Abstract
Immunization with dendritic cells pulsed ex vivo with antigens has been successfully used to elicit primary antigen-specific immune responses. We report that mouse bone marrow-derived dendritic cells pulsed with inactivated chlamydial organisms induced strong protection against live chlamydial infection in a mouse lung infection model. Either the dendritic cells or chlamydial organisms alone or macrophages similarly pulsed with chlamydial organisms failed to induce any significant protection. These observations suggest that dendritic cells can efficiently process and present chlamydial antigens to naive T cells in vivo. Mice immunized with the chlamydia-pulsed dendritic cells preferentially developed a Th1 cell-dominant response while mice immunized with the other immunogens did not, suggesting a correlation between a Th1 cell-dominant response and protection against chlamydial infection. We further found that dendritic cells produced a large amount of interleukin 12 (IL-12) upon ex vivo pulsing with inactivated chlamydial organisms, which may allow the dendritic cells to direct a Th1 cell-dominant response. Dendritic cells from mice deficient in the IL-12 p40 gene failed to produce IL-12 after a similar ex vivo pulse with chlamydial organisms, and more importantly, immunization with these dendritic cells failed to induce a Th1 cell-dominant response and did not induce strong protection against chlamydial infection. Thus, the ability of dendritic cells to efficiently process and present chlamydial antigens and to produce IL-12 upon chlamydial-organism stimulation are both required for the induction of protection against chlamydial infection. This information may be useful for the further design of effective chlamydial vaccines.
Chlamydia trachomatis is an obligately intracellular bacterial pathogen that causes various human diseases (7, 23). Urogenital-tract infection with C. trachomatis is the leading cause of many important sexually transmitted diseases. For instance, chlamydia-induced sexually transmitted diseases in women include chronic pelvic pain, life-threatening ectopic pregnancy, and pelvic inflammatory disease, which often results in involuntary sterility. Ocular infection with C. trachomatis can result in trachoma, which is one of the major causes of preventable blindness in many developing countries. Therefore, efficient immune intervention strategies for preventing chlamydial infection are badly needed. However, no successful chlamydial vaccine is yet available, despite the tremendous amount of effort that has been made by many research groups (4, 28, 32–35, 38).
The dendritic cell (DC) network is a specialized system for presenting antigen to naive T cells and consequently plays a central role in the induction of T-cell and B-cell immunity in vivo (26). One of the characteristics of DCs is their distinct development stages (1, 21). The immature dendritic cells are highly active in phagocytizing and processing microbial antigens (10, 22). During the maturation process, DCs can migrate to draining lymph node, and the matured DCs express high levels of costimulatory and major histocompatibility complex (MHC) molecules that may allow the mature DCs to efficiently present antigens and to induce primary antigen-specific immune responses. As generation of large quantities of relatively pure DCs became possible (11), DCs have been successfully used to induce antigen-specific immune responses and protective immunity against various cancers and infectious diseases (15, 19, 24), including chlamydial infections (29). In many of these studies, DCs were pulsed ex vivo with either peptide or whole-protein antigens and delivered in vivo to syngeneic hosts. The protection thus induced often correlates with a strong, antigen-specific T-cell response (12, 29). DCs were found to produce a large amount of interleukin 12 (IL-12) in both CD40 ligand-dependent and -independent manners (2, 25). The IL-12 production by DCs may allow the DCs to direct the development of Th1 cells from naive CD4+ T cells (16). A Th1 cell-dominant immune response is often required for the control of many intracellular infections (6, 9). Thus, DCs not only possess the ability to prime an antigen-specific response but also are able to direct a protective immune response against infections.
Since it has been demonstrated that a Th1 cell-mediated immune response played a critical role in controlling intracellular chlamydial infection (20, 27), we have used the DC-based immunization approach for inducing protective immunity to chlamydial infection in the present study. We found that mouse bone marrow-derived DCs pulsed ex vivo with inactivated chlamydial organisms induced strong protection against chlamydial respiratory-tract infection, which confirms a recent observation made in a genital-tract infection model by Su et al. (29). Either DCs or chlamydial organisms alone or bone marrow-derived macrophages similarly pulsed with chlamydial organisms failed to induce any significant protection. Furthermore, using DCs from IL-12 knockout mice, the present study has extended the previous observations by Su et al. (29) by demonstrating that the protection induced by the chlamydia-pulsed DCs required the donor DCs to produce IL-12 and to direct a Th1 cell-dominant immune response.
MATERIALS AND METHODS
Animals and organisms.
Six- to eight-week-old female C57BL/6 and IL-12p40 knockout mice on a C57 background were purchased from the Jackson Laboratory (Bar Harbor, Maine). The mice were housed at the University of Manitoba animal facility, and all animal procedures used in this study were approved by the Protocol Review Committee of the University of Manitoba.
