Abstract
Listeria monocytogenes secretes several proteins that have been shown to contribute to virulence. Among these is listeriolysin O (LLO), a pore-forming hemolysin that is absolutely required for virulence. Two other virulence factors are phospholipases: a phosphatidylinositol-specific phospholipase C (PI-PLC [plcA]) and a broad-range PLC (plcB). Although mutations in plcA or plcB resulted in small increases in mouse 50% lethal dose (LD50), deletions in both genes resulted in a 500-fold increase in LD50. We have examined the role of these secreted proteins in host intracellular signaling in the J774 macrophage-like cell line. Measurements of cytosolic free calcium ([Ca2+]i) have revealed a rapid spike upon exposure of these cells to wild-type L. monocytogenes. This is followed by a second peak at 5 min and a third prolonged peak with a maximal [Ca2+]i of 800 to 1,000 nM. The pattern of calcium changes was greatly altered by deletion of any of the three virulence factors. An LLO mutant produced none of these elevations in [Ca2+]i; however, a transient elevation was observed whenever these bacteria entered the cell. A PI-PLC mutant produced a diminished single elevation in [Ca2+]i at 15 to 30 min. A broad-range PLC mutant produced only the first calcium spike. Studies with inhibitors suggested that the first elevation arises from influx of calcium from the extracellular medium through plasma membrane channels and that the second and third elevations come from release of Ca2+ from intracellular stores. We observed that internalization of wild-type bacteria and the broad-range PLC mutant was delayed for 5 to 10 min, but the LLO and PI-PLC mutants were internalized rapidly upon infection. Inhibitors that affected calcium signaling changed the kinetics of association of wild-type bacteria with J774 cells, the kinetics of entry, and the efficiency of escape from the primary phagosome.
Listeria monocytogenes, a food-borne animal and human pathogen, secretes several proteins that have been shown to be either essential or contributory to virulence both in mammalian cell tissue cultures and in animal models of infection. Among these is listeriolysin O (LLO [encoded by hly]) a pore-forming hemolysin that is absolutely required for virulence (9, 15, 26, 44). Two other virulence factors are phospholipases of the C type (PLC). One, encoded by plcA, is a phosphatidylinositol-specific PLC (PI-PLC) (7, 21, 29, 38, 39), and the other, encoded by plcB, is a broad-range PLC with activity on most of the common phospholipids found in biological membranes, including sphingomyelin (16, 20, 58). Although mutations in plcA or plcB resulted in relatively small increases in mouse 50% lethal dose, deletions in both genes simultaneously resulted in a 500-fold increase (53). A fourth gene, mpl, encodes a metalloprotease required for processing of the proenzyme form of the broad-range PLC in broth cultures (45); however, activation of the proenzyme can occur in J774 cells by an Mpl-independent pathway (34). All four of these genes are contained in a small region of the L. monocytogenes chromosome and are regulated by PrfA (43).
Interactions of L. monocytogenes with host cells can be conceptually divided into four steps: association with the host cell and internalization, escape from the primary vacuole, recruitment of host cell actin, and cell-to-cell spread. Association of L. monocytogenes with the host cell may occur by means of different receptors, depending on the cell type (27). Upon internalization, a phagosome is formed which fuses with early endosomal compartments of the host cell (1). Depending on the type of host cell, a significant portion of the invading bacteria are able to escape from this early phagosomal-endosomal compartment (primary vacuole) into the cytosol, where the bacteria use host cell actin-based motility to spread to neighboring cells by forming filopodia-like protrusions (55). After cell-to-cell spread, the bacteria are located in double-membrane vacuoles from which they escape and repeat the cycle (55). Mutants lacking LLO cannot escape from the primary vacuole (4, 14, 55), mutants lacking PI-PLC escape less efficiently than wild-type L. monocytogenes (8), and mutants lacking broad-range PLC accumulate in the double-membrane secondary vacuole (53, 58).
Despite considerable progress in our understanding of the roles of these virulence factors in the intracellular life of L. monocytogenes, what are not known is when their activities are manifested in host cells and the exact biochemical mechanisms involved. Recent findings indicate that induction of phospholipid metabolism and generation of eicosanoids occur prior to entry of L. monocytogenes into host cells (50, 51). Mutants lacking LLO and PI-PLC displayed significant defects in initiation of host cell signaling in host human umbilical vein endothelial cells (HUVEC), indicating that one of these proteins alone was not enough to induce maximal phosphoinositide metabolism. Based on these findings, it was suggested that LLO allows access of the bacterial PI-PLC to host PI, thus leading to formation of inositol-P (InsP) and diacylglycerol (DAG) (50). Since changes in host cell free calcium levels have been shown to be important for various stages of microbial infection, such as internalization (18, 41), host cell actin rearrangement (31), and early endosome fusion (36), this study was undertaken to determine if infection with L. monocytogenes induces calcium signaling in host cells. Our results demonstrate that changes in cytosolic calcium occur prior to bacterial association with the host cell, and these calcium signals are significantly altered in the absence of LLO, PI-PLC, or broad-range PLC. Inhibitors of these calcium signals were shown to affect the initial association of L. monocytogenes with host cells, entry, and eventual escape from the primary vacuole.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study include the following wild-type and isogenic mutants: 10403S (wild type) (5); DP-L1552 (ΔplcA), a mutant lacking PI-PLC activity (8); DP-L1935 (ΔplcB), a mutant lacking broad-range PLC activity (53); DP-L1936 (ΔplcA ΔplcB), a mutant deficient in both phospholipase activities (53); and DP-L2161 (Δhly), a mutant lacking LLO activity (24). Heat-killed wild-type bacteria were incubated at 60°C for 1 h. All strains were grown in brain heart infusion (BHI) medium and maintained on BHI agar. Stock cultures were stored at −80°C in Luria-Bertani (LB) broth containing 40% glycerol.
FITC labeling of bacterial strains.
Bacteria were grown overnight at 30°C without shaking. The following morning, the culture was diluted 1:10 into fresh medium. This culture was grown for 1 h 45 min at 37°C with aeration, after which time, 1 ml was removed, washed once with phosphate-buffered saline (PBS [pH 7.4]), prewarmed to 37°C, resuspended in 0.8 ml of prewarmed PBS (pH 8.0) containing 0.2 ml of freshly prepared fluorescein isothiocyanate (FITC) solution (1 mg/ml in PBS), and incubated for another 45 min at 37°C (22). After this incubation, bacteria were washed three times with prewarmed PBS. The pellet was then resuspended in 1 ml of prewarmed PBS and used to infect J774 cells 3 to 5 min after resuspension.
Measurement of bacterial entry into J774 cells.
The murine macrophage cell line J774 was obtained from Daniel Portnoy, University of California, Berkely, Calif. and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 7.5% fetal calf serum (FCS), glutamine, and penicillin-streptomycin and incubated at 37°C under 5% CO2. One day prior to infection, cells were plated on glass coverslips (12-mm circular diameter; Fisher Scientific, King of Prussia, Pa.) in antibiotic-free DMEM. Five coverslips per well of a six-well tissue culture dish were used, and cells were plated at a density of 4.0 × 105 to 5.0 × 105 cells per well. To measure uptake, J774 cells were infected with log-phase bacteria (multiplicity of infection of 25 to 30) for 30 min. Cells were then washed five times with PBS prewarmed to 37°C and then returned to antibiotic-free DMEM for an additional 30 min, at which time, 50 mg of gentamicin per ml was added, and infection was allowed to progress for another 30 min. Coverslips were then added to sterile distilled water to lyse the J774 cells. Aliquots of bacteria were plated on LB agar plates, which were incubated overnight at 37°C (25).
