Abstract
Background
Genes related to the SWItch/sucrose nonfermentable (SWI/SNF) chromatin remodeling complex are frequently mutated across cancers. SWI/SNF-mutant tumors are vulnerable to synthetic lethal inhibitors. However, the landscape of SWI/SNF mutations and their associations with tumor mutational burden (TMB), microsatellite instability (MSI) status, and response to immune checkpoint inhibitors (ICIs) have not been elucidated in large real-world Chinese patient cohorts.
Methods
The mutational rates and variation types of six SWI/SNF complex genes (ARID1A, ARID1B, ARID2, SMARCA4, SMARCB1, and PBRM1) were analyzed retrospectively by integrating next-generation sequencing data of 4591 cases covering 18 cancer types. Thereafter, characteristics of SWI/SNF mutations were depicted and the TMB and MSI status and therapeutic effects of ICIs in the SWI/SNF-mutant and SWI/SNF-non-mutant groups were compared.
Results
SWI/SNF mutations were observed in 21.8% of tumors. Endometrial (54.1%), gallbladder and biliary tract (43.4%), and gastric (33.9%) cancers exhibited remarkably higher SWI/SNF mutational rates than other malignancies. Further, ARID1A was the most frequently mutated SWI/SNF gene, and ARID1A D1850fs was identified as relatively crucial. The TMB value, TMB-high (TMB-H), and MSI-high (MSI-H) proportions corresponding to SWI/SNF-mutant cancers were significantly higher than those corresponding to SWI/SNF-non-mutant cancers (25.8 vs. 5.6 mutations/Mb, 44.3% vs. 10.3%, and 16.0% vs. 0.9%, respectively; all p < 0.0001). Furthermore, these indices were even higher for tumors with co-mutations of SWI/SNF genes and MLL2/3. Regarding immunotherapeutic effects, patients with SWI/SNF variations showed significantly longer progression-free survival (PFS) rates than their SWI/SNF-non-mutant counterparts (hazard ratio [HR], 0.56 [95% confidence interval {CI} 0.44–0.72]; p < 0.0001), and PBRM1 mutations were associated with relatively better ICI treatment outcomes than the other SWI/SNF gene mutations (HR, 0.21 [95% CI 0.12–0.37]; p = 0.0007). Additionally, patients in the SWI/SNF-mutant + TMB-H (HR, 0.48 [95% CI 0.37–0.54]; p < 0.0001) cohorts had longer PFS rates than those in the SWI/SNF-non-mutant + TMB-low cohort.
Conclusions
SWI/SNF complex genes are frequently mutated and are closely associated with TMB-H status, MSI-H status, and superior ICI treatment response in several cancers, such as colorectal cancer, gastric cancer, and non-small cell lung cancer. These findings emphasize the necessity and importance of molecular-level detection and interpretation of SWI/SNF complex mutations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12935-022-02757-x.
Keywords: SWI/SNF complex genes, Mutational landscape, Tumor mutational burden, Microsatellite instability, Immune checkpoint inhibitors, Synthetic lethality
Background
Precision diagnostics are prerequisites for achieving the goal of cancer precision treatment. The concept that cancer is a genetically driven disease is widely supported by therapeutic successes directed at particular mutations or pathways. The traditional paradigm of drug development in oncology has gradually shifted to a tissue-agnostic therapeutic model, wherein patients are deemed eligible for a given treatment based on the presence of specific molecular variations rather than on the cancer type (i.e., the affected tissue) [1]. One particularly representative class of tissue-agnostic drugs is tropomyosin receptor kinase (TRK) inhibitors, which have been approved for cancer treatment owing to their durable responses in diverse adult and pediatric cancer patients with NTRK fusions; moreover, various other potential tissue-agnostic drugs are being developed [2]. Notably, genes of the SWItch/sucrose nonfermentable (SWI/SNF) chromatin remodeling complex are potential candidates for tissue-agnostic drug development, as these genes are commonly mutated in 20–25% of all human cancers [3]; this prevalence is notably higher than that of NTRK fusions (0.3%) [4].
The SWI/SNF complex is an ATP-consuming multi-subunit cellular machine that modulates chromatin compaction, thereby, regulating DNA-related processes, such as transcription, replication, and repair [5]. There are three subfamilies of the SWI/SNF complex in mammals, namely, the BRG1/BRM-associated factor (BAF), polybromo-associated BAF (PBAF), and noncanonical BAF (ncBAF) complexes [6]. Among dozens of SWI/SNF complex genes, ARID1A, ARID1B, ARID2, SMARCA4, and PBRM1 have been reported to be altered in ≥ 5% of a certain tumor and are mutated above the background mutation rate in two or more cancer types, accounting for their gene length, which suggests that mutations in these genes are “driver” rather than “passenger” variations [7]. In addition, the biallelically inactivated SMARCB1 was frequently found in malignant rhabdoid tumors, which is a clear evidence that at least one SWI/SNF subunit is indeed a tumor suppressor [8, 9]. According to our preliminary data, the median variant allele frequencies (VAFs) of these six genes were 13.3–17.2% (Additional file 1: Figure S1), consistent with the previous report. Therefore, we decided to focus on the above six genes in this study. AT-rich interactive domain 1A (ARID1A), also known as BAF250a, is a tumor suppressor that is typically mutated in Epstein-Barr virus-positive and microsatellite instability-high (MSI-H) gastric cancer [10, 11], ovarian clear cell carcinoma [12], endometrial cancer [13], and non-small cell lung cancer [14]. Further, SMARCA4 (BRG1) encodes a core catalytic component of the SWI/SNF complex and its inactivation is indicative of the presence of hypercalcemic-type small cell carcinoma of the ovary [15, 16], and loss-of-function (LOF) mutations of SMARCB1 (SNF5/INI1/BAF47), which encodes another core subunit of the SWI/SNF complex, have been identified in the majority of rhabdoid tumors [17, 18]. Furthermore, the loss of both ARID1B and SMARCB1 expression has been detected in approximately one-third of undifferentiated endometrial cancers [19]. ARID2 has also been identified as one of the most frequently altered genes in non-small cell lung cancer [20], gallbladder cancer [21], and metastatic breast cancer [22], and its deficiency can hamper DNA repair processes and enhance the sensitivity of lung cancer cells to DNA-damaging agents [23]. PBRM1 encodes polybromo 1, a specific subunit of the PBAF complex, and reportedly, in clinical practice, PBRM1 LOF variations favor the therapeutic effect of immune checkpoint inhibitors (ICIs) in renal cell carcinomas [24–26]. In addition to the aforementioned associations with various cancers, accumulating evidence suggests that SWI/SNF mutations can induce certain molecular perturbations in a synthetic lethal pattern [3, 27, 28], highlighting their potential as targets for drug development.
Next-generation sequencing (NGS) has been extensively applied as a cost-effective diagnostic tool in clinical practice and trials [29]. In the present study, we aimed to retrospectively integrate the NGS data corresponding to a large real-world Chinese patient cohort to comprehensively depict the landscape of SWI/SNF mutations and explored the associations between SWI/SNF variations and tumor mutational burden (TMB), MSI status, and therapeutic responses to ICIs across solid tumors. These findings can serve as a useful reference as well as a basis for molecular diagnostics and targeted drug development.
Methods
Study design and patient information
NGS data and clinical information corresponding to patients who visited the Department of Molecular Diagnostics of Sun Yat-sen University Cancer Center (Guangzhou, China) for NGS analysis between September 1, 2019 and June 30, 2021 were retrospectively included. All the cancer diagnoses were also confirmed via pathological examination. Cases with genomic alterations in at least one of the six SWI/SNF complex genes (ARID1A, ARID1B, ARID2, SMARCA4, SMARCB1, and PBRM1) were classified under the SWI/SNF-mutant group. Thereafter, NGS data, including variant genes, number of variants, variation types, protein changes, TMB value, TMB status, and MSI status, as well as clinical characteristics of the patients, including age, sex, smoking status, cancer type, TNM stage, ICI type, and progression-free survival (PFS) during ICI treatments, were systematically collected. The use of clinical and NGS data was approved by the Ethics Committee of the Sun Yat-Sen University Cancer Center (Approval number B2020-344-01). All the patients also provided written informed consent, and the study was performed in accordance with the Declaration of Helsinki.
NGS and data processing
The detailed experimental steps and data analysis strategies for NGS were as previously described [30–32]. For library construction, approximately 0.5 μg of DNA fragments were mixed with Illumina-indexed adapters (Illumina, San Diego, CA, USA) using the KAPA Library Preparation Kit (Kapa Biosystems, Wilmington, MA, USA). A hybrid captured-based NGS assay covering approximately 1.1 megabases (Mb) of the genomic sequences of 1021 cancer-related genes (GenePlus-Beijing, China) was used for the sequencing, which was performed using a GenePlus 2000 sequencing system (Beijing, China) with 2 × 100 bp paired-end reads. DNA samples from matched peripheral white blood cells were sequenced simultaneously to filter out benign single nucleotide polymorphisms and possible germline mutations.
The sequencing data were then analyzed by aligning the clean reads to the reference human genome (hg38) using BWA18 (version 0.7.12-r1039) [33], and small insertions and deletions (indels) and single-nucleotide variants were identified using MuTect19 (version 1.1.4) [34]. A somatic mutation was confirmed if it was consistently detected in five high-quality reads (Phred score ≥ 30, mapping quality ≥ 30, and no paired-end read bias) and had a variant allele frequency ≥ 1% [35]. Copy number variations (CNVs) were detected using the Copy Number Targeted Resequencing Analysis (CONTRA) software (http://contra-cnv.sourceforge.net/) [36], and mutations were then annotated to the genes using the ANNOVAR20 software (http://www.openbioinformatics.org/annovar/) [37].
Classification of LOF and non-LOF variations
LOF variations generally include frameshift indels, nonsense mutations, and splice site mutations. Missense mutations can result in both LOF and non-LOF consequences. To properly stratify LOF/non-LOF mutations, we assessed all of the missense mutations using prediction scores MetaLR and MetaSVM for mutation pathogenicity analysis [38]. MetaLR and MetaSVM are ensemble models based on 10 component scores (SIFT, PolyPhen-2 HDIV, PolyPhen-2 HVAR, GERP + + , MutationTaster, Mutation Assessor, FATHMM, LRT, SiPhy, and PhyloP) and the maximum frequency observed in the 1000 genomes populations. Furthermore, these two ensemble scores have been reported to outperform all of their component scores [39]. Both scores range from 0 to 1, with scores close to 1 indicating certainty that the variant is deleterious. In this study, a missense mutation with both MetaLR and MetaSVM scores of > 0.8 was classified as a LOF mutation as previously recommended [40].