A C. trachomatis murine strain designated the mouse pneumonitis (MoPn) strain was used in this study. The chlamydial organisms were grown in HeLa 229 cells, and elementary bodies (EBs) were purified on discontinuous gradients of Renografin-76 (Squibb Canada Inc., Montreal, Canada) as described elsewhere (38). The purified chlamydial EB organisms were aliquoted in a sucrose-phosphate-glutamic acid (SPG) buffer and stored at −80°C. The infectivity of the purified EBs was titrated by counting chlamydial inclusion-forming units (IFUs) on monolayers of HeLa 229 cells grown in 96-well plates. Portions of the purified EBs were inactivated by UV-light (G15T8 UV lamp; D. William Fuller Inc., Chicago, Ill.) irradiation at a distance of 5 cm for 1 h at room temperature. The UV-inactivated EBs (UV-EBs) were checked for infectivity. No chlamydial growth was detected when the UV-EBs were inoculated onto HeLa monolayers at a dose equivalent to 108 IFUs per 106 HeLa cells. For convenience, the number of UV-EBs used in all experiments was calculated based on the number of IFUs of the corresponding live EBs prior to UV-light treatment.
Generation of bone marrow-derived DCs and (Mφs).
A procedure described by Inaba et al. (11) was followed for the generation of bone marrow-derived DCs and macrophages (Mφs) from mice. Briefly, mouse bone marrow was collected from both tibias and femurs. Erythrocytes were lysed by ammonium chloride treatment, and lymphocytes, granulocytes, and MHC-positive cells were killed with a cocktail of monoclonal antibodies (MAbs) (see below) and rabbit complement (Sigma, St. Louis, Mo.). The MAbs were GK1.5 anti-CD4, 2.43 anti-CD8, RA3-3A1/6.1 anti-B220/CD45R, and 25-9-17s11 anti-MHC (TIB 207, 210, and 146 and HB26, respectively; American Type Culture Collection, Manassas, Va.) and RB6-8c5 anti-GR1 (PharMingen, San Diego, Calif.). The cells were plated in 6-well culture plates (Costar, Cambridge, Mass.) in RPMI-1640 medium (Gibco Laboratories, Grand Island, N.Y.) supplemented with 5% heat-inactivated fetal calf serum (FCS; Intergen Company, Purchase, N.Y.), 5 mM 2-mercaptoethanol, and 5 ng of recombinant mouse granulocyte-macrophage colony-stimulating factor (PharMingen)/ml. At day 3 of culture, floating cells were gently removed and fresh medium was added. Thereafter, cells were refed every 2 days. At day 6 to day 8 of culture, nonadherent cells and loosely adherent proliferating DC aggregates were collected. After DCs were harvested, the culture dishes were vigorously washed to further remove any nonadherent or loosely adherent cells. The residual adherent cells were then dislodged after incubation with 1× trypsin-EDTA solution (Gibco Laboratories) at 37°C for 30 min and collected as bone marrow derived-Mφs. The DC fraction contained 80 to 90% DCs based on the culture characteristics, morphology, and MHC class II and CD11c phenotype. More than 90% of cells in the Mφ fraction were CD11b+ and CD11c−.
Flow cytometry analysis.
Cell surface markers of the bone marrow-derived cell samples were analyzed by antibody staining and flow cytometry. All antibodies, including isotype controls, were purchased from PharMingen. The following antibodies conjugated with fluorochromes were used: red-phycoerythrin (PE)-conjugated anti-mouse I-Ab (catalog no. 6045A), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD11c (9714D), and FITC-conjugated anti-mouse CD11b (1714D). The corresponding isotype controls used were PE-mouse immunoglobulin G2A (IgG2A) (3025), FITC-hamster IgG (11144L), and FITC-rat IgG2B (11034C). After the cell samples were incubated with the appropriate antibodies for 30 to 60 min on ice, the stained cells were analyzed by using a FACScalibur equipped with CellQuest software (Becton Dickinson, Mountain View, Calif.). Propidium iodide staining and scatter gating were used to exclude dead cells and debris.
Ex vivo pulse of DCs and Mφs with chlamydial organisms.
