Abstract
BACKGROUND.
Several ordinal grading systems are employed in deciding whether to perform angioembolization or splenectomy following blunt splenic injury. The 2018 AAST Organ Injury Scale (OIS) incorporates vascular lesions but not hemoperitoneum, which is considered in the Thompson classifier. Granular and verifiable quantitative measurements of these features may have a future role in facilitating objective decision-making.
PURPOSE.
To compare performance of CT volumetry-based quantitative modeling to the 1994 and 2018 AAST OIS and Thompson classifier for the following endpoints: decision to perform splenectomy (SPY), and the composite of SPY or angioembolization (AE)
MATERIALS AND METHODS.
Adult BSI patients (age ≥ 18 years) scanned with dual-phase CT prior to intervention at a single level I trauma center from 2017-2019 were included in this retrospective study (n=174). Scoring using 2018 AAST, 1994 AAST, and Thompson systems was performed retrospectively by two radiologists and arbitrated by a third. Endpoints included 1. SPY and 2. The composite of SPY or AE. Logistic regression models were developed from segmented active bleed, contained vascular lesion, splenic parenchymal disruption, and hemoperitoneum volumes. AUCs for ordinal systems and volumetric models were compared.
RESULTS.
Forty-seven BSI patients (27%) underwent SPY, and 87 patients (50%) underwent SPY or AE. Quantitative model AUCs (0.85- SPY, 0.82-composite) were not significantly different from 2018 AAST AUCs (0.81, 0.88, p=0.66, 0.14) for both endpoints, and were significantly improved over Thompson scoring (0.76, p=0.02; 0.77, p=0.04).
CONCLUSION:
Quantitative CT volumetry can be used to model intervention for BSI with accuracy comparable to 2018 AAST scoring and significantly higher than Thompson scoring.
Study Type:
Prognostic
Level of Evidence:
IV
Keywords: CT, quantitative imaging, abdomen/GI, spleen, trauma, observer performance, outcomes analysis
Summary Statement:
CT volumetry of blunt splenic injury-related features predicts splenectomy and angioembolization in adults and identifies clinically important target features for computer vision and automation research.
Introduction
Since CT is rapid and allows concurrent monitoring by the trauma team, the modality is routinely employed after blunt trauma in both hemodynamically stable patients and transient responders (1). Short term morbidity and mortality after blunt splenic injury (BSI) is related to intracavitary hemorrhage (2). The spleen is the largest secondary lymphoid organ in the body and plays an important role in adaptive immunity and bacterial clearance (3). In the long term, splenectomy carries a lifetime risk of overwhelming post-splenectomy infection (OPSI), for which mortality ranges from 38-69% (4). Patients must be appropriately selected for any given management approach.
Therefore, BSI grading systems used to stratify risk based on admission CT should ideally discriminate between patients requiring intervention for hemorrhage control from those who do not, and between patients requiring splenectomy from those whose spleens can be salvaged (5). Various scoring systems, including the 1994 splenic organ injury scale (OIS)-originally a research tool relying on combined imaging and surgical findings but repurposed for computed tomography (6), the 2018 AAST splenic organ injury scale (OIS) update (7, 8), and the binary classifier of Thompson et al. (9) have been reported for point of care decision support based on imaging features on abdominopelvic contrast-enhanced computed tomography (CECT). These classification systems are summarized in tables 1-3 of the supplemental digital content (SDC).
A review of grading systems.—
Major differences between the aforementioned scoring systems reflect 1. Increased appreciation of the prognostic value of traumatic vascular lesions (10-12), 2. Adoption of routine dual phase arterial and portal venous scanning which differentiates between contained vascular injury (best visualized on arterial images) and active bleeding (best assessed on portal venous phase images) (11, 13, 14), and 3. Growing recognition of hemoperitoneum as an independent marker of splenic injury severity (2, 9).
The 1994 AAST splenic OIS [SDC, table 1] considers the degree of parenchymal disruption and subcapsular hematoma but does not incorporate vascular lesions in severity grading (12). The 2018 AAST splenic OIS update [SDC, table 2] stratified levels of BSI severity based additionally on the presence of contained vascular lesions (pseudoaneurysms and arteriovenous fistulas), and intracapsular or extracapsular active bleeding. The AAST Patient Assessment Committee now explicitly recommends use of arterial and portal venous phase (PVP) imaging (7).
The AAST OIS does not consider the degree of hemoperitoneum despite increasing clinical reliance among clinicians on this feature for managing BSI (2). The Thompson system [SDC, table 3] (9) is a binary classifier for predicting splenic angioembolization (AE) or splenectomy based on presence of at least one of the following features: greater than 50% splenic parenchymal disruption, contrast blush greater than 1 centimeter (cm) in diameter, and large hemoperitoneum, graded using a subjective semi-quantitative method originally described by Federle and Jeffrey (15).
Multiparametric quantitative modeling.—
CT parameters of solid organ injury and intracavitary bleeding including parenchymal disruption, subcapsular hematoma, contained vascular lesions, active hemorrhage, and hemoperitoneum, are frequently irregular and multifocal on CT (16-20). Linear caliper measurements and subjective estimation are shown to have limited correlation with results of voxelwise segmentation for abdominopelvic bleeding features (16, 21). These parameters of intracavitary bleeding and solid organ injury are amenable to voxelwise labeling and quantitative measurement and can be modeled together with the battery of clinical quantitative results including vital signs and laboratory values. High model performance could justify future development of explainable, objective, and personalized computer vision-based multiparametric BSI decision support tools.
