Abstract
Previous studies have not accounted for the close link between type 2 diabetes mellitus (T2DM) and obesity when investigating the impact of T2DM on cytochrome P450 (CYP) activities. The aim was to investigate the effect of T2DM on in vivo activities and protein expressions of CYP2C19, CYP3A, CYP1A2, and CYP2C9 in patients with obesity. A total of 99 patients from the COCKTAIL study (NCT02386917) were included in this cross‐sectional analysis; 29 with T2DM and obesity (T2DM‐obesity), 53 with obesity without T2DM (obesity), and 17 controls without T2DM and obesity (controls). CYP activities were assessed after the administration of a cocktail of probe drugs including omeprazole (CYP2C19), midazolam (CYP3A), caffeine (CYP1A2), and losartan (CYP2C9). Jejunal and liver biopsies were also obtained to determine protein concentrations of the respective CYPs. CYP2C19 activity and jejunal CYP2C19 concentration were 63% (−0.39 [95% CI: −0.82, −0.09]) and 40% (−0.09 fmol/μg protein [95% CI: −0.18, −0.003]) lower in T2DM‐obesity compared with the obesity group, respectively. By contrast, there were no differences in the in vivo activities and protein concentrations of CYP3A, CYP1A2, and CYP2C9. Multivariable regression analyses also indicated that T2DM was associated with interindividual variability in CYP2C19 activity, but not CYP3A, CYP1A2, and CYP2C9 activities. The findings indicate that T2DM has a significant downregulating impact on CYP2C19 activity, but not on CYP3A, CYP1A2, and CYP2C9 activities and protein concentrations in patients with obesity. Hence, the effect of T2DM seems to be isoform‐specific.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The current literature suggests that type 2 diabetes mellitus (T2DM) alters cytochrome P450 (CYP) activities in an iso‐specific manner. However, an important limitation of previous studies investigating the impact of T2DM on CYP activities is that the close link between T2DM and obesity has not have been accounted for.
WHAT QUESTION DID THIS STUDY ADDRESS?
Does T2DM impact in vivo activities and protein expressions of CYP2C19, CYP3A, CYP1A2, and CYP2C9 in patients with obesity?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study suggests that in vivo CYP2C19 activity is lower in patients with T2DM and obesity compared with patients with obesity only. T2DM does not seem to impact CYP3A, CYP1A2, and CYP2C9 activities and protein concentrations in patients with obesity.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The risk of treatment failure (prodrugs) and adverse effects (active drugs) should be borne in mind when prescribing drugs dependent on CYP2C19‐mediated metabolism in patients with T2DM and obesity.
INTRODUCTION
The cytochrome P450 (CYP) superfamily is involved in the metabolism of more than half of all clinically used drugs on the market, making it the most important group of drug‐metabolizing enzymes. 1 , 2 Among all CYP isoforms identified, only a small number is considered to have a central role in drug metabolism. 3 These include CYP3A, CYP2C19, CYP2C9, CYP1A2, CYP2B6, CYP2D6, and CYP2E1. CYP isoforms are particularly important for the hepatic clearance of substrate drugs, but may also contribute significantly to the first‐pass metabolism of orally administered drugs. 4 There is substantial interindividual variability in CYP‐mediated metabolism due to a combination of factors, including genetics, environment, and diseases. 3 , 5
Type 2 diabetes mellitus (T2DM) is a growing health issue worldwide, mainly caused by obesity leading to insulin resistance and impaired β‐cell function. 6 , 7 The prevalence of nonalcoholic fatty liver disease (NAFLD) is particularly high in people with obesity and/or T2DM, 8 , 9 who typically also have elevated levels of inflammatory cytokines. 8 , 10 , 11 The drug response in patients with T2DM is often variable, and different, compared with other patient populations, 12 , 13 , 14 possibly due to altered activity and expression of drug‐metabolizing enzymes.
A recent study investigating the impact of T2DM on different CYP activities showed that alterations in CYP activity were isoform‐specific. 15 For instance, CYP3A and CYP2C19 activities were lower in patients with T2DM compared with individuals without T2DM, and elevated levels of inflammatory cytokines were suggested as an important mechanism. 15 However, chronic low‐grade inflammation is also central in the pathophysiology of obesity, the main preventable cause of T2DM. 6 , 7 Hence, an important limitation of the current literature is that the obesity component has not been accounted for when the effect of T2DM on different CYP activities has been investigated. Previous studies have in fact provided evidence of lower CYP3A activity in patients with obesity compared with individuals without obesity. 16 , 17 , 18 , 19 Additionally, we have recently shown that patients with obesity (with or without T2DM) had lower CYP2C19 activity than mainly normal weight controls. 20 There is also evidence of lower CYP3A and CYP2C19 activity in patients with NAFLD, 20 , 21 and since obesity, T2DM, and NAFLD often coexist, 8 this further complicates the interpretation of previous findings.
In view of this, the primary objective was to investigate the impact of T2DM on in vivo activities and protein expressions of CYP2C19, CYP3A, CYP1A2, and CYP2C9, by comparing patients with obesity and T2DM with their respective counterparts without T2DM. Secondary, we aimed to investigate the effect of covariates such as NAFLD, body mass index (BMI), and inflammatory markers on these CYP activities within a wide body weight range.
METHODS
Participants
A total of 108 patients were included in the COCKTAIL study, an open‐label, three‐armed, single‐center, controlled study (NCT02386917). 16 Of these, six patients withdrew and two patients were excluded before study start. 16 Also, one patient was excluded from the pharmacokinetic analysis due to severe liver cirrhosis, leaving 99 patients that were included in the present cross‐sectional exploratory post hoc analysis. Inclusion and exclusion criteria of the COCKTAIL study have been published previously in a protocol article. 22 The study population included patients with obesity scheduled for weight loss treatment with Roux‐en‐Y‐gastric bypass (RYGB) or non‐surgical calorie restriction based on clinical indications, and a control group of mainly normal weight individuals scheduled for cholecystectomy. The study design included a stratification for diabetic status of patients with obesity. 22 The impact of RYGB and strict diet on the activities and protein concentrations of central CYP enzymes in the same study population have been published previously. 16 , 20 The COCKTAIL study was approved by the Regional Committee for Medical and Health Research Ethics (2013/2379/REK), performed according to the Declaration of Helsinki, and all patients gave written informed consent as part of the COCKTAIL study.