Alternatively, wild-type and mutant L. monocytogenes cells were labeled with FITC and used to infect J774 cells as described above. At various times during a 45-min infection, cells were washed five times with PBS, stained for 1 min with 25 mg of ethidium bromide per ml, which stains only extracellular bacteria (12), washed five times with PBS, and fixed with 3.3% formaldehyde. For each time point, 150 to 200 J774 cells with associated bacteria were viewed with a fluorescence microscope (Nikon Microphot FXA) under oil immersion. Images were captured and analyzed by using a Phase III imaging system (Phase III Imaging Systems, Glen Mills, Pa.). Cells and extracellular bacteria fluoresced red, and were counted with the 590-nm rhodamine filter. Total bacteria, labeled with FITC, fluoresced green and were viewed with a 520-nm fluorescein filter. The number of intracellular bacteria was determined by subtracting the number of extracellular bacteria (red) from the total number of bacteria (green). The time of incubation and concentration of ethidium bromide staining were determined to be optimal for these experiments.
Measurement of escape from the primary vacuole.
Escape of L. monocytogenes from the primary vacuole was measured by labeling bacteria with FITC and rhodamine-phalloidin as described previously (33). J774 cells were plated and infected as described in the section on measurement of bacterial entry. After the 90-min infection, cells were fixed with 3.3% formaldehyde, permeabilized with Tris-buffered saline containing 0.1% Triton X-100, and blocked with 1% bovine serum albumin. Bacteria, which had been labeled with FITC as described above, were then colabeled with rhodamine-tagged phalloidin, which stains polymerized actin associated with the cytosolic bacteria. After washing, the coverslips were mounted and viewed under a ×60 oil immersion objective using both the 520-nm fluorescein and 590-nm rhodamine filters. Images were captured and analyzed with the Phase III imaging system as described above. Bacteria viewed with both filters were counted, and escape was expressed as the percentage of total FITC-labeled bacteria that were labeled with rhodamine-labeled phalloidin.
Cytosolic Ca2+ measurements.
For cytosolic calcium measurements, J774 cells were plated on glass coverslips as described above. On the day of infection, cells were loaded with a 5 μM solution of the fluorescent calcium indicator fluo 3-AM (Sigma, St. Louis, Mo.) for 30 min. Since fluo 3-AM has only single excitation/emission wavelengths (490 and 520 nm, respectively) and is therefore nonratiometric, cells were also loaded for 30 min with 5 mM SNARF-1 AM (Molecular Probes, Inc., Eugene, Oreg.), which has an isoemissive point at 600 nm. At this wavelength, it can be used as a reference for fluo 3 fluorescence, thereby compensating for changes in fluorescence due to cell volume changes, photobleaching, and intracellular fluo 3 concentration (35, 46). By scanning across single cells, which do not show homogeneous fluorescent intensities, a range of intensities was established for SNARF-1 which remained constant when both pH and calcium concentration were altered, indicating that SNARF-1 fluorescence provided a satisfactory reference. There was no quenching or spectral overlap between the two probes. Both probes accumulated in the same intracellular compartments, as shown in Fig. 1 and further verified by merging the two images. The intracellular dye concentrations were the same for both probes after the 30-min loading period (0.6 μM). Both probes photobleached at the same rate (50% decrease in fluorescent intensity within 30 s), and the dye leakage for both probes was minimal after 90 min (<5%).
FIG. 1.
J774 cells labeled with fluo 3 and SNARF-1. J774 cells were loaded with fluo 3 and SNARF-1 as described in the text. Fluorescent micrographs show a representative group of J774 cells labeled with both fluo 3 (a) and SNARF-1 (b) 10 min after infection with wild-type L. monocytogenes.
Coverslips were placed into hanging drop microslides (Fisher) containing either HEPES-buffered Ringer medium with 10 mM glucose (pH 7.4) or HEPES-buffered DMEM with 7.5% serum (pH 7.4). The temperature of the chamber was maintained at 37°C with a Thermolyne heating plate on the microscope stage. After addition of FITC-labeled bacteria, cells were observed with the ×40 objective, rotating the filter wheel between the fluorescein (fluo 3) and rhodamine (SNARF-1) filters. Images were captured and analyzed over a 60-min time course with the Phase III imaging system. In one set of experiments, unlabeled wild-type bacteria were added to J774 cells, and calcium changes were compared to those seen in cells infected with FITC-labeled wild-type bacteria. Calcium levels in uninfected cells were also measured over the same time period. The ratio of fluo 3 (520 nm) to SNARF-1 (590 nm) was determined, and cytosolic Ca2+ levels were calculated from a calibration curve prepared according to the method of Harrison and Bers (23). A composite calibration curve is given in Fig. 2.
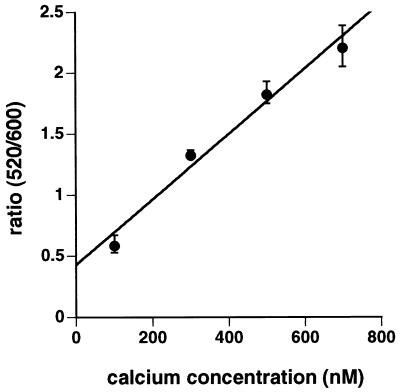
FIG. 2.
Composite calcium calibration curve. Coverslips with J774 cells were loaded with fluo 3 and SNARF-1 as described in the text. The cells were equilibrated in HEPES-buffered Ringer solution (pH 7.4) containing different concentrations of calcium, 1 mM EGTA, and the Ca2+ ionophore A23187 (1 μM) for 30 min at 37°C. The images were collected and analyzed for fluorescence intensity as described in the text. The average fluo 3/SNARF-1 ratio (520/600) was obtained for each concentration of Ca2+ and used for calculation of cytosolic Ca2+ levels in J774 cells after infection. The results are the mean ± standard error for 13 to 17 cell preparations.
Inhibitor studies.
In order to determine if cytosolic Ca2+ changes seen after infection were due to entry of calcium from the extracellular medium or release from intracellular stores, calcium levels were measured in cells treated with calcium channel blockers which inhibit calcium influx by blocking specific calcium channels in the plasma membrane or thapsigargin, a very specific inhibitor of intracellular Ca-ATPases found in calcium-sequestering organelles, such as the endoplasmic reticulum. Thapsigargin prevents the reuptake of calcium into these organelles, resulting in depletion of those stores (10, 41). SK&F 96365 (10 and 25 μM), a receptor-operated calcium channel blocker (28, 30), and verapamil (10 to 50 μM), an inhibitor of slow voltage-sensitive (L-type) calcium channels (19, 59) were added to cells 15 min prior to infection and tested for their effects on calcium signaling in J774 cells in uninfected and infected cells. Cells were also added to a HEPES-buffered calcium-free Ringer medium containing 1 mM EGTA to reduce extracellular calcium. In another set of experiments, J774 cells were pretreated for 45 min before infection with 1 μM thapsigargin. Short-term incubation (20 to 30 min) with thapsigargin produced a rise in cytosolic calcium due to depletion of intracellular stores, which is tightly coupled to entry of extracellular calcium through specific channels which open when intracellular stores need to be refilled (capacitative calcium entry) (3). Incubation for 45 min with thapsigargin prior to infection returned cytosolic free Ca2+ ([Ca2+]i) to control values as a result of Ca2+-extruding mechanisms (10). These agents were not toxic to J774 cells over a 90-min period, as determined by trypan blue exclusion in which only 2 to 4 cells in a field of 100 stained blue.