TMB and MSI evaluation
TMB was defined as the number of somatic nonsynonymous mutations/Mb of coding DNA (including small indels and single-nucleotide variants with a variant allele frequency ≥ 3%). TMB values ≥ 20 mutations/Mb in colorectal cancer were classified as TMB-high (TMB-H) [31], this cut-off was decided by the TMB values of 122 MSI-H colorectal cancer samples verified using a PCR assay by Geneplus. TMB values ≥ 7.68 mutations/Mb in all the other cancers were classified as TMB-H according to the top quartile corresponding to 3234 samples (except for colorectal cancer) in the current study [41]. The MSI status was analyzed using MSIsensor (version 0.5). Specifically, MSI scores were calculated as the percentage of unstable somatic microsatellite loci in predefined microsatellite regions covered by the NGS panel used; a sample was determined to have MSI-H if the score was > 8% (this cut-off was decided upon by Geneplus after comparing MSI results of NGS and PCR assay for five mononucleotide microsatellite loci, including NR-21, BAT-25, MONO-27, NR-24, and BAT-26).
Statistical analysis
The response to immunotherapy was characterized by determining PFS, overall response rate (ORR), and disease control rate (DCR), which were explored based on data corresponding to the subset of patients that received ICI treatment. Specifically, PFS was calculated from the start date of the ICI treatments to the date of disease progression or last follow-up. The clinical characteristics of the SWI/SNF-mutant and SWI/SNF-non-mutant groups were compared using the chi-square test. Additionally, differences in TMB values between the two groups were assessed by performing the Mann–Whitney test, while co-occurring and mutually exclusive events were detected by performing the pair-wise Fisher exact test [42]. The possible biological functions and downstream signaling pathways related to all the mutated genes in 1001 SWI/SNF-mutant samples were explored using the Gene Ontology (GO) database [43, 44]. Survival curves and estimates of the median PFS were generated using the Kaplan–Meier methods and compared across different groups by performing the log-rank tests. Hazard ratios (HR) and 95% confidence intervals (CI) were also reported. Statistical significance was based on two-tailed tests at p < 0.05. GraphPad Prism (version 8.4.0, GraphPad Software, San Diego, CA, USA) was used for statistical analyses.
Results
Patient characteristics
A total of 4591 Chinese patients with 18 types of solid tumors were included in this study, and 21.8% of them carried variants in at least one of the six selected SWI/SNF genes (ARID1A, ARID1B, ARID2, SMARCA4, SMARCB1, and/or PBRM1). Among them, 301 patients with SWI/SNF variants (SWI/SNF-mutant group) and 700 patients without SWI/SNF variants (SWI/SNF-non-mutant group) had received ICIs, including anti-PD-1, PD-L1, and CTLA4 or their combinations. The SWI/SNF-non-mutant group had a higher proportion of patients with TNM stage I than the SWI/SNF-mutant group, while age, sex, smoking status, and ICI type were not markedly different between the two groups (Table 1).
Table 1.
The clinical information of the study population grouped by whether carrying SWI/SNF variations
| Total | Treated by ICIs | |||||
|---|---|---|---|---|---|---|
| Characteristics | SWI/SNF-mutant | SWI/SNF-non-mutant | p value | SWI/SNF-mutant | SWI/SNF-non-mutant | p value |
| No. of patients | 1001 | 3590 | 301 | 700 | ||
| Age at the diagnosis, median (range, years) | 56 (14–90) | 56 (1–89) | 57 (14–87) | 55 (9–85) | ||
| ≥ 55 | 572 | 1961 | 0.1611 | 157 | 361 | 0.8904 |
| < 55 | 429 | 1629 | 144 | 339 | ||
| Sex | ||||||
| Male | 517 | 1748 | 0.1002 | 187 | 394 | 0.0938 |
| Female | 484 | 1842 | 114 | 306 | ||
| TNM stage | ||||||
| I | 140 | 629 | < 0.0001 | 12 | 30 | 0.1377 |
| II | 202 | 502 | 64 | 109 | ||
| III | 269 | 951 | 106 | 245 | ||
| IV | 364 | 1396 | 119 | 316 | ||
| Unknow | 26 | 112 | 0 | 0 | ||
| Smoking history | ||||||
| Current/Former | 178 | 668 | 0.5542 | 63 | 168 | 0.3263 |
| Never | 797 | 2810 | 238 | 532 | ||
| Unknow | 26 | 112 | 0 | 0 | ||
| ICI types | ||||||
| Anti PD-1 | 284 | 658 | 0.2511 | |||
| Anti PD-L1 | 11 | 33 | ||||
| Anti PD-1 + CTLA4 | 6 | 6 | ||||
| Anti PD-1 + PD-L1 | 0 | 3 | ||||
CTLA4 cytotoxic T lymphocyte-associated protein 4, ICIs immune checkpoint inhibitors, PD-1 programmed death-1, PD-L1 programmed death-ligand 1, SWI/SNF SWItch/sucrose nonfermentable
The most common cancer type observed in this study was non-small cell lung cancer (32.3%), followed by colorectal cancer (29.6%) and ovarian and fallopian tube cancer (7.9%). The top five malignancies with the highest SWI/SNF mutation rates were endometrial cancer (54.1%), gallbladder and biliary tract cancer (43.4%), gastric cancer (33.9%), urothelial cancer (30.6%), and ovarian and fallopian tube cancer (23.9%). The SWI/SNF mutation rate corresponding to the “Other” subset, which comprised some relatively uncommon tumors, including skin squamous cancer, urachal cancer, gastrointestinal stromal tumor, glioma, adrenal tumors, and medullary thyroid cancer, among others, was 18.6%.
Spectrum of SWI/SNF complex genomic variations
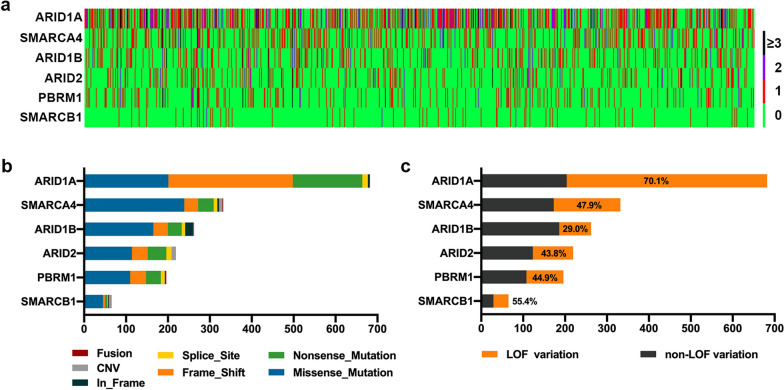
Among the six SWI/SNF genes, ARID1A and SMARCB1 were, respectively, the most and least frequently mutated genes in the majority of the cancer types (ARID1A, 10.7%; SMARCA4, 6.0%; ARID1B, 4.7%; ARID2, 4.0%; PBRM1, 3.5%; SMARCB1, 1.3%; Table 2, Additional file 1: Figure S1a). Notably, SMARCA4 mutations were slightly more common than ARID1A mutations in cases of non-small cell lung cancer, cervical cancer, and melanoma. Interestingly, up to 25.0% of the SWI/SNF-mutant tumors showed genetic aberrations at two or more SWI/SNF genes (Table 2).
Table 2.
Mutation rate of SWI/SNF complex genes in different cancer types
| Cancer type | n | n/Total (%) | SWI/SNF mutation rate (%) | ARID1A (%) | ARID1B (%) | ARID2 (%) | PBRM1 (%) | SMARCA4 (%) | SMARCB1 (%) | ≥ 2 Genes (n) | ≥ 2 Genes (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | 86 | 1.9 | 16.3 | 9.3 | 4.7 | 3.5 | 0 | 1.2 | 1.2 | 3 | 3.5 |
| Cervical cancer | 118 | 2.6 | 12.7 | 1.7 | 2.5 | 2.5 | 2.5 | 4.2 | 1.7 | 2 | 1.7 |
| colorectal cancer | 1358 | 29.6 | 23.0 | 12.1 | 6.8 | 5.0 | 5.4 | 6.8 | 1.8 | 115 | 8.5 |
| Endometrial cancer | 122 | 2.7 | 54.1 | 48.4 | 18.0 | 10.7 | 10.7 | 13.1 | 4.9 | 33 | 27.0 |
| Esophagus cancer | 37 | 0.8 | 21.6 | 8.1 | 5.4 | 5.4 | 0 | 0 | 2.7 | 2 | 5.4 |
| Gallbladder and Biliary tract cancer | 53 | 1.2 | 43.4 | 26.4 | 13.2 | 11.3 | 3.8 | 9.4 | 1.9 | 7 | 13.2 |
| Gastric cancer | 168 | 3.7 | 33.9 | 25.0 | 4.8 | 2.4 | 2.4 | 7.7 | 1.8 | 11 | 6.5 |
| Kidney cancer | 34 | 0.7 | 20.6 | 14.7 | 0 | 2.9 | 2.9 | 2.9 | 0 | 1 | 2.9 |
| Liver cancer | 84 | 1.8 | 17.9 | 8.3 | 2.4 | 2.4 | 7.1 | 3.6 | 1.2 | 3 | 3.6 |
| Melanoma | 119 | 2.6 | 12.6 | 2.5 | 3.4 | 3.4 | 3.4 | 3.4 | 0 | 3 | 2.5 |
| Non-small cell lung cancer | 1485 | 32.3 | 18.9 | 5.8 | 3.4 | 3.4 | 2.2 | 6.2 | 0.8 | 43 | 2.9 |
| Other | 242 | 5.3 | 18.6 | 7.0 | 1.2 | 4.1 | 4.5 | 6.2 | 1.7 | 11 | 4.5 |
| Ovarian and Fallopian tube cancer | 364 | 7.9 | 23.9 | 14.8 | 2.5 | 1.4 | 2.2 | 5.5 | 0.5 | 10 | 2.7 |
| Pancreatic cancer | 93 | 2.0 | 17.2 | 9.7 | 0 | 3.2 | 1.1 | 2.2 | 1.1 | 0 | 0.0 |
| Prostatic cancer | 19 | 0.4 | 15.8 | 10.5 | 5.3 | 5.3 | 0 | 5.3 | 5.3 | 1 | 5.3 |
| Small cell lung cancer | 20 | 0.4 | 15.0 | 0 | 0 | 10.0 | 0 | 5.0 | 0 | 0 | 0.0 |
| Soft tissue sarcoma | 127 | 2.8 | 11.0 | 3.9 | 1.6 | 3.9 | 0.8 | 1.6 | 0.8 | 2 | 1.6 |
| Urothelial cancer | 62 | 1.4 | 30.6 | 21.0 | 8.1 | 1.6 | 0 | 6.5 | 0 | 3 | 4.8 |
| Total | 4591 | 100.0 | 21.8 | 10.7 | 4.7 | 4.0 | 3.5 | 6.0 | 1.3 | 250 | 5.4 |
N number, SWI/SNF switch/sucrose nonfermentable
All the genetic alterations were classified under the following seven types: frameshift indels, in-frame indels, nonsense mutations, missense mutations, splice site mutations, CNVs, and fusions (gene rearrangements). Frameshift indels constituted the most common variation type in ARIDIA, whereas missense mutations were more common in the other five genes (Additional file 1: Figure S1b). The proportions of LOF mutations of the SWI/SNF complex genes were as follows: ARID1A, 70.1%; ARID2, 43.8%; PBRM1, 44.9%; ARID1B, 29.0%; SMARCA4, 47.9%; and SMARCB1, 55.4% (Fig. 1c).
