Abstract
A spiral-shaped bacterium with bipolar, single-sheathed flagella was isolated from the intestines of IL-10 (interleukin-10)-deficient (IL-10−/−) mice with inflammatory bowel disease. The organism was microaerobic, grew at 37 and 42°C, and was oxidase and catalase positive but urease negative. On the basis of 16S rRNA gene sequence analysis and biochemical and phenotypic criteria, the organism is classified as a novel helicobacter. Cesarean section-rederived IL-10−/− mice without helicobacter infection did not have histological evidence of intestinal inflammation. However, helicobacter-free IL-10−/−, SCID/NCr, and A/JNCr mice experimentally inoculated with the novel urease-negative Helicobacter sp. developed variable degrees of inflammation in the lower intestine, and in immunocompetent mice, the experimental infection was accompanied by a corresponding elevated immunoglobulin G antibody response to the novel Helicobacter sp. antigen. These data support other recent studies which demonstrate that multiple Helicobacter spp. in both naturally and experimentally infected mice can induce inflammatory bowel disease. The mouse model of helicobacter-associated intestinal inflammation should prove valuable in understanding how specific microbial antigens influence a complex disease process.
The type species of the genus Helicobacter, H. pylori, is known to cause a persistent inflammatory response in the human stomach and in some cases is directly linked to peptic ulcer disease and the development of gastric cancer (8, 13, 15). In 1994, a novel helicobacter, H. hepaticus, was isolated from the livers of A/JCr mice with a high incidence of chronic hepatitis and hepatocellular carcinoma (5, 27). Coincident with isolation of H. hepaticus from livers of infected mice, the organism was cultured from intestinal crypts of the colon and ceca (5). Shortly thereafter, we isolated H. hepaticus from inflamed lower bowel tissue of some immunodeficient strains of mice (athymic NCr-nu, BALB/c AnNcr-nu, C57BL/6 NCR-nu, and SCID/NCr) with chronic proliferative colitis and proctitis (26). When experimentally inoculated into either germfree outbred mice, defined-flora SCID mice, or specific-pathogen-free (SPF) A/JCr mice with normal microbial flora, H. hepaticus causes variable degrees of persistent inflammation of the colons and ceca of infected mice (2, 7, 28).
Inflammatory bowel disease (IBD) has also been recognized in lines of mice genetically deficient in production of the cytokines (IL-2 [interleukin-2] and IL-10) and those lacking T-cell receptor (TcR) α chain and TcR β chain (10, 14, 17). IBD is hypothesized to result from a combination of genetic and environmental factors. It is not clear whether the aberrant mucosal immune response seen in IBD is the result of a response to the microbial biota of the gastrointestinal tract or if the damage results from an immune response to self antigens (Ags). The theory favoring microbial Ags as inducing this response, in part, was substantiated in IL-10-deficient (IL-10−/−), IL-2−/−, and TcRα−/− mice, whose clinical and histologic presentation of IBD was attenuated when the mice were maintained under SPF conditions (10). In addition, IL-2−/− mice raised under germfree conditions failed to develop IBD (17). Furthermore, IL-10−/− and TcRα−/− helicobacter-free mice experimentally infected with H. hepaticus develop IBD, whereas controls do not (3, 11).
In this report we describe the isolation of a novel urease-negative Helicobacter sp. from IL-10−/− mice with IBD and document the prevention of intestinal inflammation in helicobacter-free IL-10−/− cesarean-derived mice. We also demonstrate the induction of lower bowel inflammation in selected strains of mice experimentally inoculated with this novel Helicobacter sp.
MATERIALS AND METHODS
Animals.
IL-10−/− mice on a C57BL6/129-Ola background were provided by R. Kühn and W. Müller (10). These animals were initially housed in conventional animal facilities. Clinically, the mice had a high incidence of rectal prolapse, and most of them died by 4 months of age.
Bacterial isolation.
