Abstract
The role of an extracellular cysteine protease encoded by the speB gene in group A Streptococcus (GAS) skin infection was studied with a mouse model. Mice were injected subcutaneously with a wild-type GAS serotype M3 strain or a cysteine protease-inactivated isogenic derivative grown to stationary phase. The mortality rate of mice injected with the M3 speB mutant strain was significantly decreased (P < 0.0008) compared to that of animals injected with the wild-type parental organism. The abscesses formed in animals infected with the cysteine protease mutant strain were significantly smaller (P < 0.0001) than those caused by the wild-type organism and slowly regressed over 3 to 4 weeks. In striking contrast, infection with the wild-type GAS isolate generated necrotic lesions, and in some animals the GAS disseminated widely from the injection site and produced extensive cutaneous damage. All of these animals developed bacteremia and died. GAS dissemination was accompanied by severe tissue and blood vessel necrosis. Cysteine protease expression in the infected tissue was identified by immunogold electron microscopy. These data demonstrate that cysteine protease expression contributes to soft tissue pathology, including necrosis, and is required for efficient systemic dissemination of the organism from the initial site of skin inoculation.
Invasive infections caused by group A Streptococcus (GAS) include necrotizing fasciitis, septic scarlet fever, puerperal sepsis, and toxic shock with multiorgan failure (44). The more common sources of streptococcal bacteremia are infections of the skin and soft tissue, indicating that these sites are natural entry routes for this organism (21). Local trauma, often without deep skin penetration, is sufficient to initiate infection (46). GAS strains causing severe invasive diseases produce streptococcal pyrogenic exotoxin type B (SpeB), also known as extracellular cysteine protease, and often at high levels (3, 12, 36, 42, 50).
Recently, this cysteine protease was shown to participate in GAS pathogenesis in one model of invasive disease. Inactivation of the speB gene resulted in a significant decrease in the number of mice that died after intraperitoneal injection (33). A subsequent study showed that the cysteine protease mutant was less resistant to phagocytosis and disseminated less easily to organs (32). Another recent analysis demonstrated that compared with the parent isogenic strain, the protease-negative mutant was more readily internalized, and apparently killed by, cultured human endothelial and epithelial cells (5).
Several other lines of evidence suggesting that the cysteine protease may contribute to invasive GAS infections by direct and indirect mechanisms have accumulated. Purified cysteine protease caused a cytopathic effect on cultured human endothelial cells and cleaved human extracellular matrix components, including fibronectin and vitronectin, which are involved in maintaining cell morphology and tissue integrity (7, 28). This direct tissue damage could contribute to the severe histopathologic changes observed in some patients with invasive GAS infections. One indirect cysteine protease action involves activation of a human endothelial cell matrix metalloprotease (MMP) (6). MMPs play a crucial role in maintaining proper tissue structure and function, and aberrant activation of MMPs results in tissue destruction (16, 30, 41). Recently, the streptococcal cysteine protease was shown to cleave plasma kininogen, resulting in kinin release (17). This potent proinflammatory molecule increases vascular permeability (15), a process that may facilitate systemic bacterial dissemination and host death.
Although these studies have advanced our understanding of GAS pathogenesis, they have not provided insight into the molecular mechanisms mediating soft tissue destruction. In this study, we investigated the role of the cysteine protease in a mouse model of invasive skin infection. The area and volume of the abscesses that developed following subcutaneous injection of the wild-type M3 isolate grown to stationary phase were significantly greater than those observed in mice injected with the M3 cysteine protease mutant strain. The wild-type strain produced extensive cutaneous necrosis, bacteremia, and death, whereas the mutant strain produced discrete abscesses that gradually regressed over time. Histologic analysis of skin sections confirmed the fundamental difference between the pathology caused by the two isogenic strains. We conclude that in this mouse model the GAS extracellular cysteine protease participates in the molecular pathogenesis of soft tissue disease and is required for efficient systemic spread from the subcutaneous inoculation site.
MATERIALS AND METHODS
Bacterial strains and growth.
The wild-type M3 strain, recovered from a patient with invasive GAS disease, and its isogenic mutant derivative used in these studies have been described previously (33). The isogenic M3 speB mutant lacks expression of active extracellular cysteine protease because of insertional inactivation of the structural gene.
Strains were grown in brain heart infusion broth (Remel, Lenexa, Kans.) at 37°C in a 5% CO2–20% O2 atmosphere. The M3 speB mutant strain was grown in medium supplemented with 3 μg of erythromycin per ml.
Animal inoculation.
Five-week-old (20- to 30-g) outbred, immunocompetent, hairless male mice (strain Crl:SKH1-hrBR) (Charles River, Wilmington, Mass.) were used for subcutaneous injection. Adult (20- to 25-g) male outbred CD-1 Swiss mice (Harlan, Houston, Tex.) were used for intranasal inoculation. Prior to experimental procedures, the animals were anesthetized by Metofane (Mallinckrodt Veterinary, Mundelein, Ill.) inhalation. Tissue samples were collected following humane euthanasia.
Inocula were prepared from GAS cultures as described previously (33). Since cysteine protease expression is substantially upregulated in the stationary phase of growth (8, 13), overnight GAS cultures were used. Briefly, cells were harvested and washed once with sterile ice-cold, pyrogen-free phosphate-buffered saline (PBS). The optical density at 600 nm (OD600) was adjusted to give the required inoculum. GAS cells contained in 0.1 ml were injected subcutaneously in the right flank of each animal with a tuberculin syringe. For intranasal inoculation, GAS cells contained in a 50-μl volume were applied to the nostrils of anesthetized mice with disposable tips attached to the pipette. Control mice were treated with the same volume of PBS. The number of CFU inoculated per mouse was verified for each experiment by colony counts on tryptose agar plates containing 5% sheep blood (Becton Dickinson, Cockeysville, Md.). The mice were observed for 21 days after challenge. Blood was collected from each dead animal by cardiac puncture and cultured on blood agar plates. All cultures yielded beta-hemolytic colonies with characteristic GAS morphology.
Determination of GAS inoculum size.
It was shown previously that intraperitoneal injection of 106 CFU of the wild-type M3 GAS isolate used in the present study killed at least 90% of mice by day 5 following inoculation (33). The infected animals developed bacteremia with dissemination to major organs (32).
To establish the size of the inoculum to be administered subcutaneously, serial 10-fold dilutions containing ∼104 to ∼108 CFU of the wild-type GAS M3 isolate were injected into groups of 10 animals. Inocula of ∼107 and ∼108 CFU killed 50 and 80% of the mice, respectively, as assessed at 21 days after injection. GAS was the sole organism cultured from all blood samples collected from dead animals. On the basis of these results, an inoculum of ∼107 or ∼108 CFU was used in subsequent experiments.
Tissue collection and histology.
Prior to inoculation, the animals were assigned to one of three groups (M3, M3 speB, or PBS control) with a random number generator, and blood samples were drawn to establish baseline hematologic data. Blood and tissue samples were collected at 24, 48, and 72 h after inoculation. The methods used for blood and tissue collection were identical for all time points.