Purpose.—
In this study of 174 consecutive adult patients with BSI, we compare categorical grading systems (the 2018 AAST splenic OIS update using the AAST-recommended dual phase CT protocol, 1994 AAST splenic OIS, and Thompson classifier) to multiparametric quantitative models for the endpoints of splenectomy, and a composite outcome of splenectomy and AE.
Materials and Methods
Study population.—
This HIPAA-compliant retrospective IRB-exempt study involved blinded review of 174 adult (> 18-year-old) patients with BSI admitted between July 1, 2017, and June 16, 2019. This study conforms with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and a complete checklist has been uploaded as Supplemental Digital Content (SDC Table 4). In total, 202 consecutive BSI patients with admission CT were identified during this time period. Patients were included if an admission CT was performed through the abdomen and pelvis in both arterial and portal venous phases before intervention. Patients were excluded if scanning was performed after surgery (n = 23) or after angioembolization (n = 1), or if images from the dome of the diaphragm through the greater trochanters were only included in a single phase (n = 4). Demographic characteristics, admission clinical and laboratory values, and outcomes were collected from the electronic medical record and trauma registry.
Image acquisition.—
Included patients were scanned using one of two trauma bay-adjacent scanners: a 64-section CT unit (Brilliance; Philips Healthcare, Andover, Mass.) and a 128-section dual-source CT unit (SOMATOM Force; Siemens, Erlangen Germany). All images were obtained with 100 mL of 350 mg/mL Iohexol (Omnipaque; GE healthcare; Boston, Mass.) using bolus tracking with region of interest in the descending thoracic aorta and scanner-specific optimization for the arterial phase followed by a ~60-70 second delay for the portal venous phase. Series used for interpretation were obtained at either 120 kVp tube voltage (Philips) or reconstructed from dual energy data as a 120 kVp equivalent blend (Siemens). Both scanners used tube current modulation, with 150-159 reference mAs. Images were archived at 1.5 mm section thickness. Following deidentification, all series were transferred to Aquarius iNtuition (TeraRecon; Durham, NC) for radiologist interpretation and were also saved as Neuroimaging Informatics Technology Initiative (NifTI) files for voxelwise labeling and volumetric analysis using 3D slicer (version 4.10.2, slicer.org).
Image interpretation.—
Following a training session reviewing each scoring system [see SDC tables 1-3], two trauma radiologists with 9 and 4 years of experience independently performed 1994 AAST OIS, 2018 AAST OIS, and binary Thompson scoring for each patient in four sessions spaced two months apart in randomized sequence. After completion of all interpretation sessions, discrepancies were arbitrated by a third radiologist with 13 years of experience.
A single radiologist then performed voxelwise labeling of contained vascular lesions in the arterial phase and labeled active hemorrhage, parenchymal disruption, subcapsular hematoma, and hemoperitoneum in the portal venous phase. Beginning with axial images, manual labeling was performed with an adjustable spherical threshold paint tool at ranges set to ~30-80 HU for parenchymal disruption, subcapsular hematoma, and hemoperitoneum, and ≥ 150 HU for contained vascular lesions and active hemorrhage. Further supervision and editing was performed in sagittal and coronal planes for each patient. A morphological smoothing filter was applied to reduce label artifact and ensure label uniformity despite differences in scanner makes and models. Quantitative values (in mL) were derived for each label class using the Segment Statistics slicer module.
Statistical analysis.—
Descriptive statistics for baseline clinical and demographic characteristics, categorical grading, and volumetric measurements were summarized for the study sample as a whole and across outcomes. The two endpoints assessed were 1) splenectomy versus splenic salvage, reflecting the importance of splenic preservation for adaptive immunity and bacterial clearance, and 2) a composite variable including AE or splenectomy for control of intracavitary hemorrhage. In bivariate analysis, proportions of categorical variables were compared between patients with and without the outcome of interest using Fisher’s exact test or Chi-square test as appropriate. All continuous variables were determined to have non-normal distributions and compared using the Mann-Whitney U test. For quantitative model variable selection, admission vital signs, laboratory values, and volumetric measurements significant in bivariate analysis (p < 0.1) were included in the full model. Models were developed using logistic regression with backward elimination to identify a final reduced model with all predictors that were statistically significant at a significance level of 10%. Predictor variables were analyzed for multicollinearity using the variance inflation factor (VIF). Receiver operating characteristic (ROC) curves were constructed for the grading systems and diagnostic performance for each outcome compared using chi-squared tests with Sidak adjustment for multiple comparisons.
Results
Baseline characteristics.—
Table 1 presents summary statistics for the total cohort and by outcomes. Of the 174 BSI patients, there were 121 males (69%) and 53 females (31%). The most common mechanisms of injury were motor vehicle collisions, motorcycle collisions, and falls. Forty-seven patients underwent splenectomy (27%), and 127 (73%) were managed non-operatively with successful splenic salvage. The EMR was reviewed for median arcuate ligament syndrome (MALS), which can influence the success or failure of angioembolization due to formation of collaterals (22). There were no patients with MALS.
Table 1.
