Abstract
Shotgun metagenomic sequencing is a common approach for studying the taxonomic diversity and metabolic potential of complex microbial communities. Current methods primarily use second generation short read sequencing, yet advances in third generation long read technologies provide opportunities to overcome some of the limitations of short read sequencing. Here, we compared seven platforms, encompassing second generation sequencers (Illumina HiSeq 300, MGI DNBSEQ-G400 and DNBSEQ-T7, ThermoFisher Ion GeneStudio S5 and Ion Proton P1) and third generation sequencers (Oxford Nanopore Technologies MinION R9 and Pacific Biosciences Sequel II). We constructed three uneven synthetic microbial communities composed of up to 87 genomic microbial strains DNAs per mock, spanning 29 bacterial and archaeal phyla, and representing the most complex and diverse synthetic communities used for sequencing technology comparisons. Our results demonstrate that third generation sequencing have advantages over second generation platforms in analyzing complex microbial communities, but require careful sequencing library preparation for optimal quantitative metagenomic analysis. Our sequencing data also provides a valuable resource for testing and benchmarking bioinformatics software for metagenomics.
Subject terms: Data publication and archiving, Metagenomics
| Measurement(s) | mock community • Metagenomic profiling |
| Technology Type(s) | Illumina HiSeq 3000 • ThermoFisher Ion Proton and GeneStudio S5 • MGI DNBseq G400 and T7 • Oxford Nanopore Sequencing • PacBio Sequel System • Genome mapping and assembly |
| Sample Characteristic - Organism | Synthetic microbial communities • Bacteria • Archaea |
Background & Summary
High throughput metagenomic sequencing has drastically changed our understanding of microbial ecosystems. One of the most popular approach is to use metagenomic sequencing, assembly and binning procedures1–4 to investigate the structure, functionalities and ecological interactions of microbial communities with their environment or host5–9. Most metagenomic studies rely on second generation sequencing providing billions of short sequences in a single run, with the Illumina sequencing platforms being the most widely used10. Improvement of third generation sequencing yields millions of long reads per run but are mostly used for genomic assembly procedures11–13, and further benchmarking is required to evaluate their performance for quantitative metagenomic analysis.
For this purpose, we produced three synthetic uneven DNA mocks, varying in their microbial richness (64 to 87 strains, full composition in Supplementary Table S1) and belonging to 29 prokaryotic phyla (Fig. 1). We particularly focused on combining a large spectrum of genome sizes, GC content and mixing closely related species. These mocks represent to date the most complex synthetic communities for evaluating sequencers performances compared to previous studies14–19, and not obtained from in silico simulated microbial communities20–23. We performed five short read sequencing (Ion Proton P1, Ion S5, Illumina HiSeq 3000, DNBSEQ G400, DNBSEQ T7) and two long read sequencing technologies (ONT MinION and PacBio Sequel II), making this study the one covering the widest diversity of sequencing technologies (Table 1).
Fig. 1.
Phylogenetic tree of the microbial species used in the mock microbial communities. Neighbor joining tree built using 40 universal protein markers and visualized using iTOL. On the left, colored strips referred to Phylum phylogenetic ranks using GTBD. Annotations on the right referred to genome completeness (white square, complete; red square, draft genome), genome size, genome GC percent (circle sizes proportional to the dataset range), and mocks composition (plain triangle, present; empty triangle, absent).
Table 1.
Overview of the main sequencing platform characteristics used in this study.
| Illumina HiSeq 3000 | Ion Proton P1 | Ion S5 | DNBSEQ-G400 | DNBSEQ- T7 | ONT MinION R9 | PacBio Sequel II | |
|---|---|---|---|---|---|---|---|
| Amplification type | Solid-Phase bridge | emPCR | emPCR | DNB | DNB | — | — |
| Sequencing principle | SBS | SBS | SBS | SBS | SBS | SMS | SMS |
| Average reads length ± stdv after trimming (bp) | 149 ± 4.24 | 144.041 ± 28.43 | 145.76 ± 28.12 | 99.91 ± 0.96 | 99.52 ± 2.58 | 4408.41 ± 2831.95 | 10289.7 ± 4036.27 |
| Max read length after trimming (bp) | 150 | 373 | 347 | 100 | 100 | 60869 | 40278 |
| SE/PE | PE | SE | SE | PE | PE | SE | SE |
| Average insert size ± stdv (bp) | 433.47 ± 92.37 | — | — | 245.13 ± 51.04 | 235.56 ± 54.80 | — | — |
| Total Run Time | 4d | 4 h | 4 h | 3d | 3d | 48 h* | 30 h |
emPCR = emulsion PCR; SBS: Sequencing by Synthesis; SMS: Single-Molecule Sequencing; DNB: DNA NanoBall. *Data produced by MinION are accessible few minutes after the sequencing start, however, we run the MinION for 48 h to analyze the maximal throughput. DNBSEQ-G400 platform was formerly named MGISEQ-2000. SE: Single end (Forward read only). PE: Paired End. Run Time indicates time to obtain the maximal throughput.