Blood samples were obtained from the retro-orbital sinus of the animals, and complete blood count analysis was performed with a Technicon H*1 (Tarrytown, N.Y.) hematology analyzer with species-specific software. Skin samples were collected by wide marginal excision around the abscess or the injection site. These samples always included tissue from the injection site and contiguous grossly normal tissue for comparison. Care was taken to preserve the anatomic orientation of the samples. Tissue samples were also obtained from the heart, liver, spleen, and lung.
All tissues were fixed in 10% neutral buffered formalin supplemented with zinc chloride (Antech, Ltd., Battle Creek, Mich.). Whole lungs were first infused with formalin and then, along with the other organs, fixed by submersion. The samples were placed in formalin for 18 to 24 h and then transferred to 70% ethyl alcohol prior to processing. Standard histologic methods of dehydration in ascending grades of ethyl alcohol, clearing in xylene, and paraffin infiltration were employed. The paraffin blocks were processed with a rotary microtome to obtain 4-μm sections. The histologic sections were stained with hematoxylin and eosin and mounted. Selected tissues were sectioned and stained with a tissue Gram stain (34).
Immunogold electron microscopy.
Skin samples were collected as described above, fixed in 10% phosphate-buffered formalin overnight, and dehydrated through a graded ethanol series to 95%. The specimens were infiltrated with LR White resin through a graded 95% ethanol–resin series over 3 days. The tissue was placed in fresh resin and polymerized at 60°C overnight. Thin sections were generated and placed on Formvar-coated nickel grids. Free aldehydes were blocked by floating the grids on 0.2 M glycine in PBS. The grids were washed in 1% bovine serum albumin in PBS and incubated for 1 h at room temperature with rabbit polyclonal antibody (diluted 1:25) raised against the purified 40-kDa recombinant zymogen form of the cysteine protease (14). The antibody was removed by washing with bovine serum albumin-PBS, after which the tissue was incubated for 1 h in 10-nm colloidal gold-labeled goat anti-rabbit antibody (Sigma Chemical Co., St. Louis, Mo.). The tissue sections were washed in PBS and then in water and stained in 2% aqueous uranyl acetate for 10 min. After being washed in water, the sections were examined with a JEOL 1200 transmission electron microscope at an acceleration voltage of 60 kV and a magnification of ×15,000. Negative-control tissue samples omitted the primary antibody step to exclude nonspecific labeling.
Mouse measurements.
Mice were weighed immediately before GAS inoculation. The animal weight and abscess size were measured 12 h after inoculation and daily thereafter for the first week. Animals were then observed at weekly intervals for a total of 21 days. The dimensions of the abscesses were measured with a caliper; length (L) and width (W) values were used to calculate abscess volume [V = 4/3π(L/2)2 × (W/2)] and area [A = π(L/2) × (W/2)], employing equations for a spherical ellipsoid.
Statistical analysis.
Statistical differences between the animal groups infected with either the wild-type M3 or mutant M3 speB strain were examined. Kaplan-Meier survival curves were plotted for the mouse mortality experiments and tested for statistical significance with the log rank test. Repeated-measures analysis of variance (ANOVA) was used to test for differences in the abscess areas and volumes and weight loss in the groups. A two-way ANOVA was employed, with one within-subjects and one between-subjects factor. Three-group comparisons were conducted with Duncan’s multiple comparison procedure. Nonparametric ANOVA methods (Kruskal-Wallis tests) were also used to ensure that the results did not depend on assumptions of distributional normality. Fisher’s exact test was used to compare pathologic characteristics of the vascular and cutaneous lesions in mice infected with the wild-type versus the mutant strain. Statistical significance was evaluated at the 0.05 and 0.01 levels with a two-tailed test.
RESULTS
Mouse mortality after GAS inoculation.
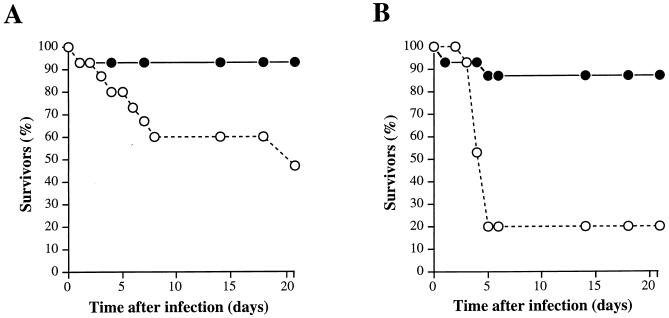
To study the effect of the cysteine protease on the ability of GAS to kill mice after subcutaneous injection, groups of 15 animals were inoculated with the wild-type M3 or the isogenic M3 speB mutant strain grown overnight and were observed for 3 weeks. An inoculum of ∼107 CFU of the wild-type strain killed significantly more mice than did the mutant derivative (P < 0.039 by the log rank test) (Fig. 1A). Systemic spread of the wild-type GAS in blood resulted in mortality occurring predominantly within the first 8 days after injection. The difference in mouse mortality between the isogenic strains was even greater (P < 0.0008 by the log rank test) when an inoculum of ∼108 CFU was used (Fig. 1B). The mice died more rapidly, usually within 5 days, when the higher inoculum was used. In striking contrast, the cysteine protease mutant strain lost virtually all ability to kill mice after subcutaneous inoculation. In the 21 days of the experiment, only one and two mice died following injection of ∼107 and ∼108 CFU of the M3 speB mutant, respectively.
FIG. 1.
Kaplan-Meier survival curves (n = 15 mice in each group) following subcutaneous inoculation with the wild-type Streptococcus pyogenes serotype M3 strain (open circles) and the cysteine protease-inactivated isogenic M3 speB derivative (solid circles) grown overnight. (A) Inoculum of ∼107 CFU; χ2 = 4.2 and P < 0.0398. (B) Inoculum of ∼108 CFU; χ2 = 11.2 and P < 0.0008.
All mice injected with ∼107 or ∼108 CFU of either the wild-type or mutant strain lost weight. There was no significant difference in mean weight loss between the mice infected with ∼107 CFU of the wild-type and mutant organisms. However, a significant difference (P < 0.007 by ANOVA) in mean weight loss was observed between the animals injected with ∼108 CFU of the wild-type and mutant strains.
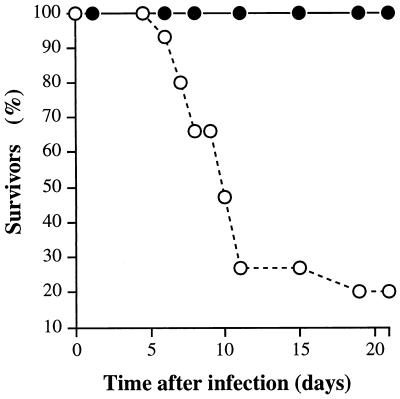
Reduced mouse mortality observed after subcutaneous (this study) and intraperitoneal (33) inoculations suggested that the cysteine protease is required for efficient pathogen dissemination. To test this hypothesis, mice were also inoculated intranasally with 107 CFU of stationary-phase organisms (Fig. 2). A significant (P < 0.0001) decrease in mouse lethality was observed in the M3 speB mutant group, indicating the importance of cysteine protease expression regardless of the site of initial infection.
