Abstract
Background
Mycoplasma pneumoniae (M. Pneumoniae) is a common pathogen of respiratory tract infections, but there is still a lack of detailed investigation on the large sample of M. Pneumoniae infection in the all age population. And patients with severe M. Pneumoniae pneumonia (SMPP) still have a certain risk of death. How to identify the clinical characteristics and population of patients with SMPP as soon as possible is still an urgent problem in clinical practice.
Methods
Demographic characteristics, patient clinical information, and laboratory data of 81,131 patients with respiratory tract infections (RTIs) in the Affiliated Suzhou Hospital of Nanjing Medical University from 2014 to 2020 were retrospectively collected from all patient records. The serum particle agglutination (PA) test was used to determine M. Pneumoniae infection by detecting specific antibodies. The white blood cell count, the proportion of neutrophils and lymphocytes, C-reactive protein (CRP) and lactate dehydrogenase (LDH) levels between children and adults with SMPP were compared by Student’s t-test; other clinical features were analyzed by χ2 test or Fisher’s exact test.
Results
A total of 81,131 patients with RTIs were included, and 21,582 (26.60%) M. Pneumoniae immunoglobulin M (IgM)-positive patients were detected. From 2014 to 2020, the annual proportions of M. Pneumoniae RTIs were 23.60%, 28.18%, 38.08%, 27.05%, 23.44%, 25.26%, and 18.33%, respectively. In terms of seasonal distribution, April–June and September–November were the peak seasons of M. Pneumoniae infection each year. Children and women have a high proportion of M. Pneumoniae infection. The peak age of M. Pneumoniae infection was between 4 and 14 years old. There were 301 cases of SMPP, including 281 children and 20 adults (8 cases of pregnant women). Children and pregnant women accounted for a high proportion of SMPP. Children with SMPP had more extrapulmonary symptoms, multilobar infiltrates, and increased CRP and LDH levels compared with adults.
Conclusions
M. Pneumoniae infection has seasonal, sex, and age distribution trends. Children and pregnant women accounted for a high proportion of SMPP. Extrapulmonary symptoms, multilobar infiltrates, and increased CRP and LDH levels may be helpful to identify SMPP in children than in adults.
Keywords: Mycoplasma pneumoniae (M. Pneumoniae), respiratory tract infection (RTI), children, pregnant women, severe Mycoplasma pneumoniae pneumonia (SMPP)
Introduction
Mycoplasma pneumoniae (M. Pneumoniae) is a common pathogen of respiratory tract infections (RTIs) worldwide. It is usually sporadic, with regional epidemics every 3 to 5 years, and each epidemic lasts for 1 to 2 years (1,2). Patients may present with a mild self-limiting clinical presentation or convert to radiologically confirmed pneumonia requiring hospitalization. M. Pneumoniae pneumonia accounts for approximately 10–30% of cases of community-acquired pneumonia (3-5). In severe cases, it can cause multiple organ damage (6,7). It is of great significance to monitor the epidemic trend of M. Pneumoniae infection and analyze the epidemic characteristics. Up to now, domestic studies on epidemiological characteristics have mainly focused on children (8-10).
However, little is known about the epidemiological data of M. Pneumoniae infection among people of all ages in China, especially in some special populations, such as pregnant women. Therefore, in order to better understand the epidemiological characteristics of M. Pneumoniae infection and clinical features of SMPP in Suzhou, we collected the total data of outpatients and inpatients infected with M. Pneumoniae in the recent 7 years of all ages in our hospital, and analyzed the distribution of factors such as gender, age, season, as well as the clinical manifestations of SMPP, in order to facilitate the treatment of M. Pneumoniae infection in clinical practice and identify the population with severe M. Pneumoniae infection. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4304/rc).
Methods
Study subjects
From January 2014 to December 2020, a total of 81,131 outpatients and inpatients with RTIs were admitted to Affiliated Suzhou Hospital of Nanjing Medical University, including 40,683 males and 40,448 females (55,658 children and 25,473 adults) aged 0–94 years old (median age: 12 years old). Demographic characteristics, patient clinical information, and laboratory data were collected retrospectively from all patient records. M. Pneumoniae RTIs included upper RTIs, bronchitis, bronchopneumonia, and pneumonia. This study protocol was approved by the Medical Research Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (No. KL901299) and strictly followed the Declaration of Helsinki (as revised in 2013). The requirement for informed consent was waived by the committee as our study was an anonymous retrospective review of patient records.
