Abstract
Objectives:
To define frequency and characteristics of acute neurologic complications in children hospitalized with infective endocarditis, and to identify risk factors for neurologic complications.
Study Design:
Retrospective cohort study of children 0-18 years-old hospitalized at a tertiary children’s hospital from 1/1/2008-12/31/2017 with infective endocarditis.
Results:
Sixty-eight children met Duke criteria for infective endocarditis (43 definite, 25 possible). Twenty-three (34%) had identified neurologic complications, including intracranial hemorrhage (25%, 17/68) and ischemic stroke (25%, 17/68). Neurologic symptoms began a median of 4.5 days after infective endocarditis symptom onset (interquartile range 1-25 days), though five children were asymptomatic and diagnosed on screening neuroimaging only. Overall, only 56% (38/68) underwent neuroimaging during acute hospitalization, so additional asymptomatic neurologic complications may have been missed. Children with identified neurologic complications compared to those without were older (48% vs 22% ≥ 13 years-old, p=0.031), more often had definite rather than possible infective endocarditis (96% vs 47%, p<0.001), mobile vegetations >10mm (30% vs 11%, p=0.048), and vegetations with the potential for systemic embolization (65% vs 29%, p=0.004). Six children died (9%), all with neurologic complications.
Conclusions:
Neurologic complications of infective endocarditis were common (34%) and associated with mortality. The true frequency of neurologic complications was likely higher because asymptomatic cases may have been missed without screening neuroimaging. Moving forward, we advocate that all children with infective endocarditis have neurologic consultation, examination, and screening neuroimaging. Additional prospective studies are needed to determine whether early identification of neurologic abnormalities may direct management and ultimately reduce neurologic morbidity and overall mortality.
Keywords: Infective endocarditis, stroke, intracranial hemorrhage, infectious intracranial aneurysm, neuroimaging, congenital heart disease
INTRODUCTION
Infective endocarditis is a life-threatening bacterial infection of the heart that affects approximately 0.3-3.3 per 100,000 children, or 5-12 per 100,000 pediatric admissions each year 1–3. Risk factors in children include congenital heart disease (CHD), previous cardiac surgery 4,5,6, the presence of central venous catheters7, and rheumatic heart disease. Mortality in pediatric infective endocarditis ranges from 5-10%. Children at highest risk for infective endocarditis-associated mortality are those with cyanotic congenital heart disease, infective endocarditis caused by Staphylococcus aureus, and children who develop cardiac or extracardiac infective endocarditis complications, such as heart failure, perivalvular abscess, or septic emboli1,3,8,9.
Neurologic complications of infective endocarditis, such as arterial ischemic stroke, infectious intracranial aneurysms, intracranial hemorrhage, and intracerebral abscesses, occur in over half of adults with infective endocarditis,10,11 likely as a direct result of septic material from cardiac vegetations embolizing to the intracranial vasculature. Risk factors for neurologic complications of infective endocarditis in adults include vegetations larger than 30 mm, left-sided vegetations, particularly those affecting the anterior leaflet of the mitral valve, anticoagulation, and infection due to Staphylococcus aureus7. The frequency of and risk factors for neurologic complications of infective endocarditis have not been well-described in children and may differ from those in adults. One recent study of 31 children under 14 years of age identified neurologic complications in 23%, with possible risk factors including lower body weight, left-sided valvular lesions, and higher C-reactive protein. However, this group did not comment on differences between children with and without congenital heart disease12. Abnormal cardiac anatomy that allows for right-to-left shunting and surgically created aberrant connections between systemic and venous vasculature (e.g., single ventricle, systemic-pulmonary artery shunts, ductus arteriosus stents), may lead to unique structural risk factors for intracranial embolization in children with CHD and infective endocarditis13. Moreover, the presence of implanted foreign materials as part of CHD repair may alter the typical microbiology associated with pediatric infective endocarditis3. A better understanding of risk factors for neurologic complications of infective endocarditis in the pediatric population in children with structurally normal hearts and congenital heart disease is critical to guide diagnostic surveillance and management strategies in this vulnerable population.
We therefore aimed to 1) define the frequency and characteristics of acute neurologic complications in a cohort of children with infective endocarditis hospitalized at a tertiary care facility, and 2) identify risk factors for development of acute neurologic complications.
PATIENTS AND METHODS
Study Design and Data Collection
We performed a retrospective review of the electronic medical record at Children’s Hospital of Philadelphia to identify patients with infective endocarditis. The Institutional Review Board approved this study. Informed consent and Health Insurance Portability and Accountability Act (HIPAA) authorization were waived. We queried the electronic medical record for all children 0 to 18 years-old hospitalized at Children’s Hospital of Philadelphia between 1/1/2008 and 12/31/2017 with an International Classification of Disease, Tenth Revision (ICD-9 or -10) discharge diagnosis code for infective endocarditis (A01.02, A18.84, A32.82, A39.51, A52.03, A54.83, B33.21, B37.6, B39, B49, I01.1, I08, I09.1, I26.01, I26.90, I33, I35.8, I38, I39, I76, M05.03, M32.11, Q24, Z86.79). To ensure complete ascertainment of all eligible cases, we cross-referenced this list with institutional contributions to national databases (Society for Thoracic Surgeons (STS), Pediatric Cardiac Critical Care Consortium (PC4), and IMproving Pediatric and Adult Congenital Treatment (IMPACT) databases). We then manually reviewed data from each child’s medical record to confirm diagnosis of infective endocarditis by Duke criteria (see Study Definitions, Table 1) and abstracted demographic and clinical data for confirmed cases.
Table 1.
Duke Clinical Criteria for Infective Endocarditis15
| Pathologic Criteria |
Definite Diagnosis if any of the following: Any pathologic criteria 2 major criteria 1 major + 2 minor criteria 5 minor criteria Possible Diagnosis if any of the following: 1 major + 1 minor criteria 3 minor criteria |
| - Histologic: vegetation or intracardiac abscess present, confirmed by histology, showing active endocarditis - Bacteria: Demonstrated by culture or histology in a vegetation, or in a vegetation that has embolized, or in an intracardiac abscess | |
| Major Criteria | |
| - Positive blood culturesa - Evidence of endocardial involvement on echocardiogram | |
| Minor Criteria | |
| - Predispositionb - Fever ≥38C - Vascular phenomenac - Immune phenomenad - Microbiologic evidencee |
Requires typical pathogens from ≥2 separate cultures
Heart condition or intravenous drug use
Major arterial emboli, septic pulmonary infarcts, infectious intracranial aneurysm, intracranial hemorrhage, conjunctival hemorrhages, or Janeway lesions
Glomerulonephritis, Osler nodes, Roth spots, or rheumatoid factor
Positive blood cultures that do not meet Duke major criteria, or serologic evidence of active infection with an organism consistent with infective endocarditis.
Study Definitions
Infective endocarditis was defined as “definite” or “possible” using the revised Duke criteria. The Duke criteria use clinical, imaging, and laboratory evidence of intracardiac infection to define endocarditis14. While originally established for the diagnosis of adult infective endocarditis, these criteria have subsequently been modified for application to the pediatric population (Table 1)15–17. Vegetations with the potential for embolization to systemic (rather than pulmonary) circulation included those present in the left-side of the heart in any child, and those present on the right-side of the heart in a child with congenital heart disease and the anatomic potential for a right-to-left shunt. Presence of foreign material included prosthetic valves, central venous catheters, conduits, or recent surgery or catheterization in the 30 days prior to endocarditis symptom onset. We defined endocarditis symptom onset as the first days of symptoms attributed to infective endocarditis.