Two of the original non-SPF IL-10−/− knockout mice, one with and one without rectal prolapse, were euthanatized with CO2. Cecal contents and feces were collected from each mouse and resuspended in brain heart infusion broth with horse serum and yeast extract; the slurry was passed through a 0.45-μm-pore-size filter with a stacked prefilter. The filtered fecal material was then inoculated onto brucella sheep blood agar with trimethoprim, vancomycin, and polymyxin (Remel Laboratories, Lenexa, Kans.) and incubated at 37 or 42°C under microaerobic conditions for up to 5 days. Bacterial isolates were tested for oxidase, catalase, and urease, and morphology was determined by reaction to Gram’s stain.
Genomic DNA extraction for 16S rRNA gene sequencing.
Bacteria isolated from the feces of two mice were cultured on blood agar plates, and the cells were harvested and washed twice with 1 ml of double-distilled H2O. The pellets were suspended in STET buffer (8% sucrose, 50 mM EDTA, 0.1% Triton X-100, 50 mM Tris-HCl [pH 8.0]), and lysozyme (hen egg white; Boehringer Mannheim Biochemicals, Indianapolis, Ind.) was added to a final concentration of 3 mg/ml. The suspension was incubated for 12 min at 37°C and then lysed with 1% sodium dodecyl sulfate. RNase A (bovine pancreas; Boehringer Mannheim) was added to a final concentration of 0.05 mg/ml, and the solution was incubated for 1 h at 37°C. Then 0.1 volume of a 5% cetyltrimethylammonium bromide–0.5 M NaCl solution (Sigma Chemical Co., St. Louis, Mo.) was added, and the solution was gently mixed and incubated at 65°C for 10 min. DNA was extracted with an equal volume of phenol-chloroform (1:1, vol/vol), precipitated overnight in 0.3 M sodium acetate with 2 volumes of absolute ethanol at −20°C, and pelleted by centrifugation at 13,000 × g for 1 h at 4°C. The ethanol was decanted, and the pellet was air dried and suspended in sterile distilled water.
16S rRNA gene sequencing.
Sequences of the 16S rRNA genes of two bacterial isolates (MIT 97-6810 and MIT 97-6811) were determined. For amplification of 16S rRNA cistrons, 16S rRNA gene sequencing, and 16S rRNA data analysis, we used the methods described by Fox et al. (6). Briefly, primers C70 and B37 (6) were used to amplify the 16S rRNA genes. The amplicons were purified and directly sequenced by using a TAQuence cycle sequencing kit (U.S. Biochemical, Cleveland, Ohio). The 16S rRNA gene sequences were entered into a program for analysis of 16S rRNA data in Microsoft Quickbasic for use with PC-compatible computers and were aligned as previously described (16). The database used contains approximately 100 Helicobacter, Wolinella, Arcobacter, and Campylobacter sequences and more than 900 sequences for other bacteria. Similarity matrices were constructed from the aligned sequences by using only those base positions for which 90% of the strains had data and were corrected for multiple base changes by the method of Jukes and Cantor (9). Phylogenetic trees were constructed by the neighbor-joining method (18).
Electron microscopy.
The novel Helicobacter sp. was examined by electron microscopy. Cells grown on blood agar plates were centrifuged and suspended in 10 mM Tris-HCl buffer (pH 7.4) at a concentration of about 108 cells per ml. Samples were negatively stained with 1% (wt/vol) phosphotungstic acid (pH 6.5) for 20 to 30 s. Specimens were examined with a JEOL model JEM-1200EX transmission electron microscope operating at 100 kV.
Experimental infection with the novel Helicobacter sp.
The non-SPF IL-10−/− mice were rederived by cesarean section at Taconic Farms (Germantown, N.Y.). Following rederivation, the mice were backcrossed 7 to 10 generations onto a C57BL/10 SgSnAi background. The SPF status of these mice is defined by failure to measure detectable immunoglobulin G (IgG) antibodies to the following murine viruses: mouse hepatitis virus, EDIM virus, MVM, MPV, Sendai virus, PVM, REO-3 GD-VII, lymphocytic choriomeningitis virus, K virus, mouse adenovirus, ectromelia virus, polyomavirus MCMV, and thymus virus. The animals were negative for ecto- and endoparasites. Fecal cultures were negative for Salmonella sp., Citrobacter rodentium, and Klebsiella sp. Fecal PCR was negative for H. hepaticus and H. bilis. Offspring produced were shipped to and maintained in sterile microisolater cages with autoclaved bedding, food, and water in an SPF-maintained Association for Assessment and Accreditation of Laboratory Animal Care-approved facility at National Institute of Allergy and Infectious Diseases, (NIAID), National Institutes of Health. These mice did not have rectal prolapse, nor did they die by 4 months of age. All mice were maintained in accordance to the Guide for the Care and Use of Laboratory Animals standards, and all protocols were approved by an NIAID Animal Care and Use Committee.