In the present analysis, patients were divided into three groups based on diabetes status and BMI: (1) patients with T2DM and obesity (BMI ≥ 30 kg/m2) labeled T2DM‐obesity, (2) patients with obesity, but not T2DM, labeled obesity, and (3) controls without obesity (BMI < 30 kg/m2) and without T2DM labeled controls. T2DM was diagnosed according to the following criteria: glycated haemoglobin (HbA1c) ≥48 mmol/mol (6.5%), or antidiabetic drug treatment, or previously diagnosed with T2DM treated with lifestyle interventions. The T2DM‐obesity group and the obesity group underwent a 3‐week low‐energy diet (<1200 kcal/day) before the in‐depth study investigation, whereas the controls did not undergo any defined diet. None of the patients received treatment with medications and/or other substances known to influence the pharmacokinetics of the probe drugs used in this study.
Study investigation and procedures
On the study day, patients first met for blood sampling at 7:30 a.m. before they received 100 mg oral caffeine (8:00 a.m.), followed by 1.5 mg oral midazolam syrup, 25 mg oral losartan, and 20 mg oral omeprazole 1 h later (9:00 a.m.). Intravenous midazolam (1.0 mg) was administered 4 h after the oral midazolam syrup (1:00 p.m.). Blood samples for analysis of omeprazole, caffeine, and their metabolites were collected 3 and 4 h after administration of the respective drug (12:00 a.m.). For midazolam the blood samples were collected before and 0.25, 0.5, 1, 1.5, 2, 3, 4, 4.25, 4.5, 5, 5.5, 6, 8, 10, 12, 23, and 24‐h after the administration of oral midazolam syrup. The blood samples were drawn in K2‐EDTA vacutainer tubes and centrifuged for 10 min at 4°C (1800 g ). Plasma was separated into Cryovials and frozen within 1 h at −70°C until analysis. Urine was collected in a container for 8 h after the administration of losartan, and the total volume was recorded. An aliquot of 10 ml urine was frozen immediately after sampling at −70°C. The pharmacokinetic investigation has been described in more detail previously. 16 , 20 Also, in the patients subjected to RYGB, paired jejunal and liver biopsies were obtained during surgery. This has been described in detail previously. 23 , 24 The biopsies were transferred into cryotubes, snap‐frozen in liquid nitrogen directly upon sampling, and stored at −70°C until analysis.
Standard clinical chemistry analyses were performed in fresh blood samples at the Department of Laboratory Medicine, Vestfold Hospital Trust, Tønsberg, Norway. Plasma concentrations of high‐sensitivity C‐reactive protein (hs‐CRP) were measured using immunoturbidimetry (Advia Chemistry XPT systems, Siemens) at Fürst Medical Laboratory, Oslo, Norway. Plasma concentration of inflammation markers representing various types of immune responses, 15 namely interferon (IFN)‐γ, interleukin (IL)‐1β, IL‐6, and tumor necrosis factor‐α (TNF‐α), were analyzed using multiplex bead‐based immunoassays (Bio‐Techne, UK) based on xMAP technology (Luminex, Austin, TX) at the Department of Medical Biochemistry, Diakonhjemmet Hospital, Oslo, Norway. Body weight (kg) and body composition were measured with the Inbody 720 Body Composition Analyzer (Biospace, Korea). Waist and hip circumference were measured with a stretch‐resistant tape parallel to the floor and midway between the 12th rib and the iliac crest, and around the widest portion of the buttocks, respectively.
Estimation of CYP activities
Absolute bioavailability and systemic clearance of midazolam and plasma concentrations of the endogenous CYP3A biomarker 4β‐hydroxycholesterol (4βOHC) were used to estimate CYP3A activity. Midazolam absolute bioavailability and systemic clearance were determined using a population pharmacokinetic model, which has been described in detail previously. 16 In short, the modeling was performed using the nonparametric adaptive grid approach implemented in Pmetrics (version 1.5.2) for R (version 3.6.2). 25 , 26 A catenary three‐compartment model with absorption lag‐time and first‐order elimination from the central compartment described the data adequately for the purpose of the present analysis. CYP1A2 activity was described by the 4‐h plasma paraxanthine/caffeine ratio, CYP2C19 activity by the 3‐h plasma 5‐hydroxyomeprazole (5‐OH‐omeprazole)/omeprazole ratio, and CYP2C9 activity by the 8‐h urine losartan/losartan carboxylic acid (LCA) ratio. For the metabolic ratios calculated as metabolite/drug ratio (CYP1A2 and CYP2C19), a higher ratio implies higher CYP activity, while for the metabolic ratio calculated as drug/metabolite ratio (CYP2C9), a higher ratio implies lower CYP activity.
Bioanalytical assays
Midazolam
A previously validated ultra‐high performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) method was used to determine plasma concentrations of midazolam and has been described in detail previously. 27 Briefly, liquid–liquid sample extraction was used as sample preparation. Eight calibrators in the range of 0.1–20 ng/ml were applied, and back‐calculated values of calibrators within 80–120% were accepted. The lower limit of quantification (LLOQ) was 0.1 ng/ml, and the upper limit of quantification (ULOQ) was 20 ng/ml. Samples with midazolam concentrations above ULOQ were diluted in blank plasma and reanalyzed. Dilution integrity with dilution factors of 1/2, 1/5, 1/10, 1/20, and 1/50 was established; mean accuracy ranged from 88.9% to 103.8%, and the imprecision was <4.5%. Within‐series and between‐series performance were assessed with the resulting coefficient of variation (CV) <12.3%, and the mean accuracy ranged from 99.3% to 104.3%.
Caffeine, omeprazole, and losartan
As previously described, plasma concentrations of caffeine, paraxanthine, omeprazole, and 5‐OH‐omeprazole, and urinary concentration of losartan and LCA were determined by Covance Laboratories (Madison, WI) using validated LC–MS/MS methods. 20 The inter‐run precisions, assessed as CVs, were <4.6% and <4.5% for caffeine and paraxanthine, respectively. The inter‐run accuracies ranged between 94.7% and 98.7% for caffeine and between 96.7% and 100.0% for paraxanthine. For losartan the inter‐run precision was <9.8% and inter‐run accuracies varied between 95.6% and 103.0%, and for LCA the inter‐run precision was <10.6% and inter‐run accuracies were between 96.9% and 100.2%. In the omeprazole and 5‐OH‐omeprazole assay, inter‐run CVs were <9.8% and <14.9% and inter‐run accuracies were between 96.9% and 101.2% and 98.0% and 102.4% for omeprazole and 5‐OH‐omeprazole, respectively.