The mock1 (71 strains) was sequenced using all technologies and mocks 2 and 3 (64 and 87 strains, respectively) were additionally sequenced to estimate the impact of various microbial richness (Table 2). After sequencing and quality control, we were able to align more than 99% of all reads for each technology back to their reference genomes, with almost 100% of uniquely mapped reads for long read technologies, down to 87% for Ion Proton and S5 technologies15,24. All technologies provided up to 99% identity, except for the MinION R9 with about 89% identity due to a high in/del errors and substitution errors25. The PacBio Sequel II provided the lowest substitution error rate and the DNBSeq G400 and T7 the lowest in/dels rate26,27.
Table 2.
Mapping summary per mock and sequencing technology.
| Sample ID | Sequencing Technology | N. reads before trimming (million) | N. reads after trimming (million) | %Mapped end-to-end | %Uniquely mapped | %Avg end-to-end best mapped identity | %Avg end-to-end best mapped substitutions | %Avg end-to-end best mapped in/dels |
|---|---|---|---|---|---|---|---|---|
| MOCK1 (N = 71 species) | Illumina HiSeq 3000 | 21.38*2 | 20.59*2 | 99.62 | 93.21 | 99.45 | 0.46 | 0.09 |
| Ion Proton P1 | 21.23 | 20.00 | 99.29 | 87.13 | 99.42 | 0.12 | 0.46 | |
| Ion S5 | 30.05 | 28.51 | 99.35 | 87.13 | 99.61 | 0.08 | 0.31 | |
| ONT Minion R9 | 0.757 | 0.696 | 99.75 | 99.63 | 89.08 | 3.37 | 7.55 | |
| PacBio Sequel II | 0.525 | 0.524 | 99.65 | 99.62 | 99.72 | 0.06 | 0.22 | |
| DNBSEQ-G400 | 36.17*2 | 35.42*2 | 99.22 | 89.16 | 99.70 | 0.30 | 0.003 | |
| DNBSEQ-T7 | 404.06*2 | 375.12*2 | 98.92 | 88.78 | 99.42 | 0.58 | 0.003 | |
| MOCK2 (N = 87 species) | Illumina HiSeq 3000 | 24.27*2 | 23.17*2 | 99.66 | 89.44 | 99.43 | 0.49 | 0.08 |
| Ion Proton P1 | 22.21 | 20.98 | 99.46 | 86.61 | 99.41 | 0.12 | 0.47 | |
| Ion S5 | 25.36 | 24.17 | 99.55 | 86.66 | 99.59 | 0.09 | 0.32 | |
| ONT Minion R9 | 0.919 | 0.831 | 99.74 | 99.61 | 89.05 | 3.39 | 7.56 | |
| DNBSEQ-G400 | 37.92*2 | 37.14*2 | 99.43 | 88.85 | 99.73 | 0.27 | 0.003 | |
| DNBSEQ-T7 | 404.99*2 | 376.04*2 | 99.03 | 88.32 | 99.46 | 0.54 | 0.003 | |
| MOCK3 (N = 64 species) | Illumina HiSeq 3000 | 63.97*2 | 62.08*2 | 99.58 | 90.36 | 99.39 | 0.52 | 0.09 |
| Ion Proton P1 | 21.50 | 20.26 | 99.15 | 88.33 | 99.44 | 0.11 | 0.45 | |
| Ion S5 | 27.32 | 25.90 | 99.31 | 88.40 | 99.62 | 0.08 | 0.30 | |
| ONT Minion R9 | 0.865 | 0.791 | 99.79 | 99.61 | 89.06 | 3.35 | 7.59 |
The number of reads before and after trimming refer to the sequencing depth (million reads) before and after quality control filtering and trimming. %Mapped end-to-end correspond to the read percentage aligned to a reference genome considering the read full length, while %Uniquely mapped reads correspond to the percentage of reads aligned to only one region of a reference genome. %Avg end-to-end refer to the best hit mean percentage for mapped identity and substitutions and insertions/deletions (in/dels) respectively. See the Method section for trimming and mapping parameters.
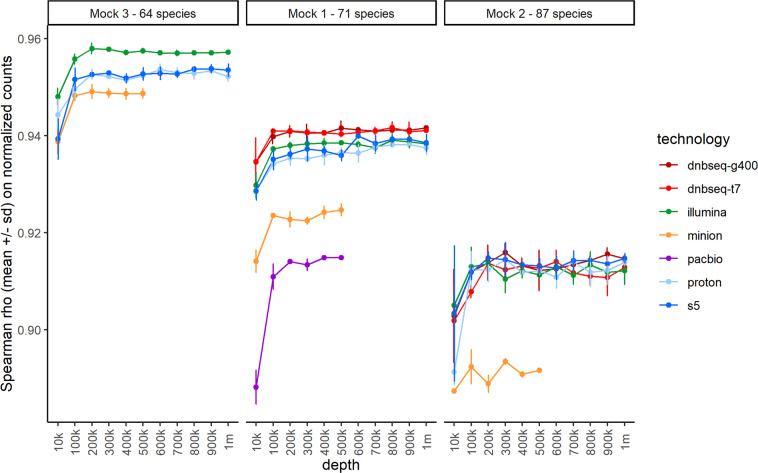
To evaluate the impact of sequencing depth, we performed a subsampling analysis and compared observed versus theoretical genome abundances (Fig. 2). In general, Spearman correlations were high for all technologies, reaching values above 0.9 when mapping at least 100,000 reads. Notably, correlations were slightly lower for mock communities with higher microbial richness, partially due to cross-matching events during mapping procedures. Whilst second generation sequencers were equivalent for taxonomic profiling28, we found more pronounced decreases for MinION and PacBio correlations, even if reads were almost entirely uniquely mapped. Although the PacBio sequencer presented the lowest error rate, these results could be explained by the DNA size filtering step performed during library preparation, which was calibrated to maximize the read length. We hypothesize that the filtering step could remove highly fragmented DNA, thus impacting strains relative abundances29–31.