Experiment 1.
Twelve 2-month-old male SPF, helicobacter-free IL-10−/− mice were infected with the urease-negative Helicobacter sp. Each mouse was inoculated with ∼106 bacteria as measured by spectrophotometry (7). The bacteria were suspended in 0.2 ml of phosphate-buffered saline (PBS) by intraperitoneal (i.p.) injection (six mice) and oral gavage (six mice). These mice were euthanized 4 to 8 weeks later (Table 1).
TABLE 1.
Incidence of cecal and colon lesions in mice infected with urease-negative Helicobacter sp.a
| Strain | Route of injection | Time (mo) p.i. | No. with intestinal lesion/no. sacrificed |
|---|---|---|---|
| IL 10−/− | i.p. | 1 | 3/3 |
| 2 | 5/5 | ||
| 4 | 7/7 | ||
| Intragastric | 1 | 3/3 | |
| 2 | 1/1 | ||
| A/JNCr | i.p. | 1 | 0/2 |
| 6 | 2/3b | ||
| SCID | i.p. | 1 | 2/2 |
| 6 | 2/2 |
Any mice not noted but injected either died or were used for culture only. None of the control mice had cecal or colon lesions.
Minimal inflammation.
Experiment 2.
Six male 2-month-old A/JNCr, 6 SCID/NCr, and 11 IL-10−/− helicobacter-free mice were inoculated i.p. with ∼106 bacteria in 0.2 ml of PBS. Three to four mice per group (Table 1) were euthanized 1 to 6 months postinfection (p.i.). Three IL-10−/−, three IL-10+/+, three SCID/NCr, and four A/JCr 6- to 7-month-old control male mice were euthanized 4 months after the experiment commenced.
In each experiment, before infection and at 4-week intervals thereafter, feces of experimentally infected as well as control mice were cultured for Helicobacter sp. At necropsy, the cecal content of each mouse was also cultured for Helicobacter sp.
Specific identification of Helicobacter sp. by PCR.
To specifically detect the novel urease-negative Helicobacter sp., two PCR primers, JGF-F1 (forward; 5′GAAACTATCACTCTAGAGTATG-3′, corresponding nucleotides 639 to 660) and JGF-R1 (reverse; 5′TGCTCCTCATTGTATGCC-3′, complementary to nucleotides 1243 to 1260), were generated from the 16S rRNA genes of the two isolates (MIT 97-6810 and MIT 97-6811), targeting a ∼0.6-kb DNA fragment. Primers C97 and C98, designed as universal primers for amplifying a ∼0.4-kb PCR product from 16S rRNA genes of all known Helicobacter sp., were previously described (20). Chromosomal DNA from positive control isolates of the novel Helicobacter sp. (MIT 97-6810 an MIT 97-6811) and six additional isolates of the novel Helicobacter sp. isolated from the ceca of experimentally infected mice 4 (98-6781 and 98-6782) and 18 (98-784, 98-6785, 98-7686, and 98-6787) weeks p.i. were obtained, along with the chromosomal DNA of H. rodentium 95-1707. Samples were prepared by using a High Pure PCR template preparation kit (Boehringer Mannheim) according to the supplier’s instructions. A 50-μl reaction mixture contained 50 ng of template DNA, 200 μg of bovine serum albumin per ml, 5 pmol of each primer, 1× commercial PCR buffer, and 2.5 U of Taq DNA polymerase (Boehringer Mannheim). The PCR was conducted with a thermocycling program of 40 cycles as follows: denaturing at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 1 min.
Preparation of U− HelAg.