4β‐Hydroxycholesterol (4βOHC)
A previously described assay, 28 with an added filtration step, 29 was used to determine plasma concentrations of 4βOHC. In brief, 4βOHC was de‐esterified from fatty acids by ethanolic sodium methoxide and isolated from plasma by liquid–liquid extraction with hexane. Extracts were evaporated by nitrogen and reconstituted in methanol before filtration. For the quantitative analysis, a UPLC followed by an MS detector (Waters, Milford, MA) was used. Chromatographic separation was achieved on a BEH C18 column RP‐shield (1.7 μm, 1 × 100 mm) from Waters with a mobile phase of water and methanol. After atmospheric pressure chemical ionization, detection was obtained with multiple reaction monitoring at m/z 385.25–>367.45 (4βOHC) and m/z 392.30–>374.50 (4βOHC‐D7; internal standard). The LLOQ was 10 ng/ml. Intra‐ and interday imprecision and inaccuracy were <15% at 10 ng/ml and <4% at 644 ng/ml (n = 6). 28
Protein quantification of CYP enzymes in liver and jejunal biopsies
The protein quantification has been described previously. 30 In brief, proteins were extracted from liver and jejunal biopsies in a sodium dodecyl sulfate (SDS)‐containing (2% w/v) lysis buffer, and processed with the multi‐enzyme digestion filter‐aided sample preparation protocol, using LysC and trypsin. Proteomics analysis was performed with Q Exactive HF or Q Exactive HF‐X. MS data were processed with MaxQuant, using the human UniProtKB. Spectral raw intensities were normalized with variance stabilization. The protein concentrations were calculated using the total protein approach.
Genotype analyses
Taqman‐based real‐time polymerase chain reaction assays implemented for routine pharmacogenetic analyses at the Center for Psychopharmacology, Diakonhjemmet Hospital, Norway were used to analyze the following variant alleles: CYP1A2; the increased induction allele *1F (rs762551), CYP2C9; the reduced function alleles *2 (rs1799853) and *3 (rs1057910), CYP2C19; the null alleles *2 (rs4244285), *3 (rs4986893), and *4 (rs28399504) and the gain‐of‐function allele *17 (rs12248560), CYP3A; the reduced function allele *22 (rs35599367) and CYP3A5; and the null allele *3 (rs776746). The following subgroups were used to describe genotype‐predicted‐phenotype: normal, ultrarapid/rapid, intermediate, and poor metabolizer. Except for CYP2C9*3, all alleles were in Hardy–Weinberg equilibrium.
Data and statistical analysis
Visual inspection of plots and Shapiro Wilk's test were used to evaluate the normality of the data. Wilcoxon rank‐sum test was used to compare differences between (1) the T2DM‐obesity group and obesity group and (2) the obesity group and the controls. Fisher's exact test was used to compare genotype‐predicted‐phenotype distribution between the groups. Multivariable regression analyses were performed to explore the effects of BMI, T2DM (yes/no), and NAFLD (yes/no) on the various CYP activities. The multivariable regression analyses were adjusted for age, sex (male/female), and genotype‐predicted‐phenotype (i.e., genotypic normal, rapid/ultrarapid, intermediate, and poor metabolizer) effects. Inflammatory markers with a p value <0.25 in the univariate regression analyses were candidates for inclusion in the final multivariable model. Potential impact of the 3‐week low‐energy diet was investigated in a subanalysis. Linear mixed‐effects models with a log‐transformed dependent variable (PK parameter) were used to estimate the between‐group difference (T2DM‐obesity vs. obesity) in change over time (weeks 0–3). Visit (time), diabetes status (yes/no), and their interaction (visit × diabetes status) were treated as fixed effects. The unique patient ID was used as a random effect, and the confidence interval (CI) was adjusted using Tukey's method. Insulin resistance was estimated using the homeostasis model assessment (HOMA) and calculated by the following equation: fasting insulin (pmol/L) × fasting glucose (mmol/L)/135. 31 The NAFLD liver fat score (NAFLD‐LFS) was calculated according to Kotronen et al. 32 Values of NAFLD‐LFS greater than −0.640 were indicative of NAFLD. A p value <0.05 was considered statistically significant, and all statistical analyses were performed using R for Windows (version 4.1.2). 26
RESULTS
Patients
A total of 99 patients (30% males) were included in the present analysis; 29 in the T2DM‐obesity group, 53 in the obesity group, and 17 in the controls. Patient characteristics are presented in Table 1. Mean age was higher in the T2DM‐obesity group compared with the obesity group (8 years [95% CI: 4, 12]), whereas mean BMI was slightly lower (−4.6 kg/m2 [95% CI: −7.0, −2.1]; Table 1). In the T2DM‐obesity group, all patients (100%) had NAFLD‐LFS indicative of NAFLD, whereas in the obesity group and the controls the proportions were 62% and 6%, respectively. Among the patients with T2DM‐obesity, 69% received treatment with one or more glucose‐lowering drugs; metformin (n = 13), sodium‐glucose co‐transporter 2 (SGLT2) inhibitors (n = 5), glucagon‐like peptide‐1 (GLP‐1) analogues (n = 4), and/or a combination of metformin and dipeptidyl peptidase 4 (DPP‐4) inhibitors (n = 8). The CYP genotype distribution is given in Table 2. There were no significant between‐group differences in genotype‐predicted‐phenotype distribution between the three groups.
TABLE 1.
Patient characteristics
| Characteristic | T2DM‐obesity (n = 29) | Obesity (n = 53) | Controls (n = 17) |
|---|---|---|---|
| Age (years) | 52 ± 8 | 45 ± 9 | 42 ± 15 |
| Sex (male/female) | 8/21 | 19/34 | 3/14 |
| Body weight (kg) | 109 ± 17 | 128 ± 23 | 70 ± 11 |
| BMI (kg/m2) | 38 ± 4.9 | 43 ± 5.6 | 24 ± 2.6 |
| Waist circumference (cm) | 119 ± 10 | 124 ± 13 | 81 ± 16 |
| Hip circumference (cm) | 120 ± 13 | 131 ± 12 | 96 ± 16 |
| Waist−hip ratio | 0.99 ± 0.09 | 0.95 ± 0.10 | 0.85 ± 0.06 |
| HbA1c (mmol/mol) | 50 ± 10 | 36 ± 3.4 | 35 ± 2.7 |
| Glucose (mmol/L) | 6.7 ± 1.4 | 4.9 ± 0.45 | 4.7 ± 0.40 |
| Insulin (pmol/L) | 122 ± 43 | 100 ± 51 | 50 ± 22 |
| HOMA‐IR | 5.9 ± 3.0 | 3.4 ± 1.9 | 1.6 ± 0.81 |
| Total cholesterol (mmol/L) | 4.0 ± 1.1 | 3.9 ± 0.8 | 4.4 ± 0.9 |
| hs‐CRP (mg/L) | 4.5 ± 4.2 | 6.3 ± 6.6 | 2.4 ± 3.9 |
| ALT (U/L) | 39 ± 23 | 37 ± 21 | 23 ± 15 |
| NAFLD | 29 (100) | 33 (62) | 1 (6) |
| Comorbidities | |||
| Hypertension | 15 (52) | 17 (32) | 0 (0) |
| Heart disease a | 2 (7) | 1 (2) | 0 (0) |
| Depression/anxiety | 9 (31) | 14 (26) | 0 (0) |
| Asthma/COPD | 8 (28) | 9 (17) | 0 (0) |
| Autoimmune diseases b | 1 (3) | 2 (4) | 0 (0) |
Note: Data are described as mean ± standard deviation or absolute numbers (%).