Fig. 1.
The mutational spectrum of six SWI/SNF genes. a The number and distribution of six SWI/SNF gene variations in 1001 tumor samples. b The composition of variation types in each SWI/SNF gene. c The ratio of loss-of-function (LOF) mutations in each SWI/SNF gene
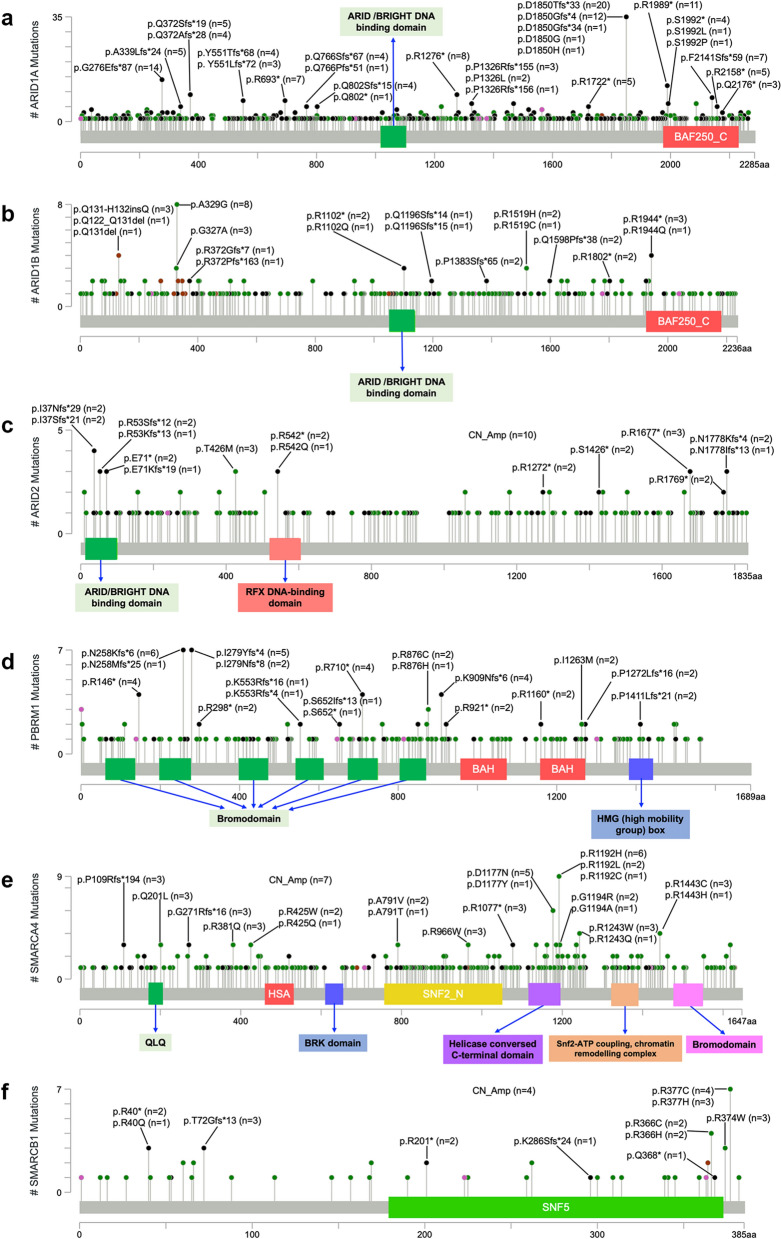
Although most variations were widely distributed along the full length of each gene, a number of frameshift indels (fs) and nonsense mutations (*), which led to the truncation of protein products, were relatively frequently detected. These included D1850fs, G276fs, R1989*, R1276*, and F2141fs in ARID1A (Fig. 2a); R1944* in ARID1B (Fig. 2b); I37fs, R53fs, and p.E71* in ARID2 (Fig. 2c); N258fs, I279fs, p.R146, R710*, and K909fs in PBRM1 (Fig. 2d); P109fs, G271fs, and R1077* in SMARCA4 (Fig. 2e); and R40*, T72fs, and R201* in SMARCB1 (Fig. 2f). In addition, several missense mutations, such as A329G in ARID1B, R1192H/L/C and D1177N/Y in SMARCA4, and R366C/H and R377C/H in SMARCB1, were detected in a relatively greater number of cases (Fig. 2b, e, and f).
Fig. 2.
The distribution of frequently-detected mutations of SWI/SNF genes. The amino acid changes and frequencies of relatively frequently-detected mutations of each gene were annotated by using MutationMapper (http://www.cbioportal.org/mutation_mapper)
Co-occurrence and mutual exclusivity
To uncover the potential pattern of SWI/SNF gene mutations, the co-occurrence and exclusivity of the mutations of the six SWI/SNF genes and the top 20 most frequently altered genes across all tumors were explored. Of the top 20 mutated genes, excluding the six hub genes, it is well known that APC, KRAS, PIK3CA, EGFR, LRP1B, BRCA2, ATM, and ROS1 are mutated in several cancer types, such as non-small cell lung cancer, colorectal cancer, and endometrial cancer. In this study, we observed that ARID1A was the second most frequently mutated gene following TP53. Furthermore, ARID1A variations were identified in a mutually exclusive pattern with variations in EGFR, TP53, ARID1B, ARID2, and SMARCA4; and ARID2 variations were identified in a mutually exclusive pattern with variations in SMARCA4. However, PBRM1 tended to co-mutate with ARID2 and SMARCB1 (Fig. 3). ARID1A, as well as the well-established tumor suppressor, PTEN, and the oncogene, PIK3CA, showed significant variations, mutually exclusive of TP53, suggesting that ARID1A may be a functional driver like PTEN and PIK3CA.
Fig. 3.
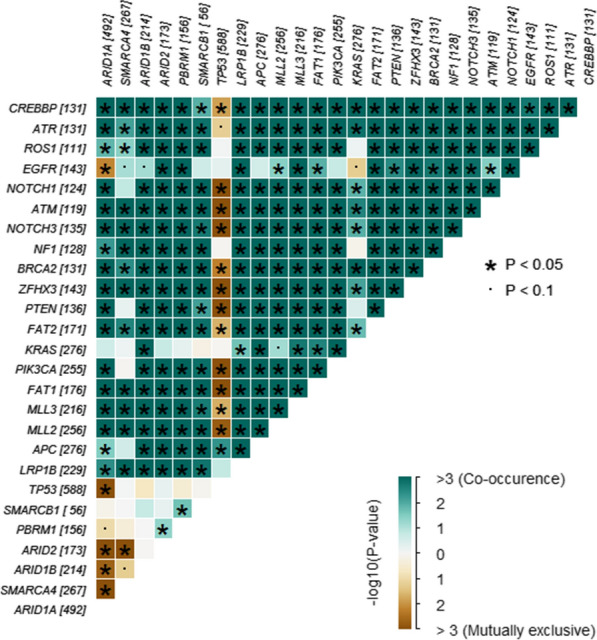
The co-occurrence and exclusivity of mutations of SWI/SNF genes and accompanied top 20 mutated genes. The value corresponding to each gene is the number of samples with mutation(s) of that gene. * p < 0.05, ·p < 0.1
Notably, MLL2 (MLL4/KMT2D) and MLL3 (KMT2C), belonging to a family of mammalian histone H3 lysine 4 (H3K4) methyltransferases [45], were frequently co-mutated with SWI/SNF genes (Fig. 3). Reportedly, KMT2D collaborates with the SWI/SNF complex to promote cell type-specific enhancer activation [46], and cancer cells with KMT2C deficiency have higher endogenous DNA damage and genomic instability [47]. The subset carrying both SWI/SNF and MLL2/3 mutations showed higher average TMB values (MLL2, 70.9 mutations/Mb; MLL3, 74.5 mutations/Mb), TMB-H ratios (MLL2, 80.5%; MLL3, 83.6%), and MSI-H ratios (MLL2, 48.6%; MLL3, 46.6%) than the whole SWI/SNF-mutant group (all p < 0.0001).