Effects of FITC and inhibitors on bacteria.
In order to determine if FITC or inhibitors used in this study were toxic to L. monocytogenes, growth rates in BHI medium were compared in control cultures and cultures to which inhibitors were added. FITC-labeled bacteria were also compared to unlabeled bacteria for effects on uptake and escape from the primary vacuole of J774 cells.
Bacterial growth was determined by measuring the A620 of control and inhibitor-treated cultures for 3 h. Concentrations of inhibitors are described in the section on inhibitor studies. Over a 3-h period, bacterial growth in BHI medium was not affected by SK&F 96365, verapamil, or thapsigargin nor by the FITC label on the bacteria.
Uptake of FITC-labeled bacteria after 90 min remained the same as that of unlabeled bacteria, as determined by viable bacterial cell count. FITC-labeled L. monocytogenes cells were also compared to antibody-tagged L. monocytogenes cells to determine if labeling had any effect on escape from the primary vacuole. Cells were infected as described previously with FITC-labeled or unlabeled bacteria. There was no difference in the total number of bacteria or the number of bacteria that stained with rhodamine-phalloidin in experiments in which FITC-labeled bacteria or bacteria labeled, after infection and permeabilization, with rabbit anti-L. monocytogenes antibody conjugated with fluorescein (Difco) were used.
Chloramphenicol studies.
To determine if new bacterial protein synthesis was necessary to initiate changes in host [Ca2+]i, calcium signaling was also measured in one set of cells infected with wild-type L. monocytogenes cells that were washed with prewarmed PBS containing 10 μg of chloramphenicol per ml and then resuspended in prewarmed PBS without chloramphenicol. In a second experiment, bacteria were washed with and then resuspended in prewarmed PBS containing 10 μg of chloramphenicol per ml with the same concentration of chloramphenicol added to the cell culture. As described previously, infections were started 3 to 5 min after resuspension.
RESULTS
Changes in cytosolic Ca2+ differ in J774 cells infected with wild-type or mutant L. monocytogenes.
To determine if cytosolic calcium levels ([Ca2+]i) change after infection of J774 cells with unlabeled or FITC-labeled wild-type or mutant L. monocytogenes, [Ca2+]i was determined in J774 cells in HEPES-buffered Ringer medium with glucose over a 60-min period postinfection (p.i.). Since it has been reported that serum contains factors, such as complement (27, 49), which may affect bacterial entry, calcium signaling was also measured in HEPES-buffered DMEM with 7.5% FCS. Three elevations in [Ca2+]i followed by a return to control levels were observed in J774 cells infected with wild-type L. monocytogenes (Fig. 3a). The first measurements appear to represent the decreasing phase of an initial peak. Since it appears the increase in [Ca2+]i occurs very rapidly after addition of bacteria, the amplitude of the [Ca2+]i spike is not known. A return to control levels at 3 min is followed by a second rise at 5 min, which then returns to control values by 10 min. The third elevation, starting 15 min after addition of the bacteria, is more sustained than either of the first two elevations, reaching 800 to 1,000 nM by 25 min and then declining to control levels by 40 min. Changes in [Ca2+]i after infection with unlabeled wild-type L. monocytogenes were identical to those detected after infection with the FITC-labeled wild-type bacteria (data not shown). Changes in [Ca2+]i in cells in HEPES-buffered DMEM with 7.5% FCS were identical to those seen for cells in HEPES-buffered Ringer medium with glucose (data not shown).
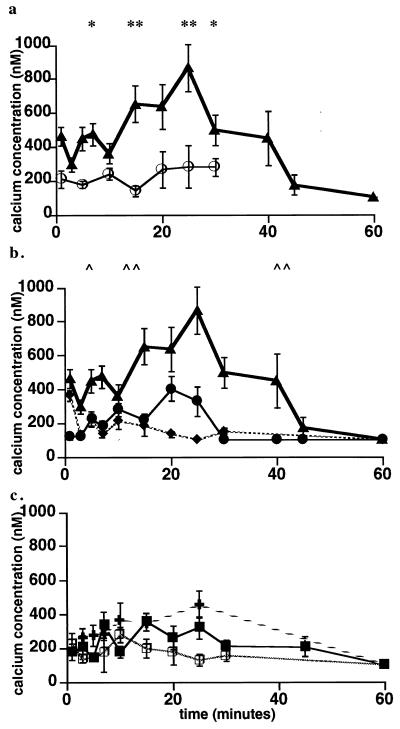
FIG. 3.
Calcium signaling in J774 cells after infection with wild-type or mutant L. monocytogenes. J774 cells were loaded with fluo 3 and SNARF-1 as described in the text. Changes in cytosolic calcium levels were measured at intervals for 60 min in wild-type-infected (▴) and uninfected (○) cells (a); wild-type (▴)-, plcB (broad-range PLC) mutant (⧫)-, and the plcA (PI-PLC) mutant (●)-infected cells (b); and double plcA plcB mutant (□)-, LLO− mutant (■)-, and heat-killed bacterium (+)-infected cells (c). Results are expressed as the mean ± standard error for three to five cell preparations. (a) ∗, P < 0.05 by Student’s paired t test for calcium levels at 1, 5, 7, and 30 min in cells infected with wild-type L. monocytogenes compared to those in uninfected cells. (b) ∗∗, P < 0.01 by Student’s t test when calcium levels measured at 15, 20, and 25 min in cells infected with wild-type L. monocytogenes were compared to uninfected cells. ∧, P < 0.05 by Student’s t test for calcium levels at 1, 5, and 7 min in cells infected with wild-type L. monocytogenes compared to those in cells infected with the plcA (PI-PLC) mutant or for calcium levels at 5 and 7 min in cells infected with wild-type L. monocytogenes compared to those in cells infected with the plcB (broad-range PLC) mutant. ∧∧, P < 0.01 by Student’s t test for calcium levels at 15, 25, 30, and 40 min in cells infected with wild-type L. monocytogenes compared to those in cells infected with the plcA (PI-PLC) mutant or calcium levels at 15, 20, 25, 30, and 40 min in cells infected with the wild type compared to those in cells infected with the plcB (broad-range PLC) mutant.
In contrast to the three elevations in [Ca2+]i seen in cells infected with wild-type L. monocytogenes, only one elevation was observed in cells infected with the PI-PLC mutant (ΔplcA) (Fig. 3b). This small, but reproducibly sustained elevation induced by the PI-PLC mutant corresponds in time to the third peak seen in cells infected with the wild type, but is only about 50 to 60% the magnitude of the signal observed after infection with the wild type. The broad-range PLC mutant (ΔplcB) produced a rise in [Ca2+]i at 1 min of a magnitude similar to that seen in cells infected with wild-type bacteria (362 ± 61 and 462 ± 56 nM, respectively) with no second and third elevations (Fig. 3b). There was no elevation in [Ca2+]i in cells infected with an LLO mutant, the double-PLC mutant (ΔplcA ΔplcB), or heat-killed bacteria (Fig. 3c).
Effects of inhibitors on calcium signaling after infection with L. monocytogenes.