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HbA1c, glycated hemoglobin; hs‐CRP, high‐sensitivity C‐reactive protein; HOMA‐IR, Homeostasis Model Assessment of Insulin Resistance; NAFLD, nonalcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
Includes coronary artery bypass surgery, myocardial infarction, and percutaneous coronary intervention.
Includes rheumatoid arthritis, Bechterew’s disease (ankylosing spondylitis), inflammatory bowel disease, coeliac disease, and polymyalgia rheumatica.
TABLE 2.
Genotype distribution in the three study groups
| Genotype (likely phenotype) | T2DM‐obesity (n = 29) | Obesity (n = 53) | Controls (n = 17) |
|---|---|---|---|
| CYP2C19 genotype | |||
| *1/*1 (NM) | 12 (41) | 21 (40) | 7 (41) |
| *17/*17 or *1/*17 (UM/RM) | 8 (28) | 22 (41) | 5 (29) |
| *1/*2 or *2/*17 (IM) | 7 (24) | 8 (15) | 5 (29) |
| *2/*2 or *2/*4 (PM) | 2 (7) | 2 (4) | 0 (0) |
| CYP3A4 genotype | |||
| *1/*1 (NM) | 27 (93) | 50 (94) | 15 (88) |
| *1/*22 (IM) | 2 (7) | 3 (6) | 2 (12) |
| CYP3A5 genotype | |||
| *1/*3 (IM) | 2 (7) | 6 (11) | 2 (12) |
| *3/*3 (PM) | 27 (93) | 47 (89) | 15 (88) |
| CYP1A2 genotype | |||
| *1/*1 or *1/*1F (NM) | 14 (48) | 25 (47) | 10 (59) |
| *1F/*1F (hyperinducer) | 15 (52) | 28 (53) | 7 (41) |
| CYP2C9 genotype | |||
| *1/*1 or *1/*2 (NM) | 26 (90) | 49 (92) | 16 (94) |
| *1/*3 or *2/*2 (IM) | 3 (10) | 4 (8) | 0 (0) |
| *3/*3 (PM) | 0 (0) | 0 (0) | 1 (6) |
Note: Data are presented as absolute numbers (%).
Abbreviations: CYP, cytochrome P450; IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; UM, ultrarapid metabolizer.
CYP2C19
Table 3 reports the activities and protein concentrations of the various CYP enzymes in the T2DM‐obesity, obesity, and control groups. The median 5‐OH‐omeprazole/omeprazole ratio was 63% lower in the T2DM‐obesity group compared with the obesity group (Figure 1a, Table 4). Jejunal CYP2C19 concentrations, available in a subset of the patients (n = 34), were 40% lower in the T2DM‐obesity group compared with the obesity group, while there was no statistically significant difference in hepatic CYP2C19 concentrations (n = 39; Figure 1b,c, Table 4). Results from the regression model including all three study groups (n = 99) suggested that interindividual variability in CYP2C19 activity (5‐OH‐omeprazole/omeprazole ratio) was associated with both T2DM and NAFLD, but not BMI (Table 5). None of the inflammatory markers were included in the final model, due to the lack of significance and/or influence of the β‐estimates. The final model explained 43% of the variability in CYP2C19 activity. Results from the univariate regression analysis of covariates are presented in Table S1.
TABLE 3.
Activities and expressions of CYP2C19, CYP3A, CYP1A2, and CYP2C9 in the three study groups
| Parameter | T2DM‐obesity (n = 29) | Obesity (n = 53) | Controls (n = 17) |
|---|---|---|---|
| CYP2C19 | |||
| 5‐OH‐omeprazole/omeprazole | 0.76 ± 0.92 | 1.5 ± 1.6 | 2.3 ± 2.3 |
| Jejunal CYP2C19 (fmol/μg protein) a , b | 0.14 ± 0.10 | 0.24 ± 0.15 | NA |
| Hepatic CYP2C19 (fmol/μg protein) c | 7.9 ± 2.5 | 9.3 ± 3.5 | 11 ± 3.9 |
| CYP3A | |||
| MDZ absolute bioavailability (%) | 25 ± 12 | 20 ± 10 | 8.4 ± 4.2 |
| MDZ systemic clearance (L/h) | 25 ± 10 | 24 ± 11 | 17 ± 9.1 |
| 4βOHC (ng/mL) | 10 ± 4.6 | 9.8 ± 3.8 | 18 ± 6.1 |
| Jejunal CYP3A4 (fmol/μg protein) a | 13 ± 4.9 | 14 ± 5.3 | NA |
| Hepatic CYP3A4 (fmol/μg protein) c | 19 ± 6.6 | 23 ± 8.1 | 26 ± 6.7 |
| CYP1A2 | |||
| Paraxanthine/caffeine | 0.41 ± 0.15 | 0.38 ± 0.14 | 0.45 ± 0.26 |
| Hepatic CYP1A2 (fmol/μg protein) c | 14 ± 5.6 | 14 ± 6.2 | 18 ± 11 |
| CYP2C9 | |||
| LCA/losartan | 1.2 ± 0.53 | 1.4 ± 0.62 | 4.3 ± 12 |
| Jejunal CYP2C9 (fmol/μg protein) a | 3.2 ± 6.1 | 1.8 ± 0.75 | NA |
| Hepatic CYP2C9 (fmol/μg protein) c | 12 ± 2.5 | 12 ± 2.2 | 12 ± 3.3 |
Note: Data are presented as mean ± standard deviation.
Abbreviations: 4βOHC, 4β‐hydroxycholesterol; CYP, cytochrome P450; MDZ, midazolam; NA, not available; T2DM, type 2 diabetes mellitus.
Jejunal biopsies were only available in 37 patients subjected to Roux‐en‐Y‐gastric bypass (RYGB).
Jejunal CYP2C19 concentration was missing in 3 patients due to technical error (n = 34).
Hepatic biopsies were only available in 56 patients subjected to RYGB or cholecystectomy.
FIGURE 1.