FIG. 2.
Kaplan-Meier survival curves (n = 15 mice in each group) following intranasal inoculation with the wild-type S. pyogenes serotype M3 strain (open circles) and the cysteine protease-inactivated isogenic M3 speB derivative (solid circles). A significant difference in mouse mortality was observed (inoculum, ∼107 CFU; χ2 = 20.2 and P < 0.0001).
Gross pathology of the cutaneous lesions in mice inoculated with wild-type GAS.
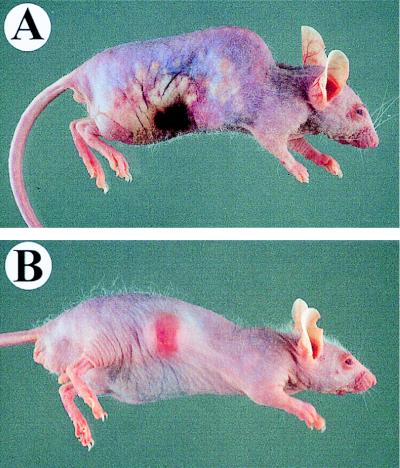
Several major differences in the character of the gross cutaneous lesions in animals injected with either the wild type or the cysteine protease mutant were observed (Fig. 3). All 10 mice infected with ∼106 CFU of the wild-type strain developed abscesses, which were accompanied in 6 animals by local erythema and ulceration (ulcerative dermatitis). All animals injected with ∼107 CFU formed cutaneous ulcers. Moreover, in eight of the mice receiving ∼107 CFU, the infection spread radially from the original injection site to cause necrotizing dermatitis 3 to 4 days after injection (Fig. 3A). These widespread necrotic skin lesions also occurred in 12 of 15 animals injected with ∼108 CFU. All mice that developed these severe skin lesions subsequently died within 1 to 2 days, and a pure culture of GAS was recovered from the heart blood of dead animals.
FIG. 3.
Cutaneous lesions in mice inoculated with wild-type and isogenic speB mutant GAS strains. (A) Mouse infected subcutaneously with 107 CFU of the wild-type M3 isolate. The infection spread radially (day 3) from the inoculation site and resulted in extensive subcutaneous and dermal necrosis involving a large portion of the lateral side of the animal. (B) Mouse inoculated with 107 CFU of the cysteine protease-inactivated M3 mutant strain. A solitary subcutaneous abscess formed and then regressed over time.
The lesion areas and volumes in mice infected with the wild-type strain depended on the inoculum size. The lesions were compared for three different inoculum sizes (∼106, ∼107, and ∼108 CFU) at 24, 48, 72, and 96 h. As assessed by ANOVA, a significant effect of group-by-time interaction on area (P < 0.0001) and volume (P < 0.0004) was identified. These results indicated that the three inocula produce different lesion area and volume changes over the duration of this experiment. Comparisons of each group to each of the other groups were conducted with Duncan’s multiple-range test. The three groups differed significantly from one another in both abscess area and volume (P < 0.01). Nonparametric tests also confirmed the group differences (P < 0.0001).
Gross pathology of the cutaneous lesions in mice receiving the speB mutant strain.
In contrast to the severe lesions observed in mice receiving the wild-type organism, the abscesses that developed in mice inoculated with the speB mutant were discrete and slowly regressed over time. For example, injection of ∼107 CFU generated abscesses but not cutaneous ulcers (Fig. 3B). The lesion areas and volumes in mice inoculated with ∼107 CFU of the wild type or speB mutant were measured over 7 days and compared by use of a repeated-measures ANOVA. Significant group differences in lesion areas (P < 0.0001) and volumes (P < 0.0001) were identified. The Kruskal-Wallis (nonparametric) test confirmed that the results did not depend on assumptions of normality of the distributions. A significant difference was also found between the two mouse groups infected with ∼108 CFU in lesion areas (ANOVA, P < 0.0007; Kruskal-Wallis, P < 0.0001) and volumes (ANOVA, P < 0.0001; Kruskal-Wallis, P < 0.0001) over time.
Histopathologic studies.
Inasmuch as the data indicated significant differences in the character of the gross skin lesions occurring in mice inoculated with the wild-type versus the mutant organism, it was important to examine the histopathology of these lesions in detail. Hence, additional experiments were performed to investigate morphologic changes in the early stages of infection that resulted in the observed differences in gross pathology. Groups of 18 mice each were injected with ∼108 CFU of wild-type M3, the M3 speB mutant, or PBS (control). Six randomly chosen mice from each group were sacrificed at 24, 48, and 72 h after inoculation and examined for histopathologic lesions in skin, liver, heart, lung, and kidney.
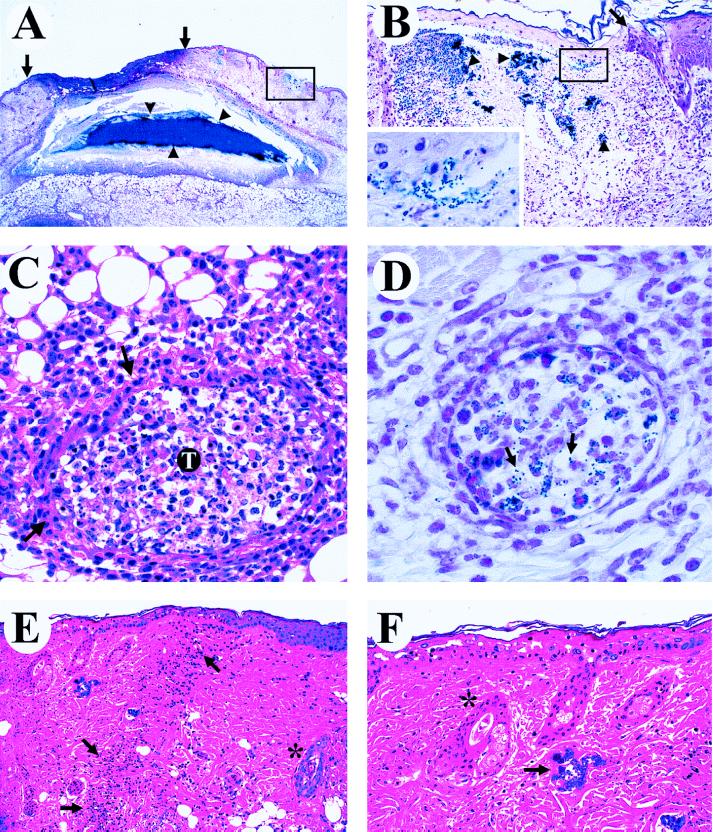
Mice inoculated with either the wild-type or mutant organisms, but not the PBS control, had one or more subcutaneous abscesses. These abscesses were located at the inoculation site and contained a central core or sheet of gram-positive cocci (Fig. 4A and B). The central abscess core was surrounded by a rim of coagulative necrosis with a serpentine or undulating border. A layer of degenerating and dying neutrophils was located exterior to this border. Adjacent to the abscesses was a wide area of subcutaneous edema containing scattered neutrophils and foci of collagen necrosis (Table 1). Blood vessels in the subcutis and dermis were often dilated and congested, and perivascular cuffing by neutrophils or fibrinoid necrosis of the vascular wall was present (Table 2). Vascular thrombosis occurred as the lesion progressed over time (Fig. 4C). Nerves located at the lesion site often were entrapped by the inflammatory process. These pyogenic lesions extended downward to the underlying musculature, a process resulting in degeneration and necrosis of individual muscle fibers. The muscle and adjacent connective tissue were infiltrated by neutrophils, and in later stages, muscle atrophy occurred. The overlying dermis contained scattered foci of suppurative inflammation, mast cells, and activated fibroblasts. Collagen degeneration and lysis was also observed in this site.
