Abstract
Background
Brazil is among the sixteen countries that conducts the most clinical trials in the world. It has a system to review research ethics with human beings made up by the National Commission on Research Ethics (CONEP) and 779 Research Ethics Committees (RECs), in 2017. The RECs are supposed to follow the same rules regarding their membership, although the RECs that review Social Science and Humanities (SSH) researches must respect Resolution 510/16. There are Brazilian RECs that review SSH and clinical trials. This study aimed to analyze the academic professional profile of the members of the CONEP and Brazilian RECs, their adequacy to the norms, and the challenges faced by the REC’s Chairs to compose their membership.
Methods
All 779 Brazilian RECs’ chairs are invited to fill in a questionnaire informing academic and professional background of the RECs members, and 92 answered. However, eight were excluded for having sent an incomplete questionnaire, leaving a total of 84 participants. The variables were described by absolute and relative frequency. The Chi-square test and ANOVA was used to analyze regional differences related difficulties to compose the committee. The significance level was 95%.
Results
The results showed a predominance of members from the biomedical area (57%), while 33% were members of the Social Sciences and Humanities and 5.5% were community representatives. As for the academic degree, there were (45.2%) PhD and (27.9%) masters. The divergences in relation to the guidelines result from the difficulties of having participants in some areas and the little interest in the work carried out by the committees.
Conclusion
The RECs are partially adequate to the norms and their performance may be compromised by the low participation of community representatives. The organization of REC’s specifics to review biomedical research could improve the ethical review process, ensuring a membership more qualified for these protocols.
Keywords: Research ethics committee, Research ethics board, Institutional review board, CONEP, Research ethics
Background
Brazil is among the sixteen countries that conducts the most clinical trials in the world [1], which highlights the importance of an adequate ethics review. Historically, the need for ethics review of research involving human beings led to the establishment of review groups independent from the research team—Research Ethics Committees (RECs). The International Guidelines for Biomedical Research Involving Human Beings (CIOMS 2016) indicate that the committees must have a composition capable of adequately evaluating research protocols in order to ensure the rights and well-being of research subjects [2].
The Nuremberg Code, the Declaration of Helsinki, the Belmont Report and the CIOMS (2016) have influenced regulatory standards on ethical issues [2–5]. However, although following a universal parameter, ethical review structures have been developed according to the specifics of each country.
In Brazil, the system of research ethics review was established by Resolution No. 196 of the National Health Council (CNS) from October 10, 1996 [6]. This resolution was derogated by Res 466/12, that reaffirms, among other aspects, the composition, structuring and performance of the RECs and CONEP, and established the CONEP linked to the National Health Council [7].
CONEP is a National advisory, normative, deliberative, educational and independent body. Its attributions include: proposal and updating of ethics guidelines and norms, final approval and monitoring of projects in special thematic areas, registration, accreditation and supervision of the RECs. It also acts as an instance of appeal for those involved in research with human beings [7].
According to CNS Res 446/11 the composition of CONEP is multidisciplinary, composed of 30 titular members. Twenty-two of them are chosen, through curriculum analysis, among the candidates nominated by the RECs and eight are nominated by CNS directly, and must be members of CNS, respecting these criteria: four community representatives, two health professionals and two nominated by federal government or by private health services [8, 9].
In 2017, the composition of CONEP presented thirteen men and fourteen women, with twenty members having a degree in Biomedical Sciences and six in Social Sciences and Humanities (SSH). One of the members had two degrees in the SSH area. Among them, twenty-one completed specialization, twenty-two master's degree and twenty-four were PhD. One of the members had two doctoral degrees. There was only one member with an engineering degree (doctorate level), who works as geneticist (Table 1).
Table 1.
Titling of CONEP members.