Baseline Characteristics
| Covariate | Total Cohort | Splenectomy | Composite (splenectomy or angioembolization) | ||||
|---|---|---|---|---|---|---|---|
| (n=174) | Yes (n=47) | No (n=127) | p-value | Yes (n=87) | No (n=87) | p-value | |
| Age | 0.181 | 0.791 | |||||
| Years, (mean[SD]) | 43.9 (17.5) | 40.7 (14.8) | 45.1 (18.4) | 43.3 (16.7) | 44.5 (18.5) | ||
| Years, (median[IQR]) | 42.0 (29.6-55.8) | 41.0 (26-50) | 42.0 (30-57.5) | 41.0 (29.5-55) | 42.0 (28.5-57) | ||
| Sex (male) (n[%]) | 121 (70) | 34 (72) | 87 (69) | 0.762 | 67 (77) | 54 (62) | 0.052 |
| Mortality*(n [%]) | 7 (4) | 3 (6.4) | 4 (3) | 0.393 | 4 (4.6) | 3 (3.5) | 1.003 |
| Injury Severity Score | <0.001 1 | <0.001 1 | |||||
| (mean[SD]) | 26.6 (11.7) | 32.7 (9.1) | 24.3 (11.8) | 30.2 (9.7) | 22.9 (12.4) | ||
| (median[IQR]) | 26.0 (17.0-34.0) | 34.0 (26.0-38.0) | 24.0 (17.0-31) | 29.0 (24.5-36.0) | 22.0 (14.0-29.0) | ||
| AIS Score, n (%) | <0.001 3 | <0.001 3 | |||||
| 2 | 62 (36) | 2 (4) | 60 (47) | 6 (7) | 56 (64) | ||
| 3 | 20 (11) | 4 (7) | 16 (13) | 11 (12) | 9 (10) | ||
| 4 | 81 (47) | 32 (68) | 49 (39) | 61 (70) | 20 (23) | ||
| 5 | 11 (6) | 9 (19) | 2 (2) | 9 (10) | 2 (2) | ||
| Mechanism of Injury, n (%) | 0.241 | 0.51 | |||||
| Assault | 6 (3) | 4 (9) | 2 (2) | 4 (5) | 2 (2) | ||
| Fall | 25 (14) | 5 (11) | 20 (16) | 15 (17) | 10 (12) | ||
| MCC | 24 (14) | 6 (13) | 18 (14) | 13 (15) | 11 (13) | ||
| MVC | 118 (68) | 32 (68) | 86 (68) | 55 (63) | 63 (72) | ||
| Admission to CT | 0.01 1 | 0.131 | |||||
| Hours, (mean[SD]) | 0.9 (0.6) | 0.8 (0.9) | 1.0 (0.5) | 0.9 (0.8) | 1.0 (0.5) | ||
| Hours, (median[IQR]) | 0.8 (0.5-1.1) | 0.6 (0.4-1.0) | 0.9 (0.6-1.2) | 0.73 (0.5-1.1) | 0.9 (0.6-1.2) | ||
| CT to intervention | |||||||
| Hours, (mean[SD]) | 12.4 (32.3) | 9.5 (37.2) | n/a | 13.0 (33.3) | n/a | ||
| Hours, (median[IQR]) | 3.1 (1.8-7.9) | 2.2 (1.6-3.4) | n/a | 3.4 (2.0 – 7.9) | n/a | ||
| LOS | <0.001 1 | 0.131 | |||||
| Days, (mean[SD]) | 13.1 (15.0) | 19.7 (20.4) | 10.7 (11.6) | 13.2 (35.5) | 8.6 (18.1) | ||
| Days, (median[IQR]) | 7.2 (4.2-15.7) | 12.6 (6-28.3) | 6.3 (3.9-12.4) | 3.6 (2.1-7.3) | 2.1 (1.6-6.8) | ||
SD - standard deviation, IQR – interquartile range, AIS – abbreviated injury scale
Mortality resulting from causes other than catastrophic closed head injury (head AIS = 6)
Bolded p-values are statistically significant (p<0.05), calculated as follows:
Mann-Whitney U Test
Chi-square test
Fisher’s exact test
Of the 174 total patients, 41 (24%) required emergent splenectomy following admission CT, and 133 patients underwent a trial of non-operative management (NOM). Of these, 80 (60%) were successfully managed with observation alone. Six (5%) patients trialed with NOM who did not undergo AE had delayed splenic rupture and were managed with splenectomy. Forty-seven (35%) NOM patients were treated with AE. Seven (15%) of these underwent splenectomy.
Of those who underwent AE, proximal angioembolization was performed in 44 patients (94%) and combined proximal and distal angioembolization was performed in 3 patients (6%).
For the purpose of analyzing the data using established binary outcomes of either splenectomy alone or the composite of splenectomy and AE (9-11), in total, 47 (27%) patients underwent splenectomy alone and 87 patients (50%) underwent splenectomy, AE, or both (composite outcome), while 87 patients (50%) were successfully managed conservatively.
Quantitative multiparametric model: bivariate analysis.—
Active bleeding, contained vascular lesion, splenic parenchymal disruption, and hemoperitoneum volumes were significantly higher in the splenectomy and composite outcome cohorts (p < 0.001) and were included in quantitative model construction. There was no significant difference in subcapsular hematoma volumes between cohorts for either outcome (p = 0.38-0.70). Clinical covariates included as candidate predictors for the quantitative models (p < 0.10) included lactate, hemoglobin, systolic blood pressure, and heart rate (Table 2).
Table 2.