Fig. 2.
Overall comparison between observed and excepted mock compositions per technology. After read mapping to the mock reference genome, a subsampling was performed 3 times at multiple sequencing depth from 10,000 to 1 million reads, except for ONT MinION and PacBio for which maximum depth was 500k. Spearman rank correlations were calculated between observed genomes abundances normalized by genome size (expressed in %) and the expected mock composition (%). Means ± SD are reported based on the 3 iterations performed per depth. PacBio sequencing was not performed on mock3 and mock2, DNBSEQ-T7 and DNBSEQ-G400 were not performed on mock3.
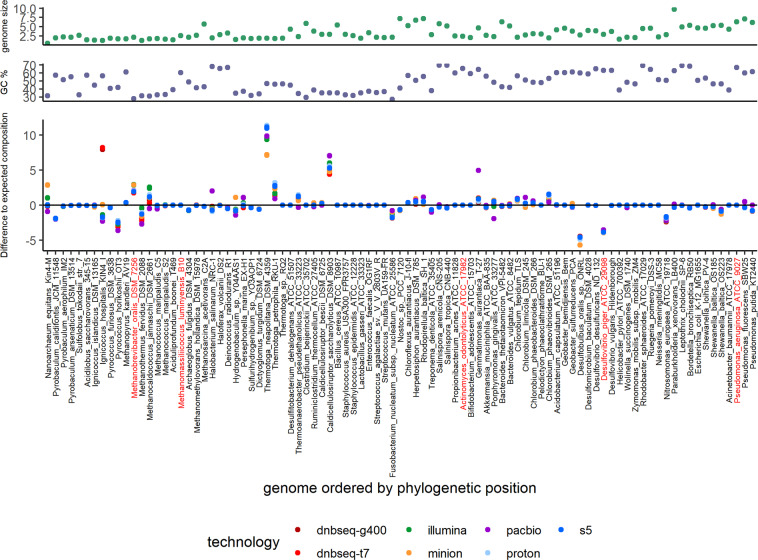
By focusing on mock1 individual genome abundances, we found that most genomes were accurately estimated for all technologies (Fig. 3). Over or under abundance estimation for most genomes was not particularly related to sequencing technology, read length, taxonomy, nor by GC-content, genome size and genome completeness, even at a low depth of 500,000 reads. These results suggest promising opportunities for affordable alternatives to high depth metagenomic sequencing, by using a limited number of reads- the so-called shallow shotgun sequencing- to explore the composition of complex microbiota32, even with third generation sequencers19.
Fig. 3.
Differential plot between observed and excepted species abundances in mock1. Abundances (%) for each genome were calculated at 500k depth for each sequencing platform and normalized by genome size. Differential abundance was determined by subtracting the excepted abundances (%) to the observed normalized abundances (%). Positive values, genomes are over-estimated; Negative values, under-estimated. Genomes colored in red indicate draft genomes. Genome size and GC percent are reported for each species.
Finally, we performed de novo metagenomic assembly and confronted assemblies with their reference genomes (Table 3). PacBio Sequel II generated the most contiguous assemblies with 36 full genomes out of 71 in mock1, followed by MinION (22 genomes), making third generation sequencers more adapted for genome reconstruction. When considering the mismatches per 100kbps, PacBio Sequel II was also providing the most accurate assemblies, followed by Illumina HiSeq 3000 and DNBSeq G400 (Table 3). However, the lower indels rates obtained with DNBSeq G400 and Illumina HiSeq suggests that hybrid procedures may provide more accurate assemblies than those obtained using long reads alone. We tested this hypothesis by generating hybrid assemblies (Supplementary Information S2) for each technology. For MinION, the hybrid assemblies improved notably the genome fraction recovery compared to MinION assembly only, while reducing the number of fully unaligned contigs, confirming our initial hypothesis. For PacBio, the hybrid assembly did not improve assembly metrics, except for Illumina and DNBSeq with a lower indels rate per 100 kpbs and an improvement in genome fraction recovery with Illumina.
Table 3.
