Abstract
Models of healthy brain function and psychiatric conditions assume that excitatory and inhibitory activity are balanced in the human brain at multiple spatial and temporal scales. In human neuroimaging, concentrations of the major excitatory (glutamate) and inhibitory (γ-aminobutyric acid, GABA) neurotransmitters are measured in vivo using magnetic resonance spectroscopy (MRS). However, despite the central importance of E/I balance to theories of brain function, a relationship between regional glutamate and GABA levels in the human brain has not been shown. We addressed this question in a large corpus of edited MRS data collected at 19 different sites (n = 220). Consistent with the notion of E/I balance, we found that levels of glutamate+glutamine (Glx) and GABA+ were highly correlated (R = 0.52, p = 2.86 x 10−14). This relationship held when controlling for site, scanner vendor, and demographics. Controlling for neurochemicals associated with neuronal density and metabolism (i.e. N-acetylaspartate and creatine) significantly reduced the correlation between GABA+ and Glx, suggesting that the levels of GABA+ and Glx may be critically linked to regional metabolism. These results are consistent with the notion that excitation and inhibition are balanced in the human brain.
Keywords: GABA, Glutamate, MRS, Excitation, Inhibition
1. Introduction
A large body of literature suggests that excitatory and inhibitory (E/I) activity is balanced in neural systems. In cortical circuits, any given neuron is thought to receive approximately equal amounts of excitation and inhibition, which precisely and dynamically counteract each other during both sensory-evoked and ongoing neural activity (Barron et al., 2017; Fishell and Kepecs, 2019; Froemke, 2015; Vogels and Abbott, 2009). This balance is thought to be crucial for enabling precise signal timing and preventing runaway excitation in the brain (Isaacson and Scanziani, 2011; Okun and Lampl, 2008; Wehr and Zador, 2003). Moreover, prominent theories of neuropsychiatric conditions, such as autism and schizophrenia, implicate an imbalance in excitatory and inhibitory activity in the neurobiology of these conditions (Marín, 2012; Rubenstein and Merzenich, 2003; Vattikuti and Chow, 2010). Yet, the mechanisms driving these putative changes are poorly understood, in part because little is known about how E/I balance manifests in the human brain.
In the human brain, the major excitatory (glutamate) and inhibitory (γ-aminobutyric acid, GABA) neurotransmitters can be measured in vivo using magnetic resonance spectroscopy (MRS). Because models of neural function posit that inhibition and excitation are balanced in cortical circuits (Vogels and Abbott, 2009), many MRS studies have quantified the ratio of GABA to glutamate (Barron et al., 2017; Gu et al., 2019; Kapogiannis et al., 2013; Koolschijn et al., 2019; Shibata et al., 2017) and GABA to glutamate+glutamine (Glx) (e.g. (Bezalel et al., 2019)) as measures of E/I balance in a given brain area. Further, studies of autism (e.g. Drenthen et al., 2016) have reported that the concentration of GABA relative to glutamate differs between patients and controls, and an altered balance between GABA and Glx has been found in schizophrenia (Chiu et al., 2018). Based on these and other studies of neuropsychiatric populations, it has been suggested that the ratio between these neurometabolites could be used as a clinical biomarker. These reports implicitly assume that, if MRS-measured glutamate (or Glx) and GABA levels reflect excitatory and inhibitory activity, the levels of these neurometabolites should be positively related within a given brain area at the population-level (i.e., individuals with greater concentration of Glx would have a higher concentration of GABA). However, whether or not MRS-measured levels of glutamate (or Glx) and GABA are correlated in the human brain has not been shown empirically.
Here, we sought to test whether the regional concentrations of GABA and Glx are correlated in the resting human brain. To investigate this question, we used data from the Big GABA dataset (Mikkelsen et al., 2019, 2017), a large, collaborative dataset designed to assess the comparability of edited MRS data across scanner vendors and research sites in a large sample of human participants. MRS data from the Big GABA dataset were acquired from a single voxel placed in each participant’s medial parietal lobe (Fig. 1, inset). The medial parietal lobe is an ideal region for assessing the correlation between GABA+ and Glx levels in the resting brain because it is a key node within the default mode network (Kapogiannis et al., 2013; Raichle, 2015) and is active during rest and mind-wandering (Fox et al., 2015; Raichle, 2015).
Fig. 1.
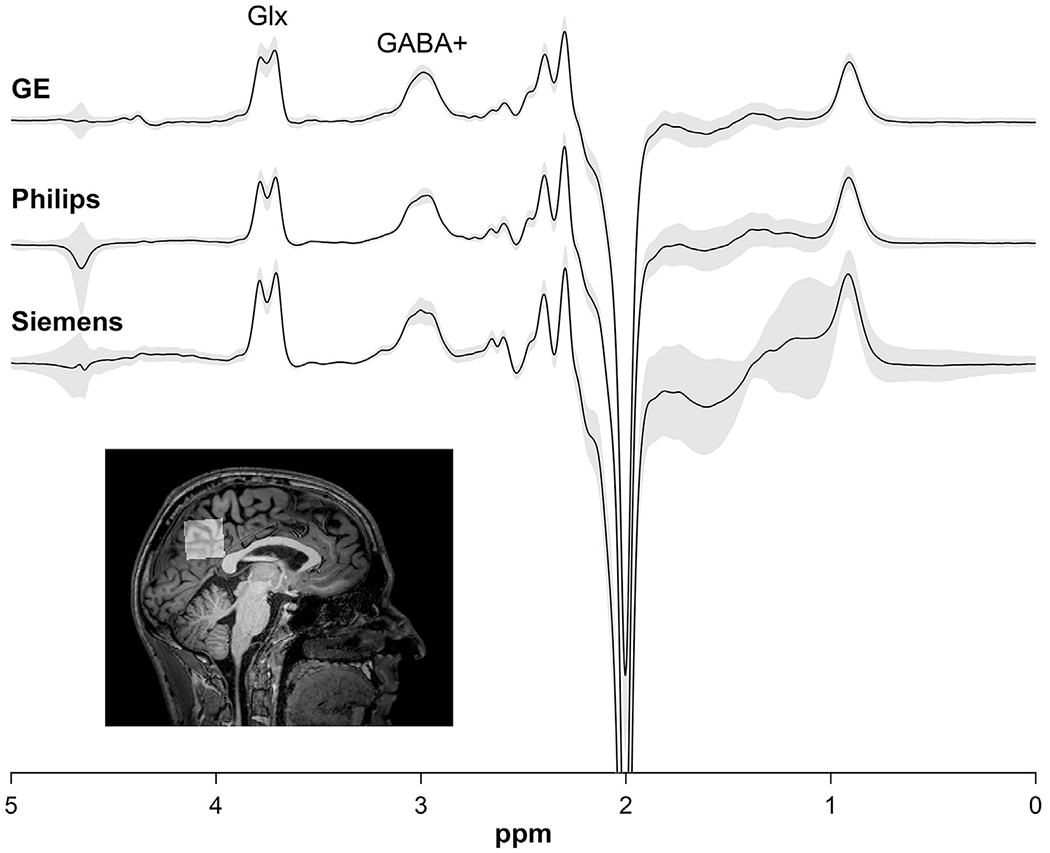
Mean processed MRS spectrum from all vendors (top). Shaded areas represent mean signal ± 1 SD. (inset) An example voxel placement from one representative subject shows voxel location and coverage within medial parietal lobe. The data used in this paper were collected as part of the Big GABA consortium (Mikkelsen et al., 2019, 2017).
