control analysis. Surg Endosc. 2015;29(11):3231–3238. doi: 10.1007/s00464-015-4065-5. [DOI] [PubMed] [Google Scholar]
- 61.Lin GL, Meng WC, Lau PY, et al. Local resection for early rectal tumours: Comparative study of transanal endoscopic microsurgery (TEM) versus posterior trans-sphincteric approach (Mason’s operation) Asian J Surg. 2006;29(4):227–232. doi: 10.1016/S1015-9584(09)60093-2. [DOI] [PubMed] [Google Scholar]
- 62.Lloyd GM, Sutton CD, Marshall LJ, et al. Transanal endoscopic microsurgery–lessons from a single UK centre series. Colorectal Dis. 2002;4(6):467–472. doi: 10.1046/j.1463-1318.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- 63.Lohsiriwat V, Anubhonganant W, Prapasrivorakul S, et al. Outcomes of local excision for early rectal cancer: a 6-year experience from the largest university hospital in Thailand. Asian Pac J Cancer Prev. 2013;14(9):5141–5144. doi: 10.7314/apjcp.2013.14.9.5141. [DOI] [PubMed] [Google Scholar]
- 64.Luglio G, Tarquini R, Sivero L, et al. Functional and oncological outcomes after transanal local excision for rectal cancer. A prospective study. Eur J Surg Oncol. 2013;26:337–340. [Google Scholar]
- 65.Madbouly KM, Remzi FH, Erkek BA et al (2005) Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum 48(4):711–719; discussion 9–21. [DOI] [PubMed]
- 66.Maeda K, Maruta M, Sato H, et al. Outcomes of novel transanal operation for selected tumors in the rectum. J Am Coll Surg. 2004;199(3):353–360. doi: 10.1016/j.jamcollsurg.2004.05.268. [DOI] [PubMed] [Google Scholar]
- 67.Márquez MF, Duarte AR, Gil FR, et al. Indications and results of transanal endoscopic microsurgery in the treatment of rectal tumours in a consecutive series of 52 patients. Cir Esp. 2011;89(8):505–510. doi: 10.1016/j.ciresp.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 68.Maslekar S, Pillinger SH, Monson JR. Transanal endoscopic microsurgery for carcinoma of the rectum. Surg Endosc. 2007;21(1):97–102. doi: 10.1007/s00464-005-0832-z. [DOI] [PubMed] [Google Scholar]
- 69.Mentges B, Buess G, Effinger G, et al. Indications and results of local treatment of rectal cancer. Br J Surg. 1997;84(3):348–351. [PubMed] [Google Scholar]
- 70.Morino M, Allaix ME, Caldart M, et al. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc. 2011;25(11):3683–3690. doi: 10.1007/s00464-011-1777-z. [DOI] [PubMed] [Google Scholar]
- 71.Nakagoe T, Ishikawa H, Sawai T, et al. Surgical technique and outcome of gasless video endoscopic transanal rectal tumour excision. Br J Surg. 2002;89(6):769–774. doi: 10.1046/j.1365-2168.2002.02097.x. [DOI] [PubMed] [Google Scholar]
- 72.Nascimbeni R, Nivatvongs S, Larson DR, et al. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773–1779. doi: 10.1007/s10350-004-0706-9. [DOI] [PubMed] [Google Scholar]
- 73.Oh BY, Yun HR, Kim SH, et al. Features of late recurrence following transanal local excision for early rectal cancer. Dis Colon Rectum. 2015;58(11):1041–1047. doi: 10.1097/DCR.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 74.O'Neill CH, Platz J, Moore JS, et al. Transanal endoscopic microsurgery for early rectal cancer: a single-center experience. Dis Colon Rectum. 2017;60(2):152–160. doi: 10.1097/DCR.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 75.Osman KA, Ryan D, Afshar S, et al. Transanal Endoscopic Microsurgery (TEM) for Rectal Cancer: University Hospital of North Tees Experience. Indian J Surg. 2015;77(Suppl 3):930–935. doi: 10.1007/s12262-014-1067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pakkastie T, Järvinen HJ. Local excision as an option in the treatment of low rectal carcinoma. Ann Chir Gynaecol. 1997;86(4):291–296. [PubMed] [Google Scholar]
- 77.Palma P, Horisberger K, Joos A, et al. Local excision of early rectal cancer: is transanal endoscopic microsurgery an alternative to radical surgery? Rev Esp Enferm Dig. 2009;101(3):172–178. doi: 10.4321/s1130-01082009000300003. [DOI] [PubMed] [Google Scholar]
- 78.Park SM, Kye BH, Kim MK, et al. Are we doing too much?: local excision before radical surgery in early rectal cancer. Int J Colorectal Dis. 2018;33(4):383–391. doi: 10.1007/s00384-018-2982-1. [DOI] [PubMed] [Google Scholar]
