Abstract
Background
Despite upscaled control efforts, deaths and hospitalization due to malaria remained high in counties of western Kenya highlands.
Objectives
This study assessed the knowledge of malaria in two rural communities, the control strategies they use, and their capacity to integrate the available control programs.
Methods
A cross-sectional survey was carried out in two rural villages in November – December 2018. Focus group discussions and a questionnaire survey were carried out in 736 households. Frequencies and proportions were used for descriptive analysis while the Chi-square test was used to determine factors that were associated with knowledge of malaria at p ≤ 0.05
Results
Ninety-seven percent of the respondents had knowledge of malaria and this was associated with the level of education attained (χ2 = 30.108; p > 0.0001). Bed net ownership was at 86% and 92% correctly identified its use. Draining stagnant water (53.9%) was the most cited environmental management practice.
Conclusion
There was awareness of the risk factors of malaria transmission in the study sites. The local communities must be mobilized and empowered through EIC for the control practises to bear fruit against malaria transmission. However, more sensitization needs to be done to optimize the use of malaria control practices.
Keywords: Malaria, control practices, Kenya highlands, Mining
Introduction
Malaria is still reported in many areas around the world even with concerted efforts to control the transmission. In 2016 Sub-Saharan Africa (SSA) reported 90% of the cases1. In Kenya, the control practices are widespread in malaria-endemic zones where malaria prevalence remains high2. Despite these control efforts, deaths from malaria and hospitalization due to the disease are still high especially in highlands west of the Rift Valley3. This suggests that there could be a stagnation in the fight against the malaria scourge. This calls for newer and more effective control tools and practices to shore up the existing ones without reversing the gains already made. The focus has been more on the long-lasting insecticidal nets (LLINs) and IRS. Other strategies can be incorporated and implemented at the individual household level to significantly limit human-vector interaction4. Besides environmental management and bed net use, perceptions and knowledge of the people enhance efforts to eliminate the vector5.
The socio-economic abilities of individuals and populations have a crucial role to play in the malaria cycle6. Poverty is a major cause of unnecessary deaths due to malaria and other public health issues7. Economic status determines the success or failure of a program by an individual or government since impoverished households would not consume services that would otherwise protect them against the malaria burden. Artisanal gold mining and farming are socio-economic activities that alter the landscape and have been implicated in malaria transmission8,9 Besides, artisanal mining is associated with the poor10 whereas sugarcane farming puts a constraint on available farmland available for other activities11. Available data suggest that the poorest people remain least likely to adopt recommended disease control practices and get treatment. Factors that prevent access to this treatment are not well documented12. Therefore, poorly managed water holding pans such as mines, fish ponds, burrow pits, and drainages in Rosterman mines and Eluche regions create ideal breeding habitats for Anopheles, thus contributing to malaria transmission. A previous study in the two sites implicated the availability of aquatic habitats to the proliferation of Anopheles13. Malaria remains a major public concern in the two sites and other parts of the Western Kenya highlands14. The missing link between the resurgence of malaria in the Western Kenya highlands and these economic activities requires investigation to assess the success of the control programs available.
People of the same economic standing live in similar house settings15. Poorly constructed dwellings enable the mosquito vector to gain access to the dwellers16 through cracks in walls/roofs, eaves, and unscreened windows and doors. Entry of the vector into human dwellings is also because of the bodily odors and heat generated from within17. Houses that are squeezed with more occupants attract more of the vector than spacious ones with few occupants18. Additionally, it has been reported that living space for poor families may not be well separated from the domestic and that this animals' body heat also attracts mosquitoes19. Therefore, structural factors complicate the control practices put in place by many households. Dwellings with more members sleeping together are characteristic of poverty levels and even with the availability of the mosquito net, proper use becomes another challenge20.
Despite ownership of the bed net rising to above 80% in the last decade21 malaria cases have remained high. Under the universal coverage and usage in SSA, the net is meant to prevent host-vector interaction by preventing access of the nocturnal anopheles vector, repelling or killing it. However, a gap exists between possession and proper use of bed nets20. Therefore, an effort is required to change people's behavior by providing accurate information. Besides the net, other control practices including traditional methods16 have to be integrated to reduce the transmission of malaria. Household and community ability to implement and sustain these malaria control strategies are of great significance to the rollback malaria initiative. Little is available on the knowledge, attitudes, and practices (KAP) among populations in mining and sugarcane growing areas about malaria and the control methods they employ in the prevention of the disease. This study endeavored to find out this missing link in the fight against malaria in two rural communities of Western Kenya highlands. The findings will give crucial information to authorities on the success of malaria control programs based on how the communities consider malaria to be a public health concern to them and if they have the skills and knowledge to participate in its prevention.