Source: authors
| Bachelor | Specialization | Master’s | PhD | |
|---|---|---|---|---|
| Area | N | N | N | N |
| Biomedicine | 20 | 16 | 12 | 17 |
| SSH | 6 | 5 | 10 | 6 |
| Engineering | – | – | – | 1 |
| No degree | – | 5 | 4 | 3 |
| Not informed | 1 | 1 | 1 | 1 |
| TOTAL | 27 | 27 | 27 | 28* |
*One of the members had two PhD degrees
Brazil had 779 RECs in 2017, and the composition of the RECs is determined by the Operational Standard 001/2013 CNS (CNS OS 001/13). The minimum composition of the RECs is seven members, multidisciplinary, with 50% of the members having proven experience in research, with a balance of members regarding sex and without a marked predominance (more than 50%) of a professional category. The term of mandate and choice of members are determined by the internal regulations of each REC [10].
The creation and organization of RECs, as well as the choice of members, were left to the institutions. The Resolution No. 240 from CNS (CNS Res 240/97) informs that community representative should be nominated by forums or non-governmental organizations that represent end users of SUS [11]. In October 2020 this resolution was revoked by CNS Resolution 647 (CNS Res 647/20), which states that each REC must have at least two community representatives, and keep one community member per seven members. And the community representative was renamed as research participant representative [12].
An accredited REC is certified by CONEP to review high risk protocols involving human beings, including all clinical trials conducted in Brazil, and the standards for the accreditation process were established in the CNS Resolution No. 506 of 2016. Among the requirements to obtain the accreditation certificate, the REC must have at least one member with curriculum experience in bioethics or research ethics in its board and prove the effective and continuous participation of the community representative in the last three years [13].
In 2016, CNS Res 510/16 established guidelines for research in the social sciences and humanities. In order to review these protocols, RECs must have a composition with equal representation of members of the SSH, and the rapporteurs must be chosen from among them [14].
The objective of the study was to analyze the academic professional profile of the members of the CONEP and Brazilian RECs, their adequacy to the norms, and analyze the challenges faced by the RECs’ Chairs to compose their membership. It is fundamental to define the decisions taken by the RECs, which has important implications on the quality of the opinions, including to clinical trials’ deadlines.
Methods
A cross-sectional study with a quantitative approach carried out with Brazilian RECs’ chairs, identified on the CONEP website, in September 2017.
Research stages
E-mails were sent to the REC, inviting their chair to participate in the study and including: an e-mail address to access the informed consent and the questionnaire to be answered by the chairs; a standard form to be filled out with data on academic and professional background of the members. After thirty days, the same material was sent by mail to the RECs, with the possibility of answering by mail without cost, so there was the option to participate in this way. After sending e-mails and correspondence, telephone contact was made with the RECs that did not respond to reiterate the invitation. The collection period lasted from September to December 2017.
Instruments
The data were collected using information provided by the REC and the questionnaire answered by the chair, containing ten closed-ended questions in which respondents could choose more than one answer. A database was built with the information obtained using EXCEL software in Portuguese. The data were analyzed quantitatively by descriptive statistics and presented in matrices according to analyzed items.
Data analysis
Two databases were created in the Microsoft Excel 2010 program—one for REC information and one for the questionnaires answers. The data were analyzed, compared with the literature and legislation in force.
The variables were described by absolute and relative frequency. The ANOVA and Chi-square test and was used to analyze regional differences, time of participation in the REC and related difficulties to compose the committee. The significance level was 95%. The statistical program used was Jasp Version 12.0.
Results
In the analyzed RECs, participation time averaged 46.09 (+ 38.72) months and there were a high number of women (85.4%). There were many members participating in the committees for a long time and another part with little REC time. Only 5.5% of members were identified as community representatives, and 48.2% had never participated in another REC. Most of the RECs in this study were from the Northeast, 36.9% (N = 31), followed by the South with 28.6% (N = 24) and the Southeast with 14.3% (N = 12). Out of the members (N = 1118), 45.2% had completed a doctorate; 27.9% had completed a master's degree and 9.8% had completed a specialty; 2.4% of the members had only high school education (Table 2).