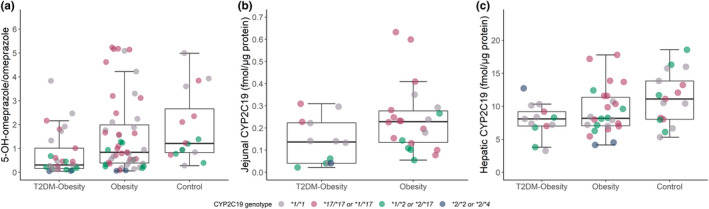
In vivo CYP2C19 activity and jejunal and hepatic protein expression in paired samples. Boxplot with individual points of (a) the 5‐OH‐omeprazole/omeprazole ratio (in vivo CYP2C19 activity), (b) jejunal CYP2C19 concentration, and (c) hepatic CYP2C19 concentration. The dots are colored according to genotype. CYP, cytochrome P450; T2DM, type 2 diabetes mellitus. Jejunal CYP2C19 concentration was only available in 34 patients subjected to Roux‐en‐Y‐gastric bypass (RYGB). Hepatic CYP2C19 concentration was only available in 56 patients subjected to RYGB or cholecystectomy.
TABLE 4.
Between‐group differences in protein expression and in vivo activities of CYP2C19, CYP3A, CYP1A2, and CYP2C9
| Parameter | T2DM‐obesity vs. Obesity |
|---|---|
| CYP2C19 a | |
| 5‐OH‐omeprazole/omeprazole | −0.39 [−0.82, −0.09] |
| Jejunal CYP2C19 (fmol/μg protein) b , c | −0.09 [−0.18, −0.003] |
| Hepatic CYP2C19 (fmol/μg protein) d | −0.83 [−3.4, 0.97] |
| CYP3A e | |
| MDZ absolute bioavailability (%) | 4.4 [−1.2, 11] |
| MDZ systemic clearance (L/h) | 1.9 [−3.7, 7.0] |
| 4βOHC (ng/ml) | 0.70 [−1.2, 2.7] |
| Jejunal CYP3A4 (fmol/μg protein) b | −1.6 [−5.6, 2.0] |
| Hepatic CYP3A4 (fmol/μg protein) d | −3.3 [−7.3, 0.81] |
| CYP1A2 f | |
| Paraxanthine/caffeine | 0.02 [−0.03, 0.08] |
| Hepatic CYP1A2 (fmol/μg protein) d | −0.11 [−3.9, 3.8] |
| CYP2C9 g | |
| LCA/losartan | −0.16 [−0.42, 0.09] |
| Jejunal CYP2C9 (fmol/μg protein) b | 1.5 [1.2, 2.0] |
| Hepatic CYP2C9 (fmol/μg protein) d | 0.04 [−1.7, 1.7] |
Note: Data are presented as median [95% confidence interval].
Wilcoxon rank‐sum test was used to compare differences between the groups. A p value <0.05 was considered statistically significant (shown in bold).
Abbreviations: 4βOHC, 4β‐hydroxycholesterol CYP; cytochrome P450; LCA, losartan carboxylic acid; MDZ, midazolam; NA, not available; T2DM, type 2 diabetes mellitus.
CYP2C19 activity was described by the 3‐h plasma 5‐OH‐omeprazole/omeprazole ratio.
Jejunal biopsies were only available in 37 patients subjected to Roux‐en‐Y‐gastric bypass (RYGB).
Jejunal CYP2C19 concentration was missing in 3 patients due to technical error (n = 34).
Hepatic biopsies were only available in 56 patients subjected to RYGB or cholecystectomy.
CYP3A activity was described by absolute bioavailability and systemic clearance of midazolam and plasma concentrations of the endogenous CYP3A4 biomarker 4βOHC.
CYP1A2 activity was described by the 4‐h plasma paraxanthine/caffeine ratio.
CYP2C9 activity was described by the 8‐h urine losartan/LCA ratio.
TABLE 5.
Multivariable analysis of CYP1A2, CYP2C9, CYP2C19, and CYP3A activities
| CYP450 isoform | Covariates | Parameter estimate (β) ± SE | p value | Adjusted R 2 |
|---|---|---|---|---|
| CYP2C19 (log 5‐OH‐omeprazole/omeprazole) | BMI (kg/m2) | −0.000 ± 0.013 | 0.99 | 0.43 |
| T2DM (yes) | −0.64 ± 0.26 | 0.016 | ||
| NAFLD (yes) | −0.63 ± 0.25 | 0.015 | ||
| CYP3A (MDZ absolute bioavailability, %) | BMI (kg/m2) | 0.030 ± 0.009 | 0.0011 | 0.19 |
| T2DM (yes) | 0.40 ± 0.18 | 0.033 | ||
| NAFLD (yes) | −0.027 ± 0.18 | 0.88 | ||
| CYP3A (MDZ systemic clearance, L/h) | BMI (kg/m2) | 0.015 ± 0.007 | 0.041 | 0.06 |
| T2DM (yes) | 0.11 ± 0.15 | 0.46 | ||
| NAFLD (yes) | −0.027 ± 0.15 | 0.86 | ||
| CYP3A (log 4βOHC, ng/ml) | BMI (kg/m2) | −0.020 ± 0.006 | 0.0017 | 0.25 |
| T2DM (yes) | −0.012 ± 0.12 | 0.92 | ||
| NAFLD (yes) | −0.27 ± 0.12 | 0.029 | ||
| CYP1A2 (log paraxanthine/caffeine) | BMI (kg/m2) | −0.007 ± 0.005 | 0.21 | 0.10 |
| T2DM (yes) | 0.14 ± 0.11 | 0.20 | ||
| NAFLD (yes) | −0.14 ± 0.11 | 0.19 | ||
| Log TNF‐α (pg/ml) | 0.074 ± 0.034 | 0.032 | ||
| CYP2C9 (log losartan/LCA) | BMI (kg/m2) | −0.001 ± 0.006 | 0.89 | 0.48 |
| T2DM (yes) | 0.013 ± 0.12 | 0.92 | ||
| NAFLD (yes) | −0.24 ± 0.12 | 0.050 | ||
| Log TNF‐α (pg/ml) | 0.081 ± 0.038 | 0.037 |
Note: The models were adjusted for age, sex (male/female), and genotype‐predicted‐phenotype (categorized according to Table 2: CYP2C19: *1/*1 (normal), *17/*17 or *1/*17 (ultrarapid/rapid), *1/*2 or *2/*17 (intermediate), and *2/*2 or *2/*4 (poor); CYP3A4: *1/*1 (normal) and *1/*22 (intermediate); CYP3A5: *1/*3 (intermediate) and *3/*3 (poor); CYP1A2: *1/*1 or *1/*1F (normal) and *1F/*1F (hyperinducer); CYP2C9: *1/*1 or *1/*2 (normal), *1/*3 or *2/*2 (intermediate), and *3/*3 (poor)).