Methods
This was a pilot cross-sectional study carried out in November – December 2018 as part of a wider study. Rosterman mines (Latitude 0.2833°N, Longitude 34.7500°E) at 1400 - 1500 meters above sea level (asl) and Eluche (Latitude 0.33511°N, Longitude 34.4864°E) at 1300 - 1400 meters asl, are two rural villages in Kakamega county, western Kenya. The communities' economic activities include mixed farming and rearing livestock, artisanal mining, sand harvesting, and small-scale business. These land modifying activities create water-holding pits in which Anopheles breed and hence sustain malaria transmission in the population13. Focus group discussions (FGDs), and a questionnaire survey were used. FGDs were composed of 3 to 7 members who were purposely selected to include village elders, opinion leaders, and community health volunteers (CHVs), and a moderator from the research team. Each group met for one hour, three times during the study period. Participants who were adult Kenyans of both sexes, residents of the two areas, and those willing to contribute, were included in the FGDs. Since the FGDs' themes were open and not gender or culturally sensitive, the data collected from them was not confidential. The questionnaire sought to find out: socio-demographic characteristics of the households, knowledge of the household head about malaria (source of information about malaria, cause, and transmission), mosquito breeding sites, preventive measures (short and long term), and availability of the net and its use. It was pre-tested, translated to the local dialect, and was verbally translated to Kiswahili to those who had difficulties with English or local dialect. The interviewers were trained on approaching, consenting, and filling the questionnaire. This was systematically done house to house giving a total of 736 households. For quality control, a supervisory meeting was held between the principal researchers and the field team after each session.the sessions were used for standardization of responses, clarity of expectations, and conformation of responses.
Data management and analysis
Collected data were entered in MS Excel spreadsheet, checked and cleaned of errors and inconsistencies. After which it was coded and processed using statistical package for social sciences (SPSS) version 20. Frequencies and proportions were used for descriptive analysis of the data. Logistic regressions were done for selected risk factors of interest. Odds ratios (OR) and the Chisquare tests were computed to determine the strength of association at 95% CI and p ≤ 0.05. Data collected from FGDs were observational and qualitative.
Ethical considerations
Ethical clearance to undertake the study was granted by MasindeMuliro University of Science and Technology Institutional Ethical Review Committee (IERC) vide approval number MMUST/IERC/090/19. Permission to proceed with the study was given by National and Kakamega County administrative authorities. The participants signed the coded consent forms before proceeding to fill the questionnaires after the objective and methodology had been explained to them. The coding and filling was done in the participants homes for confidentiality purposes. Responders were informed that there was no direct monetary benefit and absence and the unwillingness of a resident to participate excluded them from the process.
Results
Socio-demographic characteristics of the participants
The socio-demographic variables were distributed among participants as shown in Table 1. Of the participants in both FGDs and interviews, 56.5% of the cumulative total were female, 70.2% were married, 39.8% attained post-primary education, and 32.5% were in formal and informal employment.
Table 1.