Table 2.
Information on the RECs.
Source: Authors
| No | % | ||
|---|---|---|---|
| Members’ degrees | High school | 27 | 2.4 |
| Bachelor | 42 | 3.7 | |
| Specialization | 110 | 9.8 | |
| Master’s | 312 | 27.9 | |
| Doctorate | 505 | 45.2 | |
| Post-doctorate | 84 | 7.5 | |
| Sex | Male | 163 | 14.6 |
| Female | 955 | 85.4 | |
| Has been a member of another REC | YES | 50 | 4.4 |
| NO | 539 | 48.2 | |
| Community Representative | YES | 61 | 5.5 |
| NO | 508 | 45.4 | |
| Missing Values | 549 | 49.1 | |
| Members by Region | Center-west | 107 | 7.9 |
| Northeast | 463 | 41.5 | |
| North | 91 | 8.1 | |
| Southeast | 209 | 18.7 | |
| South | 248 | 22.2 |
Of the 73 identified under graduations (degrees), 57% (N = 617) were in the biomedical area; 33% (N = 358) in the social sciences and humanities and 7% (N = 65) were distributed in the remaining areas. High school graduates represented only 3%. Nursing, psychology and medicine were the most prevalent professions—12.5, 9.3, 8.6%, respectively (Fig. 1).
Fig. 1.
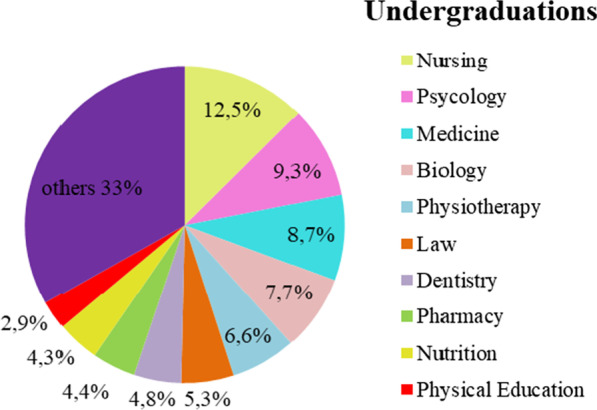
The most common degree among the RECs was a bachelor degree.
Source: Authors
The answers to the questionnaires are shown in Table 3.
Table 3.
Questionnaire’s answers.
Source: Authors
| Question | Answer | N | % |
|---|---|---|---|
| Who defines the members | Institution | 32 | 38.1 |
| Members | 16 | 19 | |
| Institution + Member + Coordinator | 12 | 14.3 | |
| Criteria for defining the members | Time availability | 16 | 19 |
| Research experience | 13 | 15.5 | |
| Time availability and research experience | 25 | 29.8 | |
| Knowledge on the REC/CONEP system | 2 | 2.4 | |
| Research experience and bioethics | 2 | 2.4 | |
| Hard to get | Community representative | 41 | 48.8 |
| Social Sciences and Humanities | 13 | 15.5 | |
| Community and Social Sciences and Humanities | 12 | 14.3 | |
| Difficulties to participate in the REC | Lack of time | 29 | 34.5 |
| Lack of time and institutional incentive | 12 | 14.3 | |
| Lack of time and interest | 8 | 9.5 | |
| No score on curriculum (associated values) | 21 | 28.8 | |
| Adapting the REC’s composition to the standards | Easy. The rules are compatible with the institutional and professional reality of the REC | 28 | 33.3 |
| Difficulty to find member for some categories | 22 | 26.2 | |
| Difficulty due to lack of interest or possibility to participate in the REC | 15 | 17.9 | |
| Meeting the deadlines of CNS OS 001/13 | Easy. Deadlines compatible with the REC’s composition and profile | 49 | 58.3 |
| Difficulty to meet deadlines in the analysis of specific areas | 14 | 16.7 | |
| Difficult. The deadlines are not compatible with the number of REC members | 8 | 9.5 | |
| Composition suitable for accreditation | Yes | 34 | 40.5 |
| No | 13 | 15.5 | |
| The REC is not interested in the accreditation | 15 | 17.9 | |
| The REC is adapting itself for the accreditation process | 14 | 16.7 | |
| Suiting the composition to CNS Res 506/16 | Easy. The rules are compatible with the institutional and professional reality of the REC | 28 | 33.3 |
| Difficult. The rules are not compatible with the institutional and professional reality of the REC | 22 | 26.2 | |
| Difficulty due to the professionals’ lack of interest or possibility of participating in the REC | 13 | 15.5 | |
| Operational and financial difficulty | 3 | 3.6 | |
| Suitable for reviewing SSH research | Yes | 64 | 76.2 |
| No | 8 | 9.5 | |
| The REC is still adapting itself to review projects in this field | 7 | 8.3 | |
| Suiting the REC’s composition to the CNS Res 510/16 | Easy. The rules are compatible with the institutional and professional reality of the members | 50 | 59.5 |
| Operational difficulty. The rules are not compatible with the institutional and professional reality of the members | 13 | 15.5 | |