The edited MRS measurements used here should be interpreted with several considerations in mind. Notably, the glutamate and glutamine signals cannot be disambiguated in the spectra comprising the Big GABA dataset (Puts and Edden, 2012; Ramadan et al., 2013). However, because glutamate and glutamine are continuously being cycled (Bak et al., 2006; Hertz and Rothman, 2016), the aggregate signal (i.e. Glx) is often taken as a proxy for excitation. Likewise, the GABA signal detected by standard, edited MRS techniques at 3T contains contribution from GABA, macromolecules, and homocarnosine (Deelchand et al., 2019; Mikkelsen et al., 2019, 2017; Mullins et al., 2014; Puts and Edden, 2012; Rothman et al., 1997). In addition, along with their roles in phasic inhibitory and excitatory neurotransmission, GABA and glutamate (along with glutamine) have several other functions in the brain, including regulation of tonic inhibition and excitability (Stagg et al., 2009a), shaping the functional architecture of the cortex (Kolasinski et al., 2017; Puts et al., 2011; Robertson et al., 2016), and regulating metabolism (Albrecht et al., 2010; Bak et al., 2006; Hertz and Rothman, 2016). Because they play multiple roles, GABA and glutamate are present in multiple tissue compartments, including the synapse, but also within vesicles, in the extracellular matrix, and within astrocytes, and it is not possible to distinguish between these sources with MRS (Gasparovic et al., 2018, 2006). Therefore, given the imperfect measurement as well as the multiple roles played by these neurometabolites, we refer to MRS measured GABA as GABA+ and glutamate+glutamine as Glx.
Our study had two specific aims. First, we sought to answer whether concentrations of GABA+ and Glx are correlated in human participants at rest. Second, we sought to determine whether any correlation between Glx and GABA+ levels could be explained by other factors, such as participant demographics, data quality, or tissue concentration.
2. Methods
Data for the present study were collected as part of the Big GABA consortium (Mikkelsen et al., 2019, 2017), a large, collaborative dataset designed to test the reliability of edited MRS data across scanner vendors and acquisition sites. A subset of the data (N = 224; 116 females, mean age = 25.95 ± 4.15 years old [mean ± s.d.]) was made publicly available on the NITRC portal (https://nitrc.org/projects/biggaba), and those data were used here. A full description of the dataset acquisition and processing methods are detailed elsewhere (Mikkelsen et al., 2019, 2017) and are reproduced here in brief.
2.1. MR data acquisition
25 sites participated in the Big GABA consortium (Mikkelsen et al., 2019, 2017). At each site, data were acquired on a 3 T scanner (see Mikkelsen et al., 2019, 2017 for scanner model details) using standard MEGA-PRESS (Mescher et al., 1998) acquisition parameters: TE/TR = 68/2000 ms; ON/OFF editing pulses = 1.9/7.46 ppm; 320 averages; 30 × 30 × 30 mm3; medial parietal lobe voxel-of-interest (VOI) (Fig. 1, inset). For accurate VOI placement and subsequent tissue segmentation, high-resolution 3D T1-weighted structural images were acquired for all participants (see (Mikkelsen et al., 2019) Table 1 for details). A full description of the acquisition parameters for the MRS and MRI data can be found in the original Big GABA publications (Mikkelsen et al., 2019, 2017).
Table 1.
List of confounding factors tested.
| Confounding factor | Model (nlme syntax) | Correlation of GABA+ and Glx residuals (corrected p-value) | Significantly different from Vendor and Site -: not significant *: significant |
|---|---|---|---|
| Vendor and Site | ~ Vendor + 1|Site | r218 = 0.400, p = 3.38e-08 | N/A |
| Demographics | ~ Vendor*Age*Sex + 1|Site | r218 = 0.384, p = 8.28e-08 | – |
| Frequency drift | ~ Vendor*Frequency Drift + 1|Site | r218 = 0.377, p = 1.404e-07 | – |
| Linewidth NAA | ~ Vendor*Linewidth NAA + 1|Site | r218 = 0.3967, p = 3.38e-08 | – |
| Linewidth Water | ~ Vendor*Linewidth Water + 1|Site | r218 = 0.327, p = 1.0478e-06 | – |
| Water area | ~ Vendor*Water Area +1|Site | r218 = 0.385, p = 8.28e-08 | – |
| Concentration Cr | ~ Vendor*Cr + 1|Site | r218 = 0.163, p = 0.01625 | * |
| Concentration NAA | ~ Vendor*NAA + 1|Site | r218 = 0.090, p = 0.184 | * |
| Concentration Cho | ~ Vendor*Cho + 1|Site | r218 = 0.330, p = 9.312875e-07 | – |
Cr Creatine level; NAA N-acetylaspartate level; Cho Choline level.
2.2. MRS data processing
Data were processed and quantified in Gannet (Edden et al., 2014) using the toolkit’s standard processing pipeline, which includes: coil combination (GE and Siemens data only), frequency-and-phase correction via spectral registration (Near et al., 2015), zero-filling to a spectral resolution of 0.061 Hz/point, and 3-Hz exponential line-broadening. Frequency drift was measured as the mean difference between the observed frequency of the residual water signal in the metabolite spectra (before frequency correction) and the nominal in vivo water frequency (4.68 pm). NAA and unsuppressed water linewidths were calculated as the full-width half-maximum of the modeled signal (see below). Fig. 1 shows the mean spectra from each vendor after processing.
The 3 ppm GABA+ and 3.75 ppm Glx signals in the difference-edited spectrum were modeled using a three-Gaussian function with a nonlinear baseline fit between 2.79 and 4.10 ppm. In the edit-OFF spectrum, the 3 ppm Cr and 3.2 ppm Cho signals were modeled using a two-Lorentzian function (2.6–3.6 ppm), while the 2 ppm NAA signal was modeled using a single Lorentzian function (1.75–2.25 ppm). The unsuppressed water signal was modeled using a Lorentzian-Gaussian function (3.8–5.6 ppm). GABA+, Glx, Cr, Cho, and NAA were quantified in institutional units (i.u.) using the unsuppressed water signal as an internal concentration reference.
The GABA+ and Glx values were not corrected for tissue-dependent signal attenuation, apart from the analyses where tissue-corrected values were explicitly considered (Section title: Tissue composition does not drive the correlation between GABA+ and Glx). For those analyses, we used three different approaches in separate analyses: 1) normalization of GABA+ and Glx values by gray matter fraction (Stagg et al., 2009a), 2) the alpha-correction method proposed by Harris and colleagues (Harris et al., 2015), 3) and the method proposed by Gasparovic and colleagues that accounts for variation in T2-relaxation times across tissue compartments (Gasparovic et al., 2006). The assumed relaxation and density parameters of water and GABA can be found in (Mikkelsen et al., 2019). The assumed longitudinal relaxation times of Glx, Cr, Cho, and NAA were 1.23 s, 1.35 s, 1.19 s, and 1.41 s, respectively (Posse et al., 2007). The assumed transverse relaxation times of these metabolites were 0.18 s, 0.154 s, 0.207s, 0.246 s, respectively (Ganji et al., 2012). Tissue fraction parameters were calculated as described below.