Mock1 metaquast assembly report.
| Sequencer | Ion Proton P1 (spades) | Ion S5 (spades) | Illumina HiSeq 3000 (spades) | DNBSeq G400 (spades) | DNBSeq T7 (spades) | ONT MinION R9 (metaflye) | PacBio Sequel II (metaflye) |
|---|---|---|---|---|---|---|---|
| Nb Reads (M) | 20 | 20 | 2 × 10 | 2 × 10 | 2 × 10 | 0.696 | 0.524 |
| Nb Contigs | 45,510 | 43,879 | 40,147 | 44,887 | 44,603 | 1,283 | 437 |
| Largest Contig (bp) | 384,996 | 794,907 | 1,599,668 | 1,063,396 | 1,002,925 | 4,324,150 | 7,147,004 |
| N50 (bp) | 7,847 | 9,089 | 13,707 | 8,519 | 8,184 | 759,940 | 2,013,697 |
| Genome Fraction(%) | 54.767 | 55.257 | 61.897 | 49.397 | 47.365 | 44.955 | 68.197 |
| Mismatches per 100kbps | 83.29 | 89.12 | 47.55 | 77.22 | 107.52 | 339.99 | 18.3 |
| Indels Per 100kbps | 77.8 | 50.03 | 3.53 | 3.23 | 3.67 | 764.45 | 11.76 |
| Fully Unaligned Contigs | 1,497 | 1,339 | 975 | 735 | 1,368 | 231 | 6 |
| Fully Unaligned Length (bp) | 900,150 | 821,545 | 620,805 | 426,856 | 711,992 | 6,279,694 | 134,713 |
| NB full genome* | 5 | 5 | 12 | 7 | 7 | 22 | 36 |
Only contigs > = 500nt were aligned to the mock1 reference genomes. Number of reads, contigs and the size of the largest contig after each assembly are reported, along with: N50 (bp), a common statistic to evaluate the assembly quality; Genome fraction (%), corresponding to the mean percentage of the genome reconstructed during the assembly; the means for Mismtaches per 100kbps and Indels (Insertions/Deletions) per 100kbps, to evaluate the distance of the reconstructed genome to the reference genome). The number of fully unaligned contigs and the respective length (pb) are reported. * Full genome: More than 99% genome recovery.
By this work, we provide a new resource with highly complex synthetic mock samples and extensive metagenomic sequencing data, using the most popular second and third generation sequencing platforms. These data could be used to benchmark or improve metagenomic assemblers, binning software and taxonomic profilers33,34.
Methods
Synthetic microbial communities’ construction
A total of 91 different strains were used in this study. For 58 strains of Archaea and Bacteria, we used archived gDNA from the Shakya et al. study14. To further increase the complexity of the constructed community, we cultured nine additional microbes for which the genomic sequence was available. High molecular weight DNA was isolated and quantified as described previously14. Purified DNA from 4 other bacteria and archaea was obtained from the laboratory of Dr. Cynthia Gilmour (Smithsonian Research Institute, Edgewater, Maryland, USA). We also used the 20 Strain Even Mix Genomic Material from ATCC (MSA-1002). Three different genomic microbial synthetic communities were assembled by mixing individual, purified DNAs. The composition of each community aimed to provide variation in the number and relative abundance of individual microbe and their represented taxonomic category. The communities achieved a diversity ranging from 64 to 87 strains, representing 29 phyla of Archaea and Bacteria, with a relative abundance distribution spanning over three orders of magnitude. The genome size distribution ranged from 0.49 to 9.7 Mbp and the G + C content was between 27 and 69%. Within the 91 strains, 21 have extrachromosomal DNA such as plasmids or additional chromosomes (Supplementary Table S1). A phylogenetic tree for all strains was constructed using 40 universal protein markers as previously described1 and taxonomic ranks were updated using gtdbtk Release 07-RS20735. The tree was visualized and annotated using iTOL36.
Library preparation and Sequencing
ThermoFisher Ion Proton P1 and Ion GeneStudio S5 library preparation and sequencing
Ion Proton P1 and Ion GeneStudio S5 libraries were built using Ion Plus Fragment Library kit (Thermo Fisher Scientific, Waltham, MD, USA). 500 ng of High Molecular Weight (HMW) DNA was sheared using Covaris E220 sonicator and AFA microtubes (Covaris, Brighton, UK) in 100 µL to achieve maximum distribution at 150pb. After shearing, Ampure XP purification and Qubit quantification were performed. Sheared DNA (100 ng) was submitted to enzymatic treatment steps (End repair, barecode ligation with IonXpress Barcode Adaptors kit and final 9 cycles of PCR amplification). Ampure XP beads were used for size selection to 150 pb after End repair reaction and for purification after the other enzymatic treatment steps. Libraries were quantified and size controlled by using High Sensitivity Small Fragment kit and Fragment Analyzer (Agilent Technologies Inc., Santa Clara, CA, USA). Libraries’ molarity was between 8.000 and 10.000 pM, before normalization at 95 pM and multiplexing for sequencing. Pipetting was performed with Biomek Fxp or Biomek 3000 Liquid handling (Beckman Coulter Inc., Brea, CA, USA). Pre-sequencing step was performed by Ion Chef (Thermo Fisher) for each sequencing device. A first sequencing was performed by Ion Proton with Ion PI HiQ Chef kit (Thermo Fisher) and Ion PI chip kit v3 (Thermo Fisher). Several run were performed including first path, any path with rebalance libraries and final path to get up to 20 million raw reads for each multiplexed sample. A second sequencing was performed by Ion GeneStudio S5 Prime with Ion 550 chef kit and Ion 550 chip kit. A single run was sufficient to obtain up to 20 million raw reads per multiplexed sample.