The collected bone marrow-derived DCs and Mφs were pulsed ex vivo with UV-EBs at a multiplicity of infection (MOI) of 1 in the RPMI culture medium described above. The cell concentration was kept at 106/ml, and the antigen pulse was carried out at 37°C for various times as specified for individual experiments. For studying the time of IL-12 production, the parallel cell samples were pulsed ex vivo with UV-EBs for 2 h, 6 h, 18 h, 2 days, and 3 days. At each time point, the supernatants were collected for IL-12 measurement as described below. For studying the duration of IL-12 production, the cells were pulsed for 18 h. The supernatants were harvested for IL-12 measurement (designated the day-0 sample), while the cells were washed to remove free UV-EBs and resuspended in the same amount of fresh medium, and culture was continued for another 24 h. The supernatants were harvested (designated the day-1 sample), and the cell pellet was again resuspended in fresh medium. After further culturing for 48 h, the supernatants were collected (day-3 sample) and the cells were resuspended in fresh medium for a final 72-h culturing. The final supernatants were collected (day-6 sample). Supernatants from DCs and Mφs similarly cultured in the absence of UV-EBs were also collected. All the supernatants collected were measured for IL-12 levels.
Mouse immunization and protection protocol.
The bone marrow-derived DCs and Mφs, with or without UV-EB ex vivo pulsing for 18 h as described above, were washed twice with phosphate-buffered saline (PBS). Cells (105 in 50 μl of sterile PBS) were injected subcutaneously into the hind footpads (25 μl for each footpad). Since the pulsed cell samples were incubated with UV-EB at an MOI of 1, 105 IFUs of UV-EBs alone in PBS was also used to immunize a group of mice as a control. In addition, a separate PBS-only control group was set up.
Ten days after the footpad injection, mice were intranasally challenged with 2 × 104 IFUs of live EBs in 50 μl of SPG buffer. After the live-organism challenge, mouse body weight was measured and mortality was monitored on a daily basis. Ten days after the challenge, the surviving mice were sacrificed and lung tissues were aseptically removed for titration of infectious chlamydial organisms. Each lung was homogenized with a cell grinder in 4 ml of cold SPG buffer. The lung tissue suspensions were centrifuged at 500 × g for 10 min at 4°C to remove coarse tissue debris and were frozen at −80°C until they were tested. For quantitation of infectious EBs, a HeLa monolayer was inoculated with a twofold serially diluted lung tissue supernatant for 2 h at 37°C. After the 2-h attachment, the HeLa cells were further cultured in Dulbecco’s modified Eagle’s medium containing 10% FCS, 1.5 μg of cycloheximide (Sigma)/ml, 100 μg of vancomycin (Sigma)/ml, and 12 μg of gentamicin (Sigma)/ml for 2 days. For visualization of the chlamydial inclusion bodies, the infected cell monolayer was stained with an anti-chlamydial lipopolysaccharide (LPS) MAb (EV1-H1, mouse IgG2A; kindly provided by Harlan Caldwell at the Rocky Mountain Laboratories, National Institutes of Health) after the appropriate fixation and permeabilization. A rabbit anti-mouse IgG conjugated with horseradish peroxidase (Cedarlane, Hornby, Ontario, Canada) and an insoluble substrate, 4-chloro-1-naphthol (Sigma), were used to probe the first-antibody binding. The number of chlamydial inclusion bodies was counted in five fields under a reverse microscope. The chlamydial infectious titers were calculated based on the number of inclusions per field, the dilution factors, and the magnification of the lenses used and were expressed as IFUs per lung (31).
Measurements of chlamydia-specific antibody in mouse sera.
An enzyme-linked immunosorbent assay (ELISA) was used for measuring serum antibody levels (31). Briefly, a 96-well ELISA plate (catalog no. 25805; Corning Glass Works, Corning, N.Y.) was coated with 105 IFUs of chlamydial EBs in 50 μl of SPG buffer at 4°C overnight. After blocking with 1% bovine serum albumin-PBS solution for 2 h at room temperature, the serially diluted sera were added to the appropriate wells and the plates were incubated for 4 h at room temperature. After washing, a biotin-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates, Inc., Birmingham, Ala.) or goat anti-mouse IgG2A (Caltag, Burlingame, Calif.) was added to the plates. The immobilized biotin was detected with an alkaline phosphatase-conjugated streptavidin (Sigma) and a soluble substrate, p-nitrophenyl phosphate (Sigma). The optical density (OD) was read at 405 nm. Triplicate wells were used in each assay.
Cytokine concentration measurement.
Various culture supernatants were collected and assayed for IL-2, IL-4, gamma interferon (IFN-γ), or IL-12. A sandwich ELISA protocol described in the PharMingen catalog was used for measuring these cytokines. The capture antibodies were R4-6A2 (for IL-2), JES6-1A12 (for IL-4), BVD4-1D11 (for IFN-γ), and C15.6 (for IL-12p40), and the biotinylated detection antibodies were XMG1.2 (for IL-2), JES6-5H4 (for IL-4), BVD6-24G2 (for IFN-γ), and C17.8 (for IL-12). All antibodies were purchased from PharMingen. The biotin was detected as described above in the serum antibody ELISA. The OD was read at 405 nm. The standard murine cytokines, including IL-2, IL-4, IFN-γ, and IL-12, were also purchased from Pharmingen. The levels of cytokines were expressed in picograms per milliliter or units per milliliter of supernatants, depending on the standards. Triplicate wells were used in each assay.