Clinical and imaging characteristics
| Covariate | Splenectomy | Composite Outcome | ||||
|---|---|---|---|---|---|---|
| Yes (n=47) | No (n=127) | p-value | Yes (n=87) | No (n= 87) | p-value | |
| Vital Signs | ||||||
| Admission HR | 0.79 | 0.65 | ||||
| (mean[SD]) | 98.3 (24.1) | 97.4 (24.7) | 97.2 (24.1) | 98.1 (25.1) | ||
| (median[IQR]) | 99 (80.5-109.5) | 95 (81.5-111.5) | 97.0 (79.5 -108.0) | 96.0 (84.5 -113.0) | ||
| Admission SBP | 0.05 | 0.49 | ||||
| (mean[SD]) | 126.0 (27.9) | 133.0 (32.6) | 130.3 (27.6) | 132.0 (35.1) | ||
| (median[IQR]) | 125 (110.0-144.0) | 132 (119.5-149.5) | 128 (112.0-145.5) | 131.0 (119.5, 147) | ||
| Initial labs | ||||||
| Lactate | 0.03 | 0.59 | ||||
| mmol/L (mean[SD]) | 4.2 (2.4) | 3.4 (2.3) | 3.6 (2.5) | 3.6 (2.3) | ||
| mmol/L (median[IQR]) | 3.8 (2.3-5.4) | 2.8 (2.1-4.3) | 3.0 (2.1 – 4.5) | 3.0 (2.2-4.3) | ||
| Hemoglobin | 0.36 | 0.95 | ||||
| g/dL mean[SD]) | 12.7 (1.8) | 13.0 (2.4) | 12.9 (1.9) | 12.9 (2.6) | ||
| g/dL (median[IQR]) | 12.8 (11.7-13.8) | 13.2 (11.4-14.5) | 13 (11.7-14.3) | 13.0 (11.4-14.4) | ||
| Imaging Finding | ||||||
| Splenic laceration | <0.001 | <0.001 | ||||
| mL mean[SD]) | 31.5 (45.2) | 8.7 (27.7) | 26.2 (45.3) | 3.5 (10.7) | ||
| mL (median[IQR]) | 16.1 (0.9-48.1) | 0.8 (0.2-4.4) | 7.5 (1.0-29.6) | 0.4 (0.1-1.5) | ||
| Subcapsular hematoma | 0.38 | 0.7 | ||||
| mL (mean[SD]) | 1.9 (8.8) | 0.1 (0.4) | 1.1 (6.5) | 0.1 (0.3) | ||
| mL (median[IQR]) | 0 (0.0-0.0) | 0 (0.0-0.0) | 0 (0.0-0.0) | 0 (0.0-0.0) | ||
| Hemoperitoneum | <0.001 | <0.001 | ||||
| mL (mean[SD]) | 502.2 (517.8) | 149.2 (297.9) | 373.1 (477.0) | 116.0 (249.3) | ||
| mL (median[IQR]) | 365.3 (79.1-712.3) | 16.6 (0.6-153.5) | 175.5 (6.2-609.4) | 11.9 (0-85.6) | ||
| PSA | <0.001 | <0.001 | ||||
| mm (mean[SD]) | 0.9 (1.9) | 0.3 (1.2) | 0.9 (2) | 0.0 (0.1) | ||
| mm (median[IQR]) | 0.1 (0-0.5) | 0 (0.0-0.0) | 0 (0.00-0.4) | 0 (0.0-0.0) | ||
| Active hemorrhage* | <0.001 | <0.001 | ||||
| mL (mean[SD]) | 1.4 (3.2) | 0 (0.2) | 0.8 (2.4) | 0.0 (0.0) | ||
| mL (median[IQR]) | 0 (0.0-0.8) | 0 (0.0-0.0) | 0 (0.0-0.2) | 0 (0.0-0.0) | ||
HR - heartrate, SBP –systolic blood pressure, LOS – length of stay, PSA – pseudoaneurysm
Mann-Whitney U test was used to compare continuous data.
Bolded p-values are statistically significant (<0.05)
CI – confidence interval, HR – heartrate, PSA – pseudoaneurysm
Quantitative multiparametric model: adjusted analysis.—
Four patients had missing lactate values and were excluded from model development. In the remaining 170 patients, VIF was < 2 for the two models for splenectomy and hemostatic intervention, indicating lack of multicollinearity. Odds ratios for individual variables and their 95% confidence intervals are shown in table 3. Example labels for CT features of BSI are shown in Figure 1.
Table 3.
Imaging variables included in models for splenectomy and the composite outcome
| Imaging Finding | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Splenectomy | |||
| Active bleed, mL | 6.592 | 1.567 – 27.726 | 0.010 |
| Pseudoaneurysm, mL | 1.262 | 0.973 – 1.636 | 0.079 |
| Lactate, mmol/L | 1.145 | 0.976 - 1.344 | 0.096 |
| Hemoperitoneum, mL | 1.002 | 1.001 - 1.003 | <0.001 |
| Composite Outcome | |||
| Pseudoaneurysm, mL | 290.961 | 7.584 – 11162.3 | 0.002 |
| Splenic laceration, mL | 1.040 | 1.018 – 1.068 | 0.005 |
| Hemoperitoneum, mL | 1.001 | 1.000 – 1.003 | 0.011 |
Bolded p-values are statistically significant at a significance level of 5% (p<0.05) for testing the null hypothesis of odds ratio = 1.