Socio-demographic characteristics of the household heads surveyed
| Variable | Category | Frequency | Percent | Cumulative Total | |||
| Rosterman | Eluche | N | % | ||||
|
|
|||||||
| N | % | N | % | ||||
| Gender | Male | 191 | 50.3 | 129 | 36.2 | 320 | 43.5 |
| Female | 189 | 49.7 | 227 | 63.8 | 416 | 56.5 | |
| Marital status | |||||||
| Unmarried | 74 | 19.5 | 49 | 13.8 | 123 | 16.7 | |
| Married | 256 | 67.4 | 261 | 73.3 | 517 | 70.2 | |
| Widowed | 43 | 11.3 | 44 | 12.4 | 87 | 11.8 | |
| Divorced | 7 | 1.8 | 2 | 0.6 | 9 | 1.2 | |
| Level of education |
|||||||
| No education | 48 | 12.6 | 62 | 17.4 | 110 | 14.9 | |
| Primary | 160 | 42.1 | 173 | 48.6 | 333 | 45.2 | |
| Secondary | 129 | 33.9 | 101 | 28.4 | 230 | 31.2 | |
| Tertiary | 43 | 11.3 | 20 | 5.6 | 63 | 8.6 | |
| Others | |||||||
| Occupation | |||||||
| Self-employed in agriculture |
72 | 18.9 | 91 | 25.6 | 163 | 22.1 | |
| Self-employed in business |
85 | 22.4 | 57 | 16.0 | 142 | 19.3 | |
| Housewife | 82 | 21.6 | 37 | 10.4 | 119 | 16.2 | |
| Employed | 101 | 26.6 | 138 | 38.8 | 239 | 32.5 | |
| Unemployed | 40 | 10.5 | 33 | 9.3 | 73 | 9.9 | |
Assessment of household's head knowledge about malaria
To assess the KAP of the head of the household on malaria, seven questions on the source of information about malaria, cause of malaria, mode of transmission, methods of prevention, mosquito breeding sites, signs of malaria, and the mosquito net were asked. Participants who gave at least three correct responses to the first five key questions were considered knowledgeable enough. Ninety-seven percent (n = 719) of the total respondents admitted to having information about malaria with the radio/television the most reported source. The role of community health workers (CHVs) was highly pronounced in the FGDs:
“Following the increase of CHVs in our village, we now have people to ask questions about malaria and other illnesses,” said Rosterman village pastor.
Plasmodium (52.9%) was the most cited cause of malaria followed by germs (17.7%), bites by the mosquito (17.0%), and dirty stagnant water (4.8%) [Table 2]. There was an association between socio-demographics and knowledge of the cause of malaria (χ2 = 30.108; p > 0.0001). On the transmission of the malaria parasite, a bite by any mosquito was 51.0% followed by a bite of a mosquito that had bitten a malaria patient at 33.8%. There was a significant association between socio-demographics and knowledge of malaria parasite transmission (χ2 = 15.663; p = 0.001). About knowledge of mosquito breeding sites, 55.0% cited bushes followed by others (water-holding containers, tanks) at 25.1%, tall grass 12.6%, and stagnant water 7.2%. There was a significant association between socio-demographics of respondents and knowledge of the mosquitoes breeding site (χ2 = 17.696; p > 0.0001).
Table 2.
Respondents' knowledge on the cause of malaria, transmission, and breeding sites of the vector
| Variables | Rosterman | Eluche | |||||
|
|
|||||||
| Response | N | % | N | % | χ2 | P-value | |
| Cause of malaria | Germs | 73 | 19.2 | 57 | 16.3 | 30.11 (3df) |
0.000 |
| Dirty stagnant water |
24 | 6.3 | 11 | 3.1 | |||
| Mosquito bites | 82 | 21.6 | 43 | 12.1 | |||
| Plasmodium | 185 | 48.7 | 204 | 57.3 | |||
| Does not know | 16 | 4.2 | 41 | 11.5 | |||
| Ways of parasite entry |
By bites of any mosquito |
204 | 53.7 | 171 | 48.0 | 15.66 (3df) |
0.001 |
| By bites of mosquito which has bitten a malaria patient |
111 | 29.2 | 138 | 38.8 | |||
| Others | 41 | 10.8 | 17 | 4.8 | |||
| Do not know | 24 | 6.3 | 30 | 8.4 | |||
| Mosquito breeding sites |
Stagnant water | 41 | 10.8 | 12 | 3.4 | 17.70 (3df) |
0.000 |
| Tall grass | 53 | 13.9 | 40 | 11.2 | |||
| Bushes | 199 | 52.4 | 206 | 57.9 | |||
| Others | 87 | 22.9 | 98 | 27.5 | |||
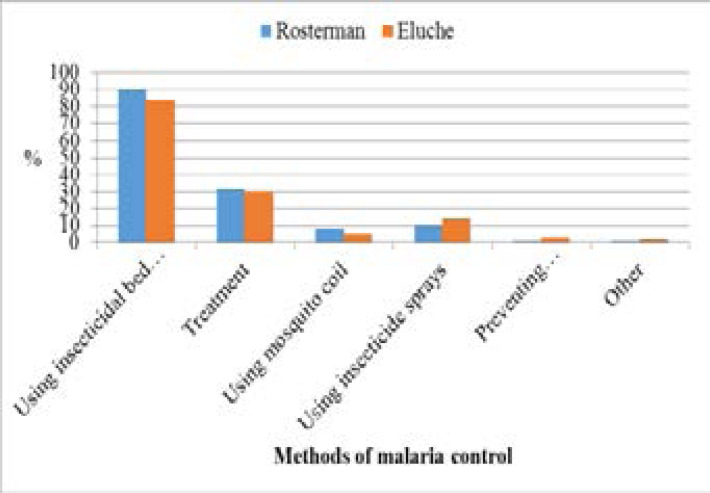
Over 95% of the respondents were able to identify at least three symptoms of malaria. They correctly identified shivering, headache, sweating, vomiting, loss of appetite, joint pains, high fever, and feeling cold as the most notable symptoms of malaria that compelled them to seek medical assistance or buy antimalarials. The majority of those interviewed had proper knowledge about how to prevent malaria, with the bed net the most mentioned method [Figure 1]. However, in the FGDs it was reported that mosquitoes bit them at odd hours:
Figure 1.