| Difficulty due to the professionals’ lack of interest or possibility of participating in the REC | 11 | 13.1 |
Discussion
The present study involved 84 RECs’ chairs. Some RECs, including those from public universities, did not participate on the grounds of data confidentiality, although information on composition is considered public. In countries such as Japan, China, Belgium and the United Kingdom, the disclosure of the names of REC’s members is usually determined [15–20]. The name of the chair and the participating REC were kept confidential and only the participants who agreed to the informed consent had access to the questionnaire.
Even though the rate response was low, the findings were not affected because the challenges for the composition of the REC are the same regardless of the location and size of the REC.
Regarding the prolonged participation in the REC of some members, there is no normative impediment in the CNS resolutions and it can be considered an advantage, since experienced members make revisions more agile. However, there is difficulty in keeping committee members for longer periods, especially the community representative, a fact observed by other works carried out in Brazil and USA [21, 22].
Even though there was a predominance of members of the biomedical area (57%), the percentage values are proportional and without hegemony of any profession, reflecting greater participation of the various categories. The higher proportion of nursing professionals differs from other studies conducted in Brazil and the number of degrees found indicates greater diversity and multidisciplinary in the RECs, a fact that raises the level of participation and understanding perspectives of the projects reviewed [21, 23]. However, this diversity may increase the difficulty to review clinical trials.
The high number of PhD (45.2%) and masters (27.9%) among REC members can be a credibility factor before the community [24], on the other hand, the hegemony of professionals with high degrees may inhibit the performance of members with less scientific knowledge, such as community representatives [22, 25].
All participating RECs had a community member, however the low participation (of members who identified themselves as) community members (5.5%) may be due to their low performance. The high number of missing values seen in Table 3 may indicate fear in disclosing that user representation is not being carried out in an ideal way and the data on participants who have only high school education (2.4%) may indicate that there are RECs in which community representation is carried out by people with higher education level.
The deliberations of a REC without the effective participation of the community member are compromised because this member represents the community's perspective on the analyzed research.
Reaching and maintaining community representatives are difficulties present in RECs and there are cases in which they are appointed only to comply with a regulation of the REC, having no distinguished action. The low participation of these members is a deficiency in the intended democratization of the committees and compromises the RECs role of social control idealized for the Brazilian system of ethical review [21, 23]. The approval of CNS Res 647/20, which states that each REC must have at least two research participant representatives [12], may result in an even more challenging situation.
The difficulty of the community representative is also observed in the Klitzman study carried out in the USA. Iijima et al. found that the external member of some RECs in Japan belonged to the institution and other committees did not even have the community member.