MGI DNBSEQ-G400 and DNBSEQ-T7 library preparation and sequencing
DNBSEQ-G400 and DNBSEQ-T7 libraries were constructed from 500 ng of HMW DNA and fragmented using Covaris sonicator E220 (Covaris, Brighton, UK). Sheared DNA underwent End repair and A-tailing steps as described in the MGI Easy Universal DNA Library Prep Set User Manual v1 (MGI Tech Co., Shenzen, China). Adapters ligation was performed following the instructions of the MGIEasy DNA Adapters kit, and cleaned up with the provided DNA Clean Beads. PCR amplification was carried out on purified adapter-ligated DNA and cleaned-up again using magnetic beads. After quality control using Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MD, USA), purified PCR products were denaturated and ligated to generate single-strand circular DNA libraries. Barcode libraries were pooled in equal amounts to make DNA Nanoballs (DNB), and sequenced using DNBSEQ-G400 and DNBSEQ-T7 sequencer technologies following the manufacturer’s recommendations.
Illumina library preparation and HiSeq 3000 sequencing
DNA libraries have been prepared using the Illumina TruSeq PCR-free HT (Illumina, San Diego, CA, USA), following the manufacturer protocol. Briefly, 2 µg of HMW genomic DNA was fragmented by sonication using Covaris sonicator (Covaris, Brighton, UK). Sheared fragments were cleaned using the Sample Purification Beads provided in the kit, before Ends repair and size selection procedures. Adapters were ligated and libraries underwent an additional cleaning step with magnetic beads. Library quality was assessed using an Advanced Analytical Fragment Analyser (Agilent Technologies Inc., Santa Clara, CA, USA) and libraries were quantified by q-PCR using the Kapa Library Quantification Kit (Illumina, San Diego, CA, US) following the manufacturer’s recommendations. Prior to multiplexing, libraries were normalized to 4 nM and equal volumes were pooled together. Final libraries were sequenced on Illumina HiSeq 3000 using a paired-end read length of 2 × 150 pb with the Illumina HiSeq 3000 Reagent Kits.
Oxford Nanopore MinION R9 library preparation and sequencing
Libraries were built with 1D Native barcoding genomic DNA kit (SQK-LSK109 rev E) from Oxford Nanopore Technologies. To increase sequencing yield, 1.5 µg DNA samples were sheared using G-Tube (Covaris) for 2 times 30 seconds at 7,200 rpm. Sheared Fragments (1 µg), of length comprised between 8 and 9 kb, underwent end repair and A-Tailing (New England Biolabs M6630L and E7546L kits). Next, 500 ng of repaired DNA was ligated with adapter barcode using Native Barcoding Expansion 1–12 kit (EXP-NBD104) and Blunt/TA Ligase (New England Biolabs, M0367L). Native barcode ligated DNA was quantified with Qubit and Fragment Analyzer. Equimolar libraries were pooled for a total quantity of 700 ng to ligate to the sequencing adapters. At this step, we choose the Long Fragment Buffer (LFB) from SQK-LSK109 kit to increase the recovery of 3 kb or longer fragments. Pooled libraries were loaded onto R9 FLO-MIN106 flowcell and sequencing was performed during 48 hours.
Pacific Biosciences Sequel II library preparation and sequencing
For Pacific Biosciences Sequel II sequencing, 500 ng of HMW genomic DNA was used to make unamplified libraries using the SMRTbell® Express Template Prep Kit 2.0. First, gDNA was sheared to a targeted fragment size of 12 kb using Megaruptor and Long Hydropores (Diagenode, Denville, NJ, USA). Sheared gDNA were concentrated using AMPure PB Beads according to the manufacturer recommendations (Pacific Biosciences, Menlo Park, CA, USA) and underwent two treatment procedures for DNA damage repair and end-repair. Barcoded overhang Hairpins adapters from the manufacturer were ligated to the fragment ends to create SMRTbell templates used for sequencing. SMRTbell templates were purified using an exonuclease procedure to remove any free ends molecules or no adapter templates. Then, size-selection was conducted using Ampure PB beads at a concentration of 0.45X to ensure the removing of short fragments. Our mock SMRTbell template was multiplexed with tree additional samples (not included in this study) to equal molarity. On the resulting template, fragments < 3 kb were removed using an additional diluted Ampure PB beads procedure. PacBio primer v2 annealing to the SMRTbell template and polymerase binding to the annealed template were achieved before being sequenced with Sequel II sequencer using Chemistry 2.0 and 30-hour movie.
Sequence QC
The raw reads were quality trimmed using software tools with similar trimming parameters to improve technical comparisons. Illumina HiSeq 3000 and DNBSeq G400 and T7 paired-end reads were trimmed with FASTP v.0.20.037, using Illumina TruSeq adapaters for the Illumina HiSeq 300 sequencer and DNBSeq adapters for the DNBSeq G400 and T7. The minimum read length after trimming was 45nt and all reads with a single N nucleotide or unpaired reads after trimming were discarded. The Ion S5 and Ion Proton reads were trimmed using AlienTrimmer v2.038 by providing the Ion S5 and Proton contaminants and using the following parameters for trimming: “-k 10 -l 45 -m 5 -p 40 -q 20”. The minION R9 reads were base called and quality trimmed using Guppy v2.3.1 + 9514fbc39 with the kit SQK-LSK109 and the barcoding kit EXP-NBD103. The PacBio CSS reads were processed through PacBio custom pipeline. Finally, all PacBio and MinION reads shorter than 500nt were discarded.