Respondents' communicated malaria control methods
“The mosquitoes bite when we are seated out selling our wares like vegetables or when milking or in the kitchen cooking,” said Mama Mboga from Eluche
However, fears about net use arose particularly from members of FGDs where they reported that they feared the bed nets would catch:
“Our bed nets can catch fire from the cooking place adjacent to the bed or from the oil lamps we use. This puts us in a dangerous situation,” opined a village elder from Rosterman.
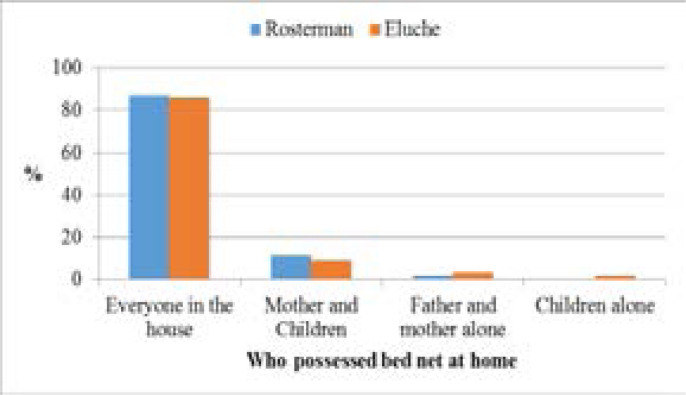
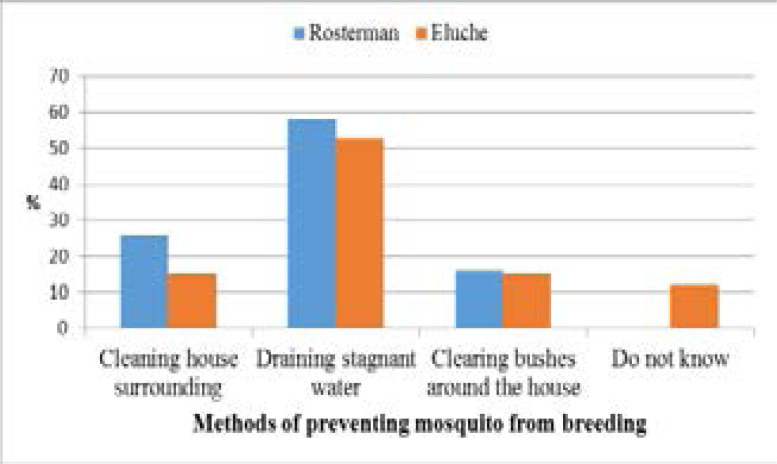
Figure 2 shows Net ownership in sampled households. Ninety-seven percent of the homesteads with the bed net had the long-lasting insecticide-treated (LLIN) type which was bought, given by health campaigners, or given at health centers (during neonatal or postnatal care). In both study sites, preventing breeding/resting places for mosquitoes received the second least responses despite featuring prominently in known environmental management methods in malaria control [Figure 3]. Draining stagnant water was the most used method outdoors to prevent the mosquito from breeding and as a result control malaria transmission. Level of education played a significant role (p = 0.046) and gender being of no notable significance (p = 0.229) in malaria control.
Figure 2.
Respondents' net ownership
Figure 3.
Known and Used Environmental Management Methods
An observed challenge to proper environment management aimed at reducing mosquito breeding sites was the socio-economic activities of the two communities. This was addressed by a member in one of the FGDs.