FIG. 4.
Photomicrographs demonstrating different aspects of the skin pathology in animals infected with the wild-type strain expressing cysteine protease. (A and B) Bacterial spread from the initial site of injection (Gram stain). (A) A large depressed cutaneous infarct is located between the solid arrows. Note the large colony of gram-positive (blue) cocci in the center of a solitary subcutaneous abscess (arrowheads). One of several microinfarcts is boxed. Magnification, ×4. (B) Photomicrograph of one microinfarct at a higher magnification (×41). The arrow identifies the junction of an epidermal infarct, with healthy tissue located on the right and a pale pink necrotizing epidermitis on the left. Note blue-stained bacterial colonies in the dermis (arrowheads). The inset shows a higher magnification (×164) of the boxed area and demonstrates the spread of gram-positive cocci into the upper dermis and epidermis. (C and D) Bacterial spreading results in vascular pathology. (C) Necrotizing vasculitis of a subcutaneous blood vessel (arrows) with a thrombus (T) (hematoxylin and eosin stain). The vascular lumen contains fibrin, neutrophils, and necrotic cellular debris. Magnification, ×82. (D) A blood vessel with early thrombosis and numerous gram-positive-cocci (arrows) located within the thrombus (Gram stain). Magnification, ×164. (E and F) Bacterial spreading causes cutaneous infarction (hematoxylin and eosin stain). (E) A line of demarcation (arrows) is located between healthy skin on the right and the necrotic zone on the left, with linear infiltration of polymorphonuclear leukocytes at the interface. Note the normal blue-stained hair follicle within a histologically normal zone (*). Magnification, ×41. (F) Higher magnification (×82) demonstrating necrosis of basal cells and pallor of the stratum granulosum and stratum spinosum in the epidermal infarct. Note blue-stained bacterial colonies in the upper dermis (arrow) and the abnormal light pink staining of the hair follicle in the infarcted area (*).
TABLE 1.
Characteristics of cutaneous lesions identified in SKH1 mice inoculated with the GAS M3 wild-type strain or cysteine protease mutanta
| Skin location and lesion character | No. positive/total at the following time (h) and treatment:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 24
|
48
|
72
|
|||||||
| PBS control | Wild-type strain | Protease mutant | PBS control | Wild-type strain | Protease mutant | PBS control | Wild-type strain | Protease mutant | |
| Epidermis, infarction | 0/6 | 0/6 | 0/6 | 0/6 | 5/6 | 0/6 | 0/6 | 6/6 | 1/6b |
| Dermis | |||||||||
| Suppurative inflammation | 0/6 | 4/6 | 5/6 | 2/6 | 6/6 | 5/6 | 2/6 | 6/6 | 6/6 |
| Collagen necrosis | 0/6 | 1/6 | 1/6 | 1/6 | 5/6 | 1/6 | 0/6 | 0/6d | 0/6 |
| Extension through panniculus | 0/6 | 2/6 | 2/6 | 0/6 | 5/6 | 1/6 | 0/6 | 6/6 | 1/6b |
| Bacterial colonies away from inoculation site | 0/6 | 1/6 | 0/6 | 0/6 | 5/6 | 1/6 | 0/6 | 4/6 | 0/6b |
| Subcutis | |||||||||
| Single abscess | 0/6 | 3/6 | 6/6 | 0/6 | 4/6 | 6/6 | 0/6 | 0/6 | 4/6c |
| Multiple abscesses | 0/6 | 3/6 | 0/6 | 0/6 | 2/6 | 0/6 | 0/6 | 6/6 | 0/6c |
| Extension to underlying muscle | 0/6 | 4/6 | 5/6 | 0/6 | 6/6 | 4/6 | 0/6 | 6/6 | 6/6 |
| Suppurative inflammation | 0/6 | 6/6 | 6/6 | 2/6 | 6/6 | 5/6 | 0/6 | 6/6 | 6/6 |
| Perineural PMNs/necrosis | 0/6 | 2/6 | 1/6 | 0/6 | 1/6 | 0/6 | 0/6 | 4/6 | 2/6 |
| Muscle, necrotizing myositis | 0/6 | 3/6 | 3/6 | 0/6 | 6/6 | 4/6 | 0/6 | 3/6 | 6/6 |
Mice were randomly assigned to be inoculated with the GAS M3 wild-type strain or cysteine protease mutant or with PBS. The statistical difference between cutaneous lesions in animals (days 2 and 3 combined) inoculated with the wild-type or cysteine protease mutant strain was calculated by using Fisher’s exact (two-tailed) test.
P < 0.01.
P < 0.05.
Collagen necrosis in the dermis on day 3 was masked by the increased size of infarcts.
TABLE 2.
Vascular lesions identified in SKH1 mice inoculated with the GAS M3 wild-type strain or cysteine protease mutanta
| Skin location and vascular lesion character | No. positive/total at the following time (h) and treatment:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 24
|
48
|
72
|
|||||||
| PBS control | Wild-type strain | Protease mutant | PBS control | Wild-type strain | Protease mutant | PBS control | Wild-type strain | Protease mutant | |
| Epidermis, infarction | 0/6 | 0/6 | 0/6 | 0/6 | 5/6 | 0/6 | 0/6 | 6/6 | 1/6b |
| Dermis | |||||||||
| Perivascular PMNs | 0/6 | 1/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 3/6 |
| Necrotizing vasculitis | 0/6 | 0/6 | 1/6 | 0/6 | 3/6 | 0/6 | 0/6 | 4/6 | 2/6c |
| Thrombosis | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 | 3/6 | 1/6 |
| Vascular congestion | 0/6 | 0/6 | 1/6 | 0/6 | 4/6 | 3/6 | 0/6 | 4/6 | 1/6 |
| Subcutis | |||||||||
| Perivascular PMNs | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 2/6 |
| Necrotizing vasculitis | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 4/6 | 2/6 |
| Thrombosis | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 3/6 | 2/6 |
| Vascular congestion | 0/6 | 0/6 | 0/6 | 0/6 | 5/6 | 3/6 | 0/6 | 1/6 | 2/6 |
Mice were randomly assigned to be inoculated with the GAS M3 wild-type strain or cysteine protease mutant or with PBS. The statistical difference between vascular lesions in animals (days 2 and 3 combined) inoculated with the wild-type or cysteine protease mutant strain was calculated by using Fisher’s exact (two-tailed) test.
P < 0.01.
P < 0.05.