Read mapping procedures
All read mapping procedures were performed on reference genomes corresponding to the expected mock composition. For Illumina, DNBSEQ G400 and T7 platforms, mapping was done with bowtie2 v2.3.5.140 using paired-end best hit end-to-end match and sensitive presets parameters. For Ion Proton and S5, bowtie2 with single-end best-hit end-to-end match and sensitive presets parameters. For MinION and PacBio, mapping was performed with minimap2 version 2.15-r915-dirty41 using default parameters, soft clipping activated and by keeping only the best hit.
Read subsampling
Subsampling was performed by a python script using the random library and differential analysis in Figs. 2 and 3 between the observed and expected mock composition at different depth, from 10k to 1 M reads were performed under R version 3.6.0 using stats, ggplot2, data.table and reshape2 packages.
Metagenomic assembly
The Illumina HiSeq 3000, DNBSeq G400 and T7 paired-end reads were assembled with SPAdes v3.14.142 with “--meta” presets and kmer iteration “--k 21,33,55” for DNBSeq, and “--k 21,33,55,77” for Illumina to account for their respective maximal read length. The Ion Proton and Ion S5 single reads were also assembled with SPADES, using the “--iontorrent” and “--careful” flag, as the “--meta” flag is not available for single reads, and a kmer iteration “--k 21,33,55,77”. MinION and Pacbio were assembled with metaFlye v2.8.1-b168843 using the “--meta” preset, “--plasmids” to recover short unassembled plasmids, a minimum overlap of 2000nt, “--pacbio-hifi--hifi-error 0.003” for Pacbio and “--nano-raw” for MinION reads. Finally, the assemblies’ quality was assessed using metaquast v4.6.344.
Hybrid metagenomic assembly
Hybrid assemblies were generated using SPAdes v3.14.142 with the same parameters previously described and by adding –pacbio and –nanopore parameters when combining with PacBio reads or MinION reads respectively.
Data Records
Shotgun metagenomes are publicly available without restriction in the EMBL-EBI European Nucleotide Archive (ENA) under accession number PRJEB5297745. All binning and taxonomy assignment results and parameters are available as a publicly shared KBase narrative (https://narrative.kbase.us/narrative/125743) and can also be seen at Figshare46.
Technical Validation
Library QC checks
Before library preparation by the different sequencing platforms, gDNA mock samples were required to pass quality and quantity controls. Initial DNA quality control included DNA quantification using Quant-iT™ dsDNA Assay Kit broad range (Q33130) reading by FiltermaxF3 (Molecular Devices, Sunnyvale, CA, USA), Qubit dsDNA Assay Kit (Q32853) and fragment analysis using HS Genomic DNA kit (DNG-488-500) on Fragment Analyzer (Agilent Technologies Inc., Santa Clara, CA, USA). The size of initial DNA peak was between 14 and 17 kb without major degradation smear. Additional specific technical validations for DNA integrity were required during each sequencing library preparation to ensure high quality of the final libraries on each platform. Depending on the sequencing technology, these validation steps typically included QC checks after DNA shearing, size selections, purifications on magnetic stands and on the pooled final libraries.
Usage Notes
Run accession numbers for all metagenomic samples, accessible in the ENA website (PRJEB52977), are fully described in Table 4.
Table 4.
Shotgun metagenomic datasets description.
| MOCK1 (71 species) | MOCK2 (87 species) | MOCK3 (64 species) | |
|---|---|---|---|
| Illumina HiSeq 3000 | ERR9765446 | ERR9765447 | ERR9765448-49 |
| Ion Proton P1 | ERR9765780-58 | ERR9765759-67 | ERR9765768-76 |
| Ion S5 | ERR9765777 | ERR9765778 | ERR9765779 |
| ONT Minion R9 | ERR9765780 | ERR9765781 | ERR9765782 |
| PacBio Sequel II | ERR9765783 | NA | NA |
| DNBSEQ-G400 | ERR9765742 | ERR9765743 | NA |
| DNBSEQ-T7 | ERR9765744 | ERR9765745 | NA |
Run accession numbers were reported for each sample and technology. Metagenomic data have been deposited under BioProject number PRJEB52977.
The protocols and datasets we are presenting in this work can be reused for different applications, in particular to benchmark and improve metagenomic assemblers, taxonomic profilers and binning software. As an example for binning applications, we used three binning software (CONCOCT47 v.1.1, MetaBAT248 v1.7 and MaxBin249 v2.2.4) by importing the mock1 Illumina HiSeq 3000 reads, assemblies and hybrid assemblies into KBase50 (https://narrative.kbase.us/narrative/125743), with minimum contig size of 1500 nt (Supplementary Table S3). The bins were then optimized using DAS Tool51 v1.1.2. We observed comparable number of binned MAGs corresponding to reference genomes using hybrid assembly and higher than using the Illumina short reads dataset alone. With all assemblies and datasets, the recovery of high quality MAGs was not successful for very low abundance genomes, present at less than 0.1% of the mock1 community (Supplementary Table S3).