Abbreviations: 4βOHC, 4β‐hydroxycholesterol; 5‐OH‐omeprazole, 5‐hydroxyomeprazole; BMI, body mass index; CYP, cytochrome P450; LCA, losartan carboxylic acid; MDZ, midazolam; NAFLD, nonalcoholic fatty liver disease; TNF‐α, tumor necrosis factor‐α; T2DM, type 2 diabetes mellitus.
CYP3A
There were no differences in any of the in vivo CYP3A metrics or in jejunal or hepatic CYP3A concentration between the T2DM‐obesity group and the obesity group (Table 4). The multivariable regression analyses showed that interindividual variability in midazolam absolute bioavailability, systemic clearance, as well as 4βOHC concentrations, were associated with BMI (Table 5). For absolute bioavailability of midazolam and 4βOHC concentrations, the interindividual variability was also associated with T2DM and NAFLD, respectively. The covariates included in the final models could only explain 19%, 6%, and 25% of the variability in midazolam absolute bioavailability, systemic clearance, and 4βOHC concentrations, respectively.
CYP1A2 and CYP2C9
No differences in the paraxanthine/caffeine ratio or losartan/LCA ratio, nor intestinal (only CYP2C9) and hepatic CYP1A2 and CYP2C9 concentrations between the T2DM‐obesity group and the obesity group were observed (Table 4). The regression analyses supported that T2DM has no relevant effect on CYP1A2 (paraxanthine/caffeine ratio) or CYP2C9 activities (losartan/LCA ratio) (Table 5). The inflammatory marker TNF‐α was associated with some of the interindividual variability in both CYP1A2 and CYP2C9 activities and was thus included in the final model. Genotype‐predicted‐phenotype also explained a significant part of the variability in CYP2C9 activity. The covariates included in the final model for CYP1A2 and CYP2C9 explained 10% and 48% of the interindividual variability, respectively.
Impact of the low‐energy diet
Before the diet intervention, the median 5‐OH‐omeprazole/omeprazole ratio was 55% lower in the T2DM‐obesity group compared with the obesity group: −0.27 [95% CI: −0.50, −0.03]. None of the other markers investigated showed any significant difference between these groups before the diet intervention (p > 0.10). During the 3‐week diet intervention, the obesity group had a significantly larger increase in the 5‐OH‐omeprazole/omeprazole ratio compared with the T2DM‐obesity group: 0.28 [95% CI: 0.05, 0.51]. For the paraxanthine/caffeine ratio, mean difference in change between the obesity group and the T2DM‐obesity group during the diet intervention was −0.05 [95% CI: −0.09, −0.01]. There was no between‐group difference in within‐group change in midazolam absolute bioavailability, systemic clearance, 4βOHC concentration, or losartan/LCA ratio between weeks 0 and 3 (all p > 0.23).
DISCUSSION
In this comprehensive analysis including data from 99 patients we were able to study the impact of T2DM on CYP2C19, CYP3A, CYP1A2, and CYP2C9 activities and protein expression in primarily an obese population with and without T2DM. The main finding was that CYP2C19 activity was lower in patients with T2DM and obesity than in patients with obesity only. By contrast, T2DM had no impact on in vivo activities and/or protein expressions of CYP3A, CYP1A2, and CYP2C9.
Our results showed that CYP2C19 activity was ~60% lower in the patients with T2DM and obesity compared with the patients with obesity only. In agreement with our finding, Gravel et al. also found approximately 50% lower CYP2C19 activity in patients with T2DM compared with non‐T2DM individuals with a wide body weight range. 15 A lower CYP2C19 activity in patients with T2DM is also supported by other studies showing an inadequate effect of clopidogrel at studied doses, a prodrug which to a large extent is activated by CYP2C19, in this patient population. 13 , 33 , 34 We have previously shown that patients with NAFLD have decreased CYP2C19 activity. 20 The regression analyses in this present analysis supported that NAFLD is involved in the downregulation of CYP2C19 activity, while BMI does not appear to be of significance. Even though higher levels of inflammatory markers have been suggested as an important mechanism for the downregulation of CYP2C19 activity in patients with T2DM, 15 none of the inflammation markers investigated in the present study could explain the additional effect of T2DM on CYP2C19 activity. The intestinal CYP2C19 protein expression was lower in the patients with T2DM and obesity than the patients with obesity only. Interestingly, there was no statistically significant difference in hepatic CYP2C19 expression. However, it may be that the study was not powered to show an effect, given that the proteomics data were only available in a subset of the patients.
We did not observe any difference in CYP3A activity or hepatic or intestinal CYP3A expression between the patients with T2DM and obesity and the patients with obesity only. The regression analyses supported that T2DM has no effect on hepatic CYP3A, but it may explain some of the interindividual variability in absolute bioavailability of midazolam. Taken together, the data in this study indicate that BMI, but not T2DM, is of importance for the downregulation of CYP3A activity in patients with obesity. By contrast, Gravel et al. showed lower CYP3A activity in patients with T2DM compared with non‐T2DM individuals. 15 Yet, in their study, the comparison was done between two groups with significantly different BMI (mean of 29.1 kg/m2 in patients with T2DM vs. 25.7 kg/m2 in non‐T2DM). Hence, the lower CYP3A activity may actually be explained by the higher BMI rather than T2DM. The regression analyses in the present analysis supported this hypothesis. That obesity may play a role in such patients has been proposed previously, 35 and several studies have shown a decreased CYP3A activity and protein expression with increasing BMI. 24 , 36 , 37 Gravel et al. have also reported that T2DM did not have any effect on CYP3A expression and ex vivo activity in intestinal biopsies compared to patients without T2DM, 38 which is in line with the results in the present analysis. Higher levels of inflammatory markers such as IL‐6, IL‐1β, and TNF‐α in obesity and T2DM have been shown previously. 6 , 11 It also appears that CYP3A is more susceptible to downregulation by inflammatory markers such as IL‐6 compared to other CYP enzymes. 39 Yet, none of the inflammatory markers in the present study could explain the variability in CYP3A activity. Nevertheless, inflammation may play an important role in the downregulation of CYP3A with increasing BMI.