Association of SWI/SNF mutations with TMB and MSI
Previous studies have revealed the existence of a potential linkage between the SWI/SNF chromatin remodeling complex and DNA repair, TMB, and MSI [6]. Thus, in this study, these relationships were further analyzed. Our results indicate that the average TMB value corresponding to SWI/SNF-mutant tumors was markedly higher than that corresponding to SWI/SNF-non-mutant tumors, regardless of the cancer type (25.8 vs. 5.6 mutations/Mb, p < 0.0001). The TMB-H and MSI-H ratios corresponding to SWI/SNF-mutant tumors were also significantly higher than those corresponding to the SWI/SNF-non-mutant tumors (TMB-H ratio: 44.3% vs. 10.3%, p < 0.0001; MSI-H ratio: 16.0% vs. 0.9%, p < 0.0001), even though the differences were not significant for certain malignancies, such as kidney cancer, pancreatic cancer, prostate cancer, and urothelial cancer. SWI/SNF-mutant colorectal cancer, endometrial cancer, and gastric cancer exhibited both higher TMB-H and MSI-H ratios than their SWI/SNF-non-mutant counterparts (Table 3). Furthermore, the patient group with mutations at two or more SWI/SNF genes had significantly higher TMB values (69.0 vs. 11.3 mutations/Mb, p < 0.0001), TMB-H ratios (86.2% vs. 40.5%, p < 0.0001), and MSI-H ratios (48.0% vs. 5.3%, p < 0.0001) than those with mutations in a single SWI/SNF gene.
Table 3.
The associations of SWI/SNF mutations with TMB and MSI status in different malignancies
| Cancer type | Average TMB value (mutations/Mb) | TMB-H proportion | MSI-H proportion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SWI/SNF-non-mutant | SWI/SNF-mutant | p | SWI/SNF-non-mutant | SWI/SNF-mutant | p | SWI/SNF-non-mutant | SWI/SNF-mutant | p | |
| Breast cancer | 4.9 | 9.3 | 0.001 | 13.9% | 57.1% | 0.001 | 0.0% | 0.0% | NA |
| Cervical cancer | 4.7 | 9.7 | 0.001 | 11.7% | 40.0% | 0.012 | 1.9% | 0.0% | 0.999 |
| Colorectal cancer | 7.2 | 44.1 | < 0.0001 | 1.6% | 39.6% | < 0.0001 | 1.2% | 33.2% | < 0.0001 |
| Endometrial cancer | 6.2 | 61.6 | < 0.0001 | 14.3% | 75.8% | < 0.0001 | 8.9% | 48.5% | < 0.0001 |
| Esophagus cancer | 6.5 | 13.9 | < 0.0001 | 24.1% | 75.0% | 0.013 | 0.0% | 0.0% | NA |
| Gallbladder and Biliary tract cancer | 6.1 | 17.6 | 0.019 | 16.7% | 43.5% | 0.063 | 3.3% | 17.4% | 0.154 |
| Gastric cancer | 4.2 | 15.3 | 0.002 | 7.2% | 31.6% | < 0.0001 | 0.0% | 12.3% | 0.0004 |
| Kidney cancer | 3.5 | 9.1 | 0.004 | 7.4% | 28.6% | 0.180 | 0.0% | 0.0% | NA |
| Liver cancer | 4.6 | 12.4 | 0.003 | 11.6% | 33.3% | 0.050 | 0.0% | 6.7% | 0.179 |
| Melanoma | 3.3 | 6.8 | < 0.0001 | 3.8% | 20.0% | 0.042 | 0.0% | 0.0% | NA |
| Non-small cell lung cancer | 5.6 | 12.7 | < 0.0001 | 18.9% | 53.0% | < 0.0001 | 0.2% | 1.1% | 0.086 |
| Other | 4.0 | 16.6 | < 0.0001 | 9.1% | 51.1% | < 0.0001 | 1.0% | 2.2% | 0.462 |
| Ovarian and Fallopian tube cancer | 3.7 | 9.1 | < 0.0001 | 3.6% | 18.4% | < 0.0001 | 1.1% | 4.6% | 0.059 |
| Pancreatic cancer | 4.0 | 6.7 | 0.006 | 3.9% | 12.5% | 0.203 | 1.3% | 0.0% | 0.999 |
| Prostatic cancer | 6.9 | 46.1 | 0.010 | 12.5% | 33.3% | 0.422 | 6.3% | 33.3% | 0.298 |
| Small cell lung cancer | 9.2 | 22.1 | 0.016 | 58.8% | 100.0% | 0.521 | 0.0% | 0.0% | NA |
| Soft tissue sarcoma | 2.5 | 9.4 | < 0.0001 | 4.4% | 42.9% | 0.0002 | 2.7% | 14.3% | 0.093 |
| Urothelial cancer | 7.3 | 15.1 | 0.005 | 32.6% | 57.9% | 0.092 | 0.0% | 5.3% | 0.306 |
| Total | 5.6 | 25.8 | < 0.0001 | 44.3% | 10.3% | < 0.0001 | 0.9% | 16.0% | < 0.0001 |
Mb megabase, MSI-H high microsatellite instability, SWI/SNF switch/sucrose nonfermentable, TMB-H high tumor mutation burden
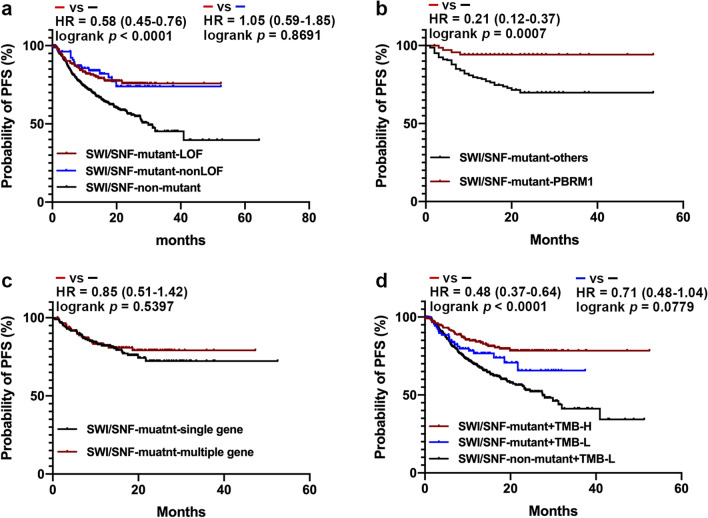
ICI treatment outcomes of patients with SWI/SNF mutations
Over the past few years, pre-clinical and clinical evidence has implicated the SWI/SNF complex as a potential predictor of response to ICIs [6]. For the ICI-treated patients, we observed that the presence of SWI/SNF LOF variants was significantly associated with a longer PFS (not reached [NR] vs. 29.9 months, HR = 0.58 [0.45–0.76]; p < 0.0001), and the presence of non-LOF variants was not inferior to the LOF variants (NR vs. NR, HR = 1.05 [0.59–1.87]; p = 0.8691; Fig. 4a). The exploration of the predicting significance of each SWI/SNF gene mutation showed that PBRM1 mutations were associated with a relatively better outcome of ICI treatments than the other SWI/SNF gene mutations (NR vs. NR, HR = 0.21 [0.12–0.37], p = 0.0007; Fig. 4b). Specifically, patients carrying mutations at two or more SWI/SNF genes did not show a superior PFS than single gene mutation carriers (NR vs. NR, HR = 0.85 [0.51–1.42], p = 0.5397; Fig. 4c). Notably, the prediction value of the SWI/SNF variants increased considerably when the TMB-H status was also considered. In particular, we observed that the SWI/SNF-mutant + TMB-low (TMB-L) cohort showed a numerically but not statistically longer PFS than the SWI/SNF-non-mutant + TMB-L cohort (NR vs. 27.5 months, HR = 0.71 [0.48–1.04], p = 0.0779), while that the SWI/SNF-mutant + TMB-H cohort showed a significantly longer PFS than the SWI/SNF-non-mutant + TMB-L cohort (NR vs. 27.5 months, HR = 0.48 [0.37–0.64], p < 0.0001; Fig. 4d). Additionally, the survival analysis for individual cancer types suggested that the PFS of the SWI/SNF-mutant group was significantly superior to that of the SWI/SNF-non-mutant group in colorectal cancer (NR vs. NR, HR = 0.33 [0.19–0.59], p = 0.0001; Additional file 2: Figure S2a) and gastric cancer (NR vs. 20.6 months, HR = 0.44 [0.19–0.97], p = 0.0437; Additional file 2: Figure S2b); the same tendency was significant numerically but not statistically in non-small cell lung cancer (NR vs. 40.9 months, HR = 0.58 [0.33–1.02], p = 0.0595; Additional file 2: Figure S2c). The PFS of SWI/SNF-mutant and SWI/SNF-non-mutant groups were not markedly different (Additional file 2: Figure S2d–h) or could not be analyzed owing to the small sample size in the other malignancies.
Fig. 4.
The progression-free survival (PFS) of patients receiving immune checkpoint inhibitor (ICI) treatment in different groups. a The PFS of patients receiving ICI treatment in SWI/SNF-mutant-loss-of-function (SWI/SNF-mutant-LOF), SWI/SNF-mutant-non-LOF, and SWI/SNF-non-mutant groups. b The PFS of patients treated by ICIs carrying PBRM1 mutations was significantly longer than that of patients carrying the other SWI/SNF gene mutations. c The PFS of patients treated by ICIs carrying mutations in two or more SWI/SNF genes was not significantly different from that of patients with mutations in single gene. d The different ICI response of the SWI/SNF-mutant + low tumor mutational burden (TMB-L), the SWI/SNF-non-mutant + TMB-L cohort, and the SWI/SNF-mutant + high tumor mutational burden (TMB-H) cohorts
Regardless of the cancer type, patients in the SWI/SNF-mutant group showed higher ORR (3.32% vs. 0.43%, p = 0.0002) and DCR (80.07% vs. 65.57%, p < 0.0001) values than their counterparts in the SWI/SNF-non-mutant group. For individual cancer types, SWI/SNF-mutant colorectal cancer (86.27% vs. 67.83%, p = 0.0014), gastric cancer (83.33% vs. 55.77%, p = 0.0222), and non-small cell lung cancer (85.07% vs. 71.58%, p = 0.0324) showed significantly higher DCR values in immunotherapy than their SWI/SNF-non-mutant counterparts (Table 4).
Table 4.