Several critical differences in the host response induced by the wild-type and mutant GAS strains were associated with dissemination of the organisms away from the original inoculation site. The pyogenic lesions present in mice infected with the wild-type strain extended outward through the panniculus carnosus and invaded the overlying dermis and epidermis. GAS colonies were located near the basal cell layer of the epidermis (Fig. 4D). This microbial invasion resulted in severe vascular embarrassment, with vascular congestion, necrotizing vasculitis, and thrombosis observed on days 2 and 3 of the experiment (Table 2). These vascular lesions resulted in single or multiple infarcts of the dermis and epidermis that were characterized by thinning of the epidermis with necrosis of basal cells and pallor of the stratum granulosum and stratum spinosum (Fig. 4E, F). These lesions had a loss of tinctorial quality in the dermis with necrosis of collagen and adnexal structures in the infarcted zone. A line of demarcation was present between the normal and infarcted tissue, and this interface was infiltrated with abundant neutrophils.
In contrast to the extensive histopathologic lesions observed in mice given wild-type GAS, mice injected with the speB mutant strain had abscesses that were smaller and more discrete. The host inflammatory response was limited to the subcutis in most animals and seldom extended through the panniculus carnosus (Table 1). Moreover, bacterial colonies located away from the injection site were observed in only one animal. Importantly, several significant differences were observed in the frequency and degree of severity of vascular lesions in mice inoculated with the cysteine protease mutant strain compared with animals inoculated with the wild-type organism (Table 2).
The heart, lung, kidneys, and liver also were investigated microscopically. No significant infection-related microscopic lesions were observed in the heart, lung, or kidney in any of the animals. Acute suppurative inflammation distributed in an apparently random pattern was seen in the livers of mice receiving either wild-type or mutant GAS. The lesions consisted of foci of neutrophils (microabscesses), and occasional animals had acute coagulative hepatocellular necrosis; these lesions were more frequent in mice in the mutant and wild-type experimental groups than in the PBS-injected control mice.
The hematologic findings for infected mice showed different patterns in the leukocyte counts during infection. Interestingly, there was an initial leukopenia (P < 0.043 by Duncan’s multiple-range test) on days 1 and 2 in animals infected with the wild-type strain, followed by a pronounced leukocytosis on day 3. A progressive neutrophilia accompanied the rise in total leukocyte count. All values obtained for mice in the PBS control group were within normal limits for this age and sex of animal.
Cysteine protease expression in infected tissue.
Humans with pharyngitis and invasive infections caused by several M protein serotypes seroconvert to cysteine protease, indicating that the virulence factor is expressed in the course of host-pathogen interactions (14, 38, 48). Although the fact that immunization of mice with streptococcal cysteine protease protects them against lethal challenge with GAS inoculated intraperitoneally is additional strong evidence that this enzyme is expressed in vivo (26), the site of cysteine protease production in a mouse model of invasive infection has not been investigated. We therefore used immunogold electron microscopy to determine whether cysteine protease was produced in the skin lesions of mice given wild-type GAS.
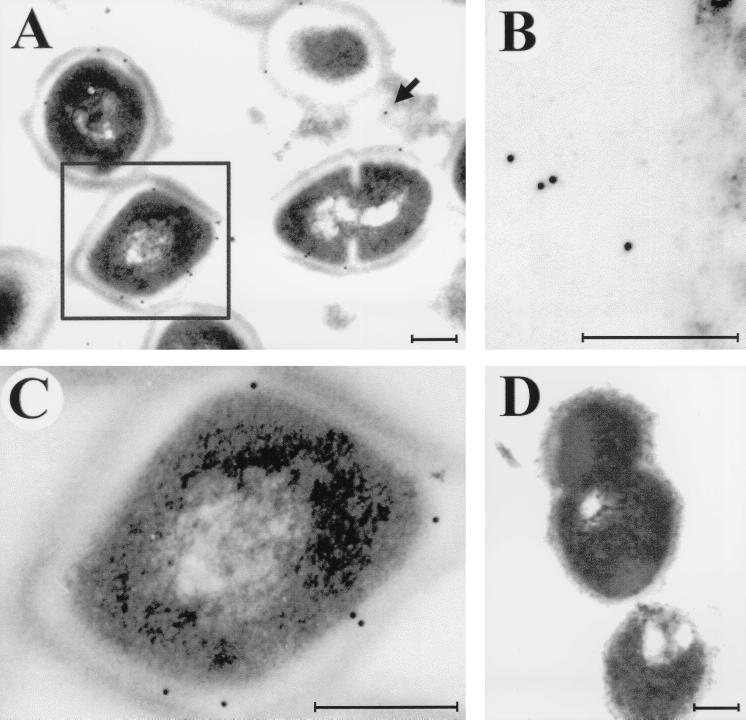
Infected skin was harvested 2 days after inoculation with the wild-type strain and examined by immunogold electron microscopy with specific anti-cysteine protease rabbit serum (Fig. 5). Immunogold staining of cysteine protease was identified in infected tissue (Fig. 5A). Free cysteine protease (not associated with GAS cells) was present in tissues, a result expected for an actively secreted product (Fig. 5B). Importantly, cysteine protease also remained associated with GAS cells in these lesions (Fig. 5C). Control immunogold staining via the same procedure, but omitting the specific anti-cysteine protease antibody, did not result in a positive signal (Fig. 5D). These data indicate that the streptococcal cysteine protease was produced in skin lesions in this mouse model of soft tissue infection.
FIG. 5.
Immunogold electron microscopy localization of the cysteine protease produced by the wild-type GAS during skin infection. (A) Cysteine protease is produced by GAS within infected tissue; the cysteine protease fraction is detected in a secreted form (solid arrow) in the tissue or as a fraction still associated with GAS cells (boxed cell). (B) Cysteine protease fraction released into surrounding tissue. (C) Cell-associated cysteine protease fraction of the boxed cell in panel A. (D) Negative control in which no nonspecific background labeling is detected. Tissue samples were collected on day 2 following inoculation with 108 CFU of the wild-type M3 strain. Bars, 200 nm.
DISCUSSION
The results of our study confirm and extend the theme that the cysteine protease, also known as pyrogenic exotoxin B, is a critical participant in the complex interaction between GAS and the host that results in pathology, morbidity, and mortality. The key findings are that expression of the cysteine protease contributes to soft tissue pathology and is required for efficient systemic dissemination from the skin.
Cysteine protease contributes to soft tissue pathology.
Several lines of evidence indicate that the cysteine protease is produced during human infection (36), including the observation that patients with GAS infections seroconvert to this virulence factor (14, 18, 38, 48). Until now, however, the location of cysteine protease production in the host with invasive disease has not been demonstrated. Our studies show that cysteine protease is expressed in injured tissue and indicate that the enzyme is released into infected tissue in a cell-free form. Immunogold labeling indicated that the cysteine protease also is located on the surface of GAS cells located in diseased tissue.
Extensive tissue destruction typically occurs in invasive streptococcal skin infections such as necrotizing fasciitis or myositis (44, 52). We observed that animals infected with the wild-type strain had significantly more tissue damage than did mice receiving the cysteine protease mutant. In principle, the difference between the wild-type and mutants strains in ability to cause tissue damage could be due to either a direct or indirect cysteine protease action. The streptococcal cysteine protease may directly damage tissue by its ability to cleave fibronectin and degrade vitronectin (28), both of which are important extracellular matrix proteins involved in maintaining connective tissue integrity (7, 51).