In order to determine if Ca2+ changes resulted from entry of extracellular calcium through plasma membrane channels or release of calcium from intracellular stores, [Ca2+]i was also measured in cells treated for 15 min with 10 or 25 μM SK&F 96365 or 10, 25, or 50 μM verapamil, both of which inhibit entry of calcium from the extracellular medium, or in cells treated for 45 min with 1 μM thapsigargin, which depletes intracellular calcium stores. Figure 4a shows the effects of the receptor-operated Ca2+ channel blocker SK&F96365 (10 and 25 μM) and intracellular Ca2+-ATPase inhibitor thapsigargin (1 μM) on cytosolic Ca2+ levels in cells infected with wild-type bacteria. With SK&F 96365, there was a concentration-dependent decrease in magnitude of the Ca2+ signals from 1 to 10 min. At 15 and 20 min, 10 μM SK&F 96365 was sufficient to reduce the signal to that seen in uninfected cells. In contrast, the L-type Ca2+ channel blocker verapamil did not reduce [Ca2+]i at concentrations from 10 to 50 μM over a 10-min period (data not shown). These results indicate that calcium entry from the extracellular medium was through receptor-operated channels rather than those which open if there is a change in membrane potential causing voltage-gated channels to open. Thapsigargin did not significantly reduce the first rise in [Ca2+]i, but eliminated the second and third elevations observed after infection with wild-type L. monocytogenes, indicating that the first elevation in calcium is not a result of capacitative calcium influx. SK&F 96365 (25 μM) also eliminated the first elevation in cytosolic Ca2+ after infection with the broad-range PLC mutant (data not shown). Cells added to the EGTA-containing Ringer medium did not show any of the three elevations in cytosolic Ca2+ after infection with wild-type L. monocytogenes (data not shown).
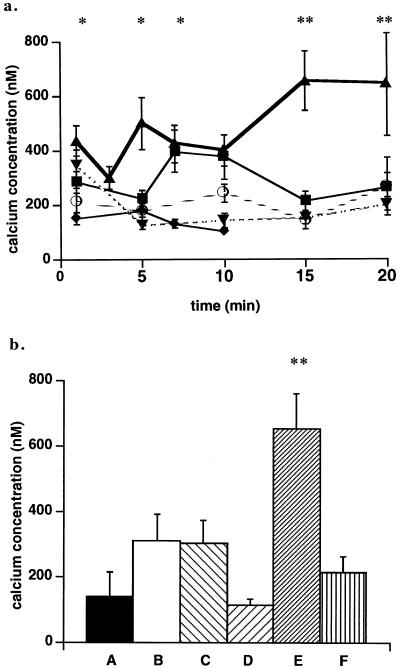
FIG. 4.
Effects of SK&F 96365 and thapsigargin on calcium signaling in J774 cells after infection with wild-type or mutant L. monocytogenes. (a) J774 cells were loaded with fluo 3 and SNARF-1 as described in the text. Changes in cytosolic calcium levels were measured at intervals for 20 min in uninfected cells (○), wild-type infected control cells (▴), and in wild-type-infected cells treated with 10 μM SK&F 96365 (■), 25 μM SK&F 96365 (⧫), or 1 μM thapsigargin (▾). Calcium levels in uninfected inhibitor-treated cells did not differ from those seen in uninfected untreated cells. These data represent the mean ± standard error of three to five cell preparations. ∗, P < 0.05 by Student’s t test for calcium levels measured at 1 and 5 min in cells infected with wild-type L. monocytogenes compared to those in uninfected cells and in infected cells pretreated with 10 and 25 μM SK&F 96365 at 1 and 5 min or thapsigargin at 5 min. At 7 and 10 min, P < 0.05 when calcium levels in infected cells pretreated with 25 μM SK&F 96365 or thapsigargin were compared to those in untreated infected cells. ∗∗, P < 0.01 by Student’s t test for calcium levels at 15 and 20 min in untreated infected cells compared to those in infected cells pretreated with 10 μM SK&F 96365 or thapsigargin. (b) Cytosolic calcium was measured in control or inhibitor-treated J774 cells loaded with fluo 3 and SNARF-1 and infected with wild-type or plcA (PI-PLC) mutant L. monocytogenes. Cytosolic calcium levels are given for 15 min p.i. for cells infected with or treated as follows: A, uninfected untreated cells; B, cells infected with the PI-PLC mutant; C, cells infected with the PI-PLC mutant and treated with 25 μM SK&F 96365; D, cells infected with the PI-PLC mutant and treated with 1 μM thapsigargin; E, cells infected with wild-type L. monocytogenes; and F, cells infected with wild-type L. monocytogenes and treated with 10 μM SK&F 96365. Calcium levels in uninfected inhibitor-treated cells did not differ from those seen in uninfected untreated cells. These data represent the mean ± standard error for three to five cell preparations. ∗∗, P < 0.01 by Student’s t test for calcium levels in cells infected with wild-type L. monocytogenes compared to those in infected cells treated with 10 μM SK&F 96365.
The effects of SK&F 96365 on the third Ca2+ elevation seen in cells infected with the wild type and the single elevation on infection with the PI-PLC mutant are shown in Fig. 4b. SK&F 96365 at 10 μM eliminated the elevation of [Ca2+]i at 15 min in wild-type-infected cells (compare bars E and F), but 25 μM inhibitor had no effect on the smaller [Ca2+]i signal observed in cells infected with the PI-PLC mutant (compare bars B and C). In cells pretreated with thapsigargin and infected with the PI-PLC mutant, Ca2+ signaling was eliminated (compare bars B and D). Together, these results indicate that Ca2+ influx is responsible for the initial signal, while both influx and release from intracellular stores contribute to the second and third signals, observed upon infection with the wild-type.
Although there was no detectable elevation in average cytosolic Ca2+ levels during a 60-min infection period with the LLO mutant or heat-killed bacteria (Fig. 3c), there was a different pattern of bacterial association of both the LLO mutant and heat-killed bacteria with the J774 cells (see below). In cells with attached or internalized green FITC-labeled bacteria a transient elevation in [Ca2+]i was observed. This elevation was insensitive to the Ca2+ channel blocker SK&F 96365 (25 μM), but it was inhibited by thapsigargin (1 μM) (data not shown).
De novo protein synthesis is required for induction of calcium signaling by L. monocytogenes.
To determine if bacteria treated with chloramphenicol, which blocks protein synthesis, affected calcium signaling, bacteria were washed with PBS containing chloramphenicol and resuspended in PBS without chloramphenicol or were resuspended in PBS plus chloramphenicol (10 μg/ml) and used to infect cells in medium containing chloramphenicol (10 μg/ml). Cells infected with bacteria which were washed only in PBS containing chloramphenicol did not show any differences in calcium signaling from cells infected with nontreated bacteria (data not shown). However, when bacteria were resuspended in PBS containing chloramphenicol and used to infect cells in medium containing chloramphenicol, Ca2+ signaling was completely eliminated (data not shown). These results indicate that de novo protein synthesis is required to initiate changes in [Ca2+]i during infection with the wild type.
LLO and PLCs modulate bacterial entry into host J774 cells.