The overall response rate and disease control rate in the SWI/SNF-mutant and SWI/SNF-non-mutant groups
| Cancer type | SWI/SNF-mutant | SWI/SNF-non-mutant | p for ORR | p for DCR | ||||
|---|---|---|---|---|---|---|---|---|
| n | ORR (%) | DCR (%) | n | ORR (%) | DCR (%) | |||
| Total | 301 | 3.32 | 80.07 | 700 | 0.43 | 65.57 | 0.0002 | < 0.0001 |
| Breast cancer | 4 | 0 | 25.00 | 15 | 0 | 40.00 | 0.9999 | 0.9999 |
| Cervical cancer | 6 | 0 | 50.00 | 25 | 4.00 | 64.00 | 0.9999 | 0.6526 |
| colorectal cancer | 102 | 4.90 | 86.27 | 115 | 0.87 | 67.83 | 0.1016 | 0.0014 |
| Endometrial cancer | 17 | 0 | 64.71 | 16 | 0 | 75.00 | 0.9999 | 0.7080 |
| Esophagus cancer | 6 | 0 | 50.00 | 17 | 0 | 47.06 | 0.9999 | 0.9999 |
| Gallbladder and Biliary tract cancer | 6 | 0 | 50.00 | 11 | 0 | 90.91 | 0.9999 | 0.0987 |
| Gastric cancer | 24 | 0 | 83.33 | 52 | 0 | 55.77 | 0.9999 | 0.0222 |
| kidney cancer | 5 | 0 | 100.00 | 7 | 0 | 71.43 | 0.9999 | 0.4697 |
| Liver cancer | 7 | 0 | 100.00 | 27 | 0 | 74.07 | 0.9999 | 0.2997 |
| Melanoma | 8 | 12.50 | 62.50 | 74 | 0 | 50.00 | 0.0976 | 0.7130 |
| Non-small cell lung cancer | 67 | 2.99 | 85.07 | 190 | 0.53 | 71.58 | 0.1674 | 0.0324 |
| Other | 16 | 12.50 | 81.25 | 62 | 0 | 69.35 | 0.0400 | 0.5345 |
| Ovarian and Fallopian tube cancer | 6 | 0 | 66.67 | 12 | 0 | 50.00 | 0.9999 | 0.6380 |
| Pancreatic cancer | 4 | 0 | 100.00 | 13 | 0 | 69.23 | 0.9999 | 0.5193 |
| Prostatic cancer | 1 | 0 | 100.00 | 5 | 0 | 60.00 | 0.9999 | 0.9999 |
| Small cell lung cancer | 1 | 0 | 100.00 | 12 | 0 | 66.67 | 0.9999 | 0.9999 |
| Soft tissue sarcoma | 4 | 0 | 50.00 | 19 | 0 | 52.63 | 0.9999 | 0.9999 |
| Urothelial cancer | 17 | 0 | 76.47 | 28 | 0 | 82.14 | 0.9999 | 0.7109 |
DCR disease control rate, n number, ORR overall response rate, SWI/SNF switch/sucrose nonfermentable
Synthetic lethality involving SWI/SNF members
In recent years, synthetic lethality has attracted considerable attention in oncology, as it may explain the sensitivity of cancer cells to certain inhibitors and provide a new angle for drug development. The previously reported synthetic lethal pairs and effective inhibitors in SWI/SNF-deficient cancers are summarized in Additional file 4: Table S1. These synthetic lethal interactions can be classified under four main categories. (a) Two subunits within the SWI/SNF complex. For example, the BRD2 inhibitor, JQ1, can suppress ARID1A-deficient ovarian clear cell cancer cells because BRD2 inhibition decreases ARID1B transcription [48]. (b) One SWI/SNF subunit with its competitor. Contrary to the chromatin relaxation-inducing function of the SWI/SNF complex, polycomb repressive complex 2 (PRC2), whose enzymatic catalytic subunit is the methyltransferase, EZH2, promotes chromatin compaction via histone H3 K27 trimethylation (H3K27me3). Thus, the inhibition of EZH2 using tazemetostat or GSK126 causes synthetic lethality in ARID1A-, SMARCA4-, SMARCB1-, PBRM1-deficient cancers [49–54]. (c) Targeting the functions of the SWI/SNF complex. The SWI/SNF chromatin remodeling complex functions in DNA double-strand break repair, transcription, replication, chromosomal segregation, and in several metabolic pathways. Therefore, SWI/SNF-deficient cancers are vulnerable to the inhibition of homologous recombination repair factor, PARP1 [20, 49], cell cycle regulator, cyclin-dependent kinase (CDK)4/CDK6 [28, 56], DNA replication checkpoint factor, ATR [57], chromosomal segregation factor, Aurora kinase A [58], and oxidative phosphorylation [59] and glutathione [60] pathways. (d) Others: PD-1/PD-L1 inhibitors have synthetic lethal effects in ARID1A- and PBRM1-deficient cancers [24, 61].
Discussion
Throughout development, chromatin architecture undergoes dynamic changes that are critical for enhancer activation and gene expression. The mammalian SWI/SNF chromatin remodeling complex plays a crucial role in cellular and tissue development, and SWI/SNF subunits have been implicated as suppressors in a variety of human cancers [7, 62]. In the present study, NGS data corresponding to 4591 solid tumors, covering 18 types of malignancies, were retrospectively integrated to depict the spectrum of SWI/SNF variations. The SWI/SNF genes, ARID1A, ARID1B, ARID2, SMARCA4, SMARCB1, and PBRM1 were mutated in up to 21.8% of all the cancers, and SWI/SNF mutation carriers had significantly higher TMB values as well as higher TMB-H and MSI-H proportions than their SWI/SNF-non-mutant counterparts in several malignancies. ARID1A was the most frequently altered SWI/SNF gene and ARID1A D1850fs was identified as a relatively hot spot. Clinically, SWI/SNF mutations were found to be closely associated with a better response to ICI treatments in colorectal cancer, gastric cancer, and non-small cell lung cancer. The PFS was not significantly different in SWI/SNF-mutant and -non-mutant groups in other cancers, which might be due to the relatively small number of cases involved in our study. Given that most SWI/SNF mutations were dispersed along the full length of each gene, NGS showed potential as the most suitable strategy for detecting SWI/SNF alterations.
ARID1A/B (BAF250a/b) contains two primary domains: an N-terminal AT-rich interacting domain (ARID, residues 1017–1104) and a C-terminal domain DUF3518, also annotated as BAF250_C (residues 1975–2231). Specifically, ARID, which is a conserved helix-turn-helix motif-containing domain, plays a role in recruiting SWI/SNF to the target gene promoters, whereas the function of the BAF250_C domain, which contains motifs, such as NES and LXXLL-motif, that putatively mediate protein–protein interactions, is still unknown [63]. A TCGA database search revealed that the R1989* nonsense mutation in the DUF3518 domain is a hotspot mutation of ARID1A across cancers [64]. In this study, we observed that R1989* was captured less frequently than D1850Tfs*33 and D1850Gfs*4 (Fig. 2a), possibly because the study included a very high proportion of colorectal cancer cases, and reportedly, D1850fs is an ARID1A hot spot in colorectal cancer [65]. Additionally, the DUF3518 domain of ARID1A was found to be functionally necessary to antagonize EZH2, and both the R1989* variant and the deletion of the DUF3518 domain could not rescue EZH2-mediated IFN-γ signaling gene repression in ARID1A-knockout ovarian cancer cells [66]. D1850Tfs*33 and D1850Gfs*4, which are frameshift truncating mutations, brought about the loss of more amino acids than did R1989*. Therefore, we concluded that D1850Tfs*33 and D1850Gfs*4 might exert their functions via the deletion of the DUF3518/BAF250_C domain. The previously reported V1067G mutation, which destabilizes the ARID domain, was not detected in any of the cases included in this study [67].
Somatic mutations in SMARCA4 and/or BRG1 (Brahma-related gene 1) loss are present in a subset of non-small cell lung carcinomas with distinct morphological features, harboring less EGFR mutations, but more KRAS, STK11, and KEAP1 mutations [68, 69]. In a study on lung cancer, the genes most frequently co-mutated with SMARCA4 were TP53 (56%), KEAP1 (41%), STK11 (39%), KRAS (36%), and EGFR (14%) [68]. Among the 58 cases of lung cancer with SMARCA4 LOF mutations in our study, the mutation rates corresponding to the above hot genes were almost consistent with the previously reported rates of 74.1%, 31.0%, 24.1%, 20.7%, and 15.5% for TP53, KEAP1, STK11, KRAS, and EGFR, respectively. In this subset, 10 of 11 patients treated with ICIs attained a stable disease state, with only one patient showing disease progression (median PFS = 17.6 month). Thus, the detection of a SMARCA4 variant via NGS was useful not only in defining the particular pathological diagnosis but also in providing important clues for the choice of treatment for SMARCA4-deficient lung cancer.
LOF variants of the SWI/SNF complex can influence the response to ICIs by increasing the infiltration of CD8 + T cells, enhancing the cytotoxicity of T cells [70], or by creating an immune-responsive milieu [24]. In the current study, the PFS of patients with SWI/SNF LOF mutations was not significantly longer than that of the SWI/SNF non-LOF mutation carriers, suggesting that at least a proportion of the SWI/SNF non-LOF mutations, the most of which are missense mutations, occurring at pivotal sites might be functional. However, further studies are required to provide additional evidence for more accurate interpretation using bioinformatics. The patients carrying mutations of two or more SWI/SNF genes did not show better responses to the ICI therapy than those with single gene mutations, indicating that the increase in the number of SWI/SNF complex mutated genes may not directly cause an accumulative effect. The immunotherapeutic effect-predicting biomarker section of several commercially available NGS panels includes positively related gene variations, such as TMB-H [71], MSI-H [72], inactivating mutations of mismatch repair-related genes (MLH1, MSH2, MSH6, PMS2) [73], homologous recombination repair-related genes (ATM, ATR, BRCA1/2, CHEK1, FANCA, PALB2, etc.) [74], and POLE and POLD1 mutations [75] as well as negatively related gene variations, including inactivating mutations of PTEN [76], B2M [77], JAK1/2 [78], DNMT3A [79], STK11 [80], copy number gain of MDM2/4 [79], and CCND1 [81]. Given that patients with SWI/SNF variations showed significantly longer PFS than their SWI/SNF-non-mutant counterparts (HR, 0.56 [95% CI 0.44–0.72]; p < 0.0001), the SWI/SNF variations could be added to the list of positively predicting biomarkers for immunotherapeutic effects.