Detection of M. Pneumoniae and protocols
As M. Pneumoniae is a common pathogen of respiratory infection, we retrospectively analyzed the situation of M. Pneumoniae infection in pediatric patients, respiratory outpatients, and inpatients with RTIs in our hospital. For inpatients, all patients suspected of RTIs were routinely tested for M. Pneumoniae-specific immunoglobulin M (IgM). For outpatients, those with dry cough (with or without fever, peripheral white blood cell count <1,000/µL) for more than 1 week (or family members with cough) were also routinely tested for M. Pneumoniae-specific IgM. M. Pneumoniae infection was confirmed by the detection of M. Pneumoniae-specific IgM, which was defined as a single serum M. Pneumoniae-specific IgM titer greater than 1:160, or a second seroconversion (a 4-fold increase in antibody titer) (11-13).
Diagnosis of RTIs
RTIs including upper tract infection, bronchitis, bronchopneumonia, and pneumonia were diagnosed based on clinical symptoms (fever, sore throat, cough, sputum, and wheezing, chest tightness) and imaging infiltrates. Severe M. Pneumoniae pneumonia (SMPP) was defined as pneumonia with one of the following criteria for severe pneumonia in children and adults with community-acquired pneumonia (14,15): persistent fever >38.5 ℃ for more than 10 days, radiological deterioration or consolidation present in >2/3 of the lung lobes, intra- and extrapulmonary complications, pulse oxygen less than 92%, and shock.
Statistical analysis
Data were expressed in terms of the mean, count, and percentage. Data analysis was performed using the SPSS 21.0 statistical software package (IBM Corp., Armonk, NY, USA). Continuous data were expressed as mean ± standard deviation. Continuous variables were analyzed by Student’s t-test, and categorical variables were analyzed by the χ2 test or Fisher’s exact test. P value <0.05 was considered significant.
Results
M. Pneumoniae epidemic characteristics from 2014–2020
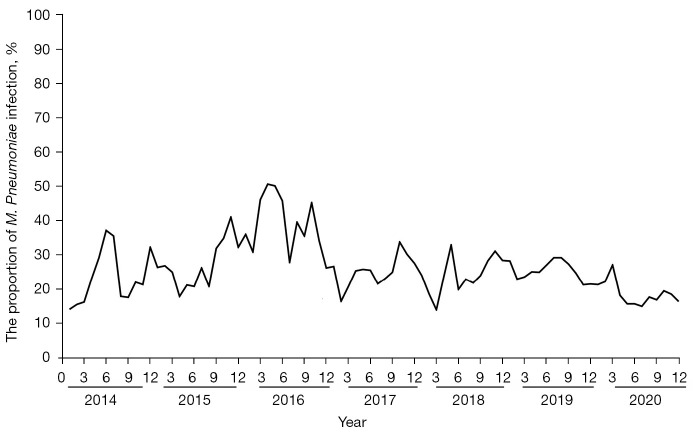
A total of 81,131 patients with RTIs were suspected of M. Pneumoniae infection, of which 21,582 cases were positive for M. Pneumoniae-IgM, and the total M. Pneumoniae infection proportion was 26.60%. The main clinical symptoms were cough, headache, sore throat, nasal symptoms, fever, malaise, and a few extrapulmonary symptoms such as skin rash, meningitis, myocarditis, hemolytic anemia, myalgia, digestive symptoms, hemolytic anemia and nephritis. The M. Pneumoniae RTI proportions from 2014 to 2020 were 23.60%, 28.18%, 38.08%, 27.05%, 23.44%, 25.26%, and 18.33%, respectively. The M. Pneumoniae infection proportion was highest in 2016 and lowest in 2020 (as shown in Figure 1 and Table 1).
Figure 1.
Epidemiological trends of M. Pneumoniae infection in adults and children from 2014 to 2020. The positive rate of M. Pneumoniae was the highest in 2016 and the lowest in 2020.
Table 1. Total epidemic characteristics of MP infection in adults and children from 2014 to 2020.