Statistics.
All data were expressed as means ± standard errors. Student’s t test was used for analysis of statistical significance (P value).
RESULTS
Bone marrow-derived DCs but not Mφs pulsed ex vivo with inactivated chlamydial organisms induced protection against chlamydial infection.
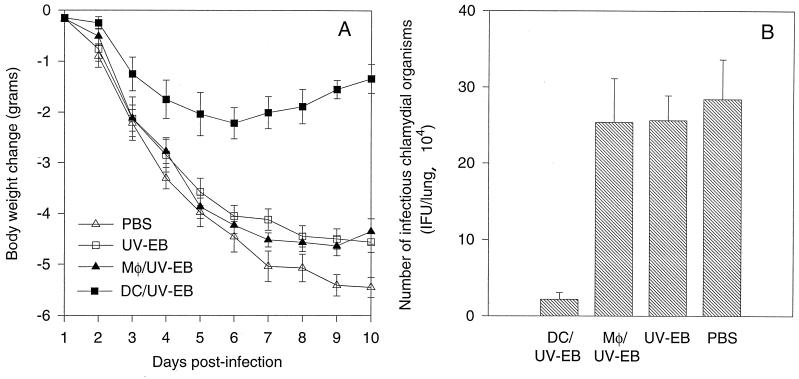
We first compared the ability of the antigen-pulsed DCs and Mφs to induce protective immune responses against chlamydial infection in a mouse lung infection model. The protection was evaluated by monitoring mouse body weight change and measuring the infectious chlamydial-organism recovery from mouse lung tissues. Mice immunized with the DCs pulsed with UV-EBs (designated DC/UV-EB) displayed significantly less body weight reduction than other treatment groups (Fig. 1A). The DC/UV-EB-immunized mice experienced only minimal body weight loss and started to regain body weight on day 7 after the chlamydial challenge infection. However, mice from other groups, including the group immunized with the chlamydial EB-pulsed Mφs (Mφ/UV-EB) displayed a progressive body weight reduction until the time of sacrifice. One mouse from the PBS treatment group died on day 7. This observation suggests that immunization with the DC/UV-EB greatly improved the overall resistance of the animals to the toxicity of the chlamydial challenge infection, while immunization with the Mφ/UV-EB failed to do so.
FIG. 1.
(A) Mouse body weight change after chlamydial challenge infection. Groups of mice were immunized with either DC/UV-EB (n = 8) or Mφ/UV-EB (n = 8), UV-EBs alone (n = 8), or PBS alone (n = 7). Ten days after the immunization, mice were challenged with a sublethal dose of live chlamydial organisms, and mouse body weight was monitored on a daily basis for 10 days. (B) Titers of chlamydial infectious particles recovered from mouse lung tissues. Groups of mice as described for panel A and shown along the x axis were sacrificed 10 days after challenge infection. The infectious chlamydial organisms recovered from lung tissues were quantitated on HeLa cell monolayers as described in Materials and Methods.
To evaluate whether this overall improvement in resistance was due to the decreased chlamydial infection as a result of the DC/UV-EB immunization, we further quantitated the infectious chlamydial organisms recovered from mouse lung tissues. As shown in Fig. 1B, mice immunized with the DC/UV-EB indeed had significantly fewer infectious chlamydial organisms in their lung tissues (2.2 × 104 IFUs) than mice treated with PBS alone (28 × 104 IFUs). This strong protection was not likely due to the effect from the free UV-EBs potentially carried in the DC/UV-EB preparation, since the DC/UV-EB preparation was washed before injection and nearly all chlamydial EB particles were localized inside DCs after the DCs were incubated with UV-EBs for 18 h (data not shown). More importantly, immunization with UV-EBs alone was not protective (Fig. 1). The DC/UV-EB immunization-induced protection may not be caused by the nonspecific cellular particle effect either, since Mφs similarly pulsed with UV-EBs did not induce any protection (Fig. 1). Furthermore, injection of a similar amount of DCs alone or DCs pulsed with unrelated antigens was found to confer no protection against chlamydial infection (data not shown). These observations together suggest that the DCs but not Mφs pulsed ex vivo with inactivated chlamydial organisms can induce a protective response against chlamydial challenge infection.
DCs but not Mφs pulsed ex vivo with inactivated chlamydial organisms induced a Th1 cell-dominated response.