2.3. Voxel co-registration and tissue segmentation
In order to control for tissue-dependent signal differences across individuals, the fraction of gray matter, white matter, and cerebrospinal fluid in the voxel were calculated for each participant. MRS voxels were co-registered to the T1w structural images (Harris et al., 2015) and segmented using the unified segmentation routine in SPM12 (Ashburner and Friston, 2005). The fractional contribution of gray matter (fGM), white matter, and cerebrospinal fluid to the total voxel volume was then calculated.
2.4. Statistical analysis
Statistical analyses were conducted using R (R Core Team, 2013). In the present study, our primary interests were to: 1) determine whether GABA+ and Glx concentrations measured using a MEGA-PRESS MRS sequence were correlated in the medial parietal cortex of the resting human brain and 2) assess whether the correlation could be explained by confounding factors, such as idiosyncratic differences in acquisition across sites, demographic data, or differing tissue volume fraction of the MRS voxel across subjects. Participants with outlying levels of either GABA+ or Glx (mean ± 2.5*std, n = 4) were excluded from analysis, leaving 220 participants for analysis.
To address our first aim, we correlated the GABA+ and Glx values derived from all scans. Next, we addressed our second aim by regressing out confounding factors that could explain the observed relationship from GABA+ and Glx using linear mixed-effects models implemented in the nlme package (Pinheiro et al., 2019); we then correlated the residual values of GABA+ and Glx after controlling for the confounding factor. If the residual values remained correlated, we inferred that the relationship held when controlling for the confounding factor. We considered each confounding factor separately. In addition, we determined whether the difference between the correlation values dropped significantly by comparing the z-scored correlation coefficients.
For all statistical tests, we used a significance threshold of p < 0.05. To correct for multiple-comparisons, we FDR-corrected across p-values obtained for each correlation test (Benjamini and Hochberg, 1995). Corrected p-values are reported.
The full list of confounding factors and the associated model used to control for these factors is provided in Table 1.
3. Results
As predicted, we found a positive relationship between the uncorrected regional GABA+ and Glx levels (r218 = 0.53, p = 2.8 x 10−14) (Fig. 2A). Because of the known impact of scanner vendor and site on GABA+ levels in this dataset, likely driven by slight differences in voxel placement or shim quality (Mikkelsen et al., 2019), next we regressed out the impact of vendor and site from the concentrations of GABA+ and Glx and re-evaluated the correlation between the residual values. We found that the correlation between GABA+ and Glx remained significant after accounting for the effects of vendor and site (r218 = 0.400, p = 3.38 x 10−8; Fig. 2b) and did not significantly differ from the model that did not include vendor and site (z = 1.159, p = 0.11). However, because site and vendor have a known effect in this dataset (Mikkelsen et al., 2019, 2017), we controlled for the impact of vendor and site in all subsequent analyses. We subsequently tested whether other factors could explain the relationship between GABA+ and Glx by iteratively regressing out the effects of several nuisance factors using linear mixed effects modeling and reevaluating the correlation between the corrected values of GABA+ and Glx (Fig. 2C–H). The correlation between the GABA+ and Glx residuals from subsequent tests are provided in Table 1.
Fig. 2.
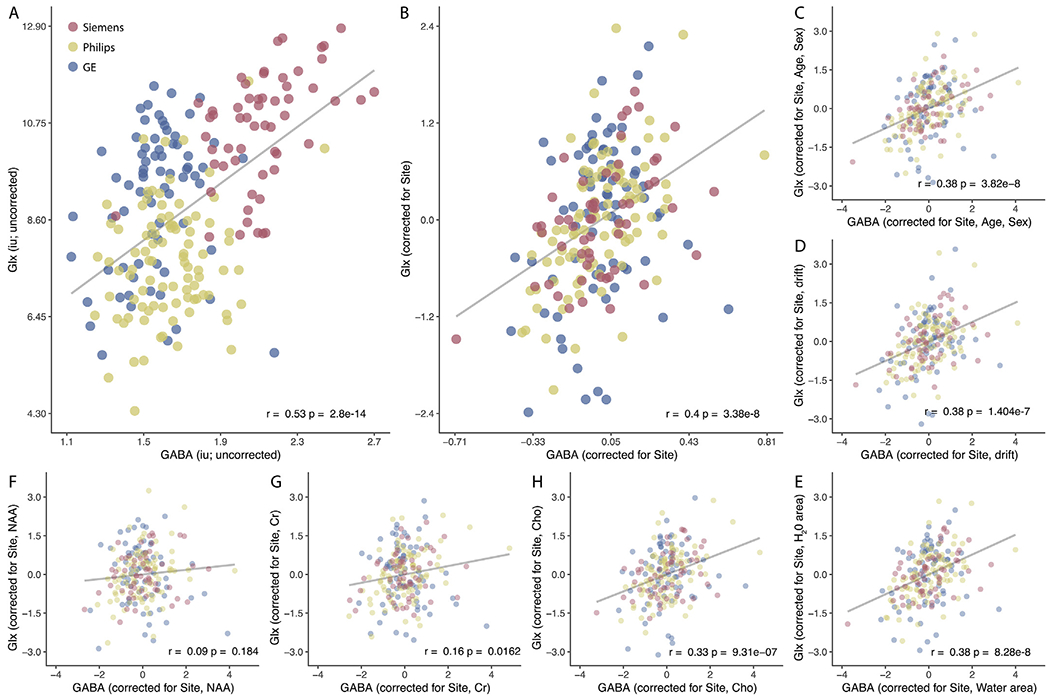
Concentrations of GABA+ and Glx are balanced in medial parietal cortex of the resting human brain. A) Across a large population of healthy adult participants (N=220), individuals with higher GABA+ concentrations also showed higher Glx concentrations. This correlation was not impacted by controlling for the B) scanner vendor (fixed effect) and site (random effect), C) subject demographics, D) frequency drift, or E) water area. We also tested whether the correlation between GABA+ and Glx was impacted by neurometabolite concentration (F-H). Correcting for the neurochemicals F) NAA and G) Cr, which are known to be involved in metabolic activity, significantly reduced the correlation between GABA+ and Glx as expected. However, the correlation between GABA+ and Glx remained significant after correcting for the concentration of H) Choline, which is not thought to be involved in metabolic processes (Öz et al., 2014). This suggests that metabolism may mediate the relationship between GABA+ and Glx. Individual data points represent an MRS measurement from medial parietal cortex in a single individual color-coded by vendor (Siemens = red, Philips = yellow, GE = blue).
3.1. Age and gender do not explain the relationship between GABA+ and Glx
GABA+ and Glx concentrations have been found to change with age, potentially due to alterations in regional tissue composition. Specifically, in adults, both GABA+ and Glx are thought to decrease with age (Cassady et al., 2019; Chang et al., 2009; Gao et al., 2013; Sailasuta et al., 2008; Simmonite et al., 2019), which could potentially drive a correlation between GABA+ and Glx concentration at the population level. However, this was not observed in our population: when age and sex were taken into account, the covariation between GABA+ and Glx was not significantly diminished (z = 0.2, p = 0.84). Further, in contrast to studies that have found GABA and Glx concentrations to decrease with age (Cassady et al., 2019; Chang et al., 2009; Gao et al., 2013; Porges et al., 2017; Sailasuta et al., 2008; Simmonite et al., 2019), we did not observe a relationship between age and concentrations of GABA+ (r218 = 0.0389, p = 0.567) or Glx (r218 = 0.020, p = 0.767) in this dataset.