Soluble urease-negative Helicobacter Ag (U− HelAg) was prepared from cultures of the novel urease-negative Helicobacter sp. as previously described (11). Briefly, the organisms were harvested and washed extensively in PBS, and the resulting suspension was sonicated at 4°C to lyse the bacteria. Cell debris was removed by centrifugation at 8,000 × g (Sorvall RC2-B, SS-34 rotor) for 30 min at 4°C. The supernatant was sterile filtered (0.22-μm-pore-size filter), protein content was determined by the Bradford method (Pierce, Rockford, Ill.), and the Ag was stored at −40°C until use.
Ab measurements.
Antibodies (Abs) reactive to the novel Helicobacter sp. Ag were measured by ELISA as described previously (11). Briefly, 96-well Immunolon 2 plates (Dynex Technologies Inc., Chantilly, Va.) were coated with U− HelAg (10 μg/ml; 50 μl/well) in 0.05 M sodium carbonate buffer (pH 9.6) at 4°C overnight. After a 1-h blocking step with 5% milk at 37°C, sera were added to the wells at different dilutions and the plates were incubated at 4°C overnight. Thereafter, peroxidase-conjugated rabbit Ab specific for mouse IgG (Zymed Laboratories, Inc., San Francisco, Calif.) was added for 3 h at 37°C. Color reactions were developed by addition of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Kirkegaard & Perry, Inc., Gaithersburg, Md.), and optical density was measured at 405 nm in an ELISA reader (Molecular Devices, Menlo Park, Calif.).
Necropsy and histopathology.
All mice were euthanized with CO2 and examined for gross tissue changes. Samples of gastrointestinal tissue were fixed in 10% neutral buffered formalin, processed by standard methods, and embedded in paraffin. Sections of 5 μm were stained with hematoxylin and eosin (H&E) or Steiner silver stain. These sections were examined by light microscopy for evidence of lesions and for the presence of a bacterium with a morphology consistent with members of the genus Helicobacter.
Nucleotide sequence accession number.
The 16S rRNA sequence for strain MIT 97-6810 has been deposited in GenBank as accession no. AF127912.
RESULTS
Characterization of the novel Helicobacter species. (i) Bacterial isolation.
Urease-negative, catalase- and oxidase-positive, gram-negative bacteria grown at 37°C under microaerobic conditions were isolated from the caca of the two IL-10−/− mice sampled from the colony with endemic IBD.
(ii) 16S rRNA analysis.
The 16S rRNA sequences determined for both urease-negative Helicobacter sp. mouse isolates were entered into our database, aligned, and compared with the over 100 Helicobacter sequences in the database to determine similarity. The sequences of the two urease-negative Helicobacter sp. strains (MIT 97-6810 and MIT 97-6811) were identical to one another and most closely related (97.5% similar) to those of H. muridarum and H. hepaticus (Fig. 1). The 2.5% difference from other described species indicates that they represent a novel species. Both Helicobacter sp. strains contain a 166-base transcribed intervening sequence in place of the 7-base stem-loop that is normally centered at position 210 (Escherichia coli numbering).
FIG. 1.
Phylogenetic tree constructed on the basis of 16S rRNA sequence similarity values, using the neighbor-joining method. Scale bar = 5% difference in nucleotide sequences as determined by measuring the lengths of the horizontal lines connecting two species.
(iii) Ultrastructure.
The novel Helicobacter sp. was motile and curved to spiral, and it measured 0.3 by 2 to 5 μm (Fig. 2). The bacterium possessed single bipolar sheathed flagella but did not have periplasmic fibers.
FIG. 2.
Phosphotungstic acid negative stain of urease-negative Helicobacter sp. depicting helical bacteria with bipolar sheathed flagellae. Magnification, ×13,650.
Experimental infections. (i) Identification of Helicobacter sp. by PCR.
A urease-negative, oxidase-positive, catalase-positive, gram-negative spiral-shaped organism was consistently isolated from feces and ceca of experimentally infected mice during the prescribed time points of the experiment and at necropsy. Helicobacter sp. was not isolated from control mice at any time point during the experiment.