“We are faced with a dilemma about draining stagnant water and our daily gold mining activities. The gold mines are our sources of our daily food and we may not fill them” opined the village elder in Rosterman.
Regression analysis showed that demographic factors had no impact on the knowledge about methods of malaria prevention and control as summarized in Table 3. Ownership and use of the bed net, the choice of the environment management practice employed by a household, and applying IRS were in no way associated with socio-demographic characteristics.
Table 3.
Demographic variables and their impact on knowledge about methods of malaria prevention and control
| Variables | N | Odds Ratio | 95% CI | P value |
|
Factors associated with knowledge of bed nets |
||||
| Age | ||||
| ≤19 | 77 | 0.114 | 0.012–1.062 | 0.056 |
| 20–39 | 340 | 0.275 | 0.026–2.924 | 0.284 |
| 40–59 | 205 | 0.485 | 0.485–4.4745 | 0.534 |
| ≥60 | 114 | 1.000 | 0.031–4.123 | 0.271 |
| Education | ||||
| No education | 110 | 1.311 | 0.127–13.498 | 0.820 |
| Primary | 333 | 1.166 | 0.138–9.824 | 0.888 |
| Secondary | 230 | 1.238 | 0.140–10.959 | 0.848 |
| Tertiary qualifications | 63 | 1.000 | 0.171–9.628 | 0.812 |
|
Factors associated with knowledge of malaria |
||||
| Gender | ||||
| Male | 320 | 0.782 | 0.518–9.212 | 0.287 |
| Female | 416 | -0.782 | 0.109–1.929 | 0.287 |
| Education | ||||
| No education | 110 | 17.205 | 0.000 | 0.848 |
| Primary | 333 | 16.467 | 0.000 | 0.996 |
| Secondary | 230 | 0.000 | 0.000 | 0.997 |
| Tertiary qualifications | 63 | 1.000 | 0.000 | 0.837 |
|
Factors associated with environmental management |
||||
| Gender | ||||
| Male | 320 | -0.015 | 0.555–1.750 | 0.959 |
| Female | 416 | 0.015 | 0.959–1.015 | 0.959 |
| Education | ||||
| No education | 110 | 1.930 | 0.868–54.680 | 0.068 |
| Primary | 333 | 1.699 | 0.730–41.013 | 0.098 |
| Secondary | 230 | 1.228 | 0.435–26.761 | 0.243 |
| Tertiary qualifications | 63 | 1.318 | 0.623–28.217 | 0.378 |
Discussion
The FGDs were good sources of information about knowledge of malaria and control practices employed in the study sites. Participants attributed transmission to a confluence of numerous factors among them was lack and inappropriate use of personal protection during the night. In the FGDs participants reported that they were normally bitten by mosquitoes while in the house during supper time when watching the news, or chatting before they slept and during sleeping. This corroborates findings of other studies in Nyabondo, Kenya16, and Yaounde, Cameroon22. The Anopheles mosquito is an efficient vector and has adapted unique behavior enabling it to feed on humans while in their dwellings23 or during their evening chores outside. Findings in Equatorial Guinea24 attributed this to indoor interventions like spraying with insecticides and the LLINs. The findings showed that the communities acknowledge that malaria remains a major threat on life.
The findings showed that education and economic levels had a significant association with knowledge of malaria, its transmission, and methods employed to control it. This supported other studies carried out in South Africa25, where individuals with low economic status had suffered from malaria in the past. In the Democratic Republic of Congo (DRC), a study showed a similar trend in which people in dire economic status and rural areas were at a higher risk of getting infected than other groups26.
In the present study, respondents acknowledged having prior information about malaria and that they were only recipients and not formulators of information about malaria control. A variety of sources were reported including radio/television, health workers, family/friends, school, religious/administrative meetings, and print media. The reception of information from multiple sources supports another study done elsewhere27. The knowledge gained by the responders was immense in understanding the interaction between the disease, the vector, the environment, and the human population. In one FGD, it was observed that the community did not participate in the formulation and ownership of the control programs or messages about malaria but they were only recipients.