The disproportion with prevalence of female (85.4%-Table 2) differs from other studies [21, 23] and indicates the need for future investigations to elucidate this finding. It may represent some conjuncture beyond the issue of sex and is a condition that can influence the decisions of RECs. On the contrary, the low participation of women was observed in the ethics committees of Iran [26] and Japan [27] where there were even committees without their presence.
The number of members per region (Table 2) was influenced by the lack of information provided by the RECs. Since the Southeast of Brazil has a larger number of educational and research institutions, and a larger number of RECs, it was expected that more participants would come from this region; however, only 18.7% were from the Southeast RECs. The low participation of RECs can be seen as a lack of interest or fear in exposing the existing reality. Anyway, this behavior is strange coming from institutions that must ensure ethics in research.
The indication of members by the institution (38.1%) and by other members of the REC (19%) is the same as in India [28]. In Brazil, members are appointed by the institution that houses the REC, in other countries such as Italy [29] and France [30] the nominations of members of ethics committees occur through regional entities such as health districts.
The lack of time cited by participants as the main difficulty for professionals to participate in RECs justifies the option "availability of time" as the main criterion for selecting members (Table 3). The lack of institutional incentive is also in line with other study [23], on the other hand, it is at odds with the regulations in our country [10], which require commitment from the institution to ensure minimum operating conditions for the REC and to provide resources for continuous maintenance and investment in personnel and infrastructure.
Resistance or little interest in being a member of a REC may occur because it requires knowledge about a huge variety of research methodologies and the guidelines on research ethics. In addition, the profile of volunteer work is a cause of dissonance among researchers, but the voluntary nature of the members may influence the performance and interest in participating in the RECs. The volume and complexity of research protocols, especially clinical trials, result in an additional workload, with many hours spent by qualified professionals, which may require a full-time dedication to the committee, making it difficult to demand voluntary work [31, 32].
The origin of the RECs-CONEP System directed to biomedical sciences [33, 34] and the finding that 39.3% of RECs are located in institutions in this specific area, such as hospitals and biomedical teaching and research institutions, justifies the majority of members belonging to this segment, however 15% of the committees presented inadequate composition, with more than 80% of biomedical area members, even though their coordinators considered ease to adapt to CNS Res 510/16. This is an aspect that must be improved in Brazil, perhaps establishing specifics RECs to review only Biomedical research.
The lack of incentive from the institution, as a factor of difficulty to align the composition of the RECs to the resolutions of the CNS, manifested only by 2.4% of the coordinators (Table 2), may demonstrate that the participant did not want to expose weaknesses in the system, considering it inconvenient to disclose institutional information, despite the commitment of confidentiality present in the study.
The objective of REC accreditation is that it can perform reviews of research involving more risk, including clinical trials that will be realized inside or outside the institution, issuing the final opinion, when forwarded by CONEP through the Brazil Platform. Although 40.5% of participants (Table 2) consider that the REC in which they participate has an adequate composition for the accreditation process, it is observed that those who are in the process of adapting to apply for the accreditation certificate will face challenges to compose their boards—either due to lack of time, or lack of interest from potential candidates for membership.
The lack of interest or possibility of professionals to participate in the committee was more highlighted by the participants than operational and financial difficulties. This reality externalized by the coordinators may reflect a greater awareness of the institutions on the importance of the REC's presence or, at least, the normative need to have a well-structured ethics committee.
The increase of work influences the performance of the committees, as seen in question six, in which it was informed that the insufficient number of members made it difficult to meet deadlines. The overload of work for REC members is identified in studies by other authors [35, 36] and the lack of interest in the accreditation certificate, admitted by 17.9% of the participating chairs, may also be because this process means additional work.