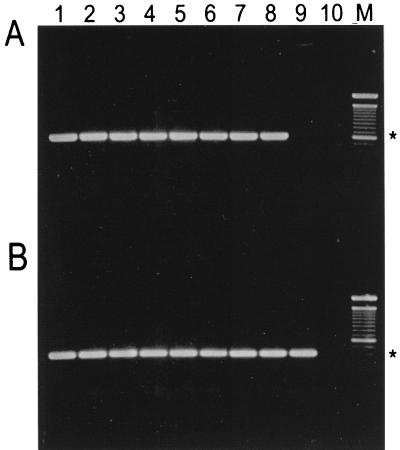
PCR products from the DNA templates were separated on a 1.5% agarose gel (Fig. 3). Primers JGF-F1 and JGF-R1 amplified a 0.6-kb PCR product from all isolates of the novel urease-negative Helicobacter sp. (Fig. 3A, lanes 1 to 8), whereas a similar product was not amplified from H. rodentium 95-1707 (Fig. 3B, lane 9). However, with the universal Helicobacter sp. primers, a 0.4-kb PCR product was amplified from both the newly isolated Helicobacter sp. and H. rodentium (Fig. 3B, lanes 1 to 9).
FIG. 3.
Agarose gel electrophoresis of DNA amplified by PCR with Helicobacter species-specific primers JGF-F1 and JGF-R1 (A) or Helicobacter genus-specific primers C97 and C98 (B). Lanes 1 to 8, novel urease-negative Helicobacter sp. isolates MIT 97-6810 and MIT 97-6811 and isolates 98-6781, 98-6782, 98-784, 98-9785, 98-7686, and 98-6787, respectively; lane 9, H. rodentium 95-1707; lane 10, control with no DNA template; M, 100-bp DNA ladder (GibcoLife Technologies, Gaithersburg, Md.). The 0.6-kb (A) and 0.4-kb (B) positions are indicated by asterisks.
(ii) ELISA.
To analyze humoral immune responses to the urease-negative Helicobacter sp., we bled A/JNCr, SCID/NCr, and IL-10−/− mice 4.5 to 5.5 months after inoculation with the urease-negative Helicobacter sp. and analyzed sera for total anti-U-HelAg IgG by ELISA. A/JNCr and IL-10−/− mice showed high titers of total anti-U−HelAg IgG, whereas, as expected, no helicobacter-reactive Ab was detected in sera from infected SCID mice (Fig. 4). Uninfected IL-10−/− mice from a separate experiment also showed no reactivity to the U− HelAg (data not shown).
FIG. 4.
U− HelAg-specific Ab in sera of urease-negative Helicobacter-infected mice. Sera from 5.5-month-infected A/J (●) and SCID (▴) mice (A) and from 4.5-month-infected IL-10−/− (■) mice (B) were analyzed for levels of total IgG reactive to U− HelAg by ELISA as described in Materials and Methods. Symbols represent means ± standard deviations of duplicate ELISA values from pools of three infected A/J, two infected SCID, and seven infected IL-10−/− mice, respectively. Uninfected IL-10−/− mice showed no reactivity to the U− HelAg (data from a separate experiment, not shown). OD, optical density.
(iii) Histopathology.
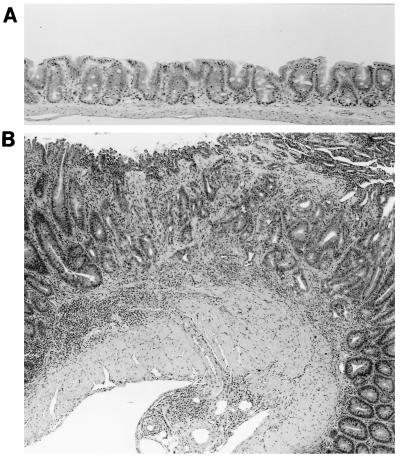
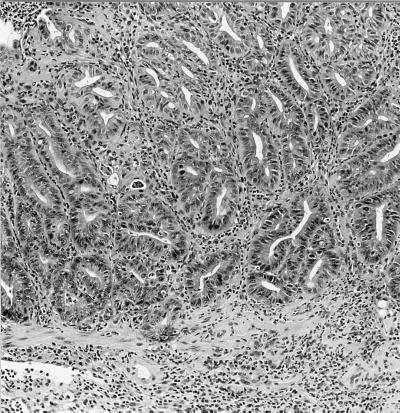
None of the experimentally infected mice developed diarrhea or rectal prolapse. Although not as severe as the intestinal lesions noted in the IL-10−/−, mice naturally infected with the urease-negative Helicobacter sp. (Fig. 5), moderate to severe large bowel lesions were noted in all IL-10−/− mice inoculated with the novel helicobacter. Route of infection did not influence lesion severity. Uninfected controls had no intestinal lesions (Fig. 6A), whereas each infected mouse had moderate to marked typhlitis, mild colitis, and moderate proctitis. Some mice, examined 6 months p.i., had focal areas of atypical hyperplasia in the cecum (Fig. 6B) and rectum (Fig. 7). Livers of the IL-10−/− infected with the novel helicobacter had few to many foci of granulomatous inflammation and mild cholangitis. With Steiner stain, many helical organisms were seen within crypts of the large bowel, especially the cecum. Organisms, however, were not observed in the livers of infected mice.