Having relevant EIC about malaria is a major cornerstone to its control. This study found out that both providers and recipients of information about malaria had relevant knowledge about appropriate control methods at a personal and communal level. The bed net ownership was above the national average and its use was reported by the majority of respondents. This corroborates findings from another study in which the bed net reduced interaction between the human and the vector during sleep28. Appropriate net use goes a significant way to reduce malaria bouts among people. The availability of the net in both regions was attributed to government and Non – G/span>overnmental Organizations' campaigns in the distribution. Getting the LLIN by the respondent was dependent on the availability of the respondent during the time of distribution. Additionally, the net was given to expectant mothers and children under 5 years during visits to the health facilities. However, it can be reported that the high LLIN availability and use did not match the knowledge of the transmission and prevention strategies for malaria by respondents. This scenario has also been reported in another study in Bangladesh29. However, it can also be reported that knowledge uptake can be hampered by indecision or misinformation. For instance, in FGDs members said they feared the nets could easily catch fire from the cooking place adjacent to the bed or the oil lamps they use as a source of light. Therefore, the community health workers must work in homes and assist in teaching the respondents on aspects like hanging and using the LLIN30. Proper use of the net is critical in reducing transmission of malaria and comprehensive information should reach the communities regularly. Other personal protection methods such as the use of mosquito coil, repellents, or spraying were sparsely mentioned by the respondents or during the FGDs. This could be attributed to a lack of proper awareness and communication. This has been reported in another study as a possible limiting factor to the success of reducing malaria transmission16.
Knowledge of the vector and proper use of vector control methods is very crucial in cutting transmission16. Availability of mosquito breeding grounds like derelict mining pits abandoned fish ponds, and poorly managed drainages that are too close to human dwellings enhance malaria transmission. Human and natural, permanent, and localized pits that hold water are known sources of vector all year round31. This study reported that there is higher awareness and use of appropriate environmental management interventions at the individual household level. In as much as draining stagnant water, clearing tall grasses and bushes, and keeping the environment around the house clean were reported to reduce considerably the number of mosquitoes available at a particular time or season, man-made and permanent water bodies in the study sites may drag the success of these interventions in combating malaria transmission as reported in FGDs. This contradicts another study that indicated that these practices are ineffective in reducing populations of Anopheles unless there is inter-sectoral collaboration in the management of the environment32. The success of these methods depends on the feeding characteristics of the species of Anopheles found in the study sites. Endophilic Anopheles rest indoors and therefore defeat the use of these malaria control practices33. In this, FGDs members noted that mosquito numbers are highest when maize plantations are blossoming and every available space is green with vegetation. This indicated a lack of knowledge that this occurrence coincided with the rainy season when breeding sites are readily avilable. As was also reported by a study in Brazil34. The success of environmental management methods of malaria control depends on engaging all the households. For instance, draining of stagnant water would be defeated if some households do not participate because mosquitoes would breed in the stagnant water in their jurisdiction. The findings of this study suggested that these methods must be owned and maintained through participation and sustained through continuous education to effectively mitigate malaria transmission.
Conclusion and Recommendations
This study reported awareness of the risk factors of malaria transmission in the two rural villages at both household and community levels. Knowledge about the connection between socio-demographic factors, environmental management, mosquito breeding, and resting sites, and malaria transmission is key in fore-stalling malaria transmission. The local communities must be mobilized and empowered through EIC for the control practises to bear fruit against malaria transmission. Therefore, efforts should be channelled at including as many people as possible in the fight against malaria to embrace individual household and communal participation. This study recommended that during education promotions and sensitization campaigns about malaria, emphasis should be laid on educating recipients on integrated control activities that result in a reduction in mosquito density enabling proper use of an individual's time and meager resources.
Acknowledgments
We acknowledge the contributions of CarolyneNjuguna, Eric Alusiola, RedemptaMboyi, and Kevin Ashuma for carrying out the interviews and filling the questionnaires. A lot of appreciation goes to village administrators/elders for mobilizing participants and the entire people of Rosterman and Eluche for according us time to carry out the study.
Competing interests
The authors declare that they have no competing interests.
Author contributions
Mukabane, Kitungulu, and Mulama conceived, planned, and designed the study, Mukabane, and Kitungulu prepared the questionnaire and conducted the interviews, Ogutu supervised the interviews, Mukabane and Kitungulu conducted the analysis and wrote the manuscript, Mukabane, Ogutu, Cheruiyot, and Mulama participated in editing the paper. All authors have read and approved the final manuscript.