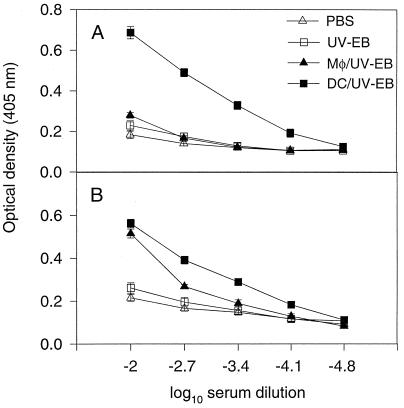
We have demonstrated that DCs but not Mφs pulsed ex vivo with chlamydial organisms can induce a strong protective response against the subsequent chlamydial infection. We next tested whether the protection induced by the DC/UV-EB immunization was due to the ability of the DCs to preferentially direct a Th1 cell-dominated response. T-cell functional phenotypes (Th1 versus Th2) can be conveniently evaluated by typing the serum Ig isotypes and directly measuring the representative cytokine levels. We found that mice immunized with DC/UV-EB preferentially developed high titers of chlamydia-specific IgG2A antibodies (Fig. 2A), while Mφ/UV-EB immunization induced high titers of chlamydia-specific IgG1 and minimal levels of IgG2A antibodies (Fig. 2B). Immunization with UV-EBs or PBS alone did not induce any significant levels of chlamydia-specific antibodies (Fig. 2). These observations suggest that DCs pulsed ex vivo with chlamydial organisms may selectively induce production of IFN-γ, since IFN-γ is responsible for promoting B-cell Ig class switching towards the IgG2A isotype. Conversely, Mφs similarly pulsed with chlamydial organisms appeared to preferentially induce an IL-4-dominant response, since IL-4 is responsible for promoting IgG1 isotype production.
FIG. 2.
Levels of chlamydia-specific IgG2A (A) and IgG1 (B) antibodies in sera of mice immunized with either DC/UV-EB (n = 15), Mφ/UV-EB (n = 8), UV-EBs alone (n = 8), or PBS alone ((n = 7) as described in the Fig. 1A legend. Ten days after immunization, mouse blood was collected from mouse tails, and the serum antibodies were titrated in an ELISA as described in Materials and Methods.
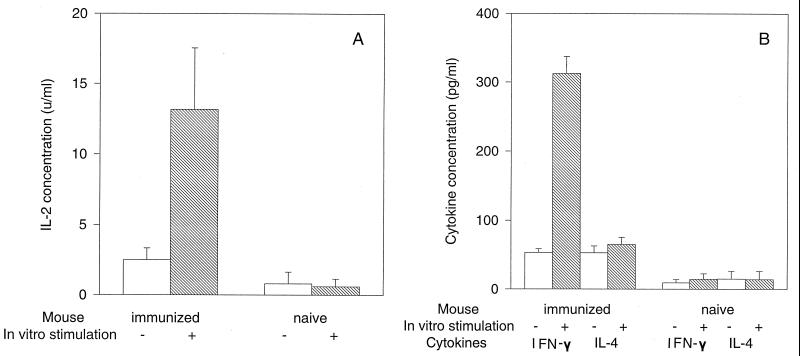
To test whether DC/UV-EB immunization can indeed selectively induce a Th1-like, IFN-γ-producing, T-cell-dominant response, we measured the cytokines produced by spleen cells from mice immunized with DC/UV-EB. The DC/UV-EB-immunized spleen cells produced high levels of IL-2 upon in vitro restimulation with chlamydial antigens, while the naive spleen cells failed to produce any measurable IL-2 regardless of the stimulation (Fig. 3A), suggesting that DC/UV-EB immunization primed chlamydial-antigen-specific T cells in vivo. More interestingly, the DC/UV-EB-immunized spleen cells produced high levels of IFN-γ but only minimal amounts of IL-4 upon in vitro chlamydial restimulation (Fig. 3B). Together, these observations clearly suggest that DCs pulsed ex vivo with inactivated chlamydial organisms can induce a chlamydial-antigen-specific and Th1 cell-dominated response in vivo.
FIG. 3.
Cytokine production by spleen cells from either naive mice (n = 5) or mice immunized with DC/UV-EB (n = 7) as shown along the x axis. IL-2 (A), IL-4 (B), and IFN-γ (B) production by the spleen cells was measured with (hatched bars) or without (open bars) in vitro restimulation by inactivated chlamydial organisms. The concentration of IL-2 was expressed in units per milliliter, while the concentrations of IL-4 and IFN-γ were expressed in picograms per milliliter based on the corresponding cytokine standards.
DCs but not Mφs pulsed ex vivo with inactivated chlamydial organisms produced IL-12.