3.2. Spectral quality does not drive the correlation between GABA+ and Glx
Because the GABA+ and Glx concentrations are derived from the same edited MRS signal, the correlation between these neurochemicals could be explained by that signal’s quality. For example, participants with lower signal-to-noise ratios may have systematically underestimated GABA+ and Glx levels. To rule out this explanation of the observed correlation between GABA+ and Glx, we tested whether overall spectral quality could explain this relationship in two ways. First, it is known that frequency drift during data acquisition can cause reduced editing efficiency and impact fitting estimates for edited metabolites such as GABA+ and Glx (Harris et al., 2014). So, the amount of frequency drift during a given scan might drive a false relationship between the two signals. We found that this was not the case: regressing out frequency drift did not significantly impact the relationship between GABA+ and Glx (z = 0.28, p = 0.78).
Second, we tested whether the linewidth of NAA could explain the correlation between GABA+ and Glx. Larger linewidths of prominent signals in the MR spectrum (e.g. NAA) are increased by factors such as subject motion, and therefore are commonly taken as proxies for poorer spectral acquisition (e.g. (Harris et al., 2014)). However, regressing out the linewidth of NAA (z = 0.05, p = 0.96) did not significantly diminish the relationship between GABA+ and Glx. Likewise, regressing out the linewidth of the water signal (used for quantification of I.U.) could not explain the relationship between GABA+ and Glx (z = 0.76, p = 0.44).
Yet another possible explanation for the relationship between GABA+ and Glx is the use of a common reference metabolite (water) for calculating the relative concentration. When we controlled for this possibility by regressing out the integral of the water peak from the GABA+ and Glx measurements, we found that the common water signal could not explain the relationship between GABA+ and Glx (z = 0.18, p = 0.85). All in all, no measures of spectral quality were observed to significantly reduce the relationship between GABA+ and Glx in our study.
3.3. Tissue composition does not drive the correlation between GABA+ and Glx
Both GABA+ and Glx are known to be present in higher concentrations in gray matter as compared with white matter at approximately a 2:1 ratio (Mikkelsen et al., 2016). Thus, we reasoned that individual differences in the tissue composition within the MRS voxel might drive the observed relationship between GABA+ and Glx.
We tested whether the tissue concentration within the voxel impacted the correlation between GABA+ and Glx. We used three different procedures for this analysis. One approach used in the literature (e.g. (Stagg et al., 2009a)) is to normalize the concentrations of GABA+ and Glx by the fraction of gray matter in the MRS voxel. Scaling by the fraction of gray matter did not significantly impact the relationship between GABA+ and Glx (r218 = 0.389, p = 1.47 x 10−8; not significantly different from the model: z = 0.14, p = 0.88). For our second approach, we applied the alpha-tissue correction method suggested by Harris and colleagues (Harris et al., 2015), which takes into account the different levels of GABA and Glx within CSF, white matter, and gray matter. We found that this did not impact the relationship between GABA+ and Glx (r218 = 0.29, p = 1.60 x 10−5; not significantly different from the model: z = 1.3, p = 0.193). Finally, for our third approach, we applied the tissue correction formula outlined in (Gasparovic et al., 2018), which accounts for differences in relaxation times across the tissue types within a voxel. Applying the Gasparovic correction to the GABA+ and Glx values and regressing out the effect of vendor and site did significantly reduce the correlation between GABA+ and Glx, although the correlation remained significant and positive (r218 = 0.183, p = 0.007; difference from model: z = 2.48, p = 0.013).
3.4. Accounting for neurochemicals associated with metabolism significantly reduce the correlation between GABA+ and Glx
Finally, we investigated whether the contributions of any other prominent metabolite signals in the MEGA-PRESS OFF spectra – Cho, NAA, and Cr – could explain our results. Cho, NAA, and Cr are each thought to serve distinct neurobiological functions. Specifically, NAA and Cr are known to be involved in energetics and metabolism of neural tissue (and NAA is often used as a marker of neuronal integrity), while Cho is not thought to be directly involved in metabolism (Andres et al., 2008; Moffett et al., 1991; Öz et al., 2014; Urenjak et al., 1992). Therefore, if NAA and Cr account for the correlation between GABA+ and Glx, it could be inferred that metabolism may mediate the relationship between GABA+ and Glx. On the other hand, if all neurochemicals explain the variation between GABA+ and Glx, one might conclude that the correlation between GABA+ and Glx is explained by their derivation from a common signal.
Our results support the former interpretation (i.e. metabolism, rather than common signal, might explain the observed correlation). We found that controlling for Cho did not significantly reduce the correlation between GABA+ and Glx (z = 0.84, p = 0.4). In contrast, regressing out the concentration of Cr and NAA reduced the correlation between GABA+ and Glx: controlling for Cr significantly reduced the relationship between GABA+ and Glx, although the correlation remained significant and positive (see Table 1; z = 2.7, p = 0.006), and controlling for NAA eliminated the relationship between GABA+ and Glx (see Table 1; z = 3.47, p = 0.0005). Together, these results suggest that the correlation between GABA+ and Glx is not due to their concentration being derived from the same spectra and raise the possibility that overall metabolic activity of the tissue within the MRS voxel might influence (or be influenced by) the concentration of GABA+ and Glx.
4. Discussion
We found that MRS measures of GABA+ and Glx are correlated in the resting human brain in a large sample of healthy adults, which supports the idea that levels of inhibition and excitation are balanced in the healthy human brain. The correlation between GABA+ and Glx could not be explained by differences in data acquisition across sites, scanner vendor, subject demographics, or spectral quality. Additionally, removing variance associated with neurochemicals known to be involved in metabolic activity (NAA and Cr) reduced the correlation between GABA+ and Glx, suggesting that the overall metabolic activity may drive the underlying concentration of GABA+ and Glx. The ratio of GABA+ and Glx is often taken as a proxy for excitatory and inhibitory neurotransmission in the human brain in MRS studies, and our study provides support for the assumption that GABA and Glx occur in balance in healthy controls.
Experimental (Wehr & Zador; Okun 2008; Issacson 2011) and theoretical (Marín, 2012; Rubenstein and Merzenich, 2003; Vogels and Abbott, 2009) studies have suggested that a balance between excitation and inhibition is critical for typical brain function. For example, in electrophysiological studies of sensory-evoked signaling, balanced inhibition is thought to sculpt excitatory signals to promote precise spike timing. Specifically, in rodent auditory cortex, tone-evoked excitatory conductances are rapidly and precisely followed by inhibitory conductances of a similar magnitude (Wehr and Zador, 2003). A tight coupling between excitation and inhibition is also observed during ongoing, spontaneous neural activity. For example, electrophysiological recordings in humans and non-human primates have demonstrated that the temporal linking of excitatory and inhibitory activity occurs in both humans (temporal lobe) and macaques (motor cortex) during both sleep and wakefulness, suggesting that the local balance of excitatory and inhibitory activity is conserved across species, brain areas, and states of consciousness (Dehghani et al., 2016). Collectively, this electrophysiological evidence suggests that the balance of excitatory and inhibitory activity is critical to the normal function of neural systems, and may be pertinent to understanding neurological and neuropsychiatric conditions (Chiu et al., 2018; Johnstone et al., 2018; Lener et al., 2017; Marín, 2012; Rubenstein and Merzenich, 2003). Using edited MRS, our study provides evidence for yet another scale at which excitation and inhibition might be in balance: the concentrations of GABA+ and Glx, which in part reflect inhibitory and excitatory neurotransmitters, appear associated across the population within a region of the resting human brain.