Abou Alaiwi et al. [6] also investigated the relationship between SWI/SNF complex gene variations and the ICI response by analyzing data from seven types of solid tumors, whereas we included a large patient cohort from China involving more than 18 cancer types. The previous study also excluded missense mutations from their study, whereas we stratified missense mutations into LOF and non-LOF mutations using two outstanding in silico predicted ensemble scores, MetaLR and MetaSVM, and showed that non-LOF mutations were not inferior to the LOF mutations in predicting PFS. Additionally, the previous study found that only patients with renal cell carcinoma and SWI/SNF-LOF mutations showed significantly improved survival in the cohort from Dana Farber Cancer Institute, which was mostly driven by PRBM1. Similarly, we found that PBRM1 mutations were associated with a better outcome of ICI treatments than the other SWI/SNF gene mutations (Fig. 4b). We also agreed with Abou Alaiwi et al. that loss of the SWI/SNF complex cannot be used as a pan-cancer biomarker of clinical benefits from ICIs. The current study demonstrated SWI/SNF complex variations were tightly associated with superior ICI response in several solid tumors, such as colorectal cancer, gastric cancer, and non-small cell lung cancer, especially when combined with TMB-H status. This may be caused by the involvement of a large number of colorectal cancer and non-small cell lung cancer cases as well as the missense mutations classification strategy in our study, and two different cohorts, respectively, from Dana Farber Cancer Institute and Memorial Sloan Kettering Cancer Center using two different NGS detection pipelines in their study.
The high mutation rate of the SWI/SNF complex across all cancers highlights its potential as a target for tissue-agnostic drugs. Synthetic lethality occurs when a combination of deficiencies in two genes leads to cell death, whereas deficiency in only one gene results in a viable phenotype [50]. Notably, PARP inhibitors targeting BRCA1/2-mutant tumors represent a notable example of such synthetic lethality [82]. A series of inhibitors, ranging from chemical probes to FDA-approved drugs, that target the synthetic lethal partners of SWI/SNF members have been shown to exhibit clear therapeutic effects in several cancers [20, 21, 25, 48–60, 81–105]. Furthermore, an overview of the possible biological functions and downstream signaling pathways using the GO database suggested that SWI/SNF genes and covariant genes were enriched in the PI3K signaling pathway (Additional file 3: Figure S3). Reportedly, ARID1A-deficient gastric cancer cells are vulnerable to the AKT inhibitor, GSK690693, and the addition of GSK690693 possibly potentiates the suppressive function of conventional chemotherapy [105]. Accordingly, the therapeutic effect of AKT inhibitors in cancers with SWI/SNF deficiencies is promising and should be explored further.
By integrating NGS data from a large real-world patient cohort, this study offers a detailed overview of the genomic alterations in SWI/SNF complex genes in various cancer types, and reveals the significant associations between SWI/SNF variants and TMB, MSI, and response to ICI treatment in colorectal cancer, gastric cancer, and non-small cell lung cancer; this could be of great significance in molecular screening and translational research. We mainly focused on six SWI/SNF genes that mutate with high frequencies, other SWI/SNF subunits, such as SMARCC1, SMARCC2, SMARCD1/D2/D3, SMARCE1, and ACTL6A/B, which are reported to be mutated infrequently in primary tumors [7], were not investigated since the targeted sequencing panels did not contain all the SWI/SNF complex members; we could not, therefore, assess the association of the other SWI/SNF complex members with the ICI response. The molecular functions and relevant signaling mechanisms involving the SWI/SNF variations were not investigated experimentally, and warrant further exploration.
Conclusions
SWI/SNF complex genes are frequently mutated in a wide range of cancers and are closely associated with TMB-H, MSI-H, and superior responses to ICIs in colorectal cancer, gastric cancer, and non-small cell lung cancer. Therefore, the detection and interpretation of genomic alterations in the SWI/SNF complex using NGS could provide new predictors of immunotherapeutic effects as well as useful data for translational research.
Supplementary Information
Additional file 1: Fig. S1 The distributions of variant allele frequencies (VAFs) of ARID1A, ARID1B, ARID2, PBRM1, SMARCA4, and SMARCB1. The median VAFs of the above genes were 16.1%, 13.4%, 13.3%, 17.2%, 15.2%, and 16.7%, respectively. Red solid line, median; black dotted line, quartiles.
Additional file 2: Fig. S2 The progression-free survival (PFS) of patients receiving immune checkpoint inhibitor (ICI) treatment based on cancer types. The survival analysis was performed for individual cancer types that contained at least 10 cases in the SWI/SNF-mutant or SWI/SNF-non-mutant groups. The PFS of the SWI/SNF-mutant group was significantly superior to that of the SWI/SNF-non-mutant group in colorectal cancer (a) and gastric cancer (b), the same tendency was significant numerically by not statistically in non-small cell lung cancer (c). The PFS of SWI/SNF-mutant and SWI/SNF-non-mutant were not markedly different in melanoma (d), soft tissue sarcoma (e), urothelial cancer (f), endometrial cancer (g) and other cancers (h).
Additional file 3: Fig. S3 The signaling pathway enrichment of the variated genes in the SWI/SNF-mutant tumors by GO analysis. The GO analysis was performed on all the mutated genes in 1001 SWI/SNF-mutant samples.
Additional file 4: Table S1. Synthetic lethal interactive pairs and chemical inhibitors involving SWI/SNF members.
Acknowledgements
Not applicable.
Abbreviations
- DCR
Disease control rate
- ICI
Immune checkpoint inhibitor
- LOF
Loss-of-function
- MSI-H
High microsatellite instability
- Mb
Megabase
- NGS
Next-generation sequencing
- ORR
Overall response rate
- PFS
Progression-free survival
- SWI/SNF
SWItch/sucrose nonfermentable
- TMB-H
High tumor mutational burden
- TMB-L
Low tumor mutational burden
Author contributions
WF and HCY designed the study; LY and ZWJ collected the clinical information; LY and YXH analyzed the data; XYX and MJJ performed the experiments; and LY and WF wrote the paper. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant number 82002561), Guangdong Basic and Applied Basic Research Foundation (Grant numbers 2020A1515010098 and 2020A1515010314), Natural Science Foundation of Guangdong Province (Grant number 2017A030310192), and Fundamental Research Funds for the Central Universities (Grant number 17ykpy84).
Availability of data and materials
The datasets supporting the conclusions of this article are available in the Research Data Deposit repository (No. RDDA2021338857, http://www.researchdata.org.cn/), and are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The use of clinical and NGS data was approved by the Ethics Committee of the Sun Yat-Sen University Cancer Center (Approval number B2020-344-01). All patients provided signed informed consent, and the study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yue Li and Xinhua Yang are contributed equally to this work
Contributor Information
Caiyun He, Email: hecy@sysucc.org.cn.
Fang Wang, Email: wangfang@sysucc.org.cn.
References
- 1.Garber K. Tissue-agnostic cancer drug pipeline grows, despite doubts. Nat Rev Drug Discov. 2018;17:227–229. doi: 10.1038/nrd.2018.6. [DOI] [PubMed] [Google Scholar]
- 2.Han SY. TRK inhibitors tissue-agnostic anti-cancer drugs. Pharmaceuticals. 2021 doi: 10.3390/ph14070632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanior M, Krämer A, Knapp S, Joerger AC. Exploiting vulnerabilities of SWI/SNF chromatin remodelling complexes for cancer therapy. Oncogene. 2021;40:3637–3654. doi: 10.1038/s41388-021-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westphalen CB, Krebs MG, Le Tourneau C, Sokol ES, Maund SL, Wilson TR, et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis Oncol. 2021 doi: 10.1038/s41698-021-00206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Haswell JR, Roberts CWM. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer—mechanisms and potential therapeutic insights. Clin Cancer Res. 2014;20:21–27. doi: 10.1158/1078-0432.CCR-13-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou Alaiwi S, Nassar AH, Xie W, Bakouny Z, Berchuck JE, Braun DA, et al. Mammalian SWI/SNF complex genomic alterations and immune checkpoint blockade in solid tumors. Cancer Immunol Res. 2020;8:1075–1084. doi: 10.1158/2326-6066.CIR-19-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45:592–601. doi: 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versteege I, Sévenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature England. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 9.Sévenet N, Sheridan E, Amram D, Schneider P, Handgretinger R, Delattre O. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet. 1999;65:1342–1348. doi: 10.1086/302639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saito M, Kono K. Landscape of EBV-positive gastric cancer. Gastric Cancer. 2021;24:983–989. doi: 10.1007/s10120-021-01215-3. [DOI] [PubMed] [Google Scholar]
- 11.Huang SC, Ng KF, Chang IYF, Chang CJ, Chao YC, Chang SC, et al. The clinicopathological significance of SWI/ SNF alterations in gastric cancer is associated with the molecular subtypes. PLoS ONE. 2021;16:1–15. doi: 10.1371/journal.pone.0245356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Takenaka M, Okamoto A, Bowtell DDL, Kohno T. Treatment strategies for ARID1A-deficient ovarian clear cell carcinoma. Cancers. 2021;13:1769. doi: 10.3390/cancers13081769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tessier-Cloutier B, Coatham M, Carey M, Nelson GS, Hamilton S, Lum A, et al. SWI/SNF-deficiency defines highly aggressive undifferentiated endometrial carcinoma. J Pathol Clin Res. 2021;7:144–153. doi: 10.1002/cjp2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D, Tian L, Zhu Y, Wo Y, Liu Q, Liu S, et al. Subunits of ARID1 serve as novel biomarkers for the sensitivity to immune checkpoint inhibitors and prognosis of advanced non-small cell lung cancer. Mol Med. 2020;26:78. doi: 10.1186/s10020-020-00208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. 2014;46:438–443. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- 16.Ramos P, Karnezis AN, Craig DW, Sekulic A, Russell ML, Hendricks WPD, et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet. 2014;46:427–429. doi: 10.1038/ng.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biegel JA, Kalpana G, Knudsen ES, Packer RJ, Roberts CWM, Thiele CJ, et al. The role of INI1 and the SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the workshop on childhood atypical teratoid/rhabdoid tumors. Cancer Res. 2002;62:323–328. [PubMed] [Google Scholar]