Of the 64 RECs (76.2%) that the coordinators found to have adequate composition to review SSH projects, 24 did not meet the requirements of CNS Res 510/16, and the difficulty of meeting deadlines in protocol review in specific areas (16.7%) may be due to problems in obtaining members for the SSH. In addition, SSH members were the second most difficult category to obtain participants for the REC, so the opinion of 76.2% chairs (Table 2) may be due to lack of knowledge about CNS Res 510/16. The biomedical training of 54.8% of the chairs may also have influenced it, indicating that this result may be related to lack of knowledge about SSH methodologies and the methodological and ethical aspects involved in these projects.
For most of the participants (59.5%), it is easy to adapt the composition of the REC to CNS Res 510/16, but this opinion does not fit with the information contained in the undergraduate profile (Fig. 1) and the challenges cited (Table 2) to obtain representatives of the SSH.
In the present study, no significant associations were found between time of participation in the REC, regions of the country and difficulties faced. The challenges such as: how to choose members, criteria to define them, difficulties in community and SSH members, time and interest in participating, institutional incentive, among others, are not current and are present in the opinion of coordinators from all regions of the country, regardless of the time of participation in the REC. It is possible that the means used to overcome the challenges have not been efficient, and the possibility that little has been done cannot be ruled out either.
Regional particularities such as socioeconomic differences, number of educational institutions, number of committees and researchers, among others, seem not to influence the composition of the REC.
Conclusion
The RECs-CONEP system has high education level members with many masters and doctorates. The committees’ profile is partially adequate to the interdisciplinary foreseen in the CNS resolutions. Currently RECs have a majority of members from the biomedical area and low participation from other areas. The role of the RECs may be compromised by the limited participation of community representatives.
The RECs-CONEP System is well structured, analyzes projects from all areas of knowledge and, unlike other countries that have their systems regulated by law, is standardized through resolutions enforced by National Health Council (CNS).
Acknowledgements
Not applicable.
Abbreviations
- CNS
National Health Council
- CNS Res
Resolution of the National Health Council
- CNS OS
Operational standard of the National Health Council
- CONEP
National Comission on Research Ethics
- REC
Research ethics committee
- SSH
Social science and humanities
- SUS
Unified Healt System
Author contributions
EPV and ICZG prepared the study protocol. EPV conducted data collection and was responsible for report writing. EPV and ICZG were involved in data analysis and preparation of the manuscript. Both authors read and approved the final manuscript.
Funding
This study was funded by the authors.
Availability of data and materials
The data-sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. This is subject to participants’ expressed permission to share transcript of the information.
Declarations
Ethics approval and consent to participate statement
Written informed consent was obtained from all participants before commencement of the study. Institutions have been anonymized to preserve confidentiality. The project of this research was submitted and approved by the Research Ethics Committee of the Faculty of Juazeiro do Norte, CAAE No. 72621717.2.000.5624, opinion No. 2.231.879, issued on 08/22/2017.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eugênio Pacelli de Veras Santos, Email: pacelliveras@yahoo.com.br.
Iara Coelho Zito Guerriero, Email: iarag@alumni.usp.br.
References
- 1.World Health Organization. Monitoring Process to R&D. Number of clinical trial by year, country, WHO region and income group (1999–2019). March 2020. https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/number-of-clinical-trials-by-year-country-who-region-and-income-group.
- 2.Council for International Organizations of Medical Sciences. 2016. International ethical guidelines for biomedical research involving human subjects. 4. ed 122p. Geneva. https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf. [PubMed]
- 3.The Nuremberg Code. Trial of war criminal before nuremberg military tribunals under control council law no. 10, v. 2, p. 181–182. Washington, D. C.; US Government Printing Office, 1949. https://www.loc.gov/rr/frd/Military_Law/p df/NT_war-criminals_Vol-II.pdf.
- 4.World Medical Associations. 2013. Declaration of Helsinki: ethical principles for medical research involving humans’ subjects. Adopted by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
- 5.The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. BELMONT REPORT: ethical principles and guidelines for the protection of human subjects of research. Washington, DHEW Publications, (OS) 78–0012, 1978. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html. [PubMed]
- 6.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 196 de 10 de outubro de 1996. Diretrizes e normas regulamentadoras de pesquisa envolvendo seres humanos. Brasília: Ministério da Saúde, 1996. http://conselho.saude.gov.br/resolucoes/reso_96.htm.