FIG. 5.
Diffuse and focal epithelial hyperplasia with marked inflammation in the cecum of a IL-10−/− mouse naturally infected with the urease-negative Helicobacter sp. H&E; magnification, ×30.
FIG. 6.
(A) Normal cecum of a 4-month-old uninfected control IL-10−/− mouse. H&E stain × 75. (B) Focal atypical hyperplasia and diffuse hyperplasia with marked chronic inflammation in cecum of an IL-10−/− mouse, 6 months after infection with the novel urease-negative Helicobacter sp. H&E; magnification, ×30.
FIG. 7.
Focal atypical hyperplasia and chronic inflammation in rectum of an IL-10−/− mouse, 6 months after injection with the novel urease-negative Helicobacter sp. H&E; magnification, ×75.
At 4 weeks p.i., mild to moderate inflammatory and hyperplastic lesions were found in the ceca but not colons of the SCID/NCr mice. Lesions at 6 months p.i. were comparable to those noted at 4 weeks p.i. The liver was normal except for a mild cholangitis. In A/JNCr mice at 4 weeks p.i., no large bowel lesions were found. One mouse had a few liver granulomas. Minimal to mild typhlitis and scattered liver granulomas and foci of cholangitis were noted in the A/JNCr mice examined at 6 months p.i.
DISCUSSION
Although IBD has been characterized in IL-10−/− mice, previous reports had implicated normal enteric bacteria as responsible for eliciting the proinflammatory response (10). In this study we isolated and characterized a novel urease-negative helicobacter in IL-10−/− mice with IBD. The development of experimentally induced IBD in several different strains of helicobacter-free mice, including IL-10−/− mice injected with the urease-negative Helicobacter sp., adds further support to the view that Helicobacter spp. can induce intestinal inflammation in murine hosts. Also, the presence of humoral IgG antibody response to the urease-negative Helicobacter sp. may indicate that the organism was responsible for the intestinal inflammation noted in these mice; however, the serological data are only inferential, not definitive.
Several other urease-negative Helicobacter spp., including H. pullorum, H. canis, H. fennelliae, and H. cinaedi, have been isolated from the feces of diarrheic humans and animals (23–25). H. rodentium, the only murine urease-negative helicobacter formally named, has been recovered from normal feces collected from a variety of mouse strains (20) as well as from diarrheic SCID mice coinfected with H. bilis (22). The novel urease-negative helicobacter in this report was identified by 16S rRNA analysis but also can be distinguished morphologically from H. rodentium because it has sheathed flagella whereas H. rodentium does not (20). Importantly for diagnostic purposes, species-specific PCR primers can differentiate between these two rodent urease-negative helicobacters. By Southern blot hybridization, we confirmed that whole genomic DNA from the novel urease-negative Helicobacter sp. and H. rodentium digested with HindIII do not hybridize with urease PCR gene products from H. pylori or H. hepaticus (data not shown).