4.1. MRS as a tool for human neuroscience
MRS techniques can be used to obtain in vivo measurements of regional concentrations of neurometabolites, including GABA+ and Glx, in the human brain. A number of findings highlight the biological relevance of MRS measurements of GABA+ and Glx for human neuroscience. First, regional GABA+ and Glx levels are remarkably stable in single-voxel test-retest reliability scans, with coefficients of variation on the order of ~8% (Gaetz et al., 2014; Greenhouse et al., 2016; O’Gorman et al., 2011; Robertson et al., 2016). Importantly, despite this high regional stability, concentrations of GABA+ and Glx are, in general, regionally specific (i.e., an individual’s motor cortex GABA concentration will not necessarily predict their visual cortex GABA concentration (Greenhouse et al., 2016; Puts et al., 2011), although bilateral areas of similar functions show high correspondence (e.g., left and right motor cortex (Bachtiar et al., 2018; Puts et al., 2018)).
Consistent with reports of regional specificity, numerous studies have reported stable relationships between GABA+ or Glx levels and psychophysical performance on tasks that are thought to depend on inhibitory or excitatory neurotransmission in specific brain areas. For example, visual cortex GABA+ levels correlate with orientation discrimination (Edden et al., 2009) and binocular rivalry dynamics (Robertson et al., 2016), somatosensory cortex GABA+ predicts tactile acuity (Kolasinski et al., 2017; Puts et al., 2011), sensorimotor cortex GABA+ correlates with motor performance and cortical excitability measurements (Cassady et al., 2019; Stagg et al., 2011c), and hippocampal GABA+ predicts retrieval performance on memory tasks (Scholl et al., 2017). Further, a number of recent studies have observed compelling changes in GABA+ signals as a result of interventions that are thought to modulate tonic levels of inhibition in specific brain regions. For example, GABA+ signals have been shown to change in response to learning both perceptual and motor tasks (Floyer-Lea et al., 2006; Frangou et al., 2019; Kolasinski et al., 2019; Shibata et al., 2017), as well as after short-term plasticity induction paradigms like eye-patching (Lunghi et al., 2015), theta-burst transcranial magnetic stimulation (Stagg et al., 2009b), and transcranial direct current stimulation (Bachtiar et al., 2018; Barron et al., 2017; Stagg et al., 2009a; Vidal-Piñeiro et al., 2015). Like GABA+, many studies have shown convincing links between regional Glx levels and performance on a variety of tasks (Fujihara et al., 2015; Lacreuse et al., 2018; Robertson et al., 2016). Additionally, studies have found that regional Glx levels are dynamically modulated by tasks. For example, Glx levels in primary visual cortex increase during visual stimulation, and the increase in Glx correlates with neural response measured using BOLD (e.g. (Apšvalka et al., 2015; Ip et al., 2017; Ip et al., 2019); for a comprehensive review and meta-analysis of studies investigating task-modulation of Glx, see (Mullins, 2018)). On the whole, this body of literature demonstrates that regional GABA and Glx levels stably predict psychophysical performance on tasks which are modeled to depend on inhibitory or excitatory neurotransmission in specific brain areas, suggesting that these measurements are relevant to cognitive processes.
The promise of this technique raises the question: what is the neurobiological basis of MRS-measured GABA+ and Glx signals in the brain? GABA, for example, plays at least three distinct roles in neuronal systems: 1) within the neuronal cell body, where it has a role in metabolism (Shulman and Rothman, 1998), 2) in the synaptic terminals, where it’s release provides intermittent (phasic) inhibitory signals (Stagg et al., 2011a), and 3) in the extracellular space between neurons outside of synapses, where it is involved in tonic inhibition (Brickley and Mody, 2012). Like GABA, glutamate and glutamine are components of cellular metabolism and also appear in high concentrations in astrocytes, where glutamine in synthesized from glutamate and ammonia (Albrecht et al., 2010; Pollen and Trachtenberg, 1970), in neurons, where glutamine is hydrolyzed to produce glutamate, and in the extracellular space between neurons outside the synapse (Albrecht et al., 2010; Shulman and Rothman, 1998). Importantly, rather than being confined to discrete roles in neurotransmission and metabolism, GABA and Glx molecules are rapidly shuttled between these roles (Bak et al., 2006; Hertz and Rothman, 2016). Thus, while the MR-visible GABA and Glx signals reflect a combination of molecules relating to metabolism and neurotransmission, the distinction between these two roles is largely uninformative with respect to the MRS-measured concentration.
4.2. Regional metabolism is linked with GABA+ and Glx
What could be the biological basis for a regional correlation between GABA+ and Glx? As discussed above, prior MRS studies have considered GABA+ and Glx as proxies for excitatory and inhibitory neural activity (e.g. (Barron et al., 2017; Bezalel et al., 2019; Koolschijn et al., 2019)). In this view, it is expected that the activity of GABA+ and Glx should co-fluctuate during normal brain function, following the ebb and flow of neural activity (Dehghani et al., 2016), and also be tightly linked to regional neuronal metabolism. Consistent with this hypothesis, in our study, we observed that the only two covariates that significantly reduced the correlation between GABA+ and Glx were two metabolites that are thought to be involved in regional metabolism, NAA and Cr. NAA is present exclusively in neurons (Moffett et al., 1991; Öz et al., 2014; Urenjak et al., 1992). Cr, on the other hand, is more broadly implicated in metabolism in a variety of cell types, both within and outside the central nervous system (Andres et al., 2008). Importantly, we found that Cho, which was also derived from the same spectral acquisition but not related to metabolism (Miller, 1991; Öz et al., 2014), did not explain the relationship between GABA+ and Glx. Together, these results support the notion that regional metabolism could be an important mediator of the relative concentrations of GABA+ and Glx.
4.3. Relationship between age and neurochemical concentrations
Surprisingly, we did not find any impact of age or sex on the relationship between GABA+ and Glx. This was unexpected given the reliably observed age-related decline in GABA (Cassady et al., 2019; Gao et al., 2013; Porges et al., 2017) and glutamate levels (Chang et al., 2009; Sailasuta et al., 2008). We note that this discrepancy might be due to the relatively restricted age range in the Big GABA dataset (18–35 years) compared to those used in prior studies investigating age-related decline. Determining whether there is an alteration in the relationship between Glx and GABA concentrations with age may be an interesting question for future research.
4.4. Limitations and considerations
Our results should be interpreted in light of the following limitations. First, in this study we only obtained one MRS measurement from one voxel location (medial parietal cortex) while participants were resting in the scanner. The medial parietal cortex is a region known to be engaged during the resting state (Murphy et al., 2018; Raichle, 2015; Sormaz et al., 2018), and therefore this single voxel is well positioned for assessing the relationship between GABA+ and Glx during rest. Whether the relationship between GABA+ and Glx holds in other brain regions and during other tasks should be addressed in future studies.