- 7.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 466 de 12 de dezembro de 2012. Diretrizes e normas regulamentadoras de pesquisa envolvendo seres humanos. Brasília: Ministério da Saúde, 2012. https://conselho.saude.gov.br/normativas-conep?view=default.
- 8.Presidência da República. Decreto presidencial nº 5.839 de 11 de julho de 2006, dispõe sobre organização, atribuições e o processo eleitoral do Conselho Nacional de Saúde. Diário Oficial da União de 12 de julho de 2006, Secção 1, p1, 2006. https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?jornal=1&pagina=1&data=12/07/2006
- 9.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 446 de 11 de agosto de 2011. Brasília: Ministério da Saúde, 2011. https://conselho.saude.gov.br/normativas-conep?view=default
- 10.Ministério da Saúde. Conselho Nacional de Saúde. Norma Operacional Nº 001 de 30 de setembro de 2013. Brasília: Ministério da Saúde, 2013. https://conselho.saude.gov.br/normativas-conep?view=default.
- 11.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 240 de 05 de junho de 1997. Brasília: Ministério da Saúde, 1997. http://conselho.saude.gov.br/resolucoes/reso_97.htm.
- 12.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 647 de 12 de outubro de 2020. Brasília: Ministério da Saúde, 2020. https://conselho.saude.gov.br/normativas-conep?view=default.
- 13.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 506 de 03 de fevereiro de 2016. Brasília: Ministério da Saúde, 2016. https://conselho.saude.gov.br/normativas-conep?view=default.
- 14.Ministério da Saúde. Conselho Nacional de Saúde. Resolução 510 de 07 de abril de 2016. Brasília: Ministério da Saúde, 2016. https://conselho.saude.gov.br/normativas-conep?view=default.
- 15.Ministry of Health, Labour and Welfare. Ethical guidelines for medical research and health involving humans. Japan, 2018. https://www.lifescience.mext.go.jp/files/pdf/n2181_01.pdf.
- 16.State Food and Drug Administration – China. Guiding principles of ethical review of drug clinical trials. China, 2010. www.gov.cn/gzdt/2010-11/08/content_1740976.htm.
- 17.National Health and Family Planning Commission. Measures for the ethical review of biomedical research involving humans. China, 2016. www.nhc.gov.cn/fzs/s3576/201610/84b33b81d8e747eaaf048f68b174f829.shtml.
- 18.Agence Fédérale des Médicaments et des Produits de Santé. Arrêté royal fixant les mesures d’exécution de la loi du 7 mai 2004 relative aux expérimentations sur la personne humaine, concernant le comité d’éthique. Moniteur Belge. No. 146, p. 39709–39724, 4 AVRIL 2014. http://www.ejustice.just.fgov.be/eli/arrete/2014/04/04/2014018155/justel.
- 19.Agence fédérale des Médicaments et des Produits de Santé. Loi relative aux essais cliniques de médicaments à usage humain. Moniteur Belge. No. 139, p. 59619–58631, 7 mai 2017. http://www.ejustice.just.fgov.be/eli/loi/2017012146/justel.
- 20.National Health Service, Health Research Authority, UK Ethics Committee Authority. Governance arrangements for research ethics committees: 2020 edition. 39p. www.hra.nhs.uk.
- 21.Freitas CBD, Novaes HMD. Lideranças de comitês de ética em pesquisa no Brasil: perfil e atuação. Rev Bioética. 2010;18(1):185–200. Available from: https://revistabioetica.cfm.org.br/index.php/revista_bioetica/article/view/544.