Neurologic complications of endocarditis were defined as neurologic signs or symptoms that were directly attributed to infective endocarditis or to a related intervention (e.g., cardiac surgery for infective endocarditis), either during acute hospitalization or follow-up at our institution (e.g., a related subsequent valve repair). Arterial ischemic stroke, intracranial hemorrhage, cerebral sinus venous thromboses, intracranial cerebral aneurysm, intracranial abscess, and other cerebral structural abnormalities were defined based on computed topography (CT) and/or magnetic resonance imaging (MRI). Endocarditis-associated seizures were defined as any clinical or electrographic seizure occurring in a child without a known seizure history, or increased seizures from baseline frequency in a child with known seizures. Encephalopathy was defined as documented parental or clinician concern for altered mental status, confusion, or irritability out of proportion to medical illness. Meningitis and encephalitis were defined as presence of pleocytosis (cerebrospinal fluid white blood count >7 cells/high powered field), or magnetic resonance imaging (MRI) findings suggestive of brain parenchymal (T2 signal abnormalities) or leptomeningeal inflammation (post-contrast T1-weighted MRI enhancement), in the absence of alternative explanations. Cranial nerve palsies were defined based on clinical examination findings. Headache, the etiology of which can be multifactorial, was not considered a neurological complication and was not used to define time of onset of neurologic symptoms unless it was temporally associated with a focal neurologic symptom or abnormal neuroimaging findings.
We classified neurological deficits at discharge based on the neurological examination documented in the discharge summary with additional input from the last neurology progress note, when available. We defined mild deficits as abnormalities on neurological examination that did not interfere with function, moderate deficits as neurological abnormalities that interfered with function, and severe deficits as abnormalities on neurological examination that resulted in significant loss of language, motor, or cognitive function.
Data Analysis and Statistical Methods
Data were analyzed using STATA version 14.2 (StataCorp, College Station, TX, USA). In order to minimize the effect of outliers on statistical associations in this small sample size, we described continuous variables using medians and interquartile ranges (IQRs) and compared inter-group differences using Wilcoxon rank-sum tests. We described categorical variables using counts and frequencies and compared inter-group differences using the χ2 test or Fisher’s exact test when n<5 in any cell of a contingency table. The Kaplan-Meier estimate of survival was calculated to determine the neurologic complication-free survival of the cohort. Survival time was calculated from date of endocarditis symptom onset or endocarditis diagnosis in those without clinical endocarditis symptoms. For those with neurologic complications, censor time was the date of neurologic complication onset or the date of imaging on which the neurologic complication was identified in those with asymptomatic neurologic complications. For those without neurologic complications, censor time was the date of hospital discharge or the date of the last examination by a neurologist, whichever was later. Statistical significance was considered a two-tailed p-value <0.05.
RESULTS
One hundred forty-one potentially eligible children were identified by electronic medical record query, 68 of whom (48%) met Duke criteria for infective endocarditis on manual chart review; 43 met criteria for “definite” infective endocarditis and 25 for “possible” infective endocarditis (Table 1 and Figure 1). The remaining 73 children had reference to infective endocarditis or infective endocarditis prophylaxis in their electronic medical record but did not meet definite or possible criteria for infective endocarditis. Demographic and clinical characteristics of the cohort and the subgroups of children with and without neurological complications are summarized in Table 2. The median age at infective endocarditis symptom onset was 9.0 years (IQR 0.3-14.6 years); about one-third of children (33%, 22/66) were less than one-year-old at infective endocarditis symptom onset. Forty-one children (60%) with infective endocarditis had congenital heart disease (CHD; Supplementary Tables 1 and 2), nine of whom had a prosthetic valve (mechanical=3 [aortic=2, mitral=1], homograft/bioprosthetic=6 [mitral=1, pulmonary=5]). There were no cases of rheumatic heart disease. Thirty-six children (53%) with infective endocarditis had foreign material present. Three children (two with CHD) had a recent dental procedure. Vegetations were present in 42 children, and twenty-eight children had a vegetation with potential for systemic embolization. Vegetations were located on the aortic valve in 8; mitral valve in 14; pulmonary valve in 4; tricuspid valve in 8; ventricle or ventricular septum in 5; atrium or atrial septum in 6; common atrioventricular valve in 1; and in other locations in 7 including 2 in right ventricle-pulmonary artery conduits, 1 in the pulmonary artery, 1 in a Sano conduit, 1 in a right pulmonary artery near a Blalock-Taussig shunt, 1 in a right ventricular outflow track, and 1 unspecified. Six children had vegetations in multiple locations. Figure 2 online shows an example of a vegetation.
Figure 1.
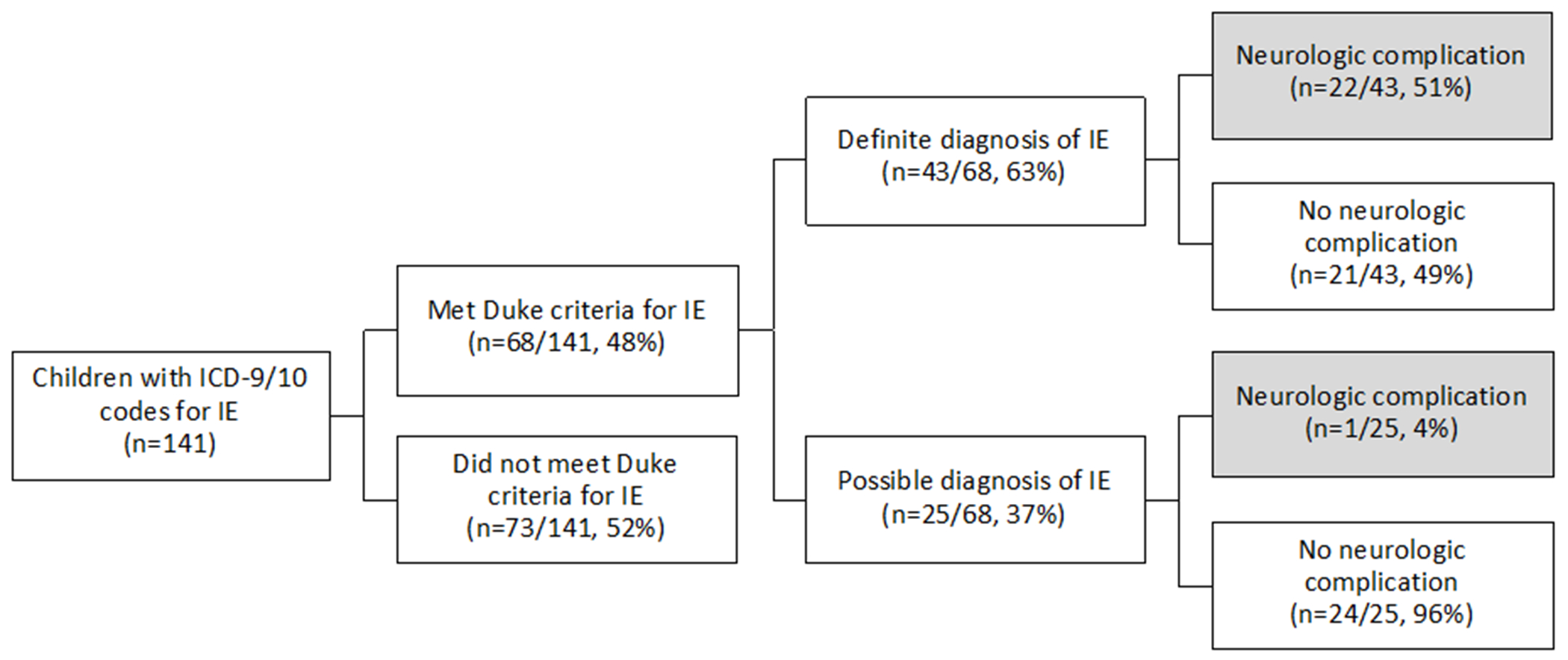
Summary of infectious endocarditis cohort.