- 18.Kim KH, Roberts CWM. Mechanisms by which SMARCB1 loss drives rhabdoid tumor growth. Cancer Genet. 2014;207:365–372. doi: 10.1016/j.cancergen.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang E, Tessier-Cloutier B, Duggan MA, Stewart CJR, Lee C, Köbel M. Loss of ARID1B and SMARCB1 expression are specific for the diagnosis of dedifferentiated/undifferentiated carcinoma in tumours of the upper gynaecological tract and cervix. Histopathology. 2021;79:160–167. doi: 10.1111/his.14333. [DOI] [PubMed] [Google Scholar]
- 20.Alessi JV, Ricciuti B, Spurr LF, Gupta H, Li YY, Glass C, et al. SMARCA4 and other SWItch/Sucrose nonfermentable family genomic alterations in NSCLC: clinicopathologic characteristics and outcomes to immune checkpoint inhibition. J Thorac Oncol. 2021;16:1176–1187. doi: 10.1016/j.jtho.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 21.D’Afonseca V, Arencibia AD, Echeverría-Vega A, Cerpa L, Cayún JP, Varela NM, et al. Identification of altered genes in gallbladder cancer as potential driver mutations for diagnostic and prognostic purposes: a computational approach. Cancer Inform. 2020;19:117693512092215. doi: 10.1177/1176935120922154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha S, Lee E, Won HH. Comprehensive characterization of distinct genetic alterations in metastatic breast cancer across various metastatic sites. NPJ Breast Cancer. 2021;7(1):1–1. doi: 10.1038/s41523-021-00303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno T, Monterde B, González-Silva L, Betancor-Fernández I, Revilla C, Agraz-Doblas A, et al. ARID2 deficiency promotes tumor progression and is associated with higher sensitivity to chemotherapy in lung cancer. Oncogene. 2021;40:2923–2935. doi: 10.1038/s41388-021-01748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359:801–806. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chabanon RM, Morel D, Eychenne T, Colmet-Daage L, Bajrami I, Dorvault N, et al. PBRM1 deficiency confers synthetic lethality to DNA repair inhibitors in cancer. Cancer Res. 2021;81:2888–2902. doi: 10.1158/0008-5472.CAN-21-0628. [DOI] [PubMed] [Google Scholar]
- 26.Brück O, Lee MH, Turkki R, Uski I, Penttilä P, Paavolainen L, et al. Spatial immunoprofiling of the intratumoral and peritumoral tissue of renal cell carcinoma patients. Mod Pathol. 2021;34:2229–2241. doi: 10.1038/s41379-021-00864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oike T, Ogiwara H, Tominaga Y, Ito K, Ando O, Tsuta K, et al. A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1. Cancer Res. 2013;73:5508–5518. doi: 10.1158/0008-5472.CAN-12-4593. [DOI] [PubMed] [Google Scholar]
- 28.Xue Y, Meehan B, Fu Z, Wang XQD, Fiset PO, Rieker R, et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat Commun. 2019;10:557. doi: 10.1038/s41467-019-08380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenquist R, Cuppen E, Buettner R, Caldas C, Dreau H, Elemento O, et al. Clinical utility of whole-genome sequencing in precision oncology. Semin Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Chang L, Yang Y, Fang W, Guan Y, Wu A, et al. The correlations of tumor mutational burden among single-region tissue, multi-region tissues and blood in non-small cell lung cancer. J Immunother Cancer. 2019;7:98. doi: 10.1186/s40425-019-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Yi Y, Xiao Y, Dong L, Liang L, Teng L, et al. Prevalence of recurrent oncogenic fusion in mismatch repair-deficient colorectal carcinoma with hypermethylated MLH1 and wild-type BRAF and KRAS. Mod Pathol. 2019;32:1053–1064. doi: 10.1038/s41379-019-0212-1. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Xi S, Yong J, Wu X, Yang X, Wang F. Morphologic, Immunohistochemical, and genetic differences between high-grade and low-grade fetal adenocarcinomas of the lung. Am J Surg Pathol. 2021;45:1464–1475. doi: 10.1097/PAS.0000000000001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhao C, Chang L, Jia R, Liu R, Zhang Y, et al. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab-resistant mechanism in advanced gastric cancer. EBioMedicine. 2019;43:261–269. doi: 10.1016/j.ebiom.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Lupat R, Amarasinghe KC, Thompson ER, Doyle MA, Ryland GL, et al. CONTRA: copy number analysis for targeted resequencing. Bioinformatics. 2012;28:1307–1313. doi: 10.1093/bioinformatics/bts146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164–e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oluwole OG, Kuivaniemi H, Abrahams S, Haylett WL, Vorster AA, Van Heerden CJ, et al. Targeted next-generation sequencing identifies novel variants in candidate genes for Parkinson’s disease in Black South African and Nigerian patients. BMC Medical Genetics. 10.1186/s12881-020-0953-1 [DOI] [PMC free article] [PubMed]
- 39.Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet. 2015;24:2125–2137. doi: 10.1093/hmg/ddu733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Wu C, Li C, Boerwinkle E. dbNSFP v3.0: a one-stop database of functional predictions and annotations for human nonsynonymous and splice-site SNVs. Hum Mutat. 2016;37:235–241. doi: 10.1002/humu.22932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun S, Liu Y, Eisfeld A-K, Zhen F, Jin S, Gao W, et al. Identification of germline mismatch repair gene mutations in lung cancer patients with paired tumor-normal next generation sequencing: a retrospective study. Front Oncol. 2019;9:550. doi: 10.3389/fonc.2019.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerstung M, Pellagatti A, Malcovati L, Giagounidis A, Della PMG, Jädersten M, et al. Combining gene mutation with gene expression data improves outcome prediction in myelodysplastic syndromes. Nat Commun. 2015 doi: 10.1038/ncomms6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 45.Fagan RJ, Dingwall AK. COMPASS ascending: emerging clues regarding the roles of MLL3/KMT2C and MLL2/KMT2D proteins in cancer. Cancer Lett Elsevier. 2019;458:56–65. doi: 10.1016/j.canlet.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park Y-K, Lee J-E, Yan Z, McKernan K, O’Haren T, Wang W, et al. Interplay of BAF and MLL4 promotes cell type-specific enhancer activation. Nat Commun. 2021 doi: 10.1038/s41467-021-21893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rampias T, Karagiannis D, Avgeris M, Polyzos A, Kokkalis A, Kanaki Z, et al. The lysine-specific methyltransferase KMT2C/MLL3 regulates DNA repair components in cancer. EMBO Rep. 2019;20:1–20. doi: 10.15252/embr.201846821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berns K, Caumanns JJ, Hijmans EM, Gennissen AMC, Severson TM, Evers B, et al. ARID1A mutation sensitizes most ovarian clear cell carcinomas to BET inhibitors. Oncogene. 2018;37:4611–4625. doi: 10.1038/s41388-018-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada L, Saito M, Thar Min AK, Saito K, Ashizawa M, Kase K, et al. Selective sensitivity of EZH2 inhibitors based on synthetic lethality in ARID1A-deficient gastric cancer. Gastric Cancer. 2021;24:60–71. doi: 10.1007/s10120-020-01094-0. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Chen SY, Karnezis AN, Colborne S, Dos SN, Lang JD, et al. The histone methyltransferase EZH2 is a therapeutic target in small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2017;242:371–383. doi: 10.1002/path.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18:316–328. doi: 10.1016/j.ccr.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gounder MM, Zhu G, Roshal L, Lis E, Daigle SR, Blakemore SJ, et al. Immunologic correlates of the abscopal effect in a SMARCB1/INI1-negative poorly differentiated chordoma after EZH2 inhibition and radiotherapy. Clin Cancer Res. 2019;25:2064–2071. doi: 10.1158/1078-0432.CCR-18-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang K, Sun R, Chen J, Yang Q, Wang Y, Zhang Y, et al. A novel EZH2 inhibitor induces synthetic lethality and apoptosis in PBRM1-deficient cancer cells. Cell Cycle. 2020;19:758–771. doi: 10.1080/15384101.2020.1729450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen J, Peng Y, Wei L, Zhang W, Yang L, Lan L, et al. ARID1A deficiency impairs the DNA damage checkpoint and sensitizes cells to PARP inhibitors. Cancer Discov. 2015;5:752–767. doi: 10.1158/2159-8290.CD-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xue Y, Meehan B, Macdonald E, Venneti S, Wang XQD, Witkowski L, et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun. 2019;10:558. doi: 10.1038/s41467-018-06958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williamson CT, Miller R, Pemberton HN, Jones SE, Campbell J, Konde A, et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun. 2016;7:13837. doi: 10.1038/ncomms13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C, Lyu J, Yang EJ, Liu Y, Zhang B, Shim JS. Targeting AURKA-CDC25C axis to induce synthetic lethality in ARID1A-deficient colorectal cancer cells. Nat Commun. 2018;9:3212. doi: 10.1038/s41467-018-05694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lissanu Deribe Y, Sun Y, Terranova C, Khan F, Martinez-Ledesma J, Gay J, et al. Mutations in the SWI/SNF complex induce a targetable dependence on oxidative phosphorylation in lung cancer article. Nat Med. 2018;24:1047–1057. doi: 10.1038/s41591-018-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogiwara H, Takahashi K, Sasaki M, Kuroda T, Yoshida H, Watanabe R, et al. Targeting the Vulnerability of glutathione metabolism in ARID1A-deficient cancers. Cancer Cell. 2019;35:177–190.e8. doi: 10.1016/j.ccell.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. 2020;8:e000438. doi: 10.1136/jitc-2019-000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson BG, Roberts CWM. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer England. 2011;11:481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 63.Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol. 2000;20:8879–8888. doi: 10.1128/mcb.20.23.8879-8888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016;34:155–163. doi: 10.1038/nbt.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tokunaga R, Xiu J, Goldberg RM, Philip PA, Seeber A, Battaglin F, et al. The impact of ARID1A mutation on molecular characteristics in colorectal cancer. Eur J Cancer. 2020;140:119–129. doi: 10.1016/j.ejca.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Wang W, Zhang Y, Cieślik M, Guo J, Tan M, et al. Epigenetic driver mutations in ARID1A shape cancer immune phenotype and immunotherapy. J Clin Invest. 2020;130:2712–2726. doi: 10.1172/JCI134402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandhya S, Maulik A, Giri M, Singh M. Domain architecture of BAF250a reveals the ARID and ARM-repeat domains with implication in function and assembly of the BAF remodeling complex. PLoS ONE. 2018;13:1–26. doi: 10.1371/journal.pone.0205267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schoenfeld AJ, Bandlamudi C, Lavery JA, Montecalvo J, Namakydoust A, Rizvi H, et al. The Genomic landscape of SMARCA4 alterations and associations with outcomes in patients with lung cancer. Clin Cancer Res. 2020;26:5701–5708. doi: 10.1158/1078-0432.CCR-20-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nambirajan A, Singh V, Bhardwaj N, Mittal S, Kumar S, Jain D. SMARCA4/BRG1–deficient non-small cell lung carcinomas: a case series and review of the literature. Arch Pathol Lab Med United States. 2021;145:90–98. doi: 10.5858/arpa.2019-0633-OA. [DOI] [PubMed] [Google Scholar]