- 22.Klitzman R. Institutional review board community members: Who are they, what do they do, and whom do they represent? Acad Med. 2012;87(7):975–981. doi: 10.1097/ACM.0b013e3182578b54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jácome MQD, Araújo TCCF, Garrafa V. Comitês de ética em pesquisa no Brasil: estudo com coordenadores. Rev Bioética. 2017 doi: 10.1590/1883-80422017251167. [DOI] [Google Scholar]
- 24.Audrey S, Brown L, Campbell R, Boyd A, Macleod J. Young people’s views about the purpose and composition of research ethics committees: findings from the PEARL qualitative study. BMC Med Eth. 2016 doi: 10.1186/s12910-016-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speers MA, Rose S. Commentary: labeling institutional review board members does not lead to better protections for research participants. Acad Med. 2012;87(7):842–844. doi: 10.1097/ACM.0b013e318257f115. [DOI] [PubMed] [Google Scholar]
- 26.Fakour Y, Eftekhari MB, Haghighi Z, Mehr NK, Hejazi F. Situation analyses of local ethical committees in medical sciences in Iran. J Res Med Sci. 2011;16(3):310–315. [PMC free article] [PubMed] [Google Scholar]
- 27.Iijima Y, Ogasawara K, Toda S, Takano T. An overview of ethical review committees in Japan: examining the certification applications of ethical review committees. Nagoya J Med Sci. 2019;81(3):501–509. doi: 10.18999/nagjms.81.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Indian Council of Medical Research. National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. 2017. Compiled & Edited by: Roli Mathur. New Delhi. https://main.icmr.nic.in/sites/default/files/guidelines/ICMR_Ethical_Guidelines_2017.pdf. [DOI] [PMC free article] [PubMed]
- 29.Ministero della Salute. Decreto 8 febbraio 2013. Critério per la composizione e il funzionamento dei comitati etici. Gazzetta Uficialle, n. 96 del 24–04–2013, p.12–21, 2013. https://www.gazzettaufficiale.it/eli/gu/2013/04/24/96/sg/pdf.
- 30.Le Service Public de La Difusion du Troit, France. 2020. Code de La Santé Publique, 2020. http://www.legifrance.gouv.fr/affichCode.do?cidTexte=LEGITEXT000006072665.
- 31.Lanzerath D. Europäische Ethikkommissionen im Wandel: Herausforderungen durch neue Rahmenbedingungen. Bundesgesundheitsblatt. 2019;62:697–705. doi: 10.1007/s00103-019-02952-8. [DOI] [PubMed] [Google Scholar]
- 32.Rates CMP, Pessalacia JDR. Conhecimento de pesquisadores acerca das normas éticas para pesquisas envolvendo humanos. Rev Bioética. 2013;21(3):566–654. doi: 10.1590/S1983-80422013000300021. [DOI] [Google Scholar]
- 33.Guerriero ICZ, Minayo MCS. O desafio de revisar aspectos éticos das pesquisas em ciências sociais e humanas: a necessidade de diretrizes específicas. Physis: Rev Saúde Coletiva. 2013;23(3):763–782. doi: 10.1590/S0103-73312013000300006. [DOI] [Google Scholar]
- 34.Costa RC, Maluf F. Estudo analítico da interdisciplinaridade na composição dos membros dos comitês de ética em pesquisa no Brasil. Rev Bioethikos. 2014;8(1):53–60. doi: 10.15343/1981-8254.20140801053060. [DOI] [Google Scholar]
- 35.Batista KT, Seidl EMF. Sistema brasileiro de revisão ética em pesquisa: percepção de pesquisadores. Rev Bras Bioética. 2019;14(15):1–18. doi: 10.26512/rbb.v14iedsup.24224. [DOI] [Google Scholar]
- 36.Petrini C. Some comments on the new regulations governing ethics committees in Italy. Ann Ist Super Sanita. 2014;50(2):160–162. doi: 10.4415/ANN_14_02_09. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. This is subject to participants’ expressed permission to share transcript of the information.