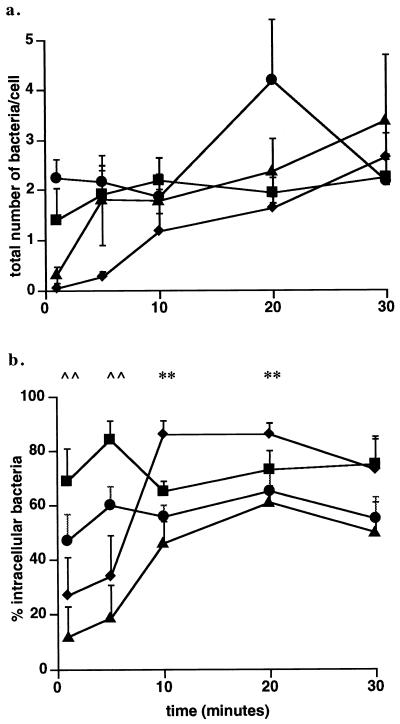
Microscopic observations of bacterial interactions with host cells suggested different kinetics of association for wild-type and mutant cells. The time course of adhesion of wild-type or mutant L. monocytogenes cells with J774 cells and the subsequent internalization over a 30-min period are given in Fig. 5. The total number of wild-type bacteria associated with the cells increased from 0.31/cell at 1 min to 3.4 by 30 min. The number of broad-range PLC mutant bacteria associated with the cells increased in a manner similar to that of the wild type from 0.04/cell at 1 min to 2.7/cell by 30 min. In contrast the number of PI-PLC mutant bacteria was 2.24/cell at 1 min and remained high for 30 min (2.2 ± 0.84), and the number of LLO mutant bacteria at 1 min was also higher than that seen with the wild type.
FIG. 5.
Association and entry of wild-type or mutant L. monocytogenes cells into J774 cells. Association and entry of the wild type (▴), the plcB (broad-range PLC) mutant (⧫), the plcA (PI-PLC) mutant (●), or the hly (LLO) mutant (■) were measured by the entry assay as described in the text. (a) Total number of bacteria (extracellular plus intracellular) associated with the cells. (b) Percentage of intracellular bacteria calculated as described in the text. These data represent the mean ± standard error for three to five cell preparations. ∧∧, P < 0.01 by Student’s t test for the percentage of intracellular bacteria (wild-type) compared to the percentage of intracellular bacteria (LLO mutant) at 1 and 5 min. , ∗∗, P < 0.01 by Student’s t test for the percentage of intracellular bacteria (wild type) compared to the percentage of intracellular bacteria (broad-range PLC mutant) at 10 and 20 min.
The percentages of intracellular wild-type L. monocytogenes and broad-range PLC mutant cells were low (12 and 27%, respectively) at 1 min, in contrast to that seen with the PI-PLC (47%) or LLO mutants (69%). The percentage of wild-type bacteria entering the cells increases over a 30-min period from 12% at 1 min to 50% at 30 min and remains at this level (Fig. 5b). A large increase in the percentage of intracellular bacteria occurs 10 min p.i. with the broad-range PLC mutant and remains elevated at a higher level than the wild type for up to 20 min. An insignificant increase occurs with the PI-PLC mutant, and no change in the percentage of intracellular bacteria was seen with the LLO mutant. The percentage of intracellular bacteria did not change for the wild type or any of the mutants between 30 and 45 min (data not shown). The kinetics of internalization of the double-PLC mutant and heat-killed bacteria entering the cells were indistinguishable from those seen with the LLO mutant (data not shown). These results suggest that virulence factors delay bacterial association and entry into J774 macrophage-like cells.
Effects of calcium signaling inhibitors on bacterial entry.
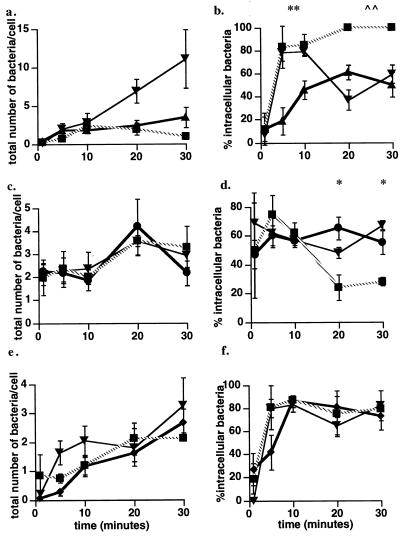
In order to determine if calcium signaling is linked to bacterial association and entry, cells were pretreated with the calcium channel blocker SK&F 96365 (25 μM) or thapsigargin (1 μM), the Ca-ATPase inhibitor, and the entry assay was carried out as described above. The presence of the calcium channel blocker, which eliminates all three [Ca2+]i elevations seen in cells after infection with wild-type L. monocytogenes (Fig. 4a), did not alter association and entry at 1 min, but at 5 and 10 min, the percentage of intracellular bacteria was greater in inhibitor-treated cells than in control cells (Fig. 6a and b). By 20 min, however, the number of bacteria associated with cells increased dramatically in cells treated with SK&F 96365 (Fig. 6a), and the percentage of intracellular bacteria had declined to 37%. The number of bacteria associated with the cell continued to rise at 30 min, and the percentage of intracellular bacteria also rose. Thapsigargin did not significantly alter the total number of wild-type bacteria associated with the cells at any time, but it increased internalization from 5 to 30 min. The calcium channel blocker had no effect on the association and entry of the PI-PLC mutant into the J774 cells (Fig. 6c and d). Thapsigargin also had no effect on the total number of bacteria associated with the cells after infection with the PI-PLC mutant (Fig. 6c), but did cause a significant reduction in the percentage of intracellular bacteria by 20 min, which did not change by 30 min (Fig. 6d). In contrast to the effects of the channel blocker and thapsigargin on wild-type association and entry, neither inhibitor altered the total number of bacteria associated with the cells or the percentage of intracellular bacteria after infection with the broad-range PLC mutant (Fig. 6e and f). There was no effect of either SK&F 96365 or thapsigargin on association with and entry into J774 cells of the LLO and double-PI-PLC broad-range PLC mutants or heat-killed bacteria (data not shown). These results show clearly that elevation of [Ca2+]i delays entry of wild-type L. monocytogenes.
FIG. 6.
Effects of SK&F 96365 and thapsigargin on bacterial association and entry. The total number of bacteria associated with cells (extracellular plus intracellular) and the percentage of intracellular bacteria were determined as described in the text. (a, c, and e) Total number of bacteria per cell for infection with the wild type (▴) (a), the plcA (PI-PLC) mutant (●) (c), the plcB (PLC) mutant (⧫) (e). (b, d, and f) Percentage of intracellular bacteria after infection with the wild type (b), the plcA (PI-PLC) mutant (d), or the plcB (PLC) mutant (f). Within each panel, either bacterial association (a, c, and e) or entry (b, d, and f) in untreated J774 cells was compared to association and entry in cells treated with 25 μM SK&F 96365 (▾) or 1 μM thapsigargin (■). These data represent the mean ± standard error for three to five cell preparations. (b) ∗∗, P < 0.01 by Student’s t test for the percentage of intracellular wild-type bacteria in untreated J774 cells compared to the percentage of intracellular wild-type bacteria in both SK&F 96365- and thapsigargin-treated cells at 5 and 10 min. ∧∧, P < 0.01 by Student’s t test for the percentage of intracellular wild-type bacteria in untreated J774 cells compared to the percentage of intracellular wild-type bacteria in thapsigargin-treated cells at 20 and 30 min. (d) ∗, P < 0.05 by Student’s t test for the percentage of intracellular bacteria (PI-PLC mutant) in untreated J774 cells compared to the percentage of intracellular bacteria in thapsigargin-treated cells at 20 and 30 min.