- 79.Patel SA, Chen YH, Hornick JL, et al. Early-stage rectal cancer: clinical and pathologic prognostic markers of time to local recurrence and overall survival after resection. Dis Colon Rectum. 2014;57(4):449–459. doi: 10.1097/DCR.0b013e3182a70709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Platell C, Denholm E, Makin G. Efficacy of transanal endoscopic microsurgery in the management of rectal polyps. J Gastroenterol Hepatol. 2004;19(7):767–772. doi: 10.1111/j.1440-1746.2004.03364.x. [DOI] [PubMed] [Google Scholar]
- 81.Ptok H, Marusch F, Meyer F et al ( 2007) Oncological outcome of local vs radical resection of low-risk pT1 rectal cancer. Arch Surg 142(7):649–655; discussion 56. [DOI] [PubMed]
- 82.Ramirez JM, Aguilella V, Valencia J et al (2011) Transanal endoscopic microsurgery for rectal cancer. Long-term oncologic results. Int J Colorectal Dis 26(4):437–443 [DOI] [PubMed]
- 83.Restivo A, Zorcolo L, D'Alia G, et al. Risk of complications and long-term functional alterations after local excision of rectal tumors with transanal endoscopic microsurgery (TEM) Int J Colorectal Dis. 2016;31(2):257–266. doi: 10.1007/s00384-015-2371-y. [DOI] [PubMed] [Google Scholar]
- 84.Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89–02. Int J Radiat Oncol Biol Phys. 2000;46(2):313–322. doi: 10.1016/s0360-3016(99)00440-x. [DOI] [PubMed] [Google Scholar]
- 85.Samalavicius N, Ambrazevicius M, Kilius A, et al. Transanal endoscopic microsurgery for early rectal cancer: single center experience. Wideochir Inne Tech Maloinwazyjne. 2014;9(4):603–607. doi: 10.5114/wiitm.2014.44138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schäfer H, Baldus SE, Gasper F, et al. Submucosal infiltration and local recurrence in pT1 low-risk rectal cancer treated by transanal endoscopic microsurgery. Chirurg. 2005;76(4):379–384. doi: 10.1007/s00104-004-0929-2. [DOI] [PubMed] [Google Scholar]
- 87.Serra-Aracil X, Vallverdú H, Bombardó-Junca J, et al. Long-term follow-up of local rectal cancer surgery by transanal endoscopic microsurgery. World J Surg. 2008;32(6):1162–1167. doi: 10.1007/s00268-008-9512-1. [DOI] [PubMed] [Google Scholar]
- 88.Speake D, Lees N, McMahon RF, et al. Who should be followed up after transanal endoscopic resection of rectal tumours? Colorectal Dis. 2008;10(4):330–335. doi: 10.1111/j.1463-1318.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 89.Stanley JD, Bell C, Hinkle N, et al. The Ferguson Operating Anoscope as a minimally invasive option for the treatment of rectal tumors. Am Surg. 2010;76(8):850–856. [PubMed] [Google Scholar]
- 90.Steinhagen E, Chang G, Guillem JG. Initial experience with transanal endoscopic microsurgery: the need for understanding the limitations. J Gastrointest Surg. 2011;15(6):958–962. doi: 10.1007/s11605-011-1496-8. [DOI] [PubMed] [Google Scholar]
- 91.Stipa F, Giaccaglia V, Burza A. Management and outcome of local recurrence following transanal endoscopic microsurgery for rectal cancer. Dis Colon Rectum. 2012;55(3):262–269. doi: 10.1097/DCR.0b013e318241ef22. [DOI] [PubMed] [Google Scholar]
- 92.Stornes T, Wibe A, Nesbakken A, et al. National Early Rectal Cancer Treatment Revisited. Dis Colon Rectum. 2016;59(7):623–629. doi: 10.1097/DCR.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 93.Sun G, Tang Y, Li X, et al. Analysis of 116 cases of rectal cancer treated by transanal local excision. World J Surg Oncol. 2014;12:202. doi: 10.1186/1477-7819-12-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175(5):360–363. doi: 10.1016/S0002-9610(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 95.Varma MG, Rogers SJ, Schrock TR et al (1999) Local excision of rectal carcinoma. Arch Surg 134(8):863–867; discussion 7–8 [DOI] [PubMed]
- 96.Vorobiev GI, Tsarkov PV, Sorokin EV. Gasless transanal endoscopic surgery for rectal adenomas and early carcinomas. Tech Coloproctol. 2006;10(4):277–281. doi: 10.1007/s10151-006-0305-y. [DOI] [PubMed] [Google Scholar]
- 97.Winde G, Blasius G, Herwig R, et al. Benefit in therapy of superficial rectal neoplasms objectivized: Transanal endoscopic microsurgery (TEM) compared to surgical standards. Minim Invasive Ther Allied Technol. 1997;6(4):315–323. [Google Scholar]