| Characteristics | Total (n, %) | Children (n, %) | Adult (n, %) | P value* |
|---|---|---|---|---|
| Year | ||||
| 2014 | 1,885/7,988 (23.60) | 1,495/5,554 (26.91) | 390/2,434 (16.02) | <0.0001 |
| 2015 | 2,985/10,593 (28.18) | 2,563/7,011 (36.56) | 422/3,582 (11.78) | <0.0001 |
| 2016 | 4,188/10,998 (38.08) | 3,574/7,723 (46.28) | 614/3,275 (18.19) | <0.0001 |
| 2017 | 3,707/13,704 (27.05) | 3,025/8,709 (37.44) | 682/4,995 (13.65) | <0.0001 |
| 2018 | 3,092/13,191 (23.44) | 2,412/8,641 (27.91) | 680/4,550 (14.95) | <0.0001 |
| 2019 | 4,392/17,386 (25.26) | 4,189/12,986 (32.25) | 203/4,400 (4.61) | <0.0001 |
| 2020 | 1,333/7,271 (18.33) | 1,244/5,034 (24.71) | 89/2,237 (3.98) | <0.0001 |
| Gender | ||||
| Male | 9,246/40,683 (22.72) | 8,113/27,261 (29.76) | 1,133/13,422 (8.44) | <0.0001 |
| Female | 12,336/40,448 (30.50) | 10,389/28,397 (36.58) | 1,947/12,051 (16.02) | <0.0001 |
| P value# | <0.0001 | <0.0001 | <0.0001 |
*, compared to children and adults; #, compared to males and females. MP, Mycoplasma pneumoniae.
Children and adults with M. Pneumoniae RTIs
As shown in Table 1, the M. Pneumoniae RTIs proportion in children was significantly higher than that in adults in almost every year. The M. Pneumoniae RTIs proportions from 2014 to 2020 in children and adults were 26.91% vs. 16.02%, 36.56% vs.11.78%, 46.28% vs. 18.19%, 37.44% vs. 13.65%, 27.91% vs. 14.95%, 32.25% vs. 4.6%, and 24.71% vs. 3.98%, respectively. The epidemic trends of the 2 groups were consistent.
Gender distribution of RTIs with M. Pneumoniae
To investigate the gender distribution of M. Pneumoniae infection, we distinguished between gender in the children and adult groups. As shown in Table 1, the male and female proportions of M. Pneumoniae infection in children were 29.76% and 36.58%, respectively. In adults, the male and female proportions were 8.44% and 16.02%, respectively. The M. Pneumoniae RTIs proportion in females was higher than that in males in both children and adults (P<0.0001).
Seasonal distribution of RTIs with M. Pneumoniae
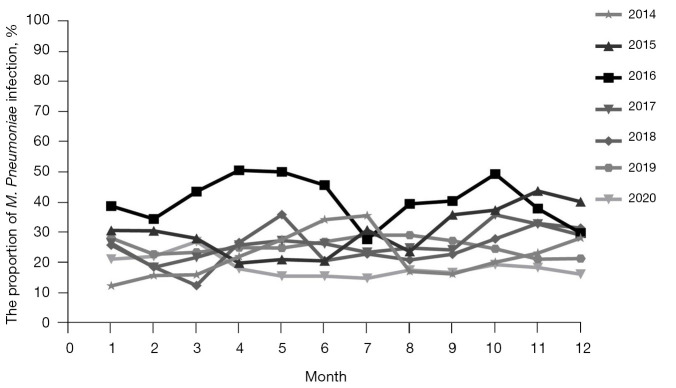
In terms of seasonal distribution, M. Pneumoniae was prevalent from January to December, with no obvious trough, but April to June and September to November were the relative peak months, especially in 2016. M. Pneumoniae RTIs accounted for 44.4–50.62% from April to June, and 37.95–49.42% from September to November in 2016 (as shown in Figure 2).
Figure 2.
Seasonal distribution of RTI with M. Pneumoniae from 2014 to 2020. April to June and September to November were peak months, especially in the epidemic year of 2016. RTI, respiratory tract infection.
Age distribution of RTIs with M. Pneumoniae
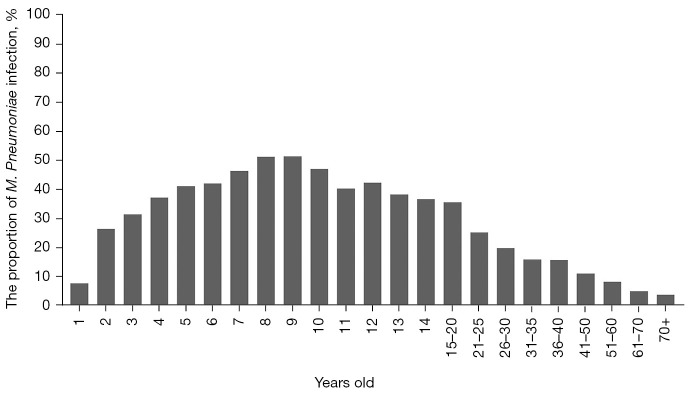
Our study included all age groups between 0–94 years old. Because M. Pneumoniae is mainly prevalent in children, we set the age group interval for children under 14 years of age at 1 year, and for people aged 14 and above were set to different age groups. From the perspective of age distribution, children and adolescents were the main population of M. Pneumoniae infection, among which 4–14-year-olds were the high-risk group. The M. Pneumoniae RTIs proportions were from 37.34% to 51.42%. In adults, the M. Pneumoniae RTIs proportion decreased gradually after 20 years old, and the proportion of M. Pneumoniae RTIs in elderly patients was very low, less than 5% (as shown in Figure 3).