Escape from the primary vacuole.
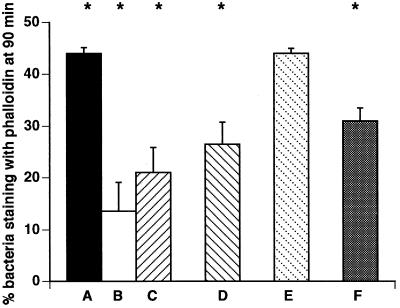
Previous studies had shown that deletion of PI-PLC resulted in diminished escape from the primary vacuole of murine bone marrow-derived macrophages (8). Figure 7 gives the percentage of wild-type or mutant bacteria that have escaped from the primary vacuole of J774 cells after a 90-min infection period. These results demonstrate that both the wild type and the broad-range PLC mutant escape with the same efficiency (44%), whereas the PI-PLC and the double-PLC mutants escape less efficiently. In J774 cells which were pretreated with SK&F 96365 (25 μM) or thapsigargin (1 μM), the percentages of wild-type bacteria that escaped decreased significantly from 44 to 13.6 ± 5.5 and 21 ± 4.9, respectively (P < 0.05 by Student’s t test for both inhibitors).
FIG. 7.
Escape of wild-type or mutant L. monocytogenes cells from the primary vacuole. The percentages of bacteria staining with phalloidin at 90 min are given for cells infected with wild-type L. monocytogenes (A), pretreated with SK&F 96365 (25 μM) and infected with wild-type L. monocytogenes (B), pretreated with thapsigargin (1 μM) and infected with wild-type L. monocytogenes (C), infected with the PI-PLC mutant (D), infected with the broad-range PLC mutant (E), and infected with the double-PLC mutant (F). Results are expressed as the mean ± standard error for three to six cell preparations. ∗, P < 0.05 for untreated cells infected with wild-type L. monocytogenes compared to wild-type-infected cells pretreated with SK&F 96365 (25 μM) or thapsigargin (1 μM) or for untreated J774 cells infected with wild-type L. monocytogenes compared to cells infected with the PI-PLC or double-PLC mutant.
DISCUSSION
Wild-type L. monocytogenes induces a rapid elevation of [Ca2+]i 1 min after addition of a washed suspension of bacteria to J774 cells. After a rapid return to basal levels, this is followed by a second elevation, 5 to 7 min p.i., again followed by a return to near basal levels. A third and more prolonged rise in [Ca2+]i begins approximately 10 min p.i. By using mutants deficient in one or more virulence factors, we have shown that this pattern of calcium signaling is either considerably altered, as was the case with both the PI-PLC and broad-range PLC mutants, or eliminated upon infection with the LLO or double-PLC mutants, thus indicating a role for each of these three virulence factors in initiating changes in host cell [Ca2+]i. This study also presents evidence that the early calcium fluxes occur before association with and entry of bacteria into the cells and that alterations in the pattern of calcium signaling by means of inhibitors can affect association of bacteria with J774 cells, entry into these cells, and eventual escape from the primary vacuole.
The finding that the first elevation in [Ca2+]i was observed only after addition of wild-type bacteria or the broad-range PLC mutant, and not with either an LLO or PI-PLC mutant, suggests that both LLO and PI-PLC are necessary for this immediate calcium signal. This signal was sensitive to the receptor-operated calcium channel blocker SK&F 96365 and to reduction of extracellular calcium, but was not affected by verapamil, an inhibitor of voltage-gated calcium channels. It was not eliminated when cells were pretreated with thapsigargin, an inhibitor of intracellular Ca-ATPases which depletes intracellular Ca2+ pools, indicating the first peak results from entry of extracellular calcium. A model to explain the effects of L. monocytogenes infection of HUVEC in the absence of bacterial uptake on lipid signaling proposes that the pore-forming ability of LLO permits access of PI-PLC to a pool of PI in the inner leaflet of the plasma membrane resulting in rapid generation of Ins-1,2-cyclic-P and DAG, (51). Localized generation of DAG would presumably lead to activation of host calcium-independent protein kinase C (PKC) δ, one of four isoforms of PKC found in host J774 cells (52). Since it has been shown that PKC is involved in opening of calcium channels in certain cell types (31, 48), we suggest that PKC activation could provide a mechanism for opening a receptor-mediated channel after infection with wild-type L. monocytogenes. It does not appear that LLO causes uncontrolled calcium influx as a result of significant membrane damage, since the first calcium elevation and subsequent return to control levels appear to be well regulated and very specific to cells infected with wild-type L. monocytogenes and the broad-range PLC mutant, both of which secrete LLO and PI-PLC.
The second increase in [Ca2+]i at 5 to 7 min p.i., which is seen only upon infection with wild-type bacteria, is sensitive to both the calcium channel blocker and thapsigargin, indicating that it requires both the initial influx of Ca2+ and its release from intracellular stores. The fact that it requires the broad-range PLC in addition to PI-PLC leads to the interesting suggestion that, as in eukaryotic signaling pathways, a second longer signal requires phospholipases with a broader substrate range than PI-PLC in order to sustain signaling (40).
The third prolonged elevation in [Ca2+]i, beginning 10 to 15 min p.i., is also sensitive to the calcium channel blocker and thapsigargin and is dependent on LLO, PI-PLC, and the broad-range PLC. Thus, it also appears to require the initial influx of calcium, release from intracellular stores, and the activity of both PLCs. A weak signal observed with the PI-PLC mutant at the same time is not sensitive to the calcium channel blocker (Fig. 4b), indicating that it arises from some late action of LLO and broad-range PLC, presumably after internalization of the bacteria.
Association with and entry of L. monocytogenes into J774 cells appear to correlate with the calcium signals produced by wild-type or mutant strains. The generation of three distinct calcium signals seen in cells after infection with wild-type bacteria appears to inhibit the binding of bacteria to J774 cells and subsequent entry compared to those of mutant bacteria (Fig. 5). The observation that both binding and entry of the LLO or PLC mutants are more rapid than those seen with wild-type infection points to a need for the combined action of LLO and both phospholipases to regulate this process. Inhibition of all three calcium signals with SK&F 96365 causes a significant increase in the percentage of intracellular wild-type bacteria during the first 10 min p.i. and produces a large increase in the total number of bacteria associated with the cell at 20 min. In contrast, thapsigargin pretreatment, which inhibits the second and third elevations of [Ca2+]i, does not increase the number of bacteria associated with the cells, but does produce an increase similar to that seen with the channel blocker in the percentage of internalized bacteria 5 min p.i. These results suggest that the second and third calcium signals are important for regulating the timing of entry and number of bacteria entering the cell. It would also be expected that elimination of the second and third calcium signals by thapsigargin would produce the same pattern of association and entry seen when the second and third elevations are eliminated by the calcium channel blocker. However, the percentages of intracellular bacteria differ at 20 and 30 min in cells treated with the two different inhibitors. These results suggest that the calcium signals are associated with two different signaling pathways, one coupled to entry of calcium from the extracellular medium and the other coupled to release of calcium from intracellular stores.