Second, with respect to our analysis methods, we have used linear modeling to control for potential confounding factors. This procedure assumes that the effect of such factors on the measured GABA+ and Glx signals is linear in nature, as opposed to non-linear effects. We have not allowed for the possibility of non-linear effects as a fundamental constraint because we have no a priori reason to assume non-linear interactions of these effects. However, we cannot rule out that non-linear effects may underly some of the unexplained variance in the relationship between GABA+ and Glx.
Third, in the previous sections, we provided details suggesting that the GABA+ and Glx signals are relevant to levels of excitation and inhibition. However, it is important to note that the GABA+ and Glx signals do not purely reflect the neurotransmitters GABA and glutamine. Specifically, the GABA+ signal reflects GABA as well as macromolecules and homocarnosine (Deelchand et al., 2019; Mikkelsen et al., 2019, 2017; Mullins et al., 2014; Puts and Edden, 2012; Rothman et al., 1997). Likewise, the Glx signal reflects both glutamate and glutamine (Puts and Edden, 2012; Ramadan et al., 2013). Therefore, the correlation between GABA+ and Glx MRS signals cannot solely be attributed to the relationship between the neurotransmitters GABA and glutamate. Future work should consider interventions that directly perturb the balance of glutamate and GABA, to more directly assess the sensitivity of MRS to the balance of these neurotransmitters.
Finally, tissue-volume fractions within the MRS voxel also pose challenges for interpreting MRS-derived neurometabolite concentrations. Partial-volume effects affect MRS interpretations in two ways. First, with respect to neural signaling, the balance of excitation and inhibition should be manifest primarily in the gray-matter (as opposed to white-matter or CSF). So, the contribution of signal from the other tissue compartments may add unwanted variance to the GABA+ and Glx signals. Second, signals emanating from gray-matter, white-matter, and CSF have distinct relaxation rates, making the neurochemicals within each compartment differentially visible, particularly at longer TEs (Gasparovic et al., 2006). This problem is exacerbated by the distributed concentration of the neurometabolites across the tissue compartments, which is unknowable (Gasparovic et al., 2018). In our investigation, we corrected for tissue-volume fraction using three different approaches from the literature (Gasparovic et al., 2006; Harris et al., 2015; Stagg et al., 2011b), and the correlation between GABA+ and Glx remained significant across all approaches. However, future work might consider utilizing a sequence with an ultra-short TE and long TR at high-field strength, which would directly mitigate the relaxation effects (rather than applying correction during data processing) (Gasparovic et al., 2018).
5. Conclusion
In conclusion, we observed a correlation between GABA+ and Glx in the human brain in a large sample of human participants, lending support to the hypothesis that excitatory and inhibitory neurotransmitter concentrations are balanced in the human brain. These results open a number of questions. First, how plastic is the relationship between GABA+ and Glx? Multiple studies have observed transient changes in GABA concentration or GABA:Glx ratio during motor (Floyer-Lea et al., 2006; Kolasinski et al., 2019) and perceptual learning (Frangou et al., 2019; Shibata et al., 2017), but whether this change represents a deviation from the normal range of excitation and inhibition is not known. Second, how do E/I ratios relate to brain network architecture? Within an individual, MRS-measured regional GABA concentrations are correlated in related brain areas (Bachtiar et al., 2018; Puts et al., 2018), but not unrelated brain areas (Greenhouse et al., 2016), but whether regional E/I balance is related to brain network architecture remains an open question. Finally, an imbalance in excitation and inhibition is implicated in the neurobiology of multiple neurological conditions (Marín, 2012; Nelson and Valakh, 2015; Robertson et al., 2016; Robertson and Baron-Cohen, 2017; Rosenberg et al., 2015; Rubenstein and Merzenich, 2003; Vattikuti and Chow, 2010). Understanding sources of variation in E/I balance in the typical human brain is an essential first step for furthering psychiatric applications of MRS.
Acknowledgements and funding
The authors would like to thank Yeo Bi Choi for helpful discussion. This work was supported by a grant from the Simons Foundation Autism Research Initiative (SFARI no. 571124) to CER. The Big GABA project was supported by NIH grants R01 EB016089, R01 EB023963 and P41 EB015909; RAEE also receives salary support from these grants.
Footnotes
Data and code availability statement
Data are freely available via nitrc.org. Code will be made available upon request.
Declaration of competing interest
The authors declare no conflict of interest.
CRediT authorship contribution statement
Adam Steel: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Mark Mikkelsen: Data curation, Writing - review & editing. Richard A.E. Edden: Writing - review & editing. Caroline E. Robertson: Conceptualization, Writing - review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.117112.
References
- Albrecht J, Sidoryk-Wȩgrzynowicz M, Zielińska M, Aschner M, 2010. Roles of glutamine in neurotransmission. Neuron Glia Biol. 6, 263–276. 10.1017/S1740925X11000093. [DOI] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR, 2008. Functions and effects of creatine in the central nervous system. Brain Res. Bull 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Apšvalka D, Gadie A, Clemence M, Mullins PG, 2015. Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. Neuroimage 118, 292–300. 10.1016/j.neuroimage.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bachtiar V, Johnstone A, Berrington A, Lemke C, Johansen-Berg H, Emir U, Stagg CJ, 2018. Modulating regional motor cortical excitability with noninvasive brain stimulation results in neurochemical changes in bilateral motor cortices. J. Neurosci 38, 7327–7336. 10.1523/JNEUROSCI.2853-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Waagepetersen HS, 2006. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem 98, 641–653. 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- Barron HC, Vogels TP, Behrens TE, Ramaswami M, Thomas Albright ED, 2017. Inhibitory engrams in perception and memory, 114. 10.1073/pnas.1701812114, 6666–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bezalel V, Paz R, Tal A, 2019. Inhibitory and excitatory mechanisms in the human cingulate-cortex support reinforcement learning: a functional Proton Magnetic Resonance Spectroscopy study. Neuroimage 184, 25–35. 10.1016/j.neuroimage.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I, 2012. Extrasynaptic GABA A receptors: their function in the CNS and implications for disease. Neuron. 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K, Gagnon H, Lalwani P, Simmonite M, Foerster B, Park D, Peltier SJ, Petrou M, Taylor SF, Weissman DH, Seidler RD, Polk TA, 2019. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 186, 234–244. 10.1016/j.neuroimage.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jiang CS, Ernst T, 2009. Effects of age and sex on brain glutamate and other metabolites. Magn. Reson. Imaging 27, 142–145. 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PW, Lui SSY, Hung KSY, Chan RCK, Chan Q, Sham PC, Cheung EFC, Mak HKF, 2018. In vivo gamma-aminobutyric acid and glutamate levels in people with first-episode schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr. Res 193, 295–303. 10.1016/j.schres.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Deelchand DK, Marjańska M, Henry P, Terpstra M, 2019. MEGA-PRESS of GABA+: influences of acquisition parameters. NMR Biomed. 10.1002/nbm.