Supplementary information
Acknowledgements
This work was supported by the European FP7 Marie Skłodowska-Curie actions AgreenSkillsPlus PCOFUND-GA-2013-609398 grant to MA. Additional funding was from the Metagenopolis grant ANR-11-DPBS-0001. MP was supported by the U.S. Department of Energy, Office of Science, Biological, and Environmental Research as part of the Genomic Science Program (the Plant Microbe Interfaces SFA), by the Subsurface Biogeochemical Research Program (Biogeochemical Transformations at Critical Interfaces SFA) and by grant R01DE024463 from the National Institute of Dental and Craniofacial Research of the US National Institutes of Health. Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract DE-AC05-00OR22725. We thank Dr. Cynthia Gilmour (Smithsonian Research Institute, Edgewater, Maryland, USA) for providing some of the purified gDNA used in this study. We also thank David Stucki, Deborah Moine and Jules Bourgon (Pacific Biosciences, Menlo Park, California, USA) for PacBio sequencing, Jihua Sun and Yong Hou (BGI, Shenzhen, Guangdong, China) for DNBseq sequencing and Clemence Genthon (Plateforme Génomique GeT INRAE Transfert, Toulouse, France) for Illumina sequencing.
Author contributions
M.A., M.P. and H.R. conceived the project. M.P. generated mock communities. M.A. and V.M. analysed the data and generated the figures. B.Q. performed library preparation and sequencing for Ion proton, DNBseq and Minion technologies. N.P. deposited the data. F.P.O., M.P. and K.D.S. contributed to data analysis. V.M., M.A., B.Q. and M.P. wrote the paper. All authors revised the manuscript.
Code availability
All reference genomes and scripts for mapping, assembly, genome coverage estimation, subsampling and correlation calculations associated with tables and figures are available at https://forgemia.inra.fr/metagenopolis/benchmark_mock.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mircea Podar, Email: podarm@ornl.gov.
Mathieu Almeida, Email: mathieu.almeida@inrae.fr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-022-01762-z.
References
- 1.Almeida, M. et al. Construction of a dairy microbial genome catalog opens new perspectives for the metagenomic analysis of dairy fermented products. BMC Genomics15 (2014). [DOI] [PMC free article] [PubMed]
- 2.Tully, B. J., Graham, E. D. & Heidelberg, J. F. The reconstruction of 2,631 draft metagenome-assembled genomes from the global oceans. Sci. Data5 (2017). [DOI] [PMC free article] [PubMed]
- 3.Pasolli E, et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell. 2019;176:649–662.e20. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida A, et al. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:199–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venter JC, et al. Environmental Genome Shotgun Sequencing of the Sargasso Sea J. Craig Venter. Science (80-.). 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 6.Tringe SG, et al. Comparative metagenomics of microbial communities. Science. 2005;308:554–7. doi: 10.1126/science.1107851. [DOI] [PubMed] [Google Scholar]
- 7.Qin N, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 8.Uritskiy G, DiRuggiero J. Applying Genome-Resolved Metagenomics to Deconvolute the Halophilic Microbiome. Genes (Basel) 2019;10:220. doi: 10.3390/genes10030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromentin S, et al. Microbiome and metabolome features of the cardiometabolic disease spectrum. Nat. Med. 2022;28:303–314. doi: 10.1038/s41591-022-01688-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segerman B. The Most Frequently Used Sequencing Technologies and Assembly Methods in Different Time Segments of the Bacterial Surveillance and RefSeq Genome Databases. Front. Cell. Infect. Microbiol. 2020;10:1–7. doi: 10.3389/fcimb.2020.527102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C. The Third Revolution in Sequencing Technology. Trends Genet. 2018;34:666–681. doi: 10.1016/j.tig.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Athanasopoulou K, et al. Third-Generation Sequencing: The Spearhead towards the Radical Transformation of Modern Genomics. Life. 2021;12:30. doi: 10.3390/life12010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logsdon GA, Vollger MR, Eichler EE. Long-read human genome sequencing and its applications. Nat. Rev. Genet. 2020;21:597–614. doi: 10.1038/s41576-020-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakya M, et al. Comparative metagenomic and rRNA microbial diversity characterization using archaeal and bacterial synthetic communities. Env. Microbiol. 2014;15:1882–1899. doi: 10.1111/1462-2920.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sevim V, et al. Shotgun metagenome data of a defined mock community using Oxford Nanopore, PacBio and Illumina technologies. Sci. Data. 2019;6:285. doi: 10.1038/s41597-019-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer E, et al. Next generation sequencing data of a defined microbial mock community. Sci. Data. 2016;3:160081. doi: 10.1038/sdata.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholls SM, Quick JC, Tang S, Loman NJ. Ultra-deep, long-read nanopore sequencing of mock microbial community standards. Gigascience. 2019;8:giz043. doi: 10.1093/gigascience/giz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tourlousse DM, et al. Characterization and Demonstration of Mock Communities as Control Reagents for Accurate Human Microbiome Community. Microbiol Spectr. 2022;10:e0191521. doi: 10.1128/spectrum.01915-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, Y., Fang, L., Nicholson, C. & Wang, K. Implications of Error-Prone Long-Read Whole- Genome Shotgun Sequencing on Characterizing Reference Microbiomes. iScience23 (2020). [DOI] [PMC free article] [PubMed]