However, the speed with which tissue destruction and endothelial cell damage progress during human invasive GAS infection suggests that more than just a direct mechanism is at work. Two indirect mechanisms of cysteine protease action may enhance tissue pathology. First, Burns et al. (6) demonstrated that the cysteine protease activates a 66-kDa human MMP (later found to be MMP-2 [unpublished data]), a process that results in increased type IV collagenase activity. Degradation of collagen and other extracellular matrix proteins by MMPs may contribute to soft tissue pathology by a mechanism analogous to that observed during tumor metastasis (41). Our mouse studies show that the wild-type GAS strain typically produced cutaneous infarcts with extensive collagen necrosis. Second, Kapur et al. (27) discovered that cysteine protease processed inactive interleukin-1β (IL-1β) precursor to form biologically active IL-1β, a major inflammatory mediator. Release of large quantities of IL-1β may also contribute to tissue necrosis (35). We believe that there is evidence to indicate that cysteine protease contributes to GAS-mediated soft tissue pathology in both direct and indirect fashions.
In addition to cutaneous lesions, we also observed more severe vascular lesions in animals infected with the wild-type organism compared with animals inoculated with the cysteine protease mutant. This vascular pathology was manifested by acute vascular congestion, perivascular cuffing of neutrophils, necrotizing vasculitis, and thrombosis. The vasculitis was characterized by fibrinoid necrosis and infiltration of neutrophils into the vascular wall and by the presence of nuclear debris in this region. Affected vessels had swollen endothelia, and a mixed cellular, fibrin thrombus was often associated with the vasculitis. These vascular lesions caused tissue anoxia and subsequent infarction of the dermis and overlying epidermis. This type of vascular disease has been classified as cutaneous leukocytoclastic vasculitis (39), has been reported to occur in humans and animals, and can be caused by sepsis (25). In this mouse model, gram-positive cocci were identified in the vascular lesions located within the lumina of affected vessels. In human invasive infection, clusters of GAS are also found in the walls and lumina of blood vessels (22), and some investigators view streptococcal gangrene as a sequel of vessel thrombosis (10).
Cysteine protease expression is required for efficient systemic dissemination.
GAS initially colonize human mucosal and skin surfaces, from where the bacteria penetrate into deeper tissues. Several investigators have reported that approximately 80% of invasive GAS episodes have either a throat or skin focus (29). We found that 80% of mice infected with 108 CFU of the wild-type strain developed bacteremia and died, versus only ∼10% of animals injected with the mutant strain (P < 0.0008). Hence, our data indicate that cysteine protease expression significantly enhances GAS dissemination from the skin to cause systemic infection and death. Inasmuch as nasopharyngeal infection is also a common source for GAS bacteremia (21), we compared the abilities of these M3 isogenic strains to cause bloodstream infections and mouse death after intranasal infection. Importantly, cysteine protease expression also was required for systemic infection and death in this model (P < 0.0001), a result consistent with the idea that cysteine protease expression is essential for GAS dissemination following colonization of several anatomic sites.
Although our studies confirmed the importance of cysteine protease as a virulence factor, other bacterial products also contribute to GAS colonization and invasive infection in mice. For example, hyaluronic acid capsule expression is critical for colonization and invasive infection after inoculation of the pharynx (49), trachea (20), and skin (1). M protein inactivation results in significant loss of virulence in mouse skin and skin air sac infection models but not after intraperitoneal inoculation (1, 4). Inactivation of the C5a peptidase (scpA) gene delays neutrophil infiltration to skin air sacs but does not significantly contribute to mouse mortality (24). In addition, the scpA mutant strain is cleared more rapidly from the nasopharynx than is the wild-type strain (23). These observations demonstrate that GAS pathogenesis is complex, with numerous gene products participating.
Cysteine protease production and human disease.
Although GAS has occasionally been recovered from diseased mice (19), it is generally considered to be a natural pathogen of humans only. Therefore, it is reasonable to consider the extent to which our findings contribute to understanding human soft tissue infection caused by GAS. Our results show that cysteine protease participates in dermatopathology in mice. On the presumption that similar molecular processes underlie the pathologic phenotypes common to infected mice and humans, three observations can be made. First, our data suggest that any process that reduces cysteine protease activity will decrease host morbidity and mortality. The results of several serologic studies support this concept. Holm et al. (18) observed that patients with serum antibody directed against cysteine protease were more likely to survive invasive GAS infection than were patients with a low level of acute-phase serum antibody directed against cysteine protease. The data imply that anti-cysteine protease antibodies have a protective effect via neutralization of enzyme activity, an implication supported by the results of active and passive mouse immunization (26, 31) and by in vitro studies showing a relationship between a low capacity of human patient sera to inhibit cysteine protease-induced T-cell mitogenesis and serious manifestation of disease (37).
Second, several histologic features identified in our mouse studies also have been reported in studies of tissues obtained from humans with GAS invasive episodes. For example, in their study of 36 patients with necrotizing fasciitis, Barker et al. (2) reported inflammation and necrosis extending from the epidermis to the subcutaneous fat. Similarly, Cockerill et al. (9) studied patients with M3 GAS infection and reported that full-thickness skin or subcutaneous biopsies showed extensive tissue edema and necrosis. In our experiments, mice given wild-type GAS clearly had more extensive skin lesions than animals receiving the protease mutant. These results imply that cysteine protease participates in skin damage in human infection.
Third, in mice infected with the wild-type organism we identified a transition zone between normal and degraded cutaneous tissue and found that infiltrated neutrophils bordered this area but were not present in diseased tissue. A lack of inflammatory cells in infected subdermal tissues in patients with necrotizing fasciitis has been reported (9). One mechanism to account for this observation postulates that degradation of fibronectin by cysteine protease detrimentally affects polymorphonuclear leukocyte (PMN) infiltration to the infection site (40, 43). We believe that taken together, our findings are relevant, in whole or in part, to human soft tissue infections caused by GAS.
At the time that this paper was ready for submission, two independent reports addressed the role of cysteine protease in other murine models of soft tissue infection (1, 31). Similar to our findings, Kuo et al. (31) showed, using the mouse air pouch model, that speB mutants of GAS serotypes M1 and M49 have a decreased ability to kill mice compared to the parental organisms. The addition of purified cysteine protease, but not a heat-inactivated preparation of the toxin, restored the ability of the speB mutant strain to cause mouse death and tissue damage. In addition, the authors reported that both active and passive immunization resulted in protection against challenge with the wild-type protease-positive strain.