Table 2.
Demographic, Historical, Clinical Features of Children with Infective Endocarditis, by Neurologic Complication
| All IE | By Neurologic Complication | |||
|---|---|---|---|---|
| (n=68) | Present (n=23) |
Not Present (n=45) |
p-valuea | |
| Age (years) at IE symptom onset (n=66) | 9.0 (0.3, 14.6) | 13.2 (1.9, 16.2) | 5.0 (0.2, 12.8) | 0.028 |
| Age ≥13 years | 21 (31) | 11 (48) | 10 (22) | 0.031 |
| Male sex | 35 (51) | 11 (48) | 24 (53) | 0.67 |
| Race | ||||
| Black (reference) | 19 (28) | 5 (22) | 14 (31) | 0.68 |
| White | 34 (50) | 12 (52) | 22 (49) | - |
| Other | 15 (22) | 6 (26) | 9 (20) | - |
| Length of hospital stay (days) | 20.5 (9, 68.5) | 21 (11, 69) | 20 (8, 57) | 0.63 |
| Medical History | ||||
| Congenital Heart Disease (CHD) | 41 (60) | 12 (52) | 29 (64) | 0.33 |
| Conduit (among those with CHD) | 15 (22) | 5 (22) | 10 (22) | 0.96 |
| Potential right-to-left shunt | 15 (22) | 2 (9) | 13 (29) | 0.07 |
| Single ventricle physiologyb | 9 (13) | 2 (9) | 7 (16) | 0.71 |
| Foreign material | 36 (53) | 11 (48) | 25 (56) | 0.55 |
| Prosthetic valve | 9 (13) | 2 (9) | 7 (16) | 0.71 |
| Mechanical | 3 (4) | 1 (4) | 2 (4) | - |
| Homograft/Bioprosthetic | 6 (9) | 1 (4) | 5 (11) | - |
| Left-sided prosthetic valve | 4 (6) | 2 (9) | 2 (4) | 0.60 |
| Central venous catheter at time of diagnosis (n=67) | 18 (27) | 6 (26) | 12 (27) | 0.92 |
| Surgery or catheterization within 30 days prior to diagnosis | 11 (16) | 3 (13) | 8 (18) | 0.74 |
| Dental procedure within 30 days prior to diagnosis | 3 (4) | 0 (0) | 3 (7) | 0.55 |
| Current antiplatelet use (n=15) | 11 (16) | 4 (17) | 7 (16) | 0.80 |
| Current anticoagulation use (n=28) | 14 (21) | 5 (22) | 9 (20) | 0.96 |
| Endocarditis Clinical Features | ||||
| Definite IE (vs possible) | 43 (63) | 22 (96) | 21 (47) | <0.001 |
| Duke pathologic criteria | ||||
| Pathologic lesions | 5 (7) | 4 (17) | 1 (2) | 0.041 |
| Micro-organisms | 6 (9) | 4 (17) | 2 (4) | 0.17 |
| Echocardiogram Findings | ||||
| Vegetation | 42 (62) | 18 (78) | 24 (53) | 0.045 |
| Mobile vegetation >10mm | 12 (18) | 7 (30) | 5 (11) | 0.048 |
| Vegetation with potential for systemic embolizationc | 28 (41) | 15 (65) | 13 (29) | 0.004 |
| Abscess | 2 (3) | 1 (4) | 1 (2) | 1.00 |
| New valvular regurgitation | 22 (32) | 12 (52) | 10 (22) | 0.012 |
| Normal | 21 (31) | 4 (17) | 17 (38) | 0.10 |
| Blood culture | ||||
| Staphylococcus aureus | 22 (32) | 9 (39) | 13 (29) | 0.39 |
| Viridans streptococci | 8 (12) | 5 (22) | 3 (7) | 0.11 |
| HACEK group | 1 (1) | 1 (4) | 0 (0) | 0.34 |
| Enterococcus | 1 (1) | 0 (0) | 1 (2) | 1.00 |
| Persistently + cultures for organisms typical of IE | 9 (13) | 3 (13) | 6 (13) | 1.00 |
| Persistently + cultures for more commonly skin contaminants | 5 (7) | 0 (0) | 5 (11) | 0.16 |
| No growth | 22 (32) | 5 (22) | 17 (38) | 0.18 |
| Fever | 60 (88) | 22 (96) | 38 (84) | 0.25 |
| Vascular phenomenad | 22 (32) | 17 (74) | 5 (11) | <0.001 |
| Immunologic phenomenae | 3 (4) | 3 (13) | 0 (0) | 0.035 |
| Microbiologic evidencef | 20 (29) | 6 (26) | 14 (31) | 0.67 |
| Neurologic Evaluation | ||||
| Neurology consult | 24 (35) | 19 (83) | 5 (11) | <0.001 |
| Neurosurgical intervention | 5 (7) | 5 (22) | 0 (0) | 0.003 |
| Neuroimaging | 38 (56) | 20 (87) | 18 (40) | <0.001 |
| HCT | 18 (26) | 15 (65) | 3 (7) | <0.001 |
| CTA head | 5 (7) | 4 (17) | 1 (2) | 0.041 |
| MRI brain | 23 (34) | 16 (70) | 7 (16) | <0.001 |
| MRA head | 14 (21) | 13 (57) | 1 (2) | <0.001 |
| MRV head | 1 (1) | 1 (4) | 0 (0) | 0.34 |
| Conventional angiogram | 5 (7) | 5 (22) | 0 (0) | 0.003 |
| Electroencephalogram | 14 (21) | 11 (48) | 3 (7) | <0.001 |
| Lumbar puncture | 5 (7) | 4 (17) | 1 (2) | 0.041 |
| Clinical Course | ||||
| Prodromal headaches | 14 (36) | 10 (63) | 4 (17) | 0.007 |
| Time from IE symptom onset to neurologic symptom onset (n=18) | 4.5 (1, 25) | |||
| Time from antibiotic start to neurologic symptom onset (n=18) | 0.5 (−2, 3) | |||
| Any cardiac surgery | 20 (29) | 11 (48) | 9 (20) | 0.017 |
| Mortality | 6 (9) | 6 (26) | 0 (0) | 0.001 |
Categorical variables are described using n (%). Continuous variables are described using median (IQR).
p-values were calculated using chi-squared tests or Fisher’s exact test for categorical variables and Wilcoxon rank-sum tests for continuous variables
Includes all children with fenestrated Fontan or stage I palliation. There were no children with stage II palliation.
Includes all children with a left-sided vegetation or those with a right-sided vegetation with potential for a right-to-left shunt and systemic embolization.
Vascular phenomena include major arterial emboli, septic pulmonary infarcts, infectious intracranial aneurysm, intracranial hemorrhage, conjunctival hemorrhages, or Janeway lesions
Immunologic phenomena include glomerulonephritis, Osler nodes, Roth spots, or rheumatoid factor
Microbiologic evidence includes positive blood cultures that do not meet Duke major criteria, or serologic evidence of active infection with organism consistent with IE
Abbreviations: IE, infective endocarditis; HACEK, Haemophilis species, Actinocacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae; HCT, head computed topography; CTA, computed topography angiogram; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; MRV, magnetic resonance venography; AHA, American Heart Association.
Figure 2.