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani N, Peyrache A, Telenczuk B, Le Van Quyen M, Halgren E, Cash SS, Hatsopoulos NG, Destexhe A, 2016. Dynamic balance of excitation and inhibition in human and monkey neocortex. Sci. Rep 6 10.1038/srep23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenthen GS, Barendse EM, Aldenkamp AP, van Veenendaal TM, Puts NAJ, Edden RAE, Zinger S, Thoonen G, Hendriks MPH, Kessels RPC, Jansen JFA, 2016. Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Res. Neuroimaging 256, 44–49. 10.1016/j.pscychresns.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD, 2009. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci 29, 15721–15726. 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ, 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imag 40, 1445–1452. 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Kepecs A, 2019. Annual review of neuroscience interneuron types as attractors and controllers. Rev. Adv. first posted 10.1146/annurev-neuro-070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM, 2006. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol 95, 1639–1644. 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K, 2015. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Frangou P, Emir UE, Karlaftis VM, Nettekoven C, Hinson EL, Larcombe S, Bridge H, Stagg CJ, Kourtzi Z, 2019. Learning to optimize perceptual decisions through suppressive interactions in the human brain. Nat. Commun 10 10.1038/s41467-019-08313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, 2015. Plasticity of cortical excitatory-inhibitory balance. 10.1146/annurev-neuro-071714-034002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara K, Narita K, Suzuki Y, Takei Y, Suda M, Tagawa M, Ujita K, Sakai Y, Narumoto J, Near J, Fukuda M, 2015. Relationship of γ-aminobutyric acid and glutamate+glutamine concentrations in the perigenual anterior cingulate cortex with performance of Cambridge Gambling Task. Neuroimage 109, 102–108. 10.1016/j.neuroimage.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, Roberts TPL, 2014. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji SK, Banerjee A, Patel AM, Zhao YD, Dimitrov IE, Browning JD, Sherwood Brown E, Maher EA, Choi C, 2012. T2 measurement of J-coupled metabolites in the human brain at 3T. NMR Biomed. 25, 523–529. 10.1002/nbm.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RAE, Li M, Puts NAJ, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, 2013. Magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78, 75–82. 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Chen H, Mullins PG, 2018. Errors in 1 H-MRS estimates of brain metabolite concentrations caused by failing to take into account tissue-specific signal relaxation. NMR Biomed. 31, e3914 10.1002/nbm.3914. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA, 2006. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med 55, 1219–1226. 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Noah S, Maddock RJ, Ivry RB, 2016. Individual differences in GABA content are reliable but are not uniform across the human cortex. Neuroimage 139, 1–7. 10.1016/j.neuroimage.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Hu Y, Chen X, He Y, Yang Y, 2019. Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage 185, 388–397. 10.1016/j.neuroimage.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Glaubitz B, Near J, John Evans C, Puts NAJ, Schmidt-Wilcke T, Tegenthoff M, Barker PB, Edden RAE, 2014. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn. Reson. Med 72, 941–948. 10.1002/mrm.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, Edden RAE, 2015. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imag 42, 1431–1440. 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Rothman DL, 2016. Glucose, lactate, β-hydroxybutyrate, acetate, GABA, and succinate as substrates for synthesis of glutamate and GABA in the glutamine-glutamate/GABA cycle. Adv. Neurobiol 13, 9–42. 10.1007/978-3-319-45096-4_2. [DOI] [PubMed] [Google Scholar]
- Ip IB, Berrington A, Hess AT, Parker AJ, Emir UE, Bridge H, 2017. Combined fMRI-MRS acquires simultaneous glutamate and BOLD-fMRI signals in the human brain. Neuroimage 155, 113–119. 10.1016/j.neuroimage.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip IB, Emir UE, Parker AJ, Campbell J, Bridge H, 2019. Comparison of neurochemical and BOLD signal contrast response functions in the human visual cortex. J. Neurosci 39, 7968–7975. 10.1523/JNEUROSCI.3021-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M, 2011. How inhibition shapes cortical activity. Neuron. 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone A, Levenstein JM, Hinson EL, Stagg CJ, 2018. Neurochemical changes underpinning the development of adjunct therapies in recovery after stroke: a role for GABA? J. Cerebr. Blood Flow Metabol 38, 1564–1583. 10.1177/0271678X17727670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP, 2013. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski J, Hinson EL, Zand APD, Rizov A, Emir UE, Stagg CJ, 2019. The dynamics of cortical GABA in human motor learning. J. Physiol 597, 271–282. 10.1113/JP276626@10.1113/(ISSN)1469-7793.EARLY-INVESTIGATOR-PRIZE-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski J, Logan JP, Hinson EL, Makin TR, Emir UE, Stagg Correspondence CJ, 2017. A mechanistic link from GABA to cortical architecture and perception. 10.1016/j.cub.2017.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn RS, Emir UE, Pantelides AC, Nili H, Behrens TEJ, Barron HC, 2019. The Hippocampus and neocortical inhibitory engrams protect against memory interference. Neuron 101, 528–541. 10.1016/j.neuron.2018.11.042 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Moore CM, LaClair M, Payne L, King JA, 2018. Glutamine/glutamate (Glx) concentration in prefrontal cortex predicts reversal learning performance in the marmoset. Behav. Brain Res 346, 11–15. 10.1016/j.bbr.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA, 2017. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatr 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C, Emir UE, Morrone MC, Bridge H, 2015. Short-Term monocular deprivation alters GABA in the adult human visual cortex. Curr. Biol 10.1016/j.cub.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, 2012. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci 13, 107–120. 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneousin vivo spectral editing and water suppression. NMR Biomed. 11, 266–272. . [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, Cecil KM, Chan KL, Chen DY-T, Craven AR, Cuypers K, Dacko M, Duncan NW, Dydak U, Edmondson DA, Ende G, Ersland L, Gao F, Greenhouse I, Harris AD, He N, Heba S, Hoggard N, Hsu T-W, Jansen JFA, Kangarlu A, Lange T, Lebel RM, Li Y, Lin C-YE, Liou J-K, Lirng J-F, Liu F, Ma R, Maes C, Moreno-Ortega M, Murray SO, Noah S, Noeske R, Noseworthy MD, Oeltzschner G, Prisciandaro JJ, Puts NAJ, Roberts TPL, Sack M, Sailasuta N, Saleh MG, Schallmo M-P, Simard N, Swinnen SP, Tegenthoff M, Truong P, Wang G, Wilkinson ID, Wittsack H-J, Xu H, Yan F, Zhang C, Zipunnikov V, Zöllner HJ, Edden RAE, 2017. Big GABA: edited MR spectroscopy at 24 research sites. Neuroimage 159, 32–45. 10.1016/J.NEUROIMAGE.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, Rimbault DL, Barker PB, Bhattacharyya PK, Brix MK, Buur PF, Cecil KM, Chan KL, Chen DYT, Craven AR, Cuypers K, Dacko M, Duncan NW, Dydak U, Edmondson DA, Ende G, Ersland L, Forbes MA, Gao F, Greenhouse I, Harris AD, He N, Heba S, Hoggard N, Hsu TW, Jansen JFA, Kangarlu A, Lange T, Lebel RM, Li Y, Lin CYE, Liou JK, Lirng JF, Liu F, Long JR, Ma R, Maes C, Moreno-Ortega M, Murray SO, Noah S, Noeske R, Noseworthy MD, Oeltzschner G, Porges EC, Prisciandaro JJ, Puts NAJ, Roberts TPL, Sack M, Sailasuta N, Saleh MG, Schallmo MP, Simard N, Stoffers D, Swinnen SP, Tegenthoff M, Truong P, Wang G, Wilkinson ID, Wittsack HJ, Woods AJ, Xu H, Yan F, Zhang C, Zipunnikov V, Zöllner HJ, Edden RAE, 2019. Big GABA II: water-referenced edited MR spectroscopy at 25 research sites. Neuroimage. 10.1016/j.neuroimage.2019.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, Singh KD, Brealy JA, Linden DEJ, Evans CJ, 2016. Quantification of γ-aminobutyric acid (GABA) 1H MRS volumes composed heterogeneously of grey and white matter. NMR Biomed. 29, 1644–1655. 10.1002/nbm.3622. [DOI] [PubMed] [Google Scholar]