Figure 3.
Age distribution of RTIs with M. Pneumoniae from 2014 to 2020. Children and adolescents comprised the main population of M. Pneumoniae infection, of which those aged 4–14 years old were the high-risk group. In adults, the positive rate of M. Pneumoniae decreased gradually after the age of 20, and the positive rate of elderly patients was very low. RTIs, respiratory tract infections.
Mycoplasma pneumoniae pneumonia (MPP) and SMPP in children and adults and pregnant and non-pregnant women
As shown in Table 2, from 2014 to 2020, there were a total of 2,003 cases of MPP, including 1,757 cases in children and 246 cases in adults. There were 301 cases of SMPP, including 281 cases in children and 20 cases in adults. During this period, 504 pregnant women and 528 non-pregnant women of reproductive age were included. The M. Pneumoniae RTIs proportions in women and non-pregnant women were 120/504 (23.81%) and 142/528 (26.90%), respectively. There were 35 cases of MPP (including 8 cases of SMPP) in pregnant women and 30 cases of MPP (including 2 cases of SMPP) in non-pregnant women. The proportion of SMPP in children and pregnant women was higher than that in adults and non-pregnant women (P=0.0403).
Table 2. Epidemic characteristics of MPP and SMPP in children, adults and pregnant/non-pregnant women.
| Characteristics | MP RTIs (n, %) | MPP (n, %) | SMPP (n, %) |
|---|---|---|---|
| Children | 18,502/55,658 (33.24) | 1,757/18,502 (9.50) | 281/1,757 (15.99) |
| Adult | 3,080/25,473 (12.09) | 246/3,080 (8.00) | 20/246 (8.13) |
| P value | <0.0001 | 0.0075 | <0.0001 |
| Pregnant women | 120/504 (23.81) | 27/120 (22.50) | 8/27 (29.62) |
| Non-pregnant women | 142/528 (26.90) | 28/142 (19.72) | 2/28 (7.14) |
| P value | 0.2551 | 0.5817 | 0.0403 |
MPP, Mycoplasma pneumoniae pneumonia; SMPP, severe Mycoplasma pneumoniae pneumonia; MP, Mycoplasma pneumoniae; RTIs, respiratory tract infections.
Clinical characteristics of SMPP in children and adults
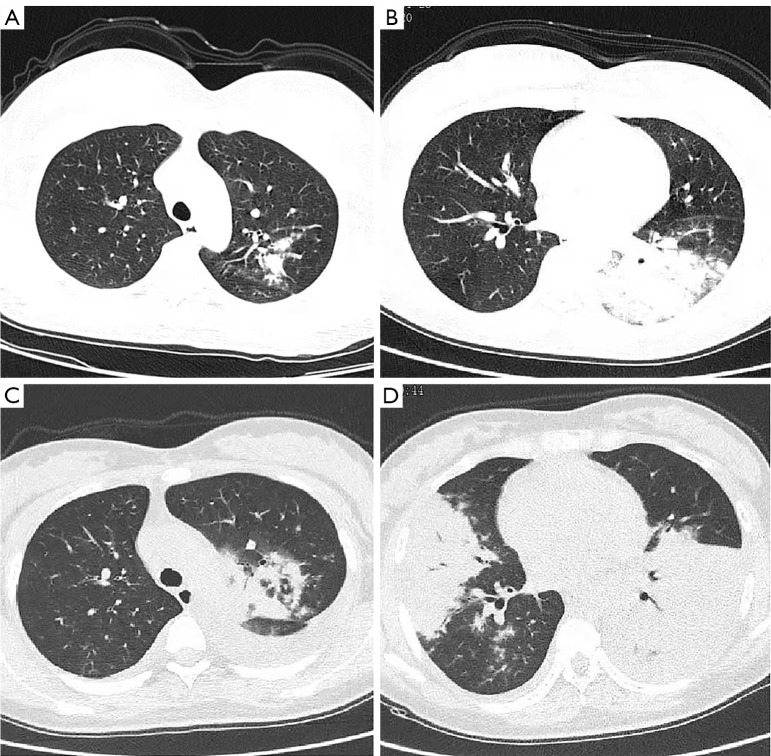
We further analyzed the clinical, laboratory, and radiographic findings of children and adults with SMPP. As shown in Table 3, children with SMPP had more extrapulmonary symptoms, lobular infiltrates, and increased C-reactive protein (CRP) and lactate dehydrogenase (LDH) levels compared with adults, which may be related to the secondary immune response of M. Pneumoniae. In addition, pregnant women made up nearly half of the adults with SMPP in our study, and their symptoms tended to progress more quickly to lobular infiltrates and pleural effusions, as well as respiratory failure (Figure 4).