Although calcium signaling starts before a significant number of wild-type bacteria associate with the cell, it appears that the bacteria are “communicating” with the cell prior to entry and do so through LLO- and PI-PLC-mediated activation of a receptor-operated calcium channel or channels. The implications for involvement of host cell signaling pathways linked to calcium signals are complex, but opening of a receptor-operated calcium channel suggests that this process occurs by means of receptor-mediated uptake and that elevations in calcium delay or prevent “general” phagocytosis. Several different receptors have been shown to be involved in phagocytosis by J774 cells, including In1A receptors (49), C3, and scavenger receptors (27). Since there was no difference in calcium signaling after infection of J774 cells which were in either HEPES-buffered Ringer solution with glucose or in HEPES-buffered DMEM with 7.5% FCS, it seems unlikely that the C3 receptor is involved in bacterial uptake (49). Receptor-mediated entry with subsequent activation of host signaling pathways may play a role in further trafficking of L. monocytogenes once it is inside the cell (6, 54, 56).
A likely mechanism for generating the second and third calcium signals observed in cells infected with wild-type L. monocytogenes is the generation of Ins-1,4,5-P3 resulting from hydrolysis of phosphatidylinositol (PtdIns)-4,5-P2 by host phospholipases and subsequent release of calcium by binding of InsP3 to specific channels in the endoplasmic reticulum membrane. We have recently demonstrated the formation of InsP3 in J774 cells infected with wild-type L. monocytogenes beginning as early as 10 min p.i. (23a). Since the bacterial PI-PLC does not hydrolyze PtdIns-4,5-P2 (21), hydrolysis by host PLC must be invoked. These phospholipases are calcium sensitive and could be activated by the initial rise in [Ca2+]i observed after infection with the wild type. The absence of the second and third calcium peaks after infection with the broad-range PLC mutant argues against this, since it would also be expected to cause activation of host PLC subsequent to the first calcium peak. However, ceramide produced upon hydrolysis of sphingomyelin by the broad-range PLC could give rise to sphingosine-P, which has also been shown to stimulate release of calcium from intracellular stores (17, 36). Thus, the broad-range PLC could contribute to release of calcium by an InsP3-independent mechanism. An additional contribution of the broad-range PLC is the continued generation of DAG from a large pool of cellular phospholipids, and the consequent activation of PKC (40) may be needed in addition to the activity of host inositide-PLC to release calcium from intracellular stores. Although general increases in DAG levels were not detected at early times after infection of J774 cells with L. monocytogenes (53), these measurements do not preclude local activity of the broad-range PLC, which, from the present work, appears to be active early in infection.
The combined activities of both LLO and PI-PLC appear to determine how efficiently wild-type or mutant L. monocytogenes cells escape from the primary vacuole. Approximately 45% of both the wild-type and the broad-range PLC mutant (plcB) cells have escaped from the primary vacuole into the cytosol by 90 min p.i. Escape by the PI-PLC mutant is 30% less than that seen with both the wild-type and broad-range PLC mutant cells, and LLO-deficient mutants do not escape from the primary vacuole (24). Both LLO and PI-PLC are needed to generate the first and second calcium fluxes after infection, and PI-PLC contributes significantly to the third prolonged elevation of [Ca2+]i.
Calcium signaling also correlates with escape from the primary vacuole, since wild-type escape at 90 min p.i. was decreased from 45 to 14% in the presence of the calcium channel blocker SK&F 96365 and to 21% in the presence of thapsigargin (Fig. 7). It is not known at present whether calcium is directly involved in mediating efficient escape or whether a calcium-dependent process or processes or signaling events occurring prior to escape are affected by both SK&F 96365 and thapsigargin. We have preliminary results that show that fusion of the wild-type L. monocytogenes phagosome with early endosomes of the J774 cells peaks 40 to 45 min subsequent to entry and may be involved in formation of the primary vacuole. Although it is not known whether fusion of the phagosome with the early endosome is calcium sensitive in J774 cells, fusion of early endosomes is mediated by calcium (37), and calcium-sensitive binding proteins (annexins) have been localized to both early endosomes and phagosomes in J774 cells (11).
Calcium signaling has been observed upon infection of host cells with gram-negative pathogens (2, 42), and these changes have been linked to fluxes in inositol phosphates by activation of host phospholipases (13). The infective trypomastigote stages of the protozoan parasite Trypanosoma cruzi contain a soluble factor that triggers rapid elevations in [Ca2+]i of mammalian cells. Prevention of calcium signaling markedly inhibits trypomastigote entry (47). These events in T. cruzi infection lead to fusion of host cell lysosomes with the plasma membrane and internalization of the parasite. With L. monocytogenes, the picture is different, in that host cell signaling appears to delay entry. This delay resulting from the activities of LLO and PI-PLC may be needed for subsequent changes in the fate of the phagosome (1).
The results of this study present evidence for the role of the L. monocytogenes virulence factors in controlling the entry and eventual escape from the primary vacuole and for the participation of host cell calcium signaling in these processes. Escape from the primary vacuole is a crucial step in cell-to-cell spread, and understanding the actions of the three virulence factors, as well as the role of calcium fluxes and the potential signaling pathways involved in this process, is extremely important for understanding and eventually intervening in L. monocytogenes infections.
ACKNOWLEDGMENTS
We thank Hao Shen and George van Rossum for careful and critical reading of the manuscript.
This research was supported by grant GM-52797 from the National Institutes of Health.
REFERENCES
- 1.Alvarez-Dominguez C, Barbieri A M, Beron W, Wandinger-Ness A, Stahl P D. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J Biol Chem. 1996;271:13834–13843. doi: 10.1074/jbc.271.23.13834. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge M J. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 5.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 6.Buchwalow I B, Emoto M, Brich M, Kaufmann S H E. Involvement of tubulin and inhibitory G proteins in the interaction of Listeria monocytogenes with mouse hepatocytes. Infect Immun. 1997;65:1095–1097. doi: 10.1128/iai.65.3.1095-1097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli A, Goldfine H, Portnoy D A. Listeria monocytogenes mutants lacking phosphatidylinositol-specific phospholipase C are avirulent. J Exp Med. 1991;173:751–754. doi: 10.1084/jem.173.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darbha S, Marchase R B. Regulation of intracellular calcium is closely linked to glucose metabolism in J774 macrophages. Cell Calcium. 1996;20:361–371. doi: 10.1016/s0143-4160(96)90042-1. [DOI] [PubMed] [Google Scholar]
- 11.Diakonova M, Gerke V, Ernst J, Liautard J P, van der Vusse G, Griffiths G. Localization of five annexins in J774 macrophages and on isolated phagosomes. J Cell Sci. 1997;110:1199–1213. doi: 10.1242/jcs.110.10.1199. [DOI] [PubMed] [Google Scholar]
- 12.Drevets D A, Campbell P A. Macrophage phagocytosis: use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J Immunol Methods. 1991;142:31–38. doi: 10.1016/0022-1759(91)90289-r. [DOI] [PubMed] [Google Scholar]
- 13.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard J-L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaillard J-L, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geoffroy C, Raveneau J, Beretti J-L, Lecroisey A, Vazquez-Boland J-A, Alouf J E, Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991;59:2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh T K, Bian J, Gill D L. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J Biol Chem. 1994;269:22628–22635. [PubMed] [Google Scholar]
- 18.Ginocchio C, Pace J, Galán J E. Identification and molecular characterization of Salmonella typhimurium gene involved in triggering the internalization of salmonellae into cultured epithelial cells. Proc Natl Acad Sci USA. 1992;89:5976–5980. doi: 10.1073/pnas.89.13.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986;38:321–416. [PubMed] [Google Scholar]
- 20.Goldfine H, Johnston N C, Knob C. Nonspecific phospholipase C of Listeria monocytogenes: activity on phospholipids in Triton X-100-mixed micelles and in biological membranes. J Bacteriol. 1993;175:4298–4306. doi: 10.1128/jb.175.14.4298-4306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldfine H, Knob C. Purification and characterization of Listeria monocytogenes phosphatidylinositol-specific phospholipase C. Infect Immun. 1992;60:4059–4067. doi: 10.1128/iai.60.10.4059-4067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinstein S, Furuya W. Assessment of Na+-H+ exchange activity in phagosomal membranes of human neutrophils. Am J Physiol. 1988;254:C272–C285. doi: 10.1152/ajpcell.1988.254.2.C272. [DOI] [PubMed] [Google Scholar]
- 23.Harrison S M, Bers D M. Correction of proton and Ca association constants of EGTA for temperature and ionic strength. Am J Physiol. 1989;256:C1250–C1256. doi: 10.1152/ajpcell.1989.256.6.C1250. [DOI] [PubMed] [Google Scholar]
- 23a.Johnston, N. C., and H. Goldfine. Unpublished observations.