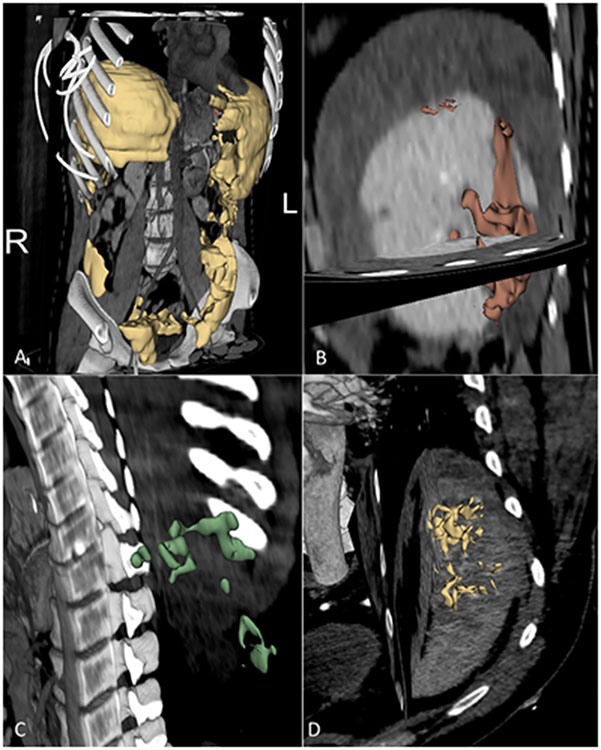
Figure 1.
Voxelwise multiclass labels of CT features associated with splenectomy and angioembolization in two patients with splenic injuries. A-C: 22-year-old male with BSI after motor vehicle collision. A volume rendered PV phase image shows segmented hemoperitoneum (yellow, Part A) throughout the abdomen and pelvis, with a total volume of 2000 mL. Sagittal oblique PV phase images in the same patient show a splenic laceration (red, Part B), measuring 71 mL, and foci of active hemorrhage (green, Part C), with volume totaling 9 mL. D: 55-year-old male status post fall. An oblique maximum intensity projection image from admission contrast enhanced arterial phase CT shows extensive pseudoaneurysms (yellow, Part D), with a total volume of 8 mL.
Significant independent imaging variables for splenectomy after all backward elimination steps were as follows: hemoperitoneum (OR: 1.002, 95% CI: 1.001-1.003, p < 0.001), contained vascular lesion (OR: 1.262, 95% CI: 0.973-1.636, p = 0.079), active bleed (OR: 6.592, 95% CI: 1.567-27.726, p = 0.010) and lactate (OR= 1.15, 95% CI: 0.98-1.64, p = 0.08). where the odds ratios (ORs) represent the change in odds per one-unit increase for a given imaging parameter (e.g., ORs of 1.001, 1.01, 1.1, and 2.0 correspond with an increase in the odds of the outcome by 0.1%, 1%, 10%, and 100% per one-mL increase in a given imaging feature respectively). Lactate was the only independently predictive clinically variable. Significant variables for the composite outcome included: hemoperitoneum (OR: 1.001, 95% CI: 1.000-1.003, p = 0.011), contained vascular lesion (OR: 291.0, 95% CI: 7.584-11162.3, p = 0.002) and splenic laceration (OR: 1.040, 95% CI: 1.018-1.068, p = 0.005).
Diagnostic performance: comparison of splenic injury grading systems with quantitative models.—
The AUCs of the quantitative models were 0.85 for splenectomy alone and 0.82 for the composite outcome of AE or splenectomy (95% CI: 0.78 – 0.91 and 0.76 – 0.89, respectively). This was not significantly different compared to the 1994 or 2018 AAST splenic OIS for splenectomy (1994 AAST- 0.75 , p = 0.08; 2018 AAST – 0.81, p = 0.66) or the composite outcome (1994 AAST- 0.80, p = 0.88; 2018 AAST – 0.88, p = 0.04) (Table 4), but was significantly higher than the AUC of Thompson scoring for both outcomes (0.77- splenectomy, p = 0.02; 0.76- composite, p = 0.04).
Table 4.
Comparison of the quantitative model with ordinal splenic injury scoring systems
| Grading system | Splenectomy | Splenectomy or AE | ||||
|---|---|---|---|---|---|---|
| AUC | SE | p-value | AUC | SE | p-value | |
| Quantitative model | 0.85 | 0.033 | 0.82 | 0.033 | ||
| 1994 AAST | 0.75 | 0.047 | 0.08 | 0.80 | 0.032 | 0.88 |
| 2018 AAST | 0.81 | 0.039 | 0.66 | 0.88 | 0.025 | 0.14 |
| Thompson | 0.77 | 0.035 | 0.02 | 0.76 | 0.032 | 0.04 |
Area under the ROC curve, SE – Standard Error of AUC
Bolded p-values are statistically significant (<0.05) for comparing the AUC of the quantitative model with the AUC of each ordinal splenic injury score system, adjusted for multiple comparisons using the Sidak method
Discussion
In 1989, Moore et al. published the first solid organ injury scales on behalf of the AAST, which were subsequently updated in 1994 (23, 24). Developed as a tool to facilitate clinical research, quality improvement measures, and trauma registry coding, the AAST scale was informally adopted as a point of care decision support tool with grading based on the extent of parenchymal injury and subcapsular hematoma. With the advent of multidetector CT, contained vascular lesions and active bleeding have since been widely recognized as major determinants of operative or endovascular management for hemorrhage control after BSI (10, 11), and are now considered in the 2018 AAST splenic OIS. Boscak (14) and Uyeda (13) previously demonstrated the utility of combining arterial and portal venous phase CT imaging to distinguish between contained vascular lesions and active bleeding after BSI, and this is reflected in the 2018 AAST recommendation for routine dual (arterial and portal venous) phase imaging (7). Neither the 1994 nor the 2018 AAST updates consider hemoperitoneum volumes, which are estimated in the Thompson method (9).