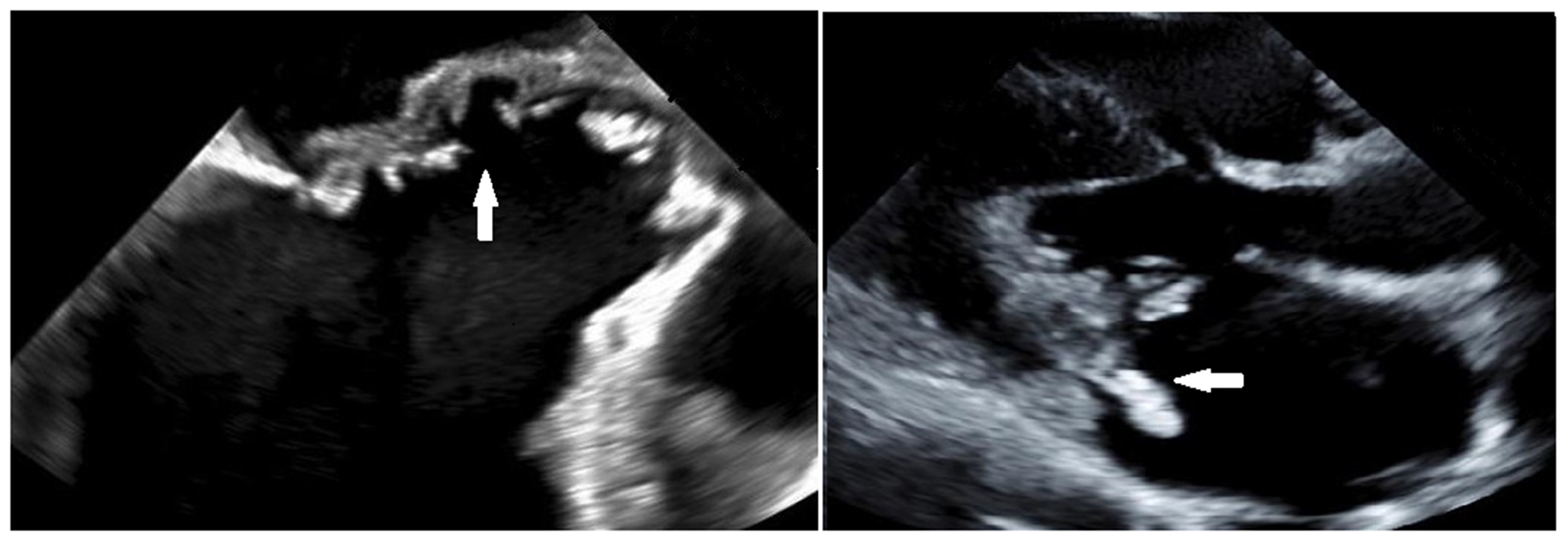
Trans-esophageal echocardiogram demonstrates erosion of the mitral to aortic fibrous continuity in a child with infective endocarditis (left). A vegetation on the posterior leaflet of the mitral valve seen on trans-thoracic echocardiogram in a different child with IE (right).
Neurologic complications.
Neurologic complications of infective endocarditis were common, identified in 23 of 68 children (34%, 95% CI 23-46%) (Tables 2 and 3). Neurologic complication-free survival was 70% at 30 days (95% CI 57%-80%), 63% at 90 days (95% CI 49%-75%), and 58% at 1 year (95% CI 42%-72%) (Figure 3). The proportion of children with neurologic complications did not differ between those with and without a diagnosis of CHD (12/41 [29%] vs 11/27 [41%], p=0.33). Neither children with CHD and a right-to-left shunt (compared to those with CHD without right-to-left shunt) nor children with a systemic prosthetic valve (compared to children without systemic prosthetic valve) had higher proportions of neurologic complications. Two children had bovine jugular vein prosthetic materials (both with Melody valves). Neither of these children had residual shunts or neurologic complications.
Table 3.
Frequency of Neurologic Complications in Children Meeting Modified Duke Criteria for Infective Endocarditis
| Neurologic Complication (n=23) | Number (%) among all IE (n=68)* | Number (%) among definite IE (n=43)* |
|---|---|---|
| Arterial ischemic stroke | 17 (25) | 16 (37) |
|
|
||
| Multiple punctate embolic | 9 (13) | 9 (21) |
|
|
||
| Middle cerebral artery | 5 (7) | 4 (9) |
|
|
||
| Single punctate embolic | 2 (3) | 2 (5) |
|
|
||
| Posterior cerebral artery | 1 (1) | 1 (2) |
|
|
||
| Anterior cerebral artery | 1 (1) | 1 (2) |
|
|
||
| Watershed | 1 (1) | 1 (2) |
|
|
||
| Intracranial hemorrhage | 17 (25) | 16 (37) |
|
|
||
| Multifocal punctate hemorrhages | 7 (13) | 7 (16) |
|
|
||
| Subarachnoid hemorrhage | 7 (13) | 6 (14) |
|
|
||
| Intraparenchymal hemorrhage | 4 (6) | 3 (7) |
|
|
||
| Intraventricular hemorrhage | 4 (6) | 3 (7) |
|
|
||
| Subdural hemorrhage | 4 (6) | 4 (9) |
|
|
||
| Hemorrhagic transformation of arterial ischemic stroke | 4 (6) | 3 (7) |
|
|
||
| Encephalopathy | 8 (12) | 8 (19) |
|
|
||
| Intact aneurysm | 5 (7) | 5 (12) |
|
|
||
| Meningitis and/or encephalitis | 5 (7) | 5 (12) |
|
|
||
| Seizure | 3 (4) | 2 (5) |
|
|
||
| Intracranial abscess | 2 (3) | 2 (5) |
|
|
||
| Cranial nerve palsy | 2 (3) | 2 (5) |
|
|
||
| Hypoxic ischemic injury+ | 1 (1) | 1 (2) |
Some children had more than one neurologic complication
Post-conduit replacement
Figure 3.
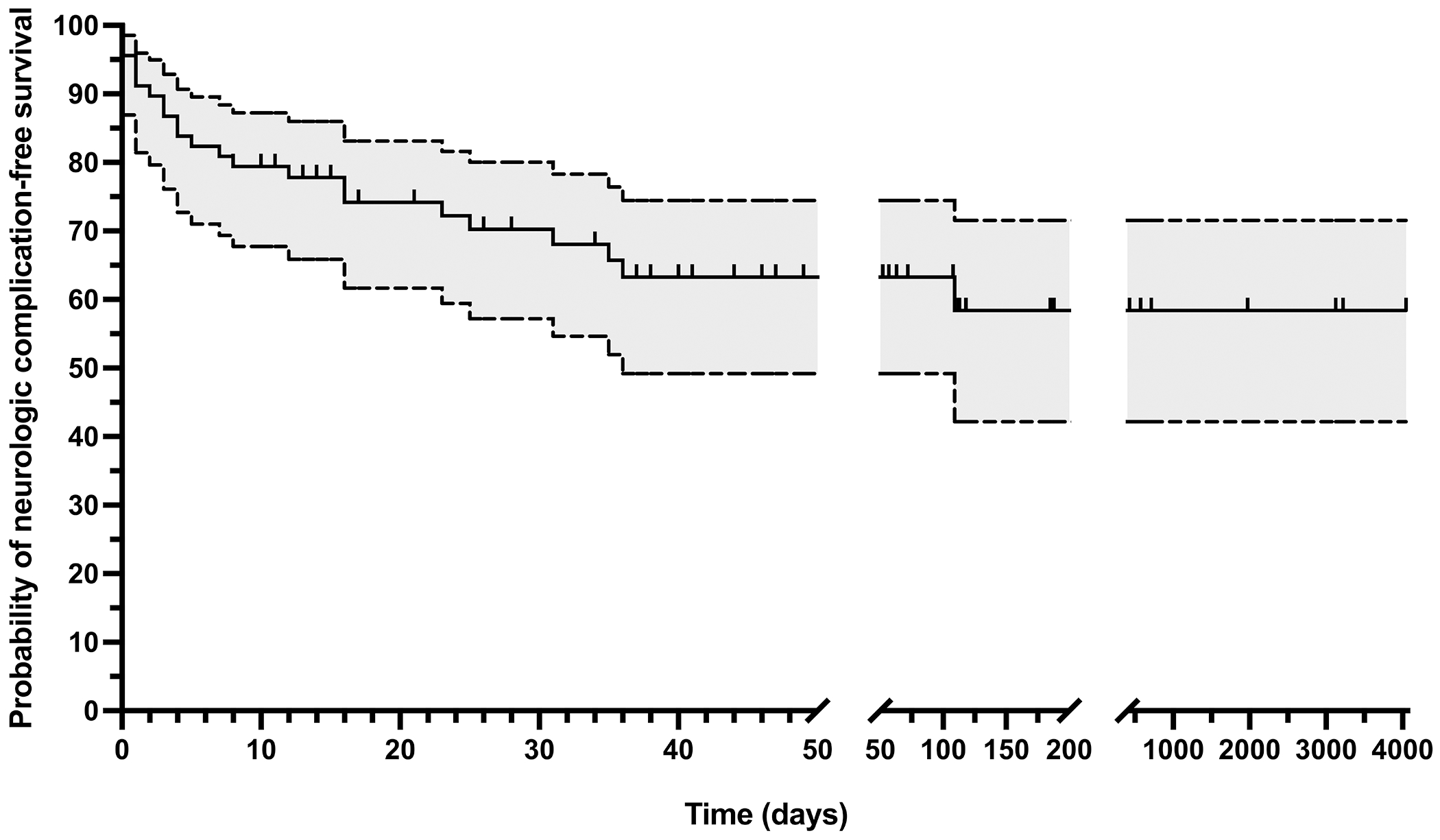
Kaplan-Meier survival curve with 95% confidence interval showing neurologic complication-free survival. Vertical tick marks indicate censor time for children without identified neurologic complications.