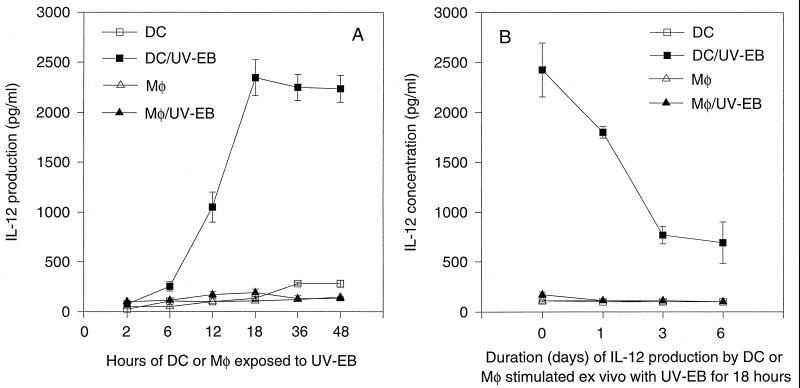
To further understand why DCs pulsed with chlamydial organisms preferentially directed a Th1 cell-dominated response while similarly pulsed Mφs failed to do so, we compared the abilities of DCs and Mφs to produce IL-12 upon chlamydial stimulation in vitro (Fig. 4). DCs produced a large amount of IL-12 (as high as 2,500 pg/ml) after ex vivo stimulation with chlamydial organisms for 18 h (Fig. 4A). However, Mφs similarly stimulated failed to produce any significant amount of IL-12 even after 48 h of stimulation (Fig. 4A). These observations confirm that only DCs but not Mφs can produce significant amounts of IL-12 upon microbial stimulation. We further evaluated the duration of IL-12 production by DCs after they were exposed to UV-EBs for 18 h. The DC/UV-EB can continue to secrete IL-12 for at least 6 additional days, although the level of IL-12 gradually declined (Fig. 4B). Since any excess chlamydial organisms were washed off the DCs after the 18-h pulse and the previously produced and accumulated IL-12 was washed off on three different days in this experiment, the continued IL-12 production on day 6 suggests that chlamydial organisms can induce sustained IL-12 production by DCs.
FIG. 4.
Production of IL-12 by bone marrow-derived DCs or Mφs alone or by DC/UV-EB or Mφ/UV-EB. (A) Time course of IL-12 production. Bone marrow-derived cells were stimulated in vitro with UV-EBs for various periods as indicated along the x axis, and the supernatants were collected at each time point for evaluation of the IL-12 concentration. (B) Duration of IL-12 production by DCs stimulated in vitro with UV-EBs for 18 h. Supernatant collected from the 18-h-stimulated culture was designated the day-0 sample. After excess chlamydial organisms were washed off and the stimulated cells were cultured again in fresh medium for another 24 h, the supernatant was collected as the day-1 sample. After washing, culturing of the cells was continued as described in Materials and Methods. Three to five separate experiments were performed; each experiment was performed in triplicate.
IL-12 production by the donor DCs is required for inducing strong protection.
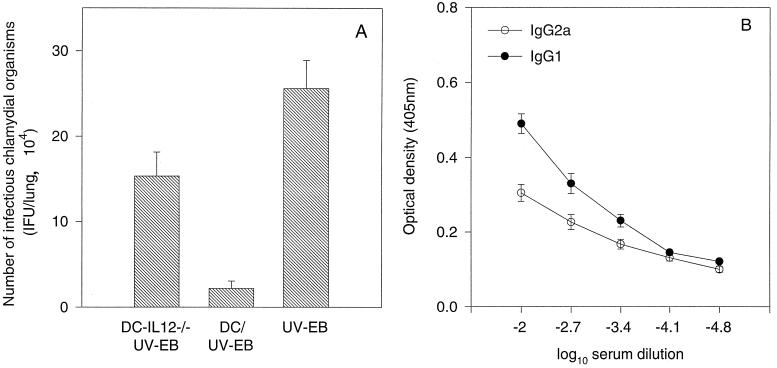
Although we have demonstrated that DCs can produce IL-12 upon ex vivo chlamydial organism stimulation, it is not known whether the IL-12 production is necessary for the DC/UV-EB-induced protection against chlamydial infection. We next evaluated the ability of DCs from mice deficient in IL-12p40 expression to induce antichlamydial immune responses. These IL-12p40-deficient DCs (designated DC-IL12p40−/−) failed to produce detectable levels of IL-12 after ex vivo stimulation with chlamydial organisms (data not shown). Immunization with DC-IL12p40−/− pulsed ex vivo with chlamydial organisms induced only minimal protection (15 × 104 IFUs per lung versus 28 × 104 IFUs per lung in the PBS control group), although it was still statistically significant (P < 0.05). However, the similarly pulsed wild-type DCs induced more than 10-fold protection compared with the PBS control group (2.2 × 104 versus 28 × 104 IFUs per lung; P < 0.001) (Fig. 5A). The reduced protection by the DC-IL12p40−/− immunization seemed to correlate with its inability to direct a Th1-like cell-dominant response, since mice thus immunized produced higher titers of chlamydia-specific IgG1 but lower titers of IgG2A antibodies (Fig. 5B).