References
- 1.WHO, author. Fact Sheet: World Malaria Report 2016. 2019. [cited 2019 Nov 5]. Available from: http://www.who.int/malaria/media/world-malaria-report-2016/en/
- 2.Snow RW, Kibuchi E, Karuri SW, Sang G, Gitonga CW, Mwandawiro C, et al. Changing Malaria Prevalence on the Kenyan Coast since 1974: Climate, Drugs and Vector Control. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128792. [cited 2020 Jan 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degefa T, Yewhalaw D, Zhou G, Lee M, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malaria Journal. 2017 Nov 6;16(1):443. doi: 10.1186/s12936-017-2098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochomo E, Chahilu M, Cook J, Kinyari T, Bayoh NM, West P, et al. Insecticide-Treated Nets and Protection against Insecticide-Resistant Malaria Vectors in Western Kenya. Emerging Infectious Diseases. 2017 May;23(5):758. doi: 10.3201/eid2305.161315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO, author. Handbook for integrated vector management. 2012. [cited 2018 Aug 17]; Available from: http://apps.who.int/iris/handle/10665/44768.
- 6.Nyarko SH, Cobblah A. Sociodemographic Determinants of Malaria among Under-Five Children in Ghana. Malaria Research and Treatment. 2014 doi: 10.1155/2014/304361. [cited 2020 Jan 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricci F. Social implications of malaria and their relationships with poverty. Mediterranean Journal of Hematology and Infectious Diseases. 2012 Aug 9;4(1) doi: 10.4084/MJHID.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez JF, Carnero AM, Rivera E, Rosales LA, Baldeviano GC, Asencios JL, et al. Unstable Malaria Transmission in the Southern Peruvian Amazon and Its Association with Gold Mining, Madre de Dios, 2001–2012. American Journal of Tropical Medicine and Hygiene. 2017 Feb 8;96(2):304–311. doi: 10.4269/ajtmh.16-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mboera LEG, Shayo EH, Senkoro KP, Rumisha SF, Mlozi MRS, Mayala BK. Knowledge, perceptions and practices of farming communities on linkages between malaria and agriculture in Mvomero District, Tanzania. ActaTropica. 2010 Feb 1;113(2):139–144. doi: 10.1016/j.actatropica.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Siegel S, Veiga MM. The myth of alternative livelihoods: artisanal mining, gold and poverty. International Journal of Environment and Pollution. 2010 Jan 1;41(3–4):272–288. [Google Scholar]
- 11.Egesah OB. The socio-economic effects of sugarcane farming on smallholders in North Bunyala, Kakamega. University of Nairobi; 1994. [Google Scholar]
- 12.Chuma J, Okungu V, Molyneux C. Barriers to prompt and effective malaria treatment among the poorest population in Kenya. Malaria Journal. 2010 May 27;9:144. doi: 10.1186/1475-2875-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholas K, Bernard G, Bryson N, Mukabane K, Kilongosi M, Ayuya S, et al. Abundance and Distribution of Malaria Vectors in Various Aquatic Habitats and Land Use Types in Kakamega County, Highlands of Western Kenya. Ethiopian Journal of Health Sciences. 2021 Mar;31(2):247–256. doi: 10.4314/ejhs.v31i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapesa A, Kweka EJ, Atieli H, Kamugisha E, Zhou G, Githeko AK, et al. Why some sites are responding better to anti-malarial interventions? A case study from western Kenya. Malaria Journal. 2017 Dec 29;16(1):498. doi: 10.1186/s12936-017-2145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lwetoijera DW, Kiware SS, Mageni ZD, Dongus S, Harris C, Devine GJ, et al. A need for better housing to further reduce indoor malaria transmission in areas with high bed net coverage. Parasites and Vectors. 2013 Dec;6(1):1–9. doi: 10.1186/1756-3305-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng'ang'a PN, Mutunga J, Oliech G, Mutero CM. Community knowledge and perceptions on malaria prevention and house screening in Nyabondo, Western Kenya. BMC Public Health. 2019 Dec;19(1):423. doi: 10.1186/s12889-019-6723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raji JI, DeGennaro M. Genetic analysis of mosquito detection of humans. Current Opinion in Insect Science. 2017 Apr 1;20:34–38. doi: 10.1016/j.cois.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, et al. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malaria Journal. 2008 Jan 7;7(1):2. doi: 10.1186/1475-2875-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GEL, Emerson PM. Changes in house design reduce exposure to malaria mosquitoes. Tropical Medicine and International Health. 2003 Jun;8(6):512–517. doi: 10.1046/j.1365-3156.2003.01059.x. [DOI] [PubMed] [Google Scholar]