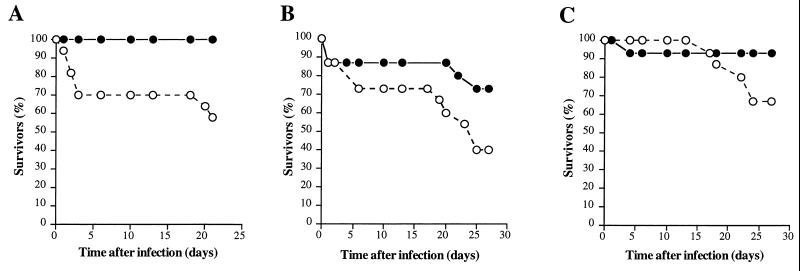
In contrast, Ashbaugh et al. (1) reported that cysteine protease does not participate in murine soft tissue damage caused by GAS serotype M3. This conclusion was based on the use of an isogenic GAS strain with the speB gene inactivated by interposon mutagenesis. No significant difference in the character of the pathology caused by the mutant and the wild-type organisms was observed. Importantly, the GAS inocula were made from early-log-phase cultures, a time when cysteine protease production is absent or negligible (8, 13). Because cysteine protease expression is known to be strikingly upregulated in the stationary phase of growth (8, 13), and because the M3 strain used in our studies produces cysteine protease only in the stationary phase, we have consistently used overnight cultures in the studies reported here and previously (32, 33). To test the possibility that the difference between our results and those reported by Ashbaugh et al. (1) resulted from the use of stationary-phase versus log-phase cultures, we performed additional experiments with our isogenic strains harvested at early (OD600 ∼ 0.25), middle (OD600 ∼ 0.4), and very late (OD600 ∼ 0.8) log phase (Fig. 6).
FIG. 6.
Kaplan-Meier survival curves (n = 15 mice in each group) following subcutaneous inoculation with the wild-type S. pyogenes serotype M3 strain (open circles) and the cysteine protease-inactivated isogenic M3 speB derivative (solid circles). (A) Inoculum prepared from early-log-phase cultures (OD600 ∼ 0.25); 1.3 × 108 CFU of the wild-type M3 and 2.1 × 108 CFU of the M3 speB mutant were injected (χ2 = 7.6 and P < 0.0005). (B) Inoculum prepared from middle-log-phase cultures (OD600 ∼ 0.4); 1.9 × 108 CFU of the wild-type M3 and 2.5 × 108 CFU of the M3 speB mutant were injected (χ2 = 2.9 and P < 0.0834). (C) Inoculum prepared from very-late-phase cultures (OD600 ∼ 0.8); 1.8 × 108 CFU of the wild-type M3 and 1.8 × 108 CFU of the M3 speB mutant were injected (χ2 = 2.9 and P < 0.0859).
When early-log-phase cells were used to prepare the inoculum, we observed a significantly higher mortality rate among animals infected with the wild-type strain than among animals infected with the speB mutant (χ2 = 7.6 and P < 0.0005 by the log rank test) (Fig. 6A). In contrast, when middle- or very-late-log-phase cells were used, the difference in mortality rate was not statistically significant (χ2 = 2.9 and P = 0.0834 or χ2 = 2.9 and P = 0.0859, respectively) (Fig. 6B and C). However, the mortality rate difference between the wild type and the speB mutant was highly significant (χ2 = 12.1 and P = 0.0005) when the results of the three experiments were combined. Based on the results shown in Fig. 1 and 6, we conclude that the growth phase of the culture used to prepare the inoculum is an important variable which may have an impact on the outcome of the experiment and interpretation of the results. We view the use of stationary-phase cultures as being particularly relevant in studies of soft tissue pathology caused by GAS. Cysteine protease expression is absent or negligible early in growth but is greatly upregulated during the stationary phase of growth (8, 13). The stationary phase of growth figures prominently in the pathogenesis of soft tissue disease in animals and humans, especially for the unusually severe forms such as myositis and necrotizing fasciitis (11, 45). The overall contribution of cysteine protease to strain virulence also depends on the entire repertoire of virulence factors expressed by a given strain. For example, in mice cysteine protease appeared to play a more important role in the virulence of strain AM3 (serotype M3) than in that of strain CS101 (M49) (33). This was also true for strain A-20 (M1) compared to strain NZ131 (M49) (31). It may also depend on the particular variant of the cysteine protease. A recent study has shown that the cysteine protease expressed by serotype M1 GAS contains an arginine-glycine-aspartic acid (RGD) motif that binds human integrins, whereas many other cysteine protease variants lack this structural motif and fail to bind integrins (47).
In summary, we have demonstrated that severe soft tissue damage and bacteremia are efficiently caused by wild-type GAS but not by an isogenic cysteine protease mutant. The difference in virulence between the wild-type and mutant strains is most prominent when the bacteria are tested at a time in the growth cycle when cysteine protease is abundantly expressed. Cysteine protease is produced during invasive infection, which adds further support to the concept that this enzyme contributes to cutaneous disease and dissemination by direct tissue destruction and MMP-2 activation (32). These data, the results of previous mouse and human studies (31, 33, 36), and the observation that cysteine protease is well conserved among all GAS isolates (28, 47) lend further credence to the potential utility of a vaccine based on this molecule.
ACKNOWLEDGMENTS
We thank S. Siddiqui and T. Landerholm for assistance with figures.
This study was supported by Public Health Service grant AI-33119 to J. M. Musser.