FIG. 5.
(A) Effect of immunization with either DC-IL12p40−/− (n = 7) or wild-type DCs (n = 8) pulsed ex vivo with UV-EBs or with UV-EBs alone (n = 8) on the recovery of infectious chlamydial organisms from mouse lung tissues following a challenge infection. Mice were immunized and challenged as described in the Fig. 1 legend. (B) Mice (n = 7) were immunized with the DC-IL12p40−/− pulsed ex vivo with UV-EBs as described above. Ten days after the immunization, mouse blood was collected and chlamydia-specific IgG2A and IgG1 antibodies in the sera were measured as described in Materials and Methods. Results are expressed in the same format as in Fig. 2.
DISCUSSION
We have demonstrated that a single immunization at the footpad with DCs pulsed ex vivo with inactivated chlamydiae induced strong protection against chlamydial respiratory-tract infection. Using a similar approach with model antigens, Inaba et al. (12) have shown that DCs pulsed ex vivo with various protein antigens can efficiently prime antigen-specific T-cell responses in draining lymph node, while other antigen-presenting cells (APCs), such as peritoneal macrophages, failed to do so when similarly pulsed, suggesting that DCs have the unique ability to prime naive T cells in vivo. This approach has since been successfully used to evaluate the roles of DCs in inducing protective immunity against various tumors and microbial infections (15, 19, 24). Su et al. (29) reported that intravenous injection of DCs pulsed ex vivo with nonviable chlamydiae induced profound protection against chlamydial genital-tract infection, which is consistent with our finding in the lung infection model. All these findings suggest that DCs are able not only to present chlamydial antigens in vitro (18) but also to prime T cells in vivo. It is interesting that in the present study, footpad injection of antigen-pulsed DCs induced a protective response against respiratory-tract infection. This can be explained by a previous finding that footpad injection of antigen-pulsed DCs induced antigen-specific T cells not only in the immediate draining lymph node but also in brachial lymph nodes (12). These observations suggest that local immunization with DCs pulsed ex vivo with a defined antigen can induce a systemic protective response, which may be useful for developing the DC-based vaccines, since in many cases, the infection sites may not be the sites convenient for delivering vaccines.
It is known that a Th1-like cell-dominant response is usually beneficial in controlling many intracellular infections (6, 20). The intracellular chlamydial infection is no exception. First, studies based on gene knockout mice have demonstrated that an MHC class II-restricted response was most critical for controlling chlamydial infection (17). Second, passive transfer of a chlamydia-specific T-cell clone that secretes Th1 types of cytokines can offer protection against chlamydial challenge infection in recipient mice (9). Third, injection of exogenous IFN-γ into mice can enhance mouse resistance to chlamydial challenge infection (36), while IFN-γ knockout mice (3) or mice treated with an anti-IFN-γ neutralizing antibody (37) displayed more severe and disseminated chlamydial infection. Fourth, IL-12, a potent Th1-promoting cytokine, was found to be important in limiting chlamydial infection (20). Finally, mouse strains that respond to chlamydial infection with preferential production of IFN-γ displayed significantly less-severe infection than the strains with preferential production of IL-10, a Th2 phenotype-promoting cytokine (30). The present study demonstrated a correlation between a Th1 cell-dominant response and protection against chlamydial infection. DC/UV-EB immunization preferentially induced a Th1 cell-dominant response and conferred protection, while Mφ/UV-EB immunization failed to induce a Th1 response and did not provide protection against chlamydial infection. A similar correlation between Th1 response and protection was also demonstrated in the study by Su et al. (29).
Why did the DC- but not the Mφ-based immunization induce a Th1 cell-dominant response? It is well known that IL-12 is very efficient in directing Th1 cell development from Th0 precursors (14). Therefore, the availability of sufficient IL-12 during the initial interactions between APCs and CD4+ T cells may determine the functional phenotypes of the T cells involved. Sousa et al. (25) have recently shown that DCs but not unprimed Mφs can produce IL-12 in a CD40 ligand-independent manner upon stimulation with microbial components such as LPS, which may allow the DCs to direct the development of a Th1 phenotype from the Th0 precursors while the DCs present microbial antigens. The chlamydial outer membrane contains LPS, and furthermore, chlamydial organisms have been found to stimulate the production of many other inflammatory cytokines. We indeed found that ex vivo stimulation of DCs but not of unprimed Mφs by inactivated chlamydial organisms can lead to a high level of production of IL-12 in the culture supernatants, which is consistent with the recent observation by Su et al. (29). The present study further demonstrated that DCs exposed to the chlamydial organisms for 18 h can continue to produce IL-12 for at least another 6 days. We therefore hypothesize that DCs pulsed ex vivo with chlamydial organisms may still be able to produce IL-12 when migrating to draining lymph node and presenting chlamydial antigens to lymph node T cells. Such direct IL-12 production may be both necessary and sufficient for promoting the development of the T cells involved towards a Th1 phenotype. This is supported by the observation that the antigen-pulsed DCs from IL-12 knockout mice failed to induce a Th1-dominant response.