Almost all children with identified neurologic complications (22/23) had these occur during the acute hospitalization. Eighteen of the 23 children with a neurologic complication (78%, 95% CI 56-93%) had clinical neurologic symptoms; five had asymptomatic neurologic diagnoses identified on screening neuroimaging only. Symptoms began a median of 4.5 days (IQR 1-25; n=18) after onset of endocarditis symptoms and a median of 0.5 days (IQR -2-3; n=18) after antibiotic initiation. Ten children had neurologic symptoms on or before the day of admission (encephalopathy [n=7], focal weakness [n=2], headache due to intracranial hemorrhage [n=2]). Two children who had neurologic complications during the acute hospitalization (one arterial ischemic stroke, one transient ischemic attack) had additional neurologic complications after discharge. One child who did not have a neurologic complication during the hospitalization had a neurologic complication after discharge.
Table 3 summarizes identified neurologic complications, including findings on neuroimaging, which was performed in only 38/68 (56%) of the cohort during their acute hospitalization. More children with definite infective endocarditis underwent neuroimaging than those with possible infective endocarditis (72% vs 28%, p<0.001), but neuroimaging was performed similarly with respect to sex (51% male vs 62% female, p=0.45), age group (57% vs 56% of children ≥13 years-old vs <13 years-old at infective endocarditis symptom onset, p=0.94), and those with and without CHD (51% vs 63%, p=0.34). Figures 4, 5, and 6 demonstrate representative neuroimaging findings. Ischemic injury and hemorrhage were the most common neurologic complications, occurring in 17 children each. Multifocal punctate embolic infarcts (n=9) and middle cerebral artery arterial ischemic strokes (n=5) were most common ischemic injuries, and multifocal punctate hemorrhage (n=7) and subarachnoid hemorrhage (n=7) were the most common types of hemorrhages. Six children had multiple different types of hemorrhage. A similar proportion of children with hemorrhage, compared to those without hemorrhage, were being treated with anticoagulation or antiplatelet therapy prior to infective endocarditis symptom onset (63% vs 76%, p=0.31). Eleven children had both hemorrhage and ischemic stroke, four of whom had hemorrhagic transformation of an ischemic injury. Intact intracranial aneurysms were visualized in five children, four of whom also had intracranial hemorrhage, two of which were subarachnoid hemorrhages. Other aneurysms may not have been detected if bleeding had already occurred at the time of vascular imaging with obliteration or compression of the aneurysm. One aneurysm was clipped, one was coiled twice, and three were managed medically. There were three children with seizures, all of whom also had intracranial hemorrhage and/or ischemic injury, suggesting these were acute symptomatic. Eight children had encephalopathy, seven of whom also had intracranial pathology including ischemic injury, intracranial hemorrhage, or meningitis, suggesting encephalopathy was symptomatic of underlying structural abnormalities or intracranial infection. The eighth child with encephalopathy had progressive deterioration of mental status to obtundation. This child had multisystem organ dysfunction and died before neuroimaging could be obtained.
Figure 4.
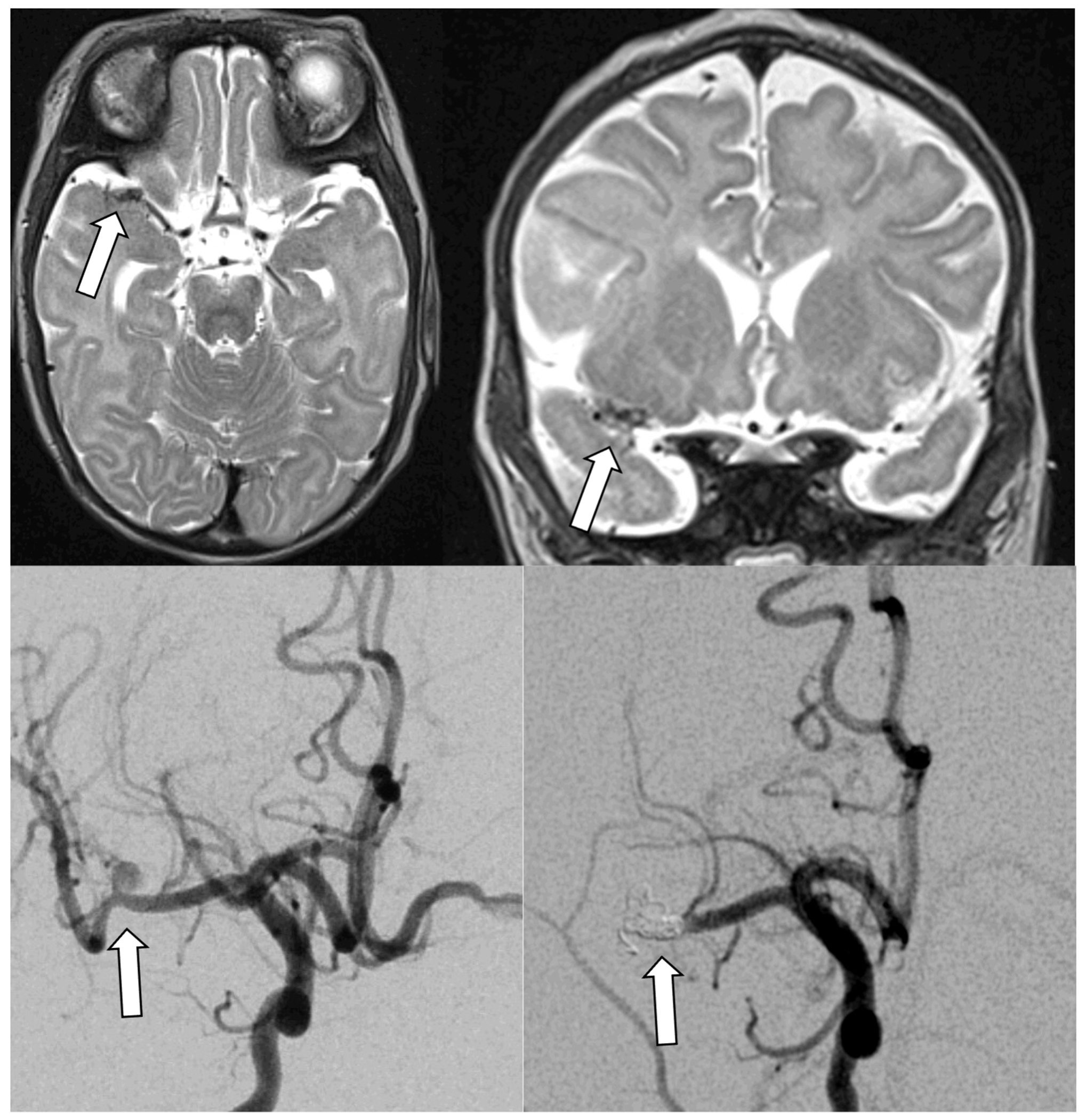
Infectious intracranial aneurysm in an infant with hypoplastic left heart syndrome and infective endocarditis with left middle cerebral artery stroke. Top panels show T2 axial (left) and coronal (right) MRIs with right middle cerebral artery infectious intracranial aneurysm. Bottom panels demonstrate the infectious intracranial aneurysm on conventional angiogram before (left) and after (right) coiling.