Table 3. Clinical characteristics of SMPP in children and adults.
| Characteristics | Children (n=281) | Adults (n=20) | P value |
|---|---|---|---|
| Clinical presentation (n, %) | |||
| Common symptoms | 281/281 (100.00) | 20/20 (100.00) | 1.0000 |
| Extrapulmonary symptoms | 116/281 (41.28) | 3/20 (15.00) | 0.0306 |
| Laboratory detection (range) | |||
| White blood cells (×109/L) | 6.3 (2.8–16.4) | 7.9 (3.9–20.1) | 0.1026 |
| Neutrophil rate (%) | 62.8 (22.4–81.7) | 65.4 (30.2–87.2) | 0.3801 |
| Lymphocyte rate (%) | 28.8 (9.5–65.0) | 31.0 (8.1–60.4) | 0.4661 |
| C-reactive protein (mg/L) | 125.4 (27.1–237.3) | 66.3 (10.2–125.6) | <0.0001 |
| LDH (U/L) | 447 [150–1,129] | 309 [170–620] | 0.0032 |
| Imaging manifestations (n, %) | |||
| Unilobar infiltration | 83/281 (29.54) | 12/20 (60.00) | 0.0105 |
| Multilobar infiltrates | 198/281 (70.46) | 8/20 (40.00) | 0.0105 |
| Pleural effusion | 115/281 (40.63) | 6/20 (30.00) | 0.4797 |
Common symptoms: cough, sputum, headache, sore throat, fever, malaise, chest pain, tachypnea; extrapulmonary symptoms: skin rash, meningitis, myocarditis, hemolytic anemia, myalgia, digestive symptoms, hemolytic anemia and nephritis. SMPP, severe Mycoplasma pneumoniae pneumonia; LDH, lactate dehydrogenase.
Figure 4.
Representative CT images of SMPP in pregnant women. (A,B) Multilobar infiltration and pleural effusion were shown on different levels. (C,D) One week later, the same level of imaging showed deteriorated multilobar infiltration, atelectasis, and increased pleural effusion. CT, computed tomography; SMPP, severe Mycoplasma pneumoniae pneumonia.
Discussion
M. Pneumoniae is a prokaryotic microorganism with a size between bacteria and viruses, which can cause epidemics worldwide. In epidemic years, M. Pneumoniae can account for 30% to 50% of RTIs (16). Since 2010, M. Pneumoniae outbreaks have been reported in several countries, including Europe, the United States, China, and Japan (2,17,18). From 2014 to 2020, we observed that 26.60% (21,582/81,131) of patients with RTIs were positive for M. Pneumoniae, which was higher than the global incidence of 12% (range, 11–15%) from the Atypical Pathogens Reference Laboratory Database (18). However, the data are basically consistent with the studies in China and Asia (2,8,9,19,20). Our research shows that the M. Pneumoniae RTIs proportion in our hospital increased significantly in 2016, and even exceeded 50% during the epidemic season. In non-epidemic years, the average M. Pneumoniae infection rate was also around 20%, which is thought to be related to the widespread presence of macrolide-resistant M. Pneumoniae in Asia. Interestingly, the proportion of M. Pneumoniae infection decreased significantly in 2020 in our hospital. We speculated that this may be due to the prevalence of novel coronavirus (2019-nCoV), resulting in people changing their living habits, such as generally maintaining social distance and wearing masks, leading to a significant decline in the M. Pneumoniae infection rate. This shows that wearing a mask is a very simple and effective way to prevent cross-infection of M. Pneumoniae.
M. Pneumoniae infection is sporadic throughout the year, but studies have shown that the epidemic of M. Pneumoniae was related to temperature and humidity (21). The study in South Korea have shown that M. Pneumoniae infection is more common in autumn and winter (22). European data indicate that June to October are the peak epidemic seasons of M. Pneumoniae (23,24). However, studies from Seattle have shown no seasonal differences in M. Pneumoniae infection (25). In China, Gao et al. and Qu et al. reported that the peak season of M. Pneumoniae was mainly in autumn and winter in the north and summer and autumn in the south (26,27). Our results showed that April to June and July to September were the peak months of the M. Pneumoniae outbreak, which is consistent with Qian et al. and Zhang (20,28). According to the epidemic situation of various countries, summer and autumn are the main peak seasons of M. Pneumoniae epidemic.