Since IL-12 can also effectively stimulate NK cells and macrophages to secrete IFN-γ (14), it is possible that the direct IFN-γ production stimulated by IL-12 secreted by DC/UV-EB contributed to the protection against chlamydial infection in the DC/UV-EB-immunized mice. This innate immunity occurs sooner than adaptive immunity and may be important in controlling infection during the early stage of the live-pathogen infections. It was demonstrated that acute infection with Toxoplasma gondii stimulated early IFN-γ synthesis, which enhanced host resistance to the infection (5). However, both the present study and the study by Su et al. (29) suggest that a DC-primed Th1 response rather than the direct early IFN-γ production was likely to be responsible for the protection, observed, since mice were challenged 10 to 14 days after the final DC immunization. From the point of view of vaccination, induction of an antigen-specific Th1 cell-dominant response is more important, since only the antigen-specific response can produce a long-lasting and recallable protection.
ACKNOWLEDGMENTS
We thank Ronald Germain for discussion and Robert Brunham for reading the manuscript.
This work was supported by the Medical Research Council (MRC) of Canada (G. Zhong). G. Zhong is the recipient of an MRC scholarship.
REFERENCES
- 1.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan J, Stephens R S. Antigen conformation dependence of Chlamydia trachomatis infectivity neutralization. J Infect Dis. 1997;176:713–721. doi: 10.1086/514095. [DOI] [PubMed] [Google Scholar]
- 5.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 6.Geginat G, Lalic M, Kretschmar M, Goebel W, Hof H, Palm D, Bubert A. Th1 cells specific for a secreted protein of Listeria monocytogenes are protective in vivo. J Immunol. 1998;160:6046–6055. [PubMed] [Google Scholar]
- 7.Grayston J T, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 8.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman R M, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 9.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 10.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inaba K, Metlay J P, Crowley M T, Steinman R M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamont A G, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- 15.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R M. Dendritic cells efficiently induce protective antiviral immunity. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 17.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojcius D M, Bravo de Alba Y, Kanellopoulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 19.Ossevoort M A, Feltkamp M C, van Veen K J, Melief C J, Kast W M. Dendritic cells as carriers for a cytotoxic T-lymphocyte epitope-based peptide vaccine in protection against a human papillomavirus type 16-induced tumor. J Immunother Emphasis Tumor Immunol. 1995;18:86–94. doi: 10.1097/00002371-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 21.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 22.Reis e Sousa C, Stahl P D, Austyn J M. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schachter J. Chlamydial infections (third of three parts) N Engl J Med. 1978;298:540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- 24.Schuler G, Steinman R M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–7. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sousa C R, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain R N, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 27.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su H, Caldwell H D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su H, Messer R, Whitmire W, Fischer E, Portis J, Caldwell H. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, HayGlass K T, Brunham R C. Genetically determined differences in IL-10 and IFN-gamma responses correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–4344. [PubMed] [Google Scholar]
- 31.Zhang D, Yang X, Berry J, Shen C, McClarty G, Brunham R C. DNA vaccination with the major outer-membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y X, Stewart S J, Caldwell H D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;57:636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong G, Berry J D, Choukri S. Mapping epitopes of neutralizing monoclonal antibodies using phage random peptide libraries. J Ind Microbiol Biotechnol. 1997;19:71–76. doi: 10.1038/sj.jim.2900364. [DOI] [PubMed] [Google Scholar]
- 34.Zhong G, Smith G P, Berry J, Brunham R C. Conformational mimicry of a chlamydial neutralization epitope on filamentous phage. J Biol Chem. 1994;269:24183–24188. [PubMed] [Google Scholar]
- 35.Zhong G, Toth I, Reid R, Brunham R C. Immunogenicity evaluation of a lipidic amino acid-based synthetic peptide vaccine for Chlamydia trachomatis. J Immunol. 1993;151:3728–3736. [PubMed] [Google Scholar]
- 36.Zhong G M, Peterson E M, Czarniecki C W, de la Maza L M. Recombinant murine gamma interferon inhibits Chlamydia trachomatis serovar L1 in vivo. Infect Immun. 1988;56:283–286. doi: 10.1128/iai.56.1.283-286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong G M, Peterson E M, Czarniecki C W, Schreiber R D, de la Maza L M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989;57:152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong G M, Reid R E, Brunham R C. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990;58:1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]