Figure 5.
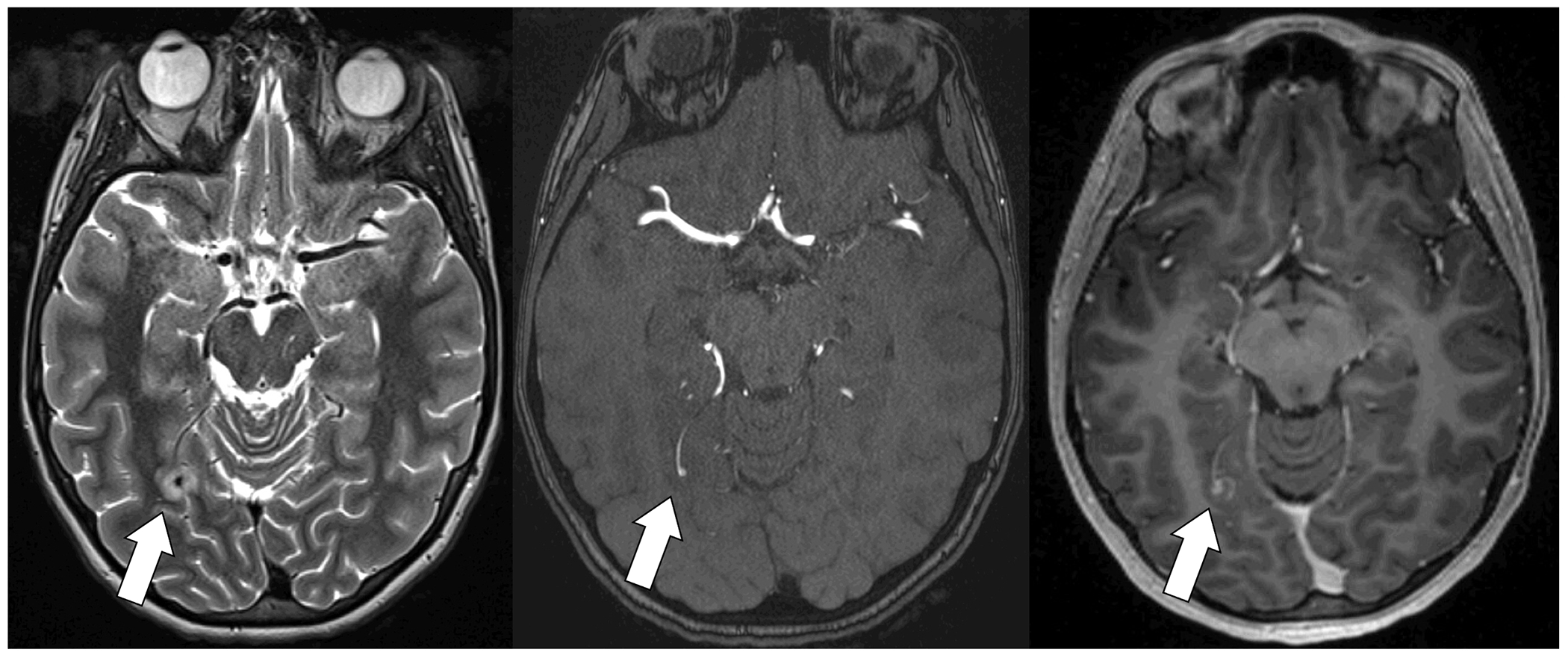
Previously healthy adolescent with structurally normal heart with infective endocarditis and small infectious intracranial aneurysm. T2 MRI (left) with edema surrounding infectious intracranial aneurysm. Time of flight MRA (middle) demonstrates small infectious intracranial aneurysm. T1 multiplanar reformation post-gadolinium image (right) demonstrates contrast enhancement.
Figure 6.
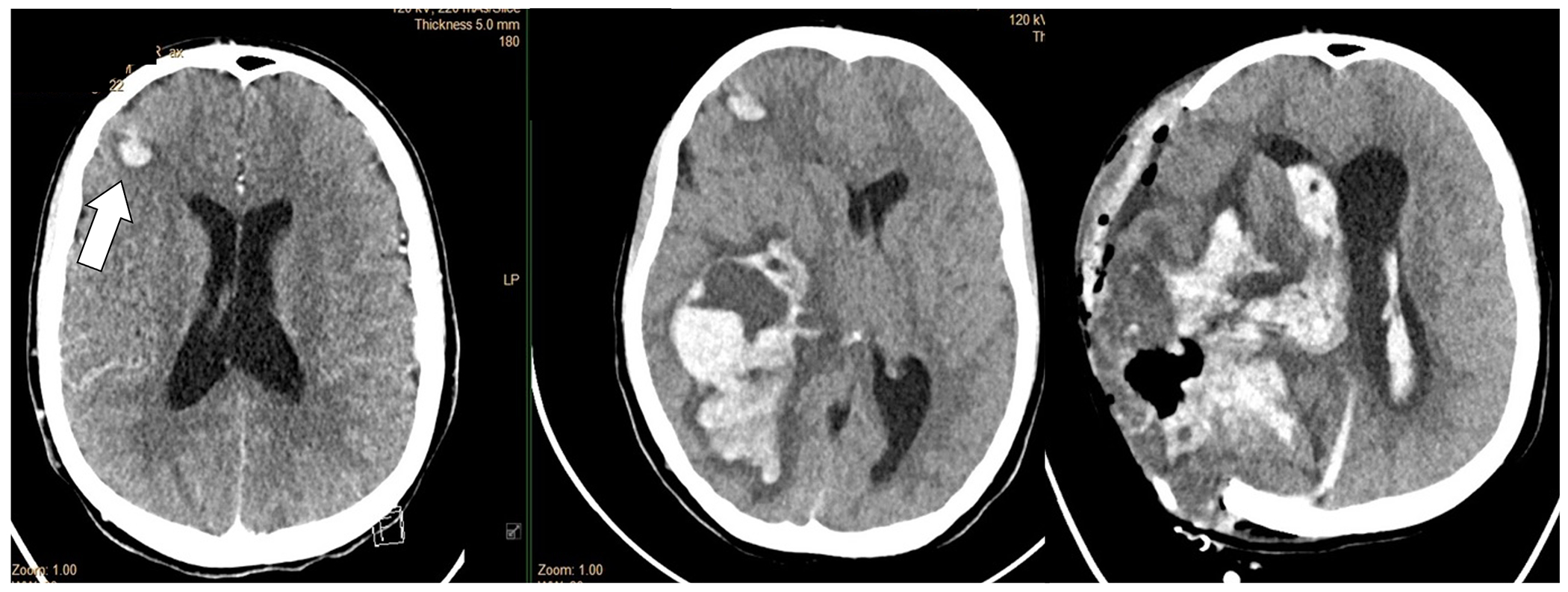
Adolescent with DiGeorge Syndrome and repaired truncus arteriosus with St. Jude’s valve in the mitral position and infective endocarditis. The head CT demonstrated multiple septic emboli with hemorrhagic transformation (small right frontal lesion demonstrated on this slice) (left panel). Head CT 48 hours later with massive hemorrhage before (middle panel) and after (right panel) craniectomy. CTA was not performed, but the large hemorrhage was presumed to be due to a ruptured infectious intracranial aneurysm.