- Miller BL, 1991. A review of chemical issues in1H NMR spectroscopy:N-acetyl-l-aspartate, creatine and choline. NMR Biomed. 4, 47–52. 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Namboodiri MA, Cangro CB, Neale JH, 1991. Immunohistochemical localization of N-acetylaspartate in rat brain. Neuroreport 2, 131–134. 10.1097/00001756-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Mullins PG, 2018. Towards a theory of functional magnetic resonance spectroscopy (fMRS): a meta-analysis and discussion of using MRS to measure changes in neurotransmitters in real time. Scand. J. Psychol 59, 91–103. 10.1111/sjop.12411. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Cardiff Symposium on MRS of GABA, C.S. on M. of, Edden, R.A.E, 2014. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 86, 43–52. 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Jefferies E, Rueschemeyer S-A, Sormaz M, Wang H, Margulies DS, Smallwood J, 2018. Distant from input: evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage 171, 393–401. 10.1016/J.NEUROIMAGE.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P, 2015. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn. Reson. Med 73, 44–50. 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Valakh V, 2015. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698. 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E, 2011. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J. Magn. Reson. Imag 33, 1262–1267. 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Lampl I, 2008. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci 11, 535–537. 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- Öz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dinçer A, Dydak U, Emir UE, Frahm J, Gilberto González R, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Hüppi PS, Hurd RE, Kantarci K, Klomp DWJ, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjańska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir N, Pan JW, Peet AC, Poptani H, Poptani H, Posse S, Pouwels PJW, Ratai EM, Ross BD, Scheenen TWJ, Schuster C, Smith ICP, Soher BJ, Tkáč I, Vigneron DB, Kauppinen RA, 2014. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 10.1148/radiol.13130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC, 2019. Nlme: Linear and Nonlinear Mixed Effects Models. [Google Scholar]
- Pollen DA, Trachtenberg MC, 1970. Neuroglia: gliosis and focal epilepsy. Science 167, 1252–1253. 10.1126/science.167.3922.1252, 80. [DOI] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Lamb DG, Williamson JB, Cohen RA, Edden RAE, Harris AD, 2017. Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage 162, 249–256. 10.1016/j.neuroimage.2017.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry P-G, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim KO, Alger JR, 2007. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn. Reson. Med 58, 236–244. 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, 2012. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog. Nucl. Magn. Reson. Spectrosc 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, John Evans C, McGlone F, McGonigle DJ, 2011. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci 31, 16556–16560. 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Heba S, Harris AD, Evans CJ, McGonigle DJ, Tegenthoff M, Schmidt-Wilcke T, Edden RAE, 2018. GABA levels in left and right sensorimotor cortex correlate across individuals. Biomedicines 6. 10.3390/biomedicines6030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computingitle. [Google Scholar]
- Raichle ME, 2015. The brain’s default mode network. Annu. Rev. Neurosci 38, 433–447. 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Ramadan S, Lin A, Stanwell P, 2013. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed. 10.1002/nbm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Baron-Cohen S, 2017. Sensory perception in autism. Nat. Rev. Neurosci 18, 671–684. 10.1038/nrn.2017.112. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Ratai E-M, Kanwisher N, 2016. Reduced GABAergic action in the autistic brain. Curr. Biol 26, 80–85. 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Rosenberg A, Patterson JS, Angelaki DE, 2015. A computational perspective on autism. Proc. Natl. Acad. Sci 112, 9158–9165. 10.1073/pnas.1510583112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Prichard JW, Petroff OAC, 1997. Homocarnosine and the measurement of neuronal pH in patients with epilepsy. Magn. Reson. Med 38, 924–929. 10.1002/mrm.1910380611. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM, 2003. Model of autism: increased ratio of excitation/inhibition in key neural systems. Gene Brain Behav. 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Ernst T, Chang L, 2008. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn. Reson. Imaging 26, 667–675. 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl J, Kolling N, Nelissen N, Stagg CJ, Harmer CJ, Rushworth MFS, 2017. Excitation and inhibition in anterior cingulate predict use of past experiences. Elife 6. 10.7554/eLife.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Sasaki Y, Bang JW, Walsh EG, Machizawa MG, Tamaki M, Chang L-H, Watanabe T, 2017. Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant. Nat. Neurosci 20, 470–475. 10.1038/nn.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, 1998. Interpreting functional imaging studies in terms of neurotransmitter cycling. Proc. Natl. Acad. Sci. U.S.A 95, 11993–11998. 10.1073/pnas.95.20.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonite M, Carp J, Foerster BR, Ossher L, Petrou M, Weissman DH, Polk TA, 2019. Age-related declines in occipital GABA are associated with reduced fluid processing ability. Acad. Radiol 26, 1053–1061. 10.1016/j.acra.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormaz M, Murphy C, Wang HT, Hymers M, Karapanagiotidis T, Poerio G, Margulies DS, Jefferies E, Smallwood J, 2018. Default mode network can support the level of detail in experience during active task states. Proc. Natl. Acad. Sci. U.S.A 115, 9318–9323. 10.1073/pnas.1721259115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011a. What are we measuring with GABA Magnetic Resonance Spectroscopy? Commun. Integr. Biol 4, 573–575. 10.4161/cib.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011b. The role of GABA in human motor learning. Curr. Biol 21, 480–484. 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kineses ZT, Morris PG, Matthews PM, Johansen-Berg H, 2009a. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci 29, 5202–5206. 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno Moreno L, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC, 2011c. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol 589, 5845–5855. 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, Bestmann S, 2009b. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J. Neurophysiol 101, 2872–2877. 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M, 1992. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J. Neurochem 59, 55–61. 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- Vattikuti S, Chow CC, 2010. A computational model for cerebral cortical dysfunction in autism spectrum disorders. Biol. Psychiatr 67, 672–678. 10.1016/j.biopsych.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Piñeiro D, Martín-Trias P, Falcón C, Bargalló N, Clemente IC, Valls-Solé J, Junqué C, Pascual-Leone A, Bartrés-Faz D, 2015. Neurochemical modulation in posteromedial default-mode network cortex induced by transcranial magnetic stimulation. Brain Stimul 8, 937–944. 10.1016/j.brs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Vogels TP, Abbott LF, 2009. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat. Neurosci 12, 483–491. 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM, 2003. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–446. 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]