- 98.Wirsing K, Lorenzo-Rivero S, Luchtefeld M, et al. Local excision of stratified T1 rectal cancer. Am J Surg. 2006;191(3):410–412. doi: 10.1016/j.amjsurg.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 99.Wykypiel H, Conrad F, Klinger A et al (2002) Local excision of rectal tumors. Coloproctology. 24(4):203–208
- 100.Xu K, Liu Y, Yu P et al (2020) Oncological outcomes of transanal endoscopic microsurgery plus adjuvant chemoradiotherapy for patients with high-risk T1 and T2 rectal cancer. J Laparoendosc Adv Surg Tech A 31(9):1006–1013 [DOI] [PubMed]
- 101.Zacharakis E, Freilich S, Rekhraj S, et al. Transanal endoscopic microsurgery for rectal tumors: the St. Mary's experience Am J Surg. 2007;194(5):694–698. doi: 10.1016/j.amjsurg.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Antonelli G, Berardi G, Rampioni Vinciguerra GL, et al. Clinical management of endoscopically resected pT1 colorectal cancer. Endosc Int Open. 2018;6(12):E1462–E1469. doi: 10.1055/a-0781-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Backes Y, de Vos Tot Nederveen Cappel WH, van Bergeijk J et al (2017) Risk for incomplete resection after macroscopic radical endoscopic resection of T1 colorectal cancer: a multicenter cohort study. Am J Gastroenterol 112(5):785–796 [DOI] [PubMed]
- 104.Cunningham KN, Mills LR, Schuman BM, et al. Long-term prognosis of well-differentiated adenocarcinoma in endoscopically removed colorectal adenomas. Dig Dis Sci. 1994;39(9):2034–2037. doi: 10.1007/BF02088143. [DOI] [PubMed] [Google Scholar]
- 105.Dang H, de Vos Tot Nederveen Cappel WH, van der Zwaan SMS et al (2019) Quality of life and fear of cancer recurrence in T1 colorectal cancer patients treated with endoscopic or surgical tumor resection. Gastrointest Endosc 89(3):533–544 [DOI] [PubMed]
- 106.Iguchi K, Mushiake H, Aoyama T, et al. Additional Surgical Resection After Endoscopic Resection for Patients With High-risk T1 Colorectal Cancer. In Vivo. 2019;33(4):1243–1248. doi: 10.21873/invivo.11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ikematsu H, Yoda Y, Matsuda T et al (2013) Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 144(3):551–559; quiz e14 [DOI] [PubMed]
- 108.Kouyama Y, Kudo SE, Miyachi H, et al. Risk factors of recurrence in T1 colorectal cancers treated by endoscopic resection alone or surgical resection with lymph node dissection. Int J Colorectal Dis. 2018;33(8):1029–1038. doi: 10.1007/s00384-018-3081-z. [DOI] [PubMed] [Google Scholar]
- 109.Levic K, Bulut O, Hansen TP et al (2019) Malignant colorectal polyps: endoscopic polypectomy and watchful waiting is not inferior to subsequent bowel resection. A nationwide propensity score-based analysis. Langenbecks Arch Surg 404(2):231–242 [DOI] [PubMed]
- 110.Meining A, von Delius S, Eames TM, et al. Risk factors for unfavorable outcomes after endoscopic removal of submucosal invasive colorectal tumors. Clin Gastroenterol Hepatol. 2011;9(7):590–594. doi: 10.1016/j.cgh.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Probst A, Ebigbo A, Märkl B, et al. Endoscopic submucosal dissection for early rectal neoplasia: experience from a European center. Endoscopy. 2017;49(3):222–232. doi: 10.1055/s-0042-118449. [DOI] [PubMed] [Google Scholar]
- 112.Probst A, Ebigbo A, Märkl B, et al. Endoscopic submucosal dissection for rectal neoplasia extending to the dentate line: European experience. Endosc Int Open. 2018;6(11):E1355–E1362. doi: 10.1055/a-0749-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Senore C, Giovo I, Ribaldone DG, et al. Management of Pt1 tumours removed by endoscopy during colorectal cancer screening: Outcome and treatment quality indicators. Eur J Surg Oncol. 2018;44(12):1873–1879. doi: 10.1016/j.ejso.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Soriani P, Tontini GE, Neumann H, et al. Endoscopic full-thickness resection for T1 early rectal cancer: a case series and video report. Endosc Int Open. 2017;5(11):E1081–E1086. doi: 10.1055/s-0043-118657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Witjes CDM, Patel AS, Shenoy A et al (2021) Oncological outcome after local treatment for early stage rectal cancer. Surg Endosc. 10.1007/s00464-021-08308-1 [DOI] [PMC free article] [PubMed]