Voxelwise CT volumetry affords granular measurements of the various imaging parameters incorporated as categorical or binary features in the AAST and Thompson systems. In our multivariable analysis, we found that the odds of splenectomy or angioembolization increased significantly with increasing volumes of hemoperitoneum, splenic laceration, and contained vascular injury. Additionally, active bleeding volume was significantly associated with splenectomy.
Multivariable modeling was comparable to the 2018 AAST system (p = 0.66 and 0.14) and improved over the Thompson classifier (p = 0.02 and 0.04) for splenectomy and the composite outcome of splenectomy and AE, with AUCs of 0.85 and 0.82. Quantitative visualization has the benefit of providing transparent, verifiable results, with label masks or contours overlayed on the native CT images and adding to the armamentarium of objective quantitative values that trauma surgeons use for decision-making.
We are not aware of any prior work demonstrating the relationship between volumetric measurements of BSI-related features and actionable outcomes. Initial validation of quantitative methods using manually-derived segmentations is a crucial first step in identifying appropriate clinical targets for resource-intensive automation research (16-18, 25). In the long term, quantitative visualization and modeling can provide a degree of standardization which is currently lacking in clinical practice. For example, a survey of AAST members by Zarzaur et al., found that only 45% of radiologists across trauma centers routinely use the splenic AAST grading scale to describe BSI (26).
Manual measurements are a research tool and not feasible at the point of care, with labeling requiring up to 30 minutes to 1 hour per CT study. However, inference times of previously described proof-of-concept deep learning-based quantitative visualization algorithms for relevant tasks including splenic vascular injury (27), active bleed (25), hematoma (17, 28) and hemoperitoneum (16) segmentation are rapid, requiring 90 seconds or less using graphics processing units (GPUs) that were state-of-the-art at the time of publication and continue to be subject to rapid technological advancement following Moore’s law (29). Once automated, computer-aided BSI decision support could be more than an order of magnitude faster than current turnaround times for trauma CT reports by expert readers, which typically range from 20 to 30 minutes (30), and vary with the number of trauma admissions, reading room distractions, and fatigue-related performance degradation (30-34).
Advancements in quantitative imaging for stroke are instructive in forecasting the future of quantitative imaging in trauma. Automated quantitative visualization software based on principles initially established using manual volumetric measurements (35, 36), is now routinely incorporated into the clinical care of stroke patients (37). Software can automatically and rapidly identify intracranial hemorrhage, calculate the Alberta Stroke Program Early CT Score (ASPECTS) on noncontrast head CTs, calculate the volume of ischemic core and penumbra on CT perfusion, and detect and localize large vessel occlusions on CT angiogram of the head (37, 38). This information can be quickly delivered to a stroke team’s mobile device, allows for rapid clinical decision making and allow for prognostication (37, 39).
Quantitative modeling of splenic injury could similarly aid surgeons by providing automated, precise, and verifiable patient-specific information for rapid decision making and prognostication, with little effort required on the part of the end-user.
Limitations of our single center study include its retrospective design and potential institutional bias. For example, at our institution, splenic embolization is almost exclusively proximal. This varies from center to center and can potentially influence outcome (40, 41). Further multicenter validation is warranted. While manual quantitative measurement of multiple parameters is impractical, automation of these features is an area of active multidisciplinary investigation at our institution (16-18, 27, 28). Additionally, the use of bolus tracking with region of interest placed in the descending thoracic aorta, while routinely employed in polytraumatized patients (42, 43), could potentially lead to missed pseudoaneurysm in high-grade BSIs and misclassification using the AAST or Thompson systems. Protocols incorporating routine angiography for grade III lesions may improve outcomes in part for this reason (41). Since splenic laceration volume was found to be an independent predictor of AE and splenectomy, increased volumes correspond with increased odds of a positive angiogram irrespective of presence or absence of pseudoaneurysm on CT, further emphasizing the potential value of quantitative imaging in better identifying high risk patients.
In conclusion, quantitative volumetric measurements of the CT features of BSI- including hemoperitoneum, splenic laceration, contained vascular injury, and active bleeding- are associated with splenectomy and AE with performance comparable to 2018 AAST grading and significantly improved over the Thompson system. Future automation of these tasks could provide rapid and verifiable objective information for point of care surgical decision-making.
Supplementary Material
Sources of Funding:
NIH K08 EB027141-01A1 (PI: David Dreizin, MD)
Accelerated Translational Incubator Pilot (ATIP) award, University of Maryland (PI: David Dreizin, MD)
Abbreviations:
- SPY
splenectomy
- BSI
Blunt splenic injury
- OPSI
Overwhelming post-splenectomy infection
- OIS
organ injury scale
- AAST
American Association for the Surgery of Trauma
- CECT
Contrast enhanced computed tomography
- NifTI
Neuroimaging Informatics Technology Initiative
- VIF
Variance inflation factor
- ROC
receiver operating characteristic
- OR
odds ratio
- AUC
area under the ROC curve
Contributor Information
David Dreizin, Department of Diagnostic Radiology and Nuclear Medicine, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, 22 S Greene St, Baltimore, MD 21201, 646-825-1229.