In our study, the proportion of female M. Pneumoniae infection is relatively high in both children and adults, which is consistent with previous research (8,9,25-28). A reasonable explanation may be related to the different levels of activity and immunity between females and males. In children, boys partake in more outdoor sports than girls, especially in school-age children, and girls are more likely to engage in indoor activities during recess, which are relatively closed. Indoor activities over a long period are more conducive to M. Pneumoniae transmission. For adults, lower immunity in women than in men may be a factor in susceptibility to M. Pneumoniae.
As shown in Table 1, The M. Pneumoniae RTIs proportion in children was significantly higher than in adults almost every month. The peak months of M. Pneumoniae infection in children and adults were April to June and September to November. Similarly, Qu et al. (26) found that the positive rate of M. Pneumoniae in children was significantly higher than that in adults (19.7% and 8.9%, P<0.001), and the positive rate in school-age children aged 7–17 was the highest. Kogoj et al. (29) reported that the proportions of M. Pneumoniae infection in children and adults were 19% and 7%, respectively, and children aged 6–16 years were at serious risk of M. Pneumoniae infection. In terms of age distribution, we found that children aged 4–14 years old were the main group, which is consistent with previous studies (26,29). The pathogenic mechanism of M. Pneumoniae infection is not only direct damage to the human body, but also damage mediated by the immune response (30). The immune system of infants and young children is immature, and the immune response level is low. Therefore, infants younger than 3 years of age show mild or subclinical infection (15). The high proportion of M. Pneumoniae infection among children over the age of 4 may be closely related to their prolonged stay in semi-enclosed settings such as kindergartens and schools.
In our study, we also included pregnant women. Pregnant women have attracted much attention because of their special physiological characteristics. A total of 504 pregnant women were included in this study over the past 7 years. There was no significant difference in the proportion of M. Pneumoniae RTIs among pregnant women compared with 528 non-pregnant women during the same period. However, the proportion of SMPP in pregnant women was higher than that in non-pregnant women (29.62% vs. 7.14%, P=0.0403).
MPP is usually mild and has a good prognosis, while SMPP is severe and has a long course of disease, with a variety of complications and sequelae, such as atelectasis, bronchiolitis obliterans, and bronchiolitis obliterans. Our study found that children with SMPP had more extrapulmonary symptoms, lobular infiltrates, and increased CRP and LDH levels compared with adults, which may be related to the secondary immune response of M. Pneumoniae. The main pathogenesis is direct damage and indirect immune responses (30,31). In addition to endothelial cells, M. Pneumoniae can adhere to other cells, such as red blood cells and macrophages, which enter multiple organs and cause tissue damage. Besides, M. Pneumoniae activates certain toll-like receptors, especially TLR2, which can also provide injury-related molecular patterns and promote the initiation of inflammatory responses (31). In addition, our research showed that the proportion of SMPP was much higher in children than in adults. Miyashita et al. (32) analyzed 227 adult patients with MPP, among whom 13 had severe SMPP, accounting for 5.7% (13/227). Gao et al. (27) reported that SMPP accounted for about 13.0% of all cases of MPP. Early identification of SMPP in young MPP patients is of urgent clinical value. Studies have shown that fever over 10 days, young children, higher D-dimer level, lung consolidation, massive pleural effusion, necrotizing pneumonia, CRP >60 mg/L, LDH >410 IU/L, and extrapulmonary complications can be used as predictors of SMPP (33-35), which is consistent with our results.
In children, atelectasis, pleural effusion, necrotizing pneumonia, or lung abscess are common in patients with severe SMPP (36). Due to the special status of pregnant women, general imaging examination is not a routine examination. Clinically, according to the recommendation of the American Department of Obstetrics and Gynecology (37), we will also recommend imaging examination for pregnant women with poor treatment response. Our retrospective analysis showed that pregnant women with SMPP also have lobular infiltration and pleural effusion, and often progress rapidly.
Due to the limitations of the study’s retrospective design, certain medical data such as medication history, efficacy, and social and economic factors could not be analyzed. In the diagnosis of M. Pneumoniae, restricted by our hospital’s own conditions, PCR detection was not carried out, and there may be some deviation in the interpretation of the results. Our hospital is also the designated treatment hospital for women and children in Suzhou. Although thousands of pregnant women and parturients are treated every year, the number of pregnant women in our study was still relatively small. More cases need to be recruited, and more fetal information needs to be collected to analyze the relationship between M. Pneumoniae infection and pregnancy outcome. However, because the study included a large number of samples and has been monitored continuously for 7 years, this will make our conclusions more reliable.