Our results also indicate that T2DM does not impact the expression and in vivo activity of CYP1A2 and CYP2C9 significantly. The literature to date has been inconclusive with respect to the effect of T2DM on CYP1A2 and CYP2C9 activity, although there have been some indications of an increased activity. 15 , 40 , 41 For CYP1A2, the inconsistent findings may be due to different gender distribution, as there are some indications of increased CYP1A2 activity in men. 3
The major strength of this study is the stratified inclusion of patients with obesity with and without T2DM and a control group of primarily normal weight individuals without T2DM. Hence, we were in the unique position to investigate the effect of T2DM in an obese population, thus limiting the confounding effect of obesity on CYP activities. Another important strength is that we have matched in vivo activity data and proteomics data from liver and intestinal samples. Limitations of this study are, first, that a single‐time point metabolic ratio for CYP2C19, CYP2C9, and CYP1A2 does not provide detailed information about pharmacokinetic processes. The absorption rate and extent may differ in the BMI range investigated, and the absorption may be delayed in patients with diabetes. 42 , 43 Thus, we cannot rule out that this has influenced the metabolic ratios. However, our data on oral midazolam did not indicate any difference in absorption rate or lag‐time in the present study. Second, as the protein concentrations from the biopsies may not be entirely representative of the CYP content in the whole organ (liver and intestine) this complicates in vitro−in vivo extrapolation. 44 Third, an important limitation of this study includes not adjusting for multiple testing, which may increase the likelihood of type 1 errors. Also, no sample size calculation was performed due to the exploratory nature of the study. Hence, the study may not have been powered to detect a statistically significant difference (alpha = 0.05), thus increasing the likelihood of type 2 errors. Fourth, the patients with obesity (with and without T2DM) were subjected to a 3‐week low‐energy diet which may have influenced the results. However, the results from the subanalysis showed that the diet intervention had no effect on CYP3A and CYP2C9 activities, whereas the effect on CYP1A2 activity was minor, but statistically significant. The diet intervention had a larger impact on CYP2C19 activity in the patients with obesity without T2DM than the patients with both obesity and T2DM. Thus, the difference in the median 5‐OH‐omeprazole/omeprazole ratio was numerically larger after the diet than prior to the diet (63% vs. 55%). Accordingly, we do not believe that the diet intervention has influenced the results to any significant degree. Finally, we used surrogate measures of NAFLD, and hence it was not possible to determine any degree of steatosis, and we only measured systemic inflammation, not local inflammation in the liver.
In conclusion, in vivo CYP2C19 activity was lower in patients with T2DM and obesity compared with patients with obesity only, while there were no differences in CYP3A, CYP1A2, and CYP2C9 in vivo activities and protein expressions. Thus, the effect of T2DM on CYP activities is isoform‐specific. It also appears that CYP2C19 activity is downregulated in patients with NAFLD. These findings should be borne in mind when prescribing drugs dependent on CYP2C19‐mediated metabolism in patients with T2DM and obesity.
AUTHOR CONTRIBUTIONS
K.E.K. and I.R wrote the manuscript. K.E.K., I.R., J.H., A.Å., S.A., C.K., H.C., E.S., R.S., and R.J.‐L. designed the research. I.R., L.K.J., J.K.H, P.A., and C.W. performed the research. K.E.K. and I.R. analyzed the data.
FUNDING INFORMATION
Study funding was received from Vestfold Hospital Trust, Norway; Department of Pharmacy, University of Oslo, Norway; and AstraZeneca, Sweden.
CONFLICT OF INTEREST
C.K., S.A., and R.J.‐L. are AstraZeneca employees and own shares in AstraZeneca, while C.W. is a former AstraZeneca employee. All other authors declared no competing interests for this work.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors would like to thank the participants, the surgical staff, and the study personnel working on the COCKTAIL study at Vestfold Hospital Trust. The authors also thank the Swedish Research Council (Approval Numbers 5715 and 01951) (C.W. and P.A.) for supporting the proteomics analyses.
Kvitne KE, Åsberg A, Johnson LK, et al. Impact of type 2 diabetes on in vivo activities and protein expressions of cytochrome P450 in patients with obesity. Clin Transl Sci. 2022;15:2685‐2696. doi: 10.1111/cts.13394
REFERENCES
- 1. Williams JA, Hyland R, Jones BC, et al. Drug–drug interactions for UDP‐glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32(11):1201‐1208. doi: 10.1124/dmd.104.000794 [DOI] [PubMed] [Google Scholar]
- 2. Rendic S, Guengerich FP. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol. 2015;28(1):38‐42. doi: 10.1021/tx500444e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103‐141. doi: 10.1016/j.pharmthera.2012.12.007 [DOI] [PubMed] [Google Scholar]
- 4. Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 "pie". Drug Metab Dispos. 2006;34(5):880‐886. doi: 10.1124/dmd.105.008672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan ET, Goralski KB, Piquette‐Miller M, et al. Regulation of drug‐metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008;36(2):205‐216. doi: 10.1124/dmd.107.018747 [DOI] [PubMed] [Google Scholar]
- 6. Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2019;10:1607. doi: 10.3389/fphys.2019.01607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet. 2022;399(10322):394‐405. doi: 10.1016/s0140-6736(21)01919-x [DOI] [PubMed] [Google Scholar]
- 8. Stefan N, Cusi K. A global view of the interplay between non‐alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284‐296. doi: 10.1016/s2213-8587(22)00003-1 [DOI] [PubMed] [Google Scholar]
- 9. Seeberg KA, Borgeraas H, Hofsø D, et al. Gastric bypass versus sleeve gastrectomy in type 2 diabetes: effects on hepatic steatosis and fibrosis: a randomized controlled trial. Ann Intern Med. 2022;175(1):74‐83. doi: 10.7326/m21-1962 [DOI] [PubMed] [Google Scholar]
- 10. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98‐107. doi: 10.1038/nri2925 [DOI] [PubMed] [Google Scholar]
- 11. Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31‐55. doi: 10.1016/j.immuni.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dostalek M, Sam WJ, Paryani KR, Macwan JS, Gohh RY, Akhlaghi F. Diabetes mellitus reduces the clearance of atorvastatin lactone: results of a population pharmacokinetic analysis in renal transplant recipients and in vitro studies using human liver microsomes. Clin Pharmacokinet. 2012;51(9):591‐606. doi: 10.2165/11632690-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 13. Angiolillo DJ, Fernandez‐Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54(8):2430‐2435. doi: 10.2337/diabetes.54.8.2430 [DOI] [PubMed] [Google Scholar]
- 14. Akhlaghi F, Dostalek M, Falck P, et al. The concentration of cyclosporine metabolites is significantly lower in kidney transplant recipients with diabetes mellitus. Ther Drug Monit. 2012;34(1):38‐45. doi: 10.1097/FTD.0b013e318241ac71 [DOI] [PubMed] [Google Scholar]