Among the seven children that had subarachnoid hemorrhages, two had an intact aneurysm visualized, one had mild fusiform prominence of distal intracranial vessels proximal to the hemorrhage but without a clear aneurysm, and one did not have vascular imaging performed (though aneurysm was suspected). While one of these seven children was asymptomatic, among the remaining six, symptoms began a median of 18.5 days (IQR 2-31) after onset of endocarditis symptoms.
Fourteen of the 68 (21%) children had a headache. Among those with headache, 10 (71%) had a neurologic complication, including encephalopathy (n=4), meningitis (n=2), seizure (n=1), ischemic injury (n=8), aneurysm (n=4), abscess (n=1), and intracranial hemorrhage (n=9). Among the four children with headache that did not have an identified neurologic complication, only two underwent neuroimaging (head CT 1, MRI brain 1), and these studies were normal.
Five children had asymptomatic neurologic complications identified on screening neuroimaging only (abscess [n=1]; aneurysm, leptomeningeal enhancement, and subarachnoid hemorrhage [n=1]; subdural hemorrhage [n=1]; punctate ischemia and/or hemorrhage [n=2]). The indications for screening neuroimaging included evaluation of fever or headache of unknown origin before diagnosis of infective endocarditis, presence of systemic embolic infarcts, concern for systemic Candida infection, and screening imaging performed following bypass. One child with an asymptomatic intracranial abscess underwent neurosurgical drainage. None of the other children required intervention for their asymptomatic neurologic complications. Only one child with asymptomatic neurologic disease (SAH and leptomeningeal enhancement) had a later additional or progressive neurologic symptoms (aneurysm identified).
On univariable analysis (Table 2), we found that children with an identified neurologic complication were older than those without (median age 13.2 [IQR 1.9, 16.2] versus 5.0 [IQR 0.2, 12.8], p=0.028), more often had a definite (rather than possible) diagnosis of infective endocarditis (96% versus 47%, p<0.001), pathologic lesions (17% versus 2%, p=0.041), large (>10mm) mobile vegetations (30% versus 11%, p=0.048), vegetations with potential for systemic embolization (65% versus 29%, p=0.004), new valve regurgitation (52% versus 22%, p=0.012), vascular phenomena by Duke criteria (74% versus 11%, p<0.001), or a headache (63% versus 17%, p=0.007). In contrast to published literature, neither Staphylococcus aureus nor use of anticoagulation was a risk factor for identified neurologic complication in this pediatric cohort.
Hospital Course.
Fifteen of the 23 children with an identified neurologic complication had additional or progressive neurologic complications during their acute hospitalizations including hemorrhage (n=10), ischemic injury (n=9), aneurysm (n=3), septic emboli (n=2), transient ischemic attack (n=2), leptomeningeal enhancement (n=2), and abscess (n=1), which occurred at a median of 18 days (IQR 10-47). Four children with symptomatic neurologic complications required neurosurgical intervention (3 extraventricular drains, 1 washout, 2 hemorrhage evacuations, 2 aneurysm interventions). Of the 23 children with neurologic complications, 6 (26%) died; there were no deaths in children without neurologic complications. Causes of death included progressive intracranial hemorrhage (n=2), septic shock (n=1), cardiac arrest in the context of multifocal infarcts and subdural hemorrhage with mass effect (n=1), cardiac arrest with diffuse systemic clotting (n=1), and withdrawal of care in an infant due to severity of global medical illness (leptomeningeal enhancement, multifocal punctate hemorrhages) and prematurity (n=1). Among survivors, there was no significant difference in total length of hospital stay between those with and without neurologic complications. Among the 17 surviving children that had a neurologic complication, 8 (47%) had residual neurological deficits. Deficits were mild in five, moderate in one, and severe in two.
Fourteen children underwent cardiac surgery during their acute hospitalization, a median of 15.5 days (IQR 6, 33 days) after admission. Six additional children underwent cardiac surgery after discharge from their acute hospitalization at a median of 57 days (IQR 36-79 days) after admission. As expected, children with an identified neurologic complication had cardiac surgery more often than children without a neurologic complication (11/23 [48%] vs 9/45 [20%], p=0.017), likely because embolic phenomena are a surgical indication. Neurological symptoms or neuroimaging abnormalities in those with asymptomatic neurologic diagnoses preceded cardiac surgery in 10 of 11 who underwent surgery (median time between neurologic symptom onset to cardiac operation 15 days, IQR 1, 34); one additional child had first neurologic symptom onset immediately postoperatively (arterial ischemic stroke with hemorrhagic transformation). Two of the 11 children with a neurologic complication who had cardiac surgery had additional neurologic symptoms 17 and 32 days after surgery, respectively; these symptoms were not considered to be attributable to surgery. Finally, one additional child had multiple new small emboli on screening repeat MRI 5 days after surgery.
DISCUSSION
To our knowledge, we present the largest pediatric cohort examining the frequency and characteristics of neurologic complications of infective endocarditis. We found that neurologic complications of infective endocarditis were common, occurring in at least 23 of 64 total children (34%, 95% CI 23-46%), or 22 of 43 children with definite infective endocarditis [51%, 95% CI 35-67%], similar estimates to the proportion of neurologic complications reported in adults11, but slightly higher than prior pediatric estimates12. At 1 year from endocarditis symptom onset, neurologic complication-free survival was only 58%. Mortality in this cohort occurred exclusively in children with neurologic complications. Risk factors for identified neurologic complications in this pediatric cohort included older age, clinical features associated with risk for embolic event (such as pathologic lesions, large and mobile vegetations, vegetations in a location with the potential for systemic embolization), and a definite (versus probable) diagnosis of infective endocarditis.
While abnormal cardiac anatomy and prosthetic material related to CHD repair could lead to unique structural risk factors for intracranial embolization of vegetations and subsequent neurologic complications in children with CHD and infective endocarditis, we did not observe a difference in neurologic complications between children with and without CHD, potential right-to-left intracardiac shunt, single ventricle physiology, or systemic prosthetic valves. This may be due to a relatively small sample size of children with heterogeneous types of CHD. Future large, prospective, multisite studies could allow construction of multivariable regression models to examine independent risk factors for neurologic complication by underlying cardiac anatomy.
Nearly a quarter of the neurologic complications documented in this pediatric cohort were asymptomatic, found incidentally on screening neuroimaging. Since our institution does not have a standard neuroimaging screening protocol for children with infective endocarditis, only 38 children (59%, 95% CI 46-71%) underwent neuroimaging during their acute hospitalization. Of the 42 children without a documented neurologic complication, only 18 (43%) underwent screening neuroimaging. Up to 80% of adults with infective endocarditis have asymptomatic neurologic complications18,19, therefore it is very possible that we underestimated the number of asymptomatic neurologic complications. Whether early identification and management of asymptomatic neurologic complications in children with infective endocarditis affects morbidity and mortality remains unknown but is probable. For example, early identification of a large infectious intracranial aneurysm may lead to stabilizing interventions which could prevent later catastrophic hemorrhage. Similarly, early identification of neurologic embolic phenomenon may impact indications for cardiac surgery6,13,14 if there is a treatable lesion. Further investigation is needed to determine whether early identification and treatment of neurologic complications leads to management changes and improved outcomes.