Kathryn Champ, University of Maryland School of Medicine, Baltimore, MD.
Matthew Dattwyler, Department of Diagnostic Radiology and Nuclear Medicine, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD.
Uttam Bodanapally, Department of Diagnostic Radiology and Nuclear Medicine, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD.
Elana B. Smith, Department of Diagnostic Radiology and Nuclear Medicine, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD.
Guang Li, Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD.
Rohan Singh, University of Maryland School of Medicine, Baltimore, MD.
Ze Wang, Department of Diagnostic Radiology and Nuclear Medicine, R Adams Cowley Shock Trauma Center, University of Maryland School of Medicine.
Yuanyuan Liang, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD.
References
- 1.Costantini TW, Coimbra R, Holcomb JB, Podbielski JM, Catalano R, Blackburn A, et al. Current management of hemorrhage from severe pelvic fractures: results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717–25. [DOI] [PubMed] [Google Scholar]
- 2.Peitzman AB, Harbrecht BG, Rivera L, Heil B, Workgroup EAftSoTMT. Failure of observation of blunt splenic injury in adults: variability in practice and adverse consequences. J Am Coll Surg. 2005;201(2):179–87. [DOI] [PubMed] [Google Scholar]
- 3.Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4(33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson R, Wall R. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7(12):657–60. [DOI] [PubMed] [Google Scholar]
- 5.Coccolini F, Montori G, Catena F, Kluger Y, Biffl W, Moore EE, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peitzman AB, Heil B, Rivera L, Federle MB, Harbrecht BG, Clancy KD, et al. Blunt splenic injury in adults: multi-institutional study of the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2000;49(2):177–89. [DOI] [PubMed] [Google Scholar]
- 7.Kozar RA, Crandall M, Shanmuganathan K, Zarzaur BL, Coburn M, Cribari C, et al. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg. 2018;85(6):1119–22. [DOI] [PubMed] [Google Scholar]
- 8.Morell-Hofert D, Primavesi F, Fodor M, Gassner E, Kranebitter V, Braunwarth E, et al. Validation of the revised 2018 AAST-OIS classification and the CT severity index for prediction of operative management and survival in patients with blunt spleen and liver injuries. Eur Radiol. 2020;30(12):6570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson BT, Munera F, Cohn SM, MacLean AA, Cameron J, Rivas L, et al. Novel computed tomography scan scoring system predicts the need for intervention after splenic injury. J Trauma Acute Care Surg. 2006;60(5):1083–6. [DOI] [PubMed] [Google Scholar]
- 10.Marmery H, Shanmuganathan K, Alexander MT, Mirvis SE. Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR Am J Roentgenol. 2007;189(6):1421–7. [DOI] [PubMed] [Google Scholar]
- 11.Saksobhavivat N, Shanmuganathan K, Chen HH, DuBose JJ, Richard H, Khan MA, et al. Blunt splenic injury: use of a Multidetector CT–based splenic injury grading system and clinical parameters for triage of patients at admission. Radiology. 2015;274(3):702–11. [DOI] [PubMed] [Google Scholar]
- 12.Federle MP, Courcoulas AP, Powell M, Ferris JV, Peitzman AB. Blunt splenic injury in adults: clinical and CT criteria for management, with emphasis on active extravasation. Radiology. 1998;206(1):137–42. [DOI] [PubMed] [Google Scholar]
- 13.Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology. 2014;270(1):99–106. [DOI] [PubMed] [Google Scholar]
- 14.Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA, Sliker CW, et al. Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology. 2013;268(1):79–88. [DOI] [PubMed] [Google Scholar]
- 15.Federle M, Jeffrey R Jr Hemoperitoneum studied by computed tomography. Radiology. 1983;148(1):187–92. [DOI] [PubMed] [Google Scholar]
- 16.Dreizin D, Zhou Y, Fu S, Wang Y, Li G, Champ K, et al. A Multiscale Deep Learning Method for Quantitative Visualization of Traumatic Hemoperitoneum at CT: Assessment of Feasibility and Comparison with Subjective Categorical Estimation. Radiol Artif Intell. 2020;2(6):e190220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreizin D, Zhou Y, Chen T, Li G, Yuille AL, McLenithan A, et al. Deep learning-based quantitative visualization and measurement of extraperitoneal hematoma volumes in patients with pelvic fractures: potential role in personalized forecasting and decision support. J Trauma Acute Care Surg. 2020;88(3):425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreizin D, Chen T, Liang Y, Zhou Y, Paes F, Wang Y, et al. Added value of deep learning-based liver parenchymal CT volumetry for predicting major arterial injury after blunt hepatic trauma: a decision tree analysis. Abdom Radiol (NY). 2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreizin D, Bodanapally U, Mascarenhas D, O’Toole RV, Tirada N, Issa G, et al. Quantitative MDCT assessment of binder effects after pelvic ring disruptions using segmented pelvic haematoma volumes and multiplanar caliper measurements. Eur Radiol. 2018;28(9):3953–62. [DOI] [PubMed] [Google Scholar]