- 20.Yang C, Chu J, Warren L, Inanc B. NanoSim: nanopore sequence read simulator based on statistical characterization. Gigascience. 2017;6:1–6. doi: 10.1093/gigascience/gix010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss EL, Maghini DG, Bhatt AS. Complete, closed bacterial genomes from microbiomes using nanopore sequencing. Nat. Biotechnol. 2020;38:701–707. doi: 10.1038/s41587-020-0422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peabody MA, Van Rossum T, Lo R, Brinkman FSL. Evaluation of shotgun metagenomics sequence classification methods using in silico and in vitro simulated communities. BMC Bioinformatics. 2015;16:1–19. doi: 10.1186/s12859-015-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alili R, et al. Exploring Semi-Quantitative Metagenomic Studies Using Oxford Nanopore Sequencing: A Computational and Experimental Protocol. Genes (Basel) 2021;12:1496. doi: 10.3390/genes12101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahens NF, et al. A comparison of Illumina and Ion Torrent sequencing platforms in the context of differential gene expression. BMC Genomics. 2017;18:602. doi: 10.1186/s12864-017-4011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyler AD, et al. Evaluation of Oxford Nanopore’s MinION Sequencing Device for Microbial Whole Genome Sequencing Applications. Sci. Rep. 2018;8:10931. doi: 10.1038/s41598-018-29334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hon T, et al. Highly accurate long-read HiFi sequencing data for five complex genomes. Sci. Data. 2020;7:399. doi: 10.1038/s41597-020-00743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H, et al. Comparative analysis of 7 short-read sequencing platforms using the Korean Reference Genome: MGI and Illumina sequencing benchmark for whole-genome sequencing. Gigascience. 2021;10:1–9. doi: 10.1093/gigascience/giab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anslan S, et al. Highly comparable metabarcoding results from MGI-Tech and Illumina sequencing platforms. PeerJ. 2021;9:e12254. doi: 10.7717/peerj.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowers, R. M. et al. Impact of library preparation protocols and template quantity on the metagenomic reconstruction of a mock microbial community. BMC Genomics16 (2015). [DOI] [PMC free article] [PubMed]
- 30.Jones MB, et al. Library preparation methodology can influence genomic and functional predictions in human microbiome research. Proc Natl Acad Sci USA. 2015;112:14024–14029. doi: 10.1073/pnas.1519288112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costea PI, et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 2017;35:1069–1076. doi: 10.1038/nbt.3960. [DOI] [PubMed] [Google Scholar]
- 32.Hillmann B, et al. Evaluating the information content of shallow shotgun metagenomics. mSystems. 2018;3:e00069–18. doi: 10.1128/mSystems.00069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sczyrba A, et al. Critical Assessment of Metagenome Interpretation — a benchmark of metagenomics software. Nat. Methods. 2017;14:1063–1071. doi: 10.1038/nmeth.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer F, et al. Critical Assessment of Metagenome Interpretation: the second round of challenges. Nat. Methods. 2022;19:429–440. doi: 10.1038/s41592-022-01431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks DH, et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022;50:785–794. doi: 10.1093/nar/gkab776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47:256–259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:884–890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Criscuolo A, Brisse S. ALIENTRIMMER: A tool to quickly and accurately trim off multiple short contaminant sequences from high-throughput sequencing reads. Genomics. 2013;102:500–506. doi: 10.1016/j.ygeno.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Wick RR, Judd LM, Holt KE. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019;20:1–10. doi: 10.1186/s13059-019-1727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–360. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34:3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolmogorov M, et al. metaFlye: scalable long-read metagenome assembly using repeat graphs. Nat. Methods. 2020;17:1103–1110. doi: 10.1038/s41592-020-00971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikheenko A, Saveliev V, Gurevich A. MetaQUAST: evaluation of metagenome assemblies. Bioinformatics. 2016;32:1088–1090. doi: 10.1093/bioinformatics/btv697. [DOI] [PubMed] [Google Scholar]
- 45.2022. ENA European Nucleotide Archive. PRJEB52977
- 46.Meslier V, 2022. Benchmarking second and third-generation sequencing platforms for microbial metagenomics. Figshare. [DOI] [PMC free article] [PubMed]
- 47.Alneberg J, et al. Binning metagenomic contigs by coverage and composition. Nat. Methods. 2014;11:1144–1146. doi: 10.1038/nmeth.3103. [DOI] [PubMed] [Google Scholar]
- 48.Kang DD, et al. MetaBAT 2: an adaptive binning algorithm for robust and ef fi cient genome reconstruction from metagenome assemblies. PeerJ. 2019;7:e7359. doi: 10.7717/peerj.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Simmons BA, Singer SW. MaxBin 2. 0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics. 2016;32:605–607. doi: 10.1093/bioinformatics/btv638. [DOI] [PubMed] [Google Scholar]
- 50.Arkin AP, et al. KBase: The United States Department of Energy Systems Biology Knowledgebase. Nat Biotechnol. 2018;36:566–569. doi: 10.1038/nbt.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sieber CMK, et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat. Microbiol. 2018;3:836–843. doi: 10.1038/s41564-018-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2022. ENA European Nucleotide Archive. PRJEB52977
- Meslier V, 2022. Benchmarking second and third-generation sequencing platforms for microbial metagenomics. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All reference genomes and scripts for mapping, assembly, genome coverage estimation, subsampling and correlation calculations associated with tables and figures are available at https://forgemia.inra.fr/metagenopolis/benchmark_mock.