- 116.Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol. 2014;12(2):292–302.e3. doi: 10.1016/j.cgh.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 117.Clancy C, Burke JP, Albert MR, et al. Transanal Endoscopic Microsurgery Versus Standard Transanal Excision for the Removal of Rectal Neoplasms: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2015;58(2):254–261. doi: 10.1097/DCR.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 118.Dekkers N, Boonstra JJ, Moons LMG, et al. Transanal minimally invasive surgery (TAMIS) versus endoscopic submucosal dissection (ESD) for resection of non-pedunculated rectal lesions (TRIASSIC study): study protocol of a European multicenter randomised controlled trial. BMC Gastroenterol. 2020;20(1):225. doi: 10.1186/s12876-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paschke S, Jafarov S, Staib L et al (2018) Are colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int J Mol Sci 19(9):2577 [DOI] [PMC free article] [PubMed]
- 120.Wang AY, Hwang JH, Bhatt A, et al. AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary. Gastroenterology. 2021;161(6):2030–40.e1. doi: 10.1053/j.gastro.2021.08.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and results (DOCX 51 kb)
Supplementary figure 1. Funnel plot for the cumulative incidence of any rectal cancer recurrence. The points correspond to the incidences of individual studies, and the vertical line in the funnel plot indicates the summary estimate. To visualize studies with 0 events, a continuity correction of +0.5 was applied (TIF 147486 kb)
Supplementary figure 2. Forest plot with cumulative incidences of any RC recurrence after local surgical resection in patients with ≥ 2 years follow-up. To visualize incidence estimates of studies with 0 events, a continuity correction of +0.5 was applied. Values of the pooled estimates, I2 and τ2 are calculated using a model without continuity correction (TIF 147486 kb)
Supplementary figure 3. Forest plot with cumulative incidences of rectal cancer-related mortality after local surgical resection in patients with ≥ 2 years follow-up. To visualize incidence estimates of studies with 0 events, a continuity correction of +0.5 was applied.Values of the pooled estimates, I2 and τ2 are calculated using a model without continuity correction (TIF 147486 kb)
Supplementary figure 4. Number of JSCCR criteria used for histological risk stratification for studies on TEM/TAMIS and endoscopic resection. TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery, JSCCR Japanese Society for Cancer of the Colon and Rectum (TIF 147486 kb)
Supplementary figure 5. Strictness of follow-up for studies on endoscopic resection and TEM/TAMIS. TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery (TIF 147486 kb)
Supplementary figure 6. Time to any rectal cancer recurrence after endoscopic resection (TIF 147486 kb)
Supplementary figure 7. Time to any rectal cancer recurrence after local surgical resection (TIF 147486 kb)
Supplementary figure 8. Treatment of recurrence after local surgical resection. a. local endoluminal recurrence, b. locoregional recurrence, c. local/locoregional + distant recurrence, d. distant recurrence. TME total mesorectal excision, CRT chemoradiotherapy (TIF 2367 kb)
Supplementary figure 9. Time between rectal cancer recurrence and rectal cancer-related mortality (TIF 831 kb)
Supplementary table 1. In- and exclusion criteria for the endoscopic sub-section. CRC colorectal cancer, RC rectal cancer (DOCX 14 kb)
Supplementary table 2. Meta-regression with study characteristics and risk of bias. Potential predictors of statistical inter-study heterogeneity for the outcome "any rectal cancer recurrence". (DOCX 21 kb)
Supplementary table 3. Meta-regression with clinical characteristics. Potential predictors of statistical inter-study heterogeneity for the outcome "any rectal cancer recurrence". LE local excision, TEM transanal endoscopic microsurgery, TAMIS transanal minimally invasive surgery, AV anal verge, DL dentate line, LVI lymphovascular invasion (DOCX 20 kb)
Supplementary file: Prisma checklist (DOCX 31 kb)
Data Availability Statement
Data file, analytic methods and study materials will be made available in the supplementary data. This meta-analysis was not pre-registered.