- 20.Battey TW, Dreizin D, Bodanapally UK, Wnorowski A, Issa G, Iacco A, et al. A comparison of segmented abdominopelvic fluid volumes with conventional CT signs of abdominal compartment syndrome in a trauma population. Abdom Radiol (NY). 2019;44(7):2648–55. [DOI] [PubMed] [Google Scholar]
- 21.Dreizin D, Bodanapally UK, Neerchal N, Tirada N, Patlas M, Herskovits E. Volumetric analysis of pelvic hematomas after blunt trauma using semi-automated seeded region growing segmentation: a method validation study. Abdom Radiol (NY). 2016;41(11):2203–8. [DOI] [PubMed] [Google Scholar]
- 22.Requarth JA, Miller PR. The splenic artery stump pressure is affected by arterial anatomy after proximal embolotherapy in blunt splenic injury. J Trauma Acute Care Surg. 2012;73(5):1221–4. [DOI] [PubMed] [Google Scholar]
- 23.Moore E, Shackford S, Pachter H, McAninch J, Browner B, Champion H, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma. 1989;29(12):1664–6. [PubMed] [Google Scholar]
- 24.Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma Acute Care Surg. 1995;38(3):323–4. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Dreizin D, Li Y, Zhang Z, Wang Y, Yuille A, editors. Multi-scale attentional network for multi-focal segmentation of active bleed after pelvic fractures. International Workshop on Machine Learning in Medical Imaging; 2019: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarzaur BL, Kozar RA, Fabian TC, Coimbra R. A survey of American Association for the Surgery of Trauma member practices in the management of blunt splenic injury. J Trauma Acute Care Surg. 2011;70(5):1026–31. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Dreizin D, Wang Y, Liu F, Shen W, Yuille AL. External Attention Assisted Multi-Phase Splenic Vascular Injury Segmentation with Limited Data. IEEE Trans Med Imaging. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreizin D, Zhou Y, Zhang Y, Tirada N, Yuille AL. Performance of a deep learning algorithm for automated segmentation and quantification of traumatic pelvic hematomas on CT. J Digit Imaging. 2020;33(1):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JY, editor GPU technology trends and future requirements. 2009 IEEE International Electron Devices Meeting (IEDM); 2009 7-9 Dec. 2009. [Google Scholar]
- 30.Banaste N, Caurier B, Bratan F, Bergerot J-F, Thomson V, Millet I. Whole-body CT in patients with multiple traumas: factors leading to missed injury. Radiology. 2018;289(2):374–83. [DOI] [PubMed] [Google Scholar]
- 31.Watchorn J, Miles R, Moore N. The role of CT angiography in military trauma. Clin Radiol. 2013;68(1):39–46. [DOI] [PubMed] [Google Scholar]
- 32.Glover IV M, Almeida RR, Schaefer PW, Lev MH, Mehan WA Jr. Quantifying the impact of noninterpretive tasks on radiology report turn-around times. J Am Coll Radiol. 2017;14(11):1498–503. [DOI] [PubMed] [Google Scholar]
- 33.Hunter TB, Taljanovic MS, Krupinski E, Ovitt T, Stubbs AY. Academic radiologists’ on-call and late-evening duties. J Am Coll Radiol. 2007;4(10):716–9. [DOI] [PubMed] [Google Scholar]
- 34.Hanna TN, Loehfelm T, Khosa F, Rohatgi S, Johnson J-O. Overnight shift work: factors contributing to diagnostic discrepancies. Emerg Radiol. 2016;23(1):41–7. [DOI] [PubMed] [Google Scholar]
- 35.Barber PA, Demchuk AM, Zhang J, Buchan AM, Group AS. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670–4. [DOI] [PubMed] [Google Scholar]
- 36.Lin K, Rapalino O, Lee B, Do KG, Sussmann AR, Law M, et al. Correlation of volumetric mismatch and mismatch of Alberta Stroke Program Early CT Scores on CT perfusion maps. Neuroradiology. 2009;51(1):17–23. [DOI] [PubMed] [Google Scholar]
- 37.Soun JE, Chow DS, Nagamine M, Takhtawala RS, Filippi CG, Yu W, et al. Artificial Intelligence and Acute Stroke Imaging. AJNR Am J Neuroradiol. 2021;42(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray NM, Unberath M, Hager GD, Hui FK. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J Neurointerv Surg. 2020;12(2):156–64. [DOI] [PubMed] [Google Scholar]
- 39.Lee EJ, Kim YH, Kim N, Kang DW. Deep into the Brain: Artificial Intelligence in Stroke Imaging. J Stroke. 2017;19(3):277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Requarth JA. Distal splenic artery hemodynamic changes during transient proximal splenic artery occlusion in blunt splenic injury patients: a mechanism of delayed splenic hemorrhage. J Trauma Acute Care Surg. 2010;69(6):1423–6. [DOI] [PubMed] [Google Scholar]
- 41.Miller PR, Chang MC, Hoth JJ, Mowery NT, Hildreth AN, Martin RS, et al. Prospective trial of angiography and embolization for all grade III to V blunt splenic injuries: nonoperative management success rate is significantly improved. J Am Coll Surg. 2014;218(4):644–8. [DOI] [PubMed] [Google Scholar]
- 42.Dreizin D, Munera F. Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics. 2012;32(3):609–31. [DOI] [PubMed] [Google Scholar]
- 43.Dreizin D, Munera F. Multidetector CT for penetrating torso trauma: state of the art. Radiology. 2015;277(2):338–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.