Lesions consistent with IBD are increasingly recognized in mice which are genetically and/or immunologically compromised. In the initial reports of IBD in mice, the animals were reported to be free of known murine pathogens (10, 17). However, as the intestinal inflammation generally was less severe under SPF conditions and absent in the germfree state, intestinal bacteria appeared to be involved in the pathogenesis of the disease. For example, when IL-2−/− mice were rederived by hysterectomy and maintained under SPF or germfree conditions, the mice exhibited no clinical signs of disease (17). The SPF mice did have mild histopathological lesions in the large intestine, but lesions were completely absent from the colonic and cecal tissue of germfree mice up to 20 weeks of age. Similar findings were recorded for IL-10−/− mice in which the IBD was less severe and delayed in onset when mice were housed under SPF conditions (10). At the time of these studies, H. hepaticus and other pathogenic helicobacters had not been identified in murine hosts. Since the description of H. hepaticus in 1994, it has been established that H. hepaticus colonizes a large number of mice, from commercial as well as academic sources (19). Indeed H. hepaticus has been isolated from multiple genetically altered mice with IBD (4). Experimental evidence confirming the relevance of H. hepaticus in the induction of IBD was described in a study where H. hepaticus inoculated into defined-flora SCID mice reconstituted with CD45RB+ T cells resulted in more severe IBD than in mice receiving T cells alone (2). This finding supported an earlier study of outbred germfree mice in which a segmental enterocolitis was noted in mice monoinfected with H. hepaticus (7). In a subsequent experiment, H. bilis inoculated into defined-flora SCID mice without reconstitution of CD45RB+ T cells also produced severe colitis and typhlitis (21). A more recent study indicates that helicobacter-free IL-10−/− mice inoculated either i.p. or orally with H. hepaticus develop severe IBD (11). In the A/JCr and SCID mice, lesions in the lower intestine produced experimentally by the urease-negative Helicobacter sp. were similar to those associated with H. hepaticus in naturally and experimentally infected A/JCr and SCID mice (7, 12, 26, 28). The milder intestinal lesions in the A/JCr mice may reflect the length of infection with the novel helicobacter. In A/JCr mice infected with H. hepaticus, the intestinal disease is much more severe 12 months after infection with H. hepaticus than it is in mice infected for shorter time periods (28). H. hepaticus induced a strong proinflammatory Th1 cytokine response and elevated gamma interferon in both IL-10−/− and A/JCr mice (11, 28). Interestingly, the novel Helicobacter sp., which unlike H. hepaticus and H. bilis lacks urease activity, elicits a similar Th1 cytokine response in experimentally infected IL-10−/− mice (10a).
Lesions developed in the large intestines of the IL-10−/− mice experimentally infected with the novel urease-negative Helicobacter sp., but they were not as severe as those in the IL-10−/− mice naturally infected with the novel urease-negative Helicobacter sp. Explanations include several possibilities; for example, the normal microbiota of the naturally infected IL-10−/− mice differed from that of the experimentally challenged mice; alternatively, the IL-10−/− mice may have been infected with additional Helicobacter sp. not recovered during the initial attempts to culture Helicobacter sp. It is also conceivable that in vitro passage of the novel helicobacter attenuated its virulence. Another likely possibility is that the IL-10−/− mice experimentally infected with the urease-negative organism were on a different genetic background than the IL-10−/− mice naturally infected with the organism. For example, Berg et al. (1) showed that inheritable factors strongly influenced the expression of IBD in IL-10−/− mice on different genetic backgrounds. In 3-month-old mutants, intestinal lesions were most severe in the 129 and BALB/c/SvEv background, intermediate severity was noted in the IL-10−/− 129 × C57BL/6J mice, and the least severe lesions were recorded for IL-10−/− C57BL/6J mice (1). Experimental infections with the urease-negative Helicobacter sp. (as well as H. hepaticus and other urease-positive murine helicobacters) in helicobacter-free mice on similar genetic backgrounds are needed to explore how host genotype influences the expression of IBD in the newly described mouse model of helicobacter-associated intestinal disease.
ACKNOWLEDGMENTS
We are grateful for the assistance of Alan Sher, Immunobiology Section, Laboratory of Parasitic Diseases, NIAID, National Institutes of Health, as well as Miriam Anver, Rhonda Anderson, and Dee Green, NCI-FCRDC.