Conclusions
In conclusion, our study indicates that M. Pneumoniae infection is common in Suzhou. The peak of M. Pneumoniae infection occurs in April to June and September to November each year. Children and adolescents aged 4–14 years are the main population of M. Pneumoniae. Among the people infected with M. Pneumoniae, the proportion of M. Pneumoniae infection and SMPP in children are relatively high. Some clinical indicators are needed for the early identification of SMPP.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The requirement for informed consent was waived by the committee as the study was an anonymous retrospective review of patient records. This study protocol was approved by the Medical Research Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (No. KL901299) and strictly followed the Declaration of Helsinki (as revised in 2013).
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4304/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4304/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4304/coif). The authors have no conflicts of interest to declare.
(English Language Editor: C. Betlazar-Maseh)
References
- 1.Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: An update. Indian J Med Microbiol 2016;34:7-16. 10.4103/0255-0857.174112 [DOI] [PubMed] [Google Scholar]
- 2.Yamazaki T, Kenri T. Epidemiology of Mycoplasma pneumoniae Infections in Japan and Therapeutic Strategies for Macrolide-Resistant M. pneumoniae. Front Microbiol 2016;7:693. 10.3389/fmicb.2016.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao B, Zhao CJ, Yin YD, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 2010;51:189-94. 10.1086/653535 [DOI] [PubMed] [Google Scholar]
- 4.Eibach D, Casalegno JS, Escuret V, et al. Increased detection of Mycoplasma pneumoniae infection in children, Lyon, France, 2010 to 2011. Euro Surveill 2012;17:20094. 10.2807/ese.17.08.20094-en [DOI] [PubMed] [Google Scholar]
- 5.Brown RJ, Macfarlane-Smith L, Phillips S, et al. Detection of macrolide resistant Mycoplasma pneumoniae in England, September 2014 to September 2015. Euro Surveill 2015;20:30078. 10.2807/1560-7917.ES.2015.20.48.30078 [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Sheng Y, Yang L, et al. Immunological Features of Pediatric Interstitial Pneumonia Due to Mycoplasma pneumoniae. Front Pediatr 2021;9:651487. 10.3389/fped.2021.651487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thangaraju S, Bagri N, Gupta V, et al. Mycoplasma-Induced Rash and Mucositis or Steven-Johnson Syndrome. Indian J Pediatr 2021;88:802-4. 10.1007/s12098-021-03658-z [DOI] [PubMed] [Google Scholar]
- 8.Yan C, Sun H, Zhao H. Latest Surveillance Data on Mycoplasma pneumoniae Infections in Children, Suggesting a New Epidemic Occurring in Beijing. J Clin Microbiol 2016;54:1400-1. 10.1128/JCM.00184-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Li S, Cao L, et al. Surveillance of Mycoplasma pneumoniae infection among children in Beijing from 2007 to 2012. Chin Med J (Engl) 2014;127:1244-8. [PubMed] [Google Scholar]
- 10.Gao J, Xu L, Xu B, et al. Human adenovirus Coinfection aggravates the severity of Mycoplasma pneumoniae pneumonia in children. BMC Infect Dis 2020;20:420. 10.1186/s12879-020-05152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Respiratory Branch of Chinese Pediatric Society of Editorial Board of Chinese Journal of Applied Clinical Pediatrics . Expert consensus on diagnosis and treatment of MP pneumonia in children. Chinese Journal of Applied Clinical Pediatrics 2015;30:1304-8. [Google Scholar]
- 12.Meyer Sauteur PM, Unger WWJ, van Rossum AMC, et al. The Art and Science of Diagnosing Mycoplasma pneumoniae Infection. Pediatr Infect Dis J 2018;37:1192-5. 10.1097/INF.0000000000002171 [DOI] [PubMed] [Google Scholar]
- 13.Clinical Laboratory Science Group of Pediatrics Branch of Chinese Medical Association . Consensus of Chinese experts on laboratory diagnosis of MP respiratory tract infection in children. Chin J Lab Med 2019;42:507-13. [Google Scholar]
- 14.Salih W, Schembri S, Chalmers JD. Simplification of the IDSA/ATS criteria for severe CAP using meta-analysis and observational data. Eur Respir J 2014;43:842-51. 10.1183/09031936.00089513 [DOI] [PubMed] [Google Scholar]
- 15.Editorial Board, Chinese Journal of Pediatrics. Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (I). Zhonghua Er Ke Za Zhi 2013;51:745-52. [PubMed] [Google Scholar]