- 70.Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, et al. A major chromatin regulator determines resistance of tumor cells to T cell–mediated killing. Science. 2018;359:770–775. doi: 10.1126/science.aao1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34:2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 74.Jiang M, Jia K, Wang L, Li W, Chen B, Liu Y, et al. Alterations of DNA damage response pathway: biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B. 2021;11:2983–2994. doi: 10.1016/j.apsb.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang F, Zhao Q, Wang Y-N, Jin Y, He M-M, Liu Z-X, et al. Evaluation of POLE and pold1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019;5:1504. doi: 10.1001/jamaoncol.2019.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Challa-Malladi M, Lieu YK, Califano O, Holmes AB, Bhagat G, Murty VV, et al. Combined genetic inactivation of β2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20:728–740. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marabelle A, Aspeslagh S, Postel-Vinay S, Soria J-C. JAK mutations as escape mechanisms to Anti–PD-1 therapy. Cancer Discov United States. 2017;7:128–130. doi: 10.1158/2159-8290.CD-16-1439. [DOI] [PubMed] [Google Scholar]
- 79.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non–small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36:633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y, Huang Y, Gao X, Li Y, Lin J, Chen L, et al. CCND1 amplification contributes to immunosuppression and is associated with a poor prognosis to immune checkpoint inhibitors in solid tumors. Front Immunol. 2020;11:1620. doi: 10.3389/fimmu.2020.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Januario T, Ye X, Bainer R, Alicke B, Smith T, Haley B, et al. PRC2-mediated repression of SMARCA2 predicts EZH2 inhibitor activity in SWI/SNF mutant tumors. Proc Natl Acad Sci. 2017;114:12249–12254. doi: 10.1073/pnas.1703966114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim KH, Kim W, Howard TP, Vazquez F, Tsherniak A, Wu JN, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015;21:1491–1496. doi: 10.1038/nm.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan-Penebre E, Armstrong K, Drew A, Grassian AR, Feldman I, Knutson SK, et al. Selective Killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: in vitro and in vivo preclinical models. Mol Cancer Ther. 2017;16:850–860. doi: 10.1158/1535-7163.MCT-16-0678. [DOI] [PubMed] [Google Scholar]
- 86.Miller RE, Brough R, Bajrami I, Williamson CT, McDade S, Campbell J, et al. Synthetic lethal targeting of ARID1A-mutant ovarian clear cell tumors with dasatinib. Mol Cancer Ther. 2016;15:1472–1484. doi: 10.1158/1535-7163.MCT-15-0554. [DOI] [PubMed] [Google Scholar]
- 87.Caumanns JJ, Wisman GBA, Berns K, van der Zee AGJ, de Jong S. ARID1A mutant ovarian clear cell carcinoma: a clear target for synthetic lethal strategies. Biochim Biophys Acta-Rev Cancer. 2018;1870:176–184. doi: 10.1016/j.bbcan.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Kawahara N, Yamada Y, Kobayashi H. CCNE1 is a putative therapeutic target for ARID1A-mutated ovarian clear cell carcinoma. Int J Mol Sci. 2021;22:5869. doi: 10.3390/ijms22115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schick S, Rendeiro AF, Runggatscher K, Ringler A, Boidol B, Hinkel M, et al. Systematic characterization of BAF mutations provides insights into intracomplex synthetic lethalities in human cancers. Nat Genet. 2019;51:1399–1410. doi: 10.1038/s41588-019-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romero OA, Vilarrubi A, Alburquerque-Bejar JJ, Gomez A, Andrades A, Trastulli D, et al. SMARCA4 deficient tumours are vulnerable to KDM6A/UTX and KDM6B/JMJD3 blockade. Nat Commun. 2021;12:1–14. doi: 10.1038/s41467-021-24618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howard TP, Arnoff TE, Song MR, Giacomelli AO, Wang X, Hong AL, et al. MDM2 and MDM4 are therapeutic vulnerabilities in malignant rhabdoid tumors. Cancer Res. 2019;79:2404–2414. doi: 10.1158/0008-5472.CAN-18-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lo Y-H, Kolahi KS, Du Y, Chang C-Y, Krokhotin A, Nair A, et al. A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 2021;11:1562–1581. doi: 10.1158/2159-8290.CD-20-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loe AKH, Francis R, Seo J, Du L, Wang Y, Kim J-E, et al. Uncovering the dosage-dependent roles of Arid1a in gastric tumorigenesis for combinatorial drug therapy. J Exp Med. 2021;218:e20200219. doi: 10.1084/jem.20200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bitler BG, Wu S, Park PH, Hai Y, Aird KM, Wang Y, et al. ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol. 2017;19:962–973. doi: 10.1038/ncb3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fukumoto T, Park PH, Wu S, Fatkhutdinov N, Karakashev S, Nacarelli T, et al. Repurposing Pan-HDAC inhibitors for ARID1A-mutated ovarian cancer. Cell Rep. 2018;22:3393–3400. doi: 10.1016/j.celrep.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dong X, Song S, Li Y, Fan Y, Wang L, Wang R, et al. Loss of ARID1A activates mTOR signaling and SOX9 in gastric adenocarcinoma—rationale for targeting ARID1A deficiency. Gut England. 2022;71:467–478. doi: 10.1136/gutjnl-2020-322660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen J, Ju Z, Zhao W, Wang L, Peng Y, Ge Z, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556–562. doi: 10.1038/s41591-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang L, Yang G, Ding Y, Huang Y, Liu S, Zhou L, et al. Combined treatment with PI3K inhibitor BKM120 and PARP inhibitor olaparib is effective in inhibiting the gastric cancer cells with ARID1A deficiency. Oncol Rep Greece. 2018;40:479–487. doi: 10.3892/or.2018.6445. [DOI] [PubMed] [Google Scholar]
- 99.Tagal V, Wei S, Zhang W, Brekken RA, Posner BA, Peyton M, et al. SMARCA4-inactivating mutations increase sensitivity to aurora kinase a inhibitor VX-680 in non-small cell lung cancers. Nat Commun. 2017;8:14098. doi: 10.1038/ncomms14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding Y, Li N, Dong B, Guo W, Wei H, Chen Q, et al. Chromatin remodeling ATPase BRG1 and PTEN are synthetic lethal in prostate cancer. J Clin Invest. 2019;129:759–773. doi: 10.1172/JCI123557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee SJ, Cimica V, Ramachandra N, Zagzag D, Kalpana GV. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71:3225–3235. doi: 10.1158/0008-5472.CAN-10-2167. [DOI] [PubMed] [Google Scholar]
- 102.Smith ME, Cimica V, Chinni S, Jana S, Koba W, Yang Z, et al. Therapeutically targeting cyclin D1 in primary tumors arising from loss of Ini1. Proc Natl Acad Sci. 2011;108:319–324. doi: 10.1073/pnas.0913297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kerl K, Moreno N, Holsten T, Ahlfeld J, Mertins J, Hotfilder M, et al. Arsenic trioxide inhibits tumor cell growth in malignant rhabdoid tumors in vitro and in vivo by targeting overexpressed Gli1. Int J Cancer United States. 2014;135:989–995. doi: 10.1002/ijc.28719. [DOI] [PubMed] [Google Scholar]
- 104.Hong AL, Tseng Y-Y, Wala JA, Kim W-J, Kynnap BD, Doshi MB, et al. Renal medullary carcinomas depend upon SMARCB1 loss and are sensitive to proteasome inhibition. Elife. 2019;8:e44161. doi: 10.7554/eLife.44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee D, Yu EJ, Ham I-H, Hur H, Kim Y-S. AKT inhibition is an effective treatment strategy in ARID1A-deficient gastric cancer cells. Onco Targets Ther. 2017;10:4153–4159. doi: 10.2147/OTT.S139664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 The distributions of variant allele frequencies (VAFs) of ARID1A, ARID1B, ARID2, PBRM1, SMARCA4, and SMARCB1. The median VAFs of the above genes were 16.1%, 13.4%, 13.3%, 17.2%, 15.2%, and 16.7%, respectively. Red solid line, median; black dotted line, quartiles.
Additional file 2: Fig. S2 The progression-free survival (PFS) of patients receiving immune checkpoint inhibitor (ICI) treatment based on cancer types. The survival analysis was performed for individual cancer types that contained at least 10 cases in the SWI/SNF-mutant or SWI/SNF-non-mutant groups. The PFS of the SWI/SNF-mutant group was significantly superior to that of the SWI/SNF-non-mutant group in colorectal cancer (a) and gastric cancer (b), the same tendency was significant numerically by not statistically in non-small cell lung cancer (c). The PFS of SWI/SNF-mutant and SWI/SNF-non-mutant were not markedly different in melanoma (d), soft tissue sarcoma (e), urothelial cancer (f), endometrial cancer (g) and other cancers (h).
Additional file 3: Fig. S3 The signaling pathway enrichment of the variated genes in the SWI/SNF-mutant tumors by GO analysis. The GO analysis was performed on all the mutated genes in 1001 SWI/SNF-mutant samples.
Additional file 4: Table S1. Synthetic lethal interactive pairs and chemical inhibitors involving SWI/SNF members.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the Research Data Deposit repository (No. RDDA2021338857, http://www.researchdata.org.cn/), and are available from the corresponding author on reasonable request.