This work was supported in part by NIH grants R01CA67529, PO1CA26731, RR010146, and R01DK52413, all to J.G.F., and NIH contract N01-CO-5000 (P.L.G. and J.M.W.).
REFERENCES
- 1.Berg O J, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L. Enterocolitis and colon cancer in interleukin 10 deficient mice are associated with aberrant cytokine production and CD4 + Th1-like response. J Clin Investig. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill R J, Foltz C J, Fox J G, Dangler C A, Powrie F, Schauer D B. Inflammatory bowel disease: an immune-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin E Y, Jha J, Dangler C A, Schauer D B. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Infectious colitis in T cell receptor knockout mice, abstr. B436. [Google Scholar]
- 4.Foltz C J, Fox J G, Cahill R J, Murphy R C, Yan L, Shames B, Schauer D B. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter. 1998;3:69–78. doi: 10.1046/j.1523-5378.1998.08006.x. [DOI] [PubMed] [Google Scholar]
- 5.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox J G, Yan L, Dewhirst F E, Paster B J, Shames B, Murphy J C, Hayward A, Belcher J C, Mendes E N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mouse strains. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox J G, Yan L, Shames B, Campbell J, Murphy J C, Li X. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun. 1996;64:3673–3681. doi: 10.1128/iai.64.9.3673-3681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham D Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989;96:615–623. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- 9.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 10.Kühn R, Lohler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 10a.Kullberg, M. Unpublished observations.
- 11.Kullberg M C, Ward J M, Gorelick P L, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Fox J G, Whary M, Yan L, Shames B, Zhao Z. Scid/NCr mice naturally infected with Helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect Immun. 1998;66:5477–5484. doi: 10.1128/iai.66.11.5477-5484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall B J. Treatment strategies for Helicobacter pylori infection. Gastroenterol Clin North Am. 1993;22:183–198. [PubMed] [Google Scholar]
- 14.Mombaerts P, Mizoguchi E, Grusby M J, Glimcher L H, Bhan A K, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet J. Bacterial infection as a cause of cancer. Environ Health Perspect. 1995;103:263–268. doi: 10.1289/ehp.95103s8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paster B J, Dewhirst F E. Phylogeny of Campylobacter, wolinellas, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int J Syst Bacteriol. 1988;38:56–62. [Google Scholar]
- 17.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 18.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 19.Shames B, Fox J G, Dewhirst F E, Yan L, Shen Z, Taylor N S. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Z, Fox J G, Dewhirst F E, Paster B J, Foltz C J, Yan L, Shames B, Perry L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol. 1997;47:627–634. doi: 10.1099/00207713-47-3-627. [DOI] [PubMed] [Google Scholar]
- 21.Shomer N H, Dangler C A, Fox J G. Helicobacter bilis-induced inflammatory bowel disease in defined flora scid mice. Infect Immun. 1997;65:4858–4864. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shomer N H, Dangler C A, Marini R, Fox J G. Helicobacter bilis/Helicobacter rodentium co-infection associated with diarrhea in a colony of scid mice. Lab Anim Sci. 1998;48:455–459. [PubMed] [Google Scholar]
- 23.Stanley J, Linton D, Burens A P, Dewhirst F E, On S L, Porter A, Owen R J, Costas M. Helicobacter pullorum sp. nov.—genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology. 1994;140:3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 24.Stanley J, Linton D, Burens A P, Dewhirst F E, Owen R J. Helicobacter canis sp. nov., a new species from dogs: an integrated study of phenotype and genotype. J Gen Microbiol. 1993;139:2495–2504. doi: 10.1099/00221287-139-10-2495. [DOI] [PubMed] [Google Scholar]
- 25.Totten P A, Fennel C L, Tenover F C. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): two new Campylobacter species associated with enteric disease in homosexual men. J Infect Dis. 1985;151:131–139. doi: 10.1093/infdis/151.1.131. [DOI] [PubMed] [Google Scholar]
- 26.Ward J M, Anver M R, Haines D C, Melhorn J M, Gorelick P, Yan L, Fox J G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 27.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, Tully J G, Russell R J, Benveniste R E, Paster B J, Dewhirst F E, Donovan J C, Anderson L M, Rice J M. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 28.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]