- 20.Toé LP, Skovmand O, Dabiré KR, Diabaté A, Diallo Y, Guiguemdé TR, et al. Decreased motivation in the use of insecticide-treated nets in a malaria endemic area in Burkina Faso. Malaria Journal. 2009 Jul 29;8:175. doi: 10.1186/1475-2875-8-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndenga BA, Mulaya NL, Musaki SK, Shiroko JN, Dongus S, Fillinger U. Malaria vectors and their bloodmeal sources in an area of high bed net ownership in the western Kenya highlands. Malaria Journal. 2016 Feb 9;15(1):76. doi: 10.1186/s12936-016-1115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talipouo A, Ngadjeu CS, Doumbe-Belisse P, Djamouko-Djonkam L, Sonhafouo-Chiana N, Kopya E, et al. Malaria prevention in the city of Yaoundé: knowledge and practices of urban dwellers. Malaria Journal. 2019 Dec;18(1):167. doi: 10.1186/s12936-019-2799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pates H, Curtis C. Mosquito behavior and vector control. Annuals Review of Entomology. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 24.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malaria Journal. 2011 Jul 7;10(1):184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutegeki E, Chimbari MJ, Mukaratirwa S. Assessment of individual and household malaria risk factors among women in a South African village. ActaTropica. 2017 Nov;175:71–77. doi: 10.1016/j.actatropica.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Ngatu NR, Kanbara S, Renzaho A, Wumba R, Mbelambela EP, Muchanga SMJ, et al. Environmental and sociodemographic factors associated with household malaria burden in the Congo. Malaria Journal. 2019 Feb 26;18(1):53. doi: 10.1186/s12936-019-2679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umeano-Enemuoh JC, Uzochukwu B, Ezumah N, Mangham-Jefferies L, Wiseman V, Onwujekwe O. A qualitative study on health workers' and community members' perceived sources, role of information and communication on malaria treatment, prevention and control in southeast Nigeria. BMC Infectious Diseases. 2015 Oct 22;15(1):437. doi: 10.1186/s12879-015-1187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst KC, Hayden MH, Olsen H, Cavanaugh JL, Ruberto I, Agawo M, et al. Comparing ownership and use of bed nets at two sites with differential malaria transmission in western Kenya. Malaria Journal. 2016 Apr 14;15(1):217. doi: 10.1186/s12936-016-1262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed SM, Hossain S, Kabir MM, Roy S. Free distribution of insecticidal bed nets improves possession and preferential use by households and is equitable: findings from two cross-sectional surveys in thirteen malaria endemic districts of Bangladesh. Malaria Journal. 2011 Dec 13;10(1):357. doi: 10.1186/1475-2875-10-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos EM, Coalson JE, Jacobs ET, Klimentidis YC, Munga S, Agawo M, et al. Bed net care practices and associated factors in western Kenya. Malaria Journal. 2019 Aug 14;18(1):274. doi: 10.1186/s12936-019-2908-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mala AO, Irungu LW, Shililu JI, Muturi EJ, Mbogo CC, Njagi JK, et al. Dry season ecology of Anopheles gambiae complex mosquitoes at larval habitats in two traditionally semi-arid villages in Baringo, Kenya. Parasites and Vectors. 2011 Feb 28;4(1):25. doi: 10.1186/1756-3305-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S. Community-based environmental management for malaria control: evidence from a small-scale intervention in Dar es Salaam, Tanzania. Malaria Journal. 2009 Apr 8;8(1):57. doi: 10.1186/1475-2875-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pålsson K, Jaenson TGT, Dias F, Laugen AT, Björkman A. Endophilic Anopheles Mosquitoes in Guinea Bissau, West Africa, in Relation to Human Housing Conditions. Journal of Medical Entomology. 2004 Jul 1;41(4):746–752. doi: 10.1603/0022-2585-41.4.746. [DOI] [PubMed] [Google Scholar]
- 34.Galardo AKR, Zimmerman RH, Lounibos LP, Young LJ, Galardo CD, Arruda M, et al. Seasonal abundance of anopheline mosquitoes and their association with rainfall and malaria along the Matapí River, Amapí, Brazil. Medical and Veterinary Entomology. 2009;23(4):335–349. doi: 10.1111/j.1365-2915.2009.00839.x. [DOI] [PubMed] [Google Scholar]