- 15. Gravel S, Chiasson JL, Turgeon J, Grangeon A, Michaud V. Modulation of CYP450 activities in patients with type 2 diabetes. Clin Pharmacol Ther. 2019;106(6):1280‐1289. doi: 10.1002/cpt.1496 [DOI] [PubMed] [Google Scholar]
- 16. Kvitne KE, Robertsen I, Skovlund E, et al. Short‐ and long‐term effects of body weight loss following calorie restriction and gastric bypass on CYP3A‐activity – a non‐randomized three‐armed controlled trial. Clin Transl Sci. 2022;15(1):221‐233. doi: 10.1111/cts.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brill MJ, van Rongen A, Houwink AP, et al. Midazolam pharmacokinetics in morbidly obese patients following semi‐simultaneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet. 2014;53(10):931‐941. doi: 10.1007/s40262-014-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brill MJ, Välitalo PA, Darwich AS, et al. Semiphysiologically based pharmacokinetic model for midazolam and CYP3A mediated metabolite 1‐OH‐midazolam in morbidly obese and weight loss surgery patients. CPT Pharmacometrics Syst Pharmacol. 2016;5(1):20‐30. doi: 10.1002/psp4.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodríguez‐Morató J, Goday A, Langohr K, et al. Short‐ and medium‐term impact of bariatric surgery on the activities of CYP2D6, CYP3A4, CYP2C9, and CYP1A2 in morbid obesity. Sci Rep. 2019;9(1):20405. doi: 10.1038/s41598-019-57002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kvitne KE, Krogstad V, Wegler C, et al. Short‐ and long‐term effects of body weight, calorie restriction and gastric bypass on CYP1A2, CYP2C19 and CYP2C9 activity. Br J Clin Pharmacol. 2022;88:4121‐4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab Dispos. 2015;43(10):1484‐1490. doi: 10.1124/dmd.115.065979 [DOI] [PubMed] [Google Scholar]
- 22. Hjelmesaeth J, Asberg A, Andersson S, et al. Impact of body weight, low energy diet and gastric bypass on drug bioavailability, cardiovascular risk factors and metabolic biomarkers: protocol for an open, non‐randomised, three‐armed single centre study (COCKTAIL). BMJ Open. 2018;8(5):e021878. doi: 10.1136/bmjopen-2018-021878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krogstad V, Peric A, Robertsen I, et al. A comparative analysis of cytochrome P450 activities in paired liver and small intestinal samples from patients with obesity. Drug Metab Dispos. 2020;48(1):8‐17. doi: 10.1124/dmd.119.087940 [DOI] [PubMed] [Google Scholar]
- 24. Krogstad V, Peric A, Robertsen I, et al. Correlation of body weight and composition with hepatic activities of cytochrome P450 enzymes. J Pharm Sci. 2021;110(1):432‐437. doi: 10.1016/j.xphs.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 25. Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467‐476. doi: 10.1097/FTD.0b013e31825c4ba6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 27. Egeland EJ, Witczak BJ, Zaré HK, Christensen H, Åsberg A, Robertsen I. Chronic inhibition of CYP3A is temporarily reduced by each hemodialysis session in patients with end‐stage renal disease. Clin Pharmacol Ther. 2020;108(4):866‐873. doi: 10.1002/cpt.1875 [DOI] [PubMed] [Google Scholar]
- 28. Gjestad C, Huynh DK, Haslemo T, Molden E. 4β‐Hydroxycholesterol correlates with dose but not steady‐state concentration of carbamazepine: indication of intestinal CYP3A in biomarker formation? Br J Clin Pharmacol. 2016;81(2):269‐276. doi: 10.1111/bcp.12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Størset E, Hole K, Midtvedt K, Bergan S, Molden E, Åsberg A. The CYP3A biomarker 4β‐hydroxycholesterol does not improve tacrolimus dose predictions early after kidney transplantation. Br J Clin Pharmacol. 2017;83(7):1457‐1465. doi: 10.1111/bcp.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wegler C, Wiśniewski JR, Robertsen I, et al. Drug disposition protein quantification in matched human jejunum and liver from donors with obesity. Clin Pharmacol Ther. 2022;111:1142‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. doi: 10.1007/bf00280883 [DOI] [PubMed] [Google Scholar]
- 32. Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non‐alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865‐872. doi: 10.1053/j.gastro.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 33. Erlinge D, Varenhorst C, Braun OO, et al. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol. 2008;52(24):1968‐1977. doi: 10.1016/j.jacc.2008.07.068 [DOI] [PubMed] [Google Scholar]
- 34. Schuette C, Steffens D, Witkowski M, et al. The effect of clopidogrel on platelet activity in patients with and without type‐2 diabetes mellitus: a comparative study. Cardiovasc Diabetol. 2015;14:15. doi: 10.1186/s12933-015-0182-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dostalek M, Court MH, Yan B, Akhlaghi F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br J Pharmacol. 2011;163(5):937‐947. doi: 10.1111/j.1476-5381.2011.01270.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ulvestad M, Skottheim IB, Jakobsen GS, et al. Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther. 2013;93(3):275‐282. doi: 10.1038/clpt.2012.261 [DOI] [PubMed] [Google Scholar]
- 37. Woolsey SJ, Beaton MD, Choi YH, et al. Relationships between endogenous plasma biomarkers of constitutive cytochrome P450 3A activity and single‐time‐point oral midazolam microdose phenotype in healthy subjects. Basic Clin Pharmacol Toxicol. 2016;118(4):284‐291. doi: 10.1111/bcpt.12492 [DOI] [PubMed] [Google Scholar]
- 38. Gravel S, Panzini B, Belanger F, Turgeon J, Michaud V. A pilot study towards the impact of type 2 diabetes on the expression and activities of drug metabolizing enzymes and transporters in human duodenum. Int J Mol Sci. 2019;20(13):3257. doi: 10.3390/ijms20133257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Jong LM, Jiskoot W, Swen JJ, Manson ML. Distinct effects of inflammation on cytochrome P450 regulation and drug metabolism: lessons from experimental models and a potential role for pharmacogenetics. Genes (Basel). 2020;11(12):1509. doi: 10.3390/genes11121509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Urry E, Jetter A, Landolt HP. Assessment of CYP1A2 enzyme activity in relation to type‐2 diabetes and habitual caffeine intake. Nutr Metab (Lond). 2016;13:66. doi: 10.1186/s12986-016-0126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adithan C, Sriram G, Swaminathan RP, Krishnan M, Bapna JS, Chandrasekar S. Effect of type II diabetes mellitus on theophylline elimination. Int J Clin Pharmacol Ther Toxicol. 1989;27(5):258‐260. [PubMed] [Google Scholar]
- 42. Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet. 2012;51(8):481‐499. doi: 10.2165/11631900-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 43. Smit C, De Hoogd S, Brüggemann RJM, Knibbe CAJ. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14(3):275‐285. doi: 10.1080/17425255.2018.1440287 [DOI] [PubMed] [Google Scholar]
- 44. Wegler C, Matsson P, Krogstad V, et al. Influence of proteome profiles and intracellular drug exposure on differences in CYP activity in donor‐matched human liver microsomes and hepatocytes. Mol Pharm. 2021;18(4):1792‐1805. doi: 10.1021/acs.molpharmaceut.1c00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