- 16.Waites KB, Xiao L, Liu Y, et al. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin Microbiol Rev 2017;30:747-809. 10.1128/CMR.00114-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue G, Li M, Wang N, et al. Comparison of the molecular characteristics of Mycoplasma pneumoniae from children across different regions of China. PLoS One 2018;13:e0198557. 10.1371/journal.pone.0198557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold FW, Summersgill JT, Lajoie AS, et al. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 2007;175:1086-93. 10.1164/rccm.200603-350OC [DOI] [PubMed] [Google Scholar]
- 19.Lee E, Cho HJ, Hong SJ, et al. Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr 2017;60:151-7. 10.3345/kjp.2017.60.5.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XX. Epidemiological analysis of MP infection in children with respiratory tract diseases in Suzhou area from 2005 to 2014. China Medical Abstracts (Internal Medicine) 2016;33:15-6. [Google Scholar]
- 21.Onozuka D, Hashizume M, Hagihara A. Impact of weather factors on Mycoplasma pneumoniae pneumonia. Thorax 2009;64:507-11. 10.1136/thx.2008.111237 [DOI] [PubMed] [Google Scholar]
- 22.Eun BW, Kim NH, Choi EH, et al. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect 2008;56:326-31. 10.1016/j.jinf.2008.02.018 [DOI] [PubMed] [Google Scholar]
- 23.Defilippi A, Silvestri M, Tacchella A, et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med 2008;102:1762-8. 10.1016/j.rmed.2008.06.022 [DOI] [PubMed] [Google Scholar]
- 24.Beeton ML, Zhang XS, Uldum SA, et al. Mycoplasma pneumoniae infections, 11 countries in Europe and Israel, 2011 to 2016. Euro Surveill 2020;25:1900112. 10.2807/1560-7917.ES.2020.25.2.1900112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foy HM, Kenny GE, Cooney MK, et al. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis 1979;139:681-7. 10.1093/infdis/139.6.681 [DOI] [PubMed] [Google Scholar]
- 26.Qu J, Yang C, Bao F, et al. Epidemiological characterization of respiratory tract infections caused by Mycoplasma pneumoniae during epidemic and post-epidemic periods in North China, from 2011 to 2016. BMC Infect Dis 2018;18:335. 10.1186/s12879-018-3250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect 2019;147:e192. 10.1017/S0950268819000839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian Q, Ji W. Epidemiological features of children hospitalized with mycoplasma pneumoniae infection from the year of 2006 to 2014. Chongqing Med 2016;45:4113-6. [Google Scholar]
- 29.Kogoj R, Mrvic T, Praprotnik M, et al. Prevalence, genotyping and macrolide resistance of Mycoplasma pneumoniae among isolates of patients with respiratory tract infections, Central Slovenia, 2006 to 2014. Euro Surveill 2015. 10.2807/1560-7917.ES.2015.20.37.30018 [DOI] [PubMed] [Google Scholar]
- 30.He J, Liu M, Ye Z, et al. Insights into the pathogenesis of Mycoplasma pneumoniae (Review). Mol Med Rep 2016;14:4030-6. 10.3892/mmr.2016.5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol 2018;30:380-7. 10.1097/BOR.0000000000000494 [DOI] [PubMed] [Google Scholar]
- 32.Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol 2007;56:1625-9. 10.1099/jmm.0.47119-0 [DOI] [PubMed] [Google Scholar]
- 33.Yan C, Xue G, Zhao H, et al. Molecular and clinical characteristics of severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 2019;54:1012-21. 10.1002/ppul.24327 [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Xu X, Zhao W, et al. Refractory Mycoplasma Pneumonia in Children: A Systematic Review and Meta-analysis of Laboratory Features and Predictors. J Immunol Res 2022;2022:9227838. 10.1155/2022/9227838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Hua L, Zhao Q, et al. The Level of D-Dimer Is Positively Correlated With the Severity of Mycoplasma pneumoniae Pneumonia in Children. Front Cell Infect Microbiol 2021;11:687391. 10.3389/fcimb.2021.687391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robnik B, Keše D, Rojko T, et al. Unilateral brachial plexopathy, a rare complication of Mycoplasma pneumoniae infection. J Infect Chemother 2018;24:309-11. 10.1016/j.jiac.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 37.American College of Obstetricians and Gynecologists' Committee on Obstetric Practice . Committee Opinion No. 656: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 2016;127:e75-80. 10.1097/AOG.0000000000001316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as