- 24.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones S, Portnoy D A. Intracellular growth of bacteria. Methods Enzymol. 1994;236:463–467. doi: 10.1016/0076-6879(94)36034-0. [DOI] [PubMed] [Google Scholar]
- 26.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn M, Goebel W. Host cell signalling during Listeria monocytogenes infection. Trends Microbiol. 1998;6:11–15. doi: 10.1016/S0966-842X(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 28.Lagaud G J, Stoclet J C, Andriantsitohaina R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J Physiol (London) 1996;492:689–703. doi: 10.1113/jphysiol.1996.sp021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leimeister-Wächter M, Domann E, Chakraborty T. Detection of a gene encoding a phosphatidylinositol-specific phospholipase C that is coordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991;5:361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 30.Leis H J, Zach D, Huber E, Ziermann L, Gleispach H, Windischhofer W. On the inhibition of prostanoid formation by SK&F 96365, a blocker of receptor-operated calcium entry. Br J Pharmacol. 1995;114:598–601. doi: 10.1111/j.1476-5381.1995.tb17181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin R, Braiman A, Priel Z. Protein kinase C induced calcium influx and sustained enhancement of ciliary beating by extracellular ATP. Cell Calcium. 1997;21:103–113. doi: 10.1016/s0143-4160(97)90034-8. [DOI] [PubMed] [Google Scholar]
- 32.Lin K M, Wenegieme E, Lu P J, Chen C S, Yin H L. Gelsolin binding to phosphatidylinositol 4,5-bisphosphate is modulated by calcium and pH. J Biol Chem. 1997;272:20443–20450. doi: 10.1074/jbc.272.33.20443. [DOI] [PubMed] [Google Scholar]
- 33.Marquis H, Doshi V, Portnoy D A. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquis H, Goldfine H, Portnoy D A. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J Cell Biol. 1997;137:1381–1392. doi: 10.1083/jcb.137.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Zaguilan R, Gurule M W, Lynch R M. Simultaneous measurement of intracellular pH and Ca2+ in insulin-secreting cells by spectral imaging microscopy. Am J Physiol. 1996;270:C1438–C1446. doi: 10.1152/ajpcell.1996.270.5.C1438. [DOI] [PubMed] [Google Scholar]
- 36.Mattie M, Brooker G, Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994;269:3181–3188. [PubMed] [Google Scholar]
- 37.Mayorga L S, Beron W, Sarrouf M N, Colombo M I, Creutz C, Stahl P D. Calcium-dependent fusion among endosomes. J Biol Chem. 1994;269:30927–30934. [PubMed] [Google Scholar]
- 38.Mengaud J, Braun-Breton C, Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991;5:367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 39.Mengaud J, Vicente M F, Cossart P. Transcriptional mapping and nucleotide sequence of the Listeria monocytogenes hlyA region reveal structural features that may be involved in regulation. Infect Immun. 1989;57:3695–3701. doi: 10.1128/iai.57.12.3695-3701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 41.Nofer J R, Tepel M, Walter M, Seedorf U, Assmann G, Zidek W. Phosphatidylcholine-specific phospholipase C regulates thapsigargin-induced calcium influx in human lymphocytes. J Biol Chem. 1997;272:32861–32868. doi: 10.1074/jbc.272.52.32861. [DOI] [PubMed] [Google Scholar]
- 42.Pace J, Hayman M J, Galan J E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 43.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poyart C, Abachin E, Razafimanantsoa I, Berche P. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect Immun. 1993;61:1576–1580. doi: 10.1128/iai.61.4.1576-1580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rijkers G T, Justement L B, Griffioen A W, Cambier J C. Improved method for measuring intracellular Ca++ with fluo-3. Cytometry. 1990;11:923–927. doi: 10.1002/cyto.990110813. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez A, Rioult M G, Ora A, Andrews N W. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurai T, Hell J W, Woppmann A, Miljanich G P, Catterall W A. Immunochemical identification and differential phosphorylation of alternatively spliced forms of the alpha 1A subunit of brain calcium channels. J Biol Chem. 1995;270:21234–21242. doi: 10.1074/jbc.270.36.21234. [DOI] [PubMed] [Google Scholar]
- 49.Sawyer R T, Drevets D A, Campbell P A, Potter T A. Internalin A can mediate phagocytosis of Listeria monocytogenes by mouse macrophage cell lines. J Leukoc Biol. 1996;60:603–610. doi: 10.1002/jlb.60.5.603. [DOI] [PubMed] [Google Scholar]
- 50.Sibelius U, Chakraborty T, Krogel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 51.Sibelius U, Rose F, Chakraborty T, Darji A, Wehland J, Weiss S, Seeger W, Grimminger F. Listeriolysin is a potent inducer of the phosphatidylinositol response and lipid mediator generation in human endothelial cells. Infect Immun. 1996;64:674–676. doi: 10.1128/iai.64.2.674-676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith E R, Jones P L, Boss J M, Merrill A H., Jr Changing J774A.1 cells to new medium perturbs multiple signaling pathways, including the modulation of protein kinase C by endogenous sphingoid bases. J Biol Chem. 1997;272:5640–5646. doi: 10.1074/jbc.272.9.5640. [DOI] [PubMed] [Google Scholar]
- 53.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang P, Rosenshine I, Cossart P, Finlay B B. Listeriolysin O activates mitogen-activated protein kinase in eucaryotic cells. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Langendonck N, Velge P, Bottreau E. Host cell protein kinases are activated during the entry of Listeria monocytogenes. Possible role of pp60c-src family protein kinases. FEMS Microbiol Lett. 1998;162:169–176. doi: 10.1111/j.1574-6968.1998.tb12995.x. [DOI] [PubMed] [Google Scholar]
- 57.Vazquez G, de Boland A R. Involvement of protein kinase C in the modulation of 1alpha,25- dihydroxy-vitamin D3-induced 45Ca2+ uptake in rat and chick cultured myoblasts. Biochim Biophys Acta. 1996;1310:157–162. doi: 10.1016/0167-4889(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 58.Vazquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zobrist R H, Giacomini K M, Nelson W L, Giacomini J C. The interaction of phenylalkylamine calcium channel blockers with the 1,4-dihydropyridine binding site. J Mol Cell Cardiol. 1986;18:963–974. doi: 10.1016/s0022-2828(86)80010-4. [DOI] [PubMed] [Google Scholar]