Our study has several limitations. First, the study was conducted at a single pediatric tertiary care referral center that may introduce referral bias. Therefore, generalizing these findings to different care contexts should be undertaken cautiously. Next, our data is from a retrospective cohort. Some details of the clinical courses may not have been available, there may have been more asymptomatic neurologic complications in this cohort that were not identified by their care team which could lead to misclassification bias, and we may have underestimated neurological deficits as certain deficits like cognitive problems may become apparent after discharge. Furthermore, we were unable to determine whether the timing of surgeries for infective endocarditis were affected by concerns about extension of existing hemorrhage or risks of anticoagulation or embolization during the procedure. Also, our cohort size limits our ability to evaluate all potential risk factors for neurologic complications and to construct appropriate multivariable models. Finally, we lack standardized long-term outcome data.
In conclusion, neurologic complications of infective endocarditis were common in this pediatric cohort with infective endocarditis (34%) and were associated with increased mortality. The true frequency of neurologic complications was likely higher than detected because asymptomatic neurologic complications may have been missed in the absence of routine screening neuroimaging. Older age, a diagnosis of definite infective endocarditis by Duke criteria, and clinical features associated with risk of embolic event were all associated with the presence of identified neurologic complications. Additional prospective studies are required to determine whether risk factors for infective endocarditis may vary based on underlying cardiac anatomy.
All children with infective endocarditis should have a multidisciplinary team approach to their care, by a specialized “endocarditis team.” In addition to early involvement of general cardiology, cardiac surgery, and infectious diseases, we suggest that all children with infective endocarditis have neurologic consultation, neurologic examination, and screening neuroimaging within the first 24-72 hours of their hospitalization, if clinically stable to do so, and serially if IE treatment is extended over a long period of time. Additional prospective studies with screening neurologic examinations and neurovascular imaging are needed to determine whether early identification of neurologic abnormalities like infectious intracranial aneurysms and microemboli may direct management and ultimately reduce neurologic morbidity and overall mortality.
Supplementary Material
FINANCIAL SUPPORT:
Dr. McGuire receives support from NIH K23 NS094069. Dr. Licht receives support from NIH R01-NS072338, R01-NS60653, and the June and Steve Wolfson Family Foundation.
Abbreviations:
- CT
computed topography
- CHD
congenital heart disease
- MRI
magnetic resonance imaging
Footnotes
Data Statement: A deidentified data set is available upon request.
CONFLICTS OF INTEREST: Dr. Beslow has consulted for Biogen; this consulting is unrelated to this manuscript. The other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pasquali SK, He X, Mohamad Z, McCrindle BW, Newburger JW, Li JS, et al. Trends in endocarditis hospitalizations at US children’s hospitals: impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J. 2012;163(5):894–899. doi: 10.1016/j.ahj.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in Infective Endocarditis in California and New York State, 1998-2013. JAMA. 2017;317(16):1652–1660. doi: 10.1001/jama.2017.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Sakhuja A, McGrath E, Asmar B. Trends, microbiology, and outcomes of infective endocarditis in children during 2000-2010 in the United States. Congenit Heart Dis. 2017;12(2):196–201. doi: 10.1111/chd.12425 [DOI] [PubMed] [Google Scholar]
- 4.Rushani D, Kaufman JS, Ionescu-Ittu R, Mackie AS, Pilote L, Therrien J, et al. Infective endocarditis in children with congenital heart disease: cumulative incidence and predictors. Circulation. 2013;128(13):1412–1419. doi: 10.1161/CIRCULATIONAHA.113.001827 [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal LB, Feja KN, Levasseur SM, Alba LR, Gersony W, Saiman L. The changing epidemiology of pediatric endocarditis at a children’s hospital over seven decades. Pediatr Cardiol. 2010;31(6):813–820. doi: 10.1007/s00246-010-9709-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association [published correction appears in Circulation. 2015 Oct 27;132(17):e215] [published correction appears in Circulation. 2016 Aug 23;134(8):e113] [published correction appears in Circulation. 2018 Jul 31;138(5):e78-e79]. Circulation. 2015;132(15):1435–1486. doi: 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 7.Stockheim JA, Chadwick EG, Kessler S, Amer M, Abdel-Haq N, Dajani AS, et al. Are the Duke criteria superior to the Beth Israel criteria for the diagnosis of infective endocarditis in children? Clin Infect Dis. 1998;27(6):1451–1456. doi: 10.1086/515021 [DOI] [PubMed] [Google Scholar]
- 8.Day MD, Gauvreau K, Shulman S, Newburger JW. Characteristics of children hospitalized with infective endocarditis [published correction appears in Circulation. 2010 Nov 23;122(21):e560]. Circulation. 2009;119(6):865–870. doi: 10.1161/CIRCULATIONAHA.108.798751 [DOI] [PubMed] [Google Scholar]
- 9.Ware AL, Tani LY, Weng HY, Wilkes J, Menon SC. Resource utilization and outcomes of infective endocarditis in children. J Pediatr. 2014;165(4):807–12.e1. doi: 10.1016/j.jpeds.2014.06.026 [DOI] [PubMed] [Google Scholar]
- 10.Snygg-Martin U, Gustafsson L, Rosengren L, Alsio A, Ackerholm P, Andersson R, et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. 2008;47(1):23–30. doi: 10.1086/588663 [DOI] [PubMed] [Google Scholar]
- 11.Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Arch Intern Med. 2000;160(18):2781–2787. doi: 10.1001/archinte.160.18.2781 [DOI] [PubMed] [Google Scholar]
- 12.AlBassri T, Sheikho M, Chaikhouni F, Al Habshan F, Kabbani MS. Neurological complications in children with infective endocarditis: Incidence, risk factors, and outcome: A 10-year single-center experience. Int J Pediatr Adolesc Med. 2021;8(3):198–202. doi: 10.1016/j.ijpam.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer AS, Bolger AF, Taubert KA, Wilson W, Steckelberg J, Karchmer AW, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98(25):2936–2948. doi: 10.1161/01.cir.98.25.2936 [DOI] [PubMed] [Google Scholar]
- 14.Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, et al. Infective Endocarditis in Childhood: 2015 Update: A Scientific Statement From the American Heart Association. Circulation. 2015;132(15):1487–1515. doi: 10.1161/CIR.0000000000000298 [DOI] [PubMed] [Google Scholar]
- 15.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–209. doi: 10.1016/0002-9343(94)90143-0 [DOI] [PubMed] [Google Scholar]
- 16.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 17.Bendig EA, Singh J, Butler TJ, Arrieta AC. The impact of the central venous catheter on the diagnosis of infectious endocarditis using Duke criteria in children with Staphylococcus aureus bacteremia. Pediatr Infect Dis J. 2008;27(7):636–639. doi: 10.1097/INF.0b013e31816b78c8 [DOI] [PubMed] [Google Scholar]
- 18.Duval X, Iung B, Klein I, Brochet E, Thabut G, Arnoult F, et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med. 2010;152(8):497–W175. doi: 10.7326/0003-4819-152-8-201004200-00006 [DOI] [PubMed] [Google Scholar]
- 19.Hess A, Klein I, Iung B, Lavallee P, Ilic-Habensus E, Dornic Q, et al. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. AJNR Am J Neuroradiol. 2013;34(8):1579–1584. doi: 10.3174/ajnr.A3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