REFERENCES
- 1.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker F G, Leppard B J, Seal D V. Streptococcal necrotising fasciitis: comparison between histological and clinical features. J Clin Pathol. 1987;40:335–341. doi: 10.1136/jcp.40.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belani K, Schlievert P M, Kaplan E L, Ferrieri P. Association of exotoxin-producing group A streptococci and severe disease in children. Pediatr Infect Dis J. 1991;10:351–354. doi: 10.1097/00006454-199105000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Boyle M D P, Raeder R, Flosdorff A, Podbielski A. Role of emm and mrp genes in the virulence of group A streptococcal isolate 64/14 in a mouse model of skin infection. J Infect Dis. 1998;177:991–997. doi: 10.1086/515241. [DOI] [PubMed] [Google Scholar]
- 5.Burns E H, Jr, Lukomski S, Rurangirwa J, Podbielski A, Musser J M. Genetic inactivation of the extracellular cysteine protease enhances in vitro internalization of group A streptococci by human epithelial and endothelial cells. Microb Pathog. 1998;24:333–339. doi: 10.1006/mpat.1998.0204. [DOI] [PubMed] [Google Scholar]
- 6.Burns E H, Jr, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey D J. Control of growth and differentiation of vascular cells by extracellular matrix proteins. Annu Rev Physiol. 1991;53:161–177. doi: 10.1146/annurev.ph.53.030191.001113. [DOI] [PubMed] [Google Scholar]
- 8.Chaussee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockerill F R, III, Thompson R L, Musser J M, Schlievert P M, Talbot J, Holley K E, Harmsen W S, Ilstrup D M, Kohner P C, Kim M H, Frankfort B, Manahan J M, Steckelberg J M, Roberson F, Wilson W R the Southeastern Minnesota Streptococcal Working Group. Molecular, serological, and clinical features of 16 consecutive cases of invasive streptococcal disease. Clin Infect Dis. 1998;26:1448–1458. doi: 10.1086/516376. [DOI] [PubMed] [Google Scholar]
- 10.Collins R N, Nadel M S. Gangrene due to the hemolytic streptococcus: a rare but treatable disease. N Engl J Med. 1965;272:578–580. doi: 10.1056/NEJM196503182721109. [DOI] [PubMed] [Google Scholar]
- 11.Eagle H. Experimental approach to the problem of treatment failure with penicillin. Group A streptococcal infection in mice. Am J Med. 1952;13:389–399. doi: 10.1016/0002-9343(52)90293-3. [DOI] [PubMed] [Google Scholar]
- 12.Ferrieri P. Microbiological features of current virulent strains of group A streptococci. Pediatr Infect Dis J. 1991;10:S20–S24. doi: 10.1097/00006454-199110001-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gerlach D, Knoll H, Kohler W, Ozegowski J H, Hribalova V. Isolation and characterization of erythrogenic toxins. V. Communication: identity of erythrogenic toxin type B and streptococcal proteinase precursor. Zentbl Bakteriol Hyg Abt 1 Orig A. 1983;255:221–233. [PubMed] [Google Scholar]
- 14.Gubba S, Low D E, Musser J M. Expression and characterization of group A Streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human disease episodes. Infect Immun. 1998;66:765–770. doi: 10.1128/iai.66.2.765-770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall J M. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992;56:131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 16.Hanemaaijer R, Koolwijk P, le Clercq L, de Vree W J, van Hinsbergh V W. Regulation of matrix metalloproteinase expression in human vein and microvascular endothelial cells: effects of tumour necrosis factor α, interleukin 1, and phorbol ester. Biochem J. 1993;296:803–809. doi: 10.1042/bj2960803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herwald H, Collin M, Muller-Ester W, Bjorck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988–1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 19.Hook E W, Wagner R R, Lancefield R C. An epizootic in Swiss mice caused by a group A streptococcus, newly designed type 50. Am J Hyg. 1960;72:111–119. doi: 10.1093/oxfordjournals.aje.a120127. [DOI] [PubMed] [Google Scholar]
- 20.Husmann L K, Yung D-L, Hollingshead S K, Scott J R. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect Immun. 1997;65:1422–1430. doi: 10.1128/iai.65.4.1422-1430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ispahani P, Donald F E, Aveline A J. Streptococcus pyogenes bacteraemia: an old enemy subdued, but not defeated. J Infect. 1988;16:37–46. doi: 10.1016/s0163-4453(88)96073-2. [DOI] [PubMed] [Google Scholar]
- 22.Jevon G P, Dunne W M, Jr, Hawkins H K, Armstrong D L, Musser J M. Fatal group A streptococcal meningitis and toxic shock-like syndrome: case report. Clin Infect Dis. 1994;18:91–93. doi: 10.1093/clinids/18.1.91. [DOI] [PubMed] [Google Scholar]
- 23.Ji Y, Carlson B, Kondagunta A, Cleary P P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect Immun. 1997;65:2080–2087. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones T C, Hunt R D, King N W. Veterinary pathology. Baltimore, Md: Williams and Wilkins; 1997. [Google Scholar]
- 26.Kapur V, Maffei J T, Greer R S, Li L L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 27.Kapur V, Majesky M W, Li L L, Black R A, Musser J M. Cleavage of interleukin 1 β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapur V, Topouzis S, Majesky M W, Li L L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 29.Kaul R, McGeer A, Low D E, Green K, Schwartz B, Simor A E the Ontario GAS Study Project. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Am J Med. 1997;103:18–24. doi: 10.1016/s0002-9343(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner D E, Jr, Stetler-Stevenson W G. Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- 31.Kuo C-F, Wu J-J, Lin K-Y, Tsai P-J, Lee S-C, Jin Y-T, Lei H-Y, Lin Y-S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukomski S, Burns E H, Jr, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacCallum W G. A stain for influenza bacilli in tissues. JAMA. 1919;72:193. [Google Scholar]
- 35.Mizutani H, Black R, Kupper T S. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. J Clin Investig. 1991;87:1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musser J M. Streptococcal superantigen, mitogenic factor, and pyrogenic exotoxin B expressed by Streptococcus pyogenes. In: Leung D Y M, Huber B T, Schlievert P M, editors. Superantigens. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 281–310. [DOI] [PubMed] [Google Scholar]
- 37.Norrby-Teglund A, Pauksens K, Holm S E, Norgren M. Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J Infect Dis. 1994;170:585–591. doi: 10.1093/infdis/170.3.585. [DOI] [PubMed] [Google Scholar]
- 38.Ogburn C A, Harris T N, Harris S. The determination of streptococcal antiproteinase titers in sera of patients with rheumatic fever and streptococcal infection. J Immunol. 1958;81:396–403. [PubMed] [Google Scholar]
- 39.Olsen T G. Vasculitis. Norwalk, Va: Appleton and Lange; 1990. pp. 176–177. [Google Scholar]
- 40.Proctor R A. Fibronectin: an enhancer of phagocyte function. Rev Infect Dis. 1987;9:S412–S419. doi: 10.1093/clinids/9.supplement_4.s412. [DOI] [PubMed] [Google Scholar]
- 41.Ray J M, Stetler-Stevenson W G. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis, and angiogenesis. Eur Respir J. 1994;7:2062–2072. [PubMed] [Google Scholar]
- 42.Simor A E, Louie L, Schwartz B, McGeer A, Scriver S, Low D E the Ontario GAS Study Project. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Association of protease activity with group A streptococcal (GAS) necrotizing fasciitis (NF), abstr. 1164; p. 332. [Google Scholar]
- 43.Simpson W A, Hasty D L, Mason J M, Beachey E H. Fibronectin-mediated binding of group A streptococci to human polymorphonuclear leukocytes. Infect Immun. 1982;37:805–810. doi: 10.1128/iai.37.2.805-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens D L. Invasive group A streptococcus infections. Clin Infect Dis. 1992;14:2–11. doi: 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 45.Stevens D L, Gibbons A E, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158:23–28. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]
- 46.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 47.Stockbauer K E, Magoun L, Liu M, Burns E H, Jr, Gubba S, Renish S, Pan X, Bodary S C, Baker E, Coburn J, Leong J M, Musser J M. A natural variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc Natl Acad Sci USA. 1999;96:242–247. doi: 10.1073/pnas.96.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Todd E W. A study of the inhibition of streptococcal proteinase by sera of normal and immune animals and of patients infected with group A hemolytic streptococci. J Exp Med. 1947;85:591–606. doi: 10.1084/jem.85.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wessels M R, Bronze M S. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci USA. 1994;91:12238–12242. doi: 10.1073/pnas.91.25.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wheeler M C, Roe M H, Kaplan E L, Schlievert P M, Todd J K. Outbreak of group A streptococcus septicemia in children: clinical, epidemiologic, and microbiological correlates. JAMA. 1991;266:533–537. [PubMed] [Google Scholar]
- 51.Yamada K M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- 52.Yoder E L, Mendez J, Khatib R. Spontaneous gangrenous myositis induced by Streptococcus pyogenes: case report and review of the literature. Rev Infect Dis. 1987;9:382–385. doi: 10.1093/clinids/9.2.382. [DOI] [PubMed] [Google Scholar]