Abstract
Francisella tularensis LVS is an effective live vaccine strain used for cutaneous vaccination against tularemia in man. In mice, injection of LVS causes invasive disease and subsequent development of immunity that is characterized by effective control of otherwise lethal doses of the organism. In the present investigation, it is shown that LVS-immune mice controlled an intradermal infection much more effectively than did naive mice; bacterial counts in skin samples were 1.5 to 2.0 log10 lower 24 h after injection and 6 log10 lower 72 h after injection in immune mice. Moreover, in contrast to naive mice, no bacteria were demonstrated in samples from livers and spleens of immune mice. By immunohistochemistry, skin samples from immune mice showed an intense staining for interleukin-12 (IL-12) and a moderate staining for tumor necrosis factor alpha (TNF-α) at 24 h postinoculation, after which staining for both cytokines faded. In naive mice, the staining for IL-12 was weak at all time points and no staining for TNF-α was observed. No staining for gamma interferon (IFN-γ) was observed in any group before 72 h. At that time point, skin samples from immune mice showed moderate staining and skin samples from naive mice showed weak staining. Reverse transcriptase PCR showed an induction of mRNA of the three cytokines in the skin within the first day after injection. A quantitative analysis demonstrated higher IFN-γ and TNF-α mRNA levels in immune mice at 24 h postinoculation. In conclusion, immunization with F. tularensis LVS conferred a capability to respond to cutaneous reinfection, with rapid local expression of IL-12, TNF-α, and IFN-γ, and this expression was paralleled by containment and mitigation of the infection. The cytokine response may be part of a local barrier function of the skin, important to host protection against tularemia.
Tularemia is caused by Francisella tularensis, a facultative intracellular bacterium. The disease is endemic in rodents and lagomorphs, which are believed to be the main sources for the spread of tularemia to humans. In man, tularemia presents in different ways, depending on the route of entry (reviewed in reference 26). Inhalation of infected dust leads to the respiratory form of the disease, while transmission by arthropods or direct contact with an infected animal causes the ulceroglandular form of the disease. The respiratory form of the disease is more severe, and in the United States, where the highly virulent type A strains are prevalent, the fatality rate before the use of antibiotics was 20 to 30%, considerably higher than the same figure for ulceroglandular tularemia, <10%. Irrespective of host species, the resulting diseases have many features in common, and experimental infection in the mouse is generally held to be an appropriate model for understanding the host-parasite interaction of human infection.
Work on the murine model of tularemia has disclosed mechanisms of host resistance that are similar to those relevant for intracellular bacteria in general. During the first few days of a primary infection, neutrophils play a critical role, as evidenced by a dramatic exacerbation of infection following their depletion or the prevention of their recruitment (3, 23). Later, an immunospecific αβ T-cell response develops which is crucial for the eradication of F. tularensis (4, 30).
Immunization against F. tularensis affords effective protection. For example, only a few cases of human reinfection have been reported (26). Similarly, mice given a sublethal inoculum of the live vaccine strain F. tularensis LVS develop long-lasting immunity and can survive reinfection with up to 100 LD50 doses (23, 24).
Work on other models of intracellular infections, e.g., murine listeriosis, the prototypic model of intracellular bacterial infection, has revealed a critical requirement for certain cytokines in innate and acquired host resistance. Requirements for resistance to tularemia are similar. At an early stage of murine tularemia, interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) are expressed by infected mononuclear phagocytes (9), and these cytokines are believed to trigger the production of gamma interferon (IFN-γ) by NK cells. The latter cytokine mediates the activation of mononuclear phagocytes and is vital for them to kill F. tularensis (7). In support of a critical role for these cytokines, neutralization of the biological effects of IFN-γ and TNF-α during the early phase of primary tularemia leads to lethal exacerbation of infection (1, 6, 14, 24). These cytokines also play important roles after reinfection (24). Although the requirement of IL-12 for host resistance against tularemia has not been established, there is ample evidence from other models of intracellular infections that IL-12 drives differentiation of the protective Th1 immune response, i.e., the differentiation into IFN-γ-secreting T cells (10, 15, 16, 27–29). Knowledge about mechanisms of host resistance to F. tularensis derives from experimental infection in normal and immunodeficient mice, with the focus on lymphoid organs. There is, however, little information on the possible local presence of cytokines and other immunoregulators within anatomic barriers, such as skin, penetrated by F. tularensis.
Host protective mechanisms in the skin need to be effective, since they often constitute a first line of defense and the skin is a principal target of infections caused by highly infectious agents. The murine model of tularemia is a striking example of the effectiveness of cutaneous immune mechanisms, since a thousand- to a million-fold-more bacteria are required to generate a lethal infection by the dermal route versus other routes of inoculation, e.g., intranasal, intravenous, or intraperitoneal (8). Thus, experimental tularemia may serve as a model to elucidate (i) cutaneous immune mechanisms that confer the host with a means to locally control and contain infection and (ii) the very effective host resistance mechanisms expressed in an immune individual. Such a characterization is of special relevance to understand how host resistance against F. tularensis is triggered in humans, since vaccination with the attenuated strain F. tularensis LVS is administered by scarification and the ulceroglandular form of infection is the most prevalent variant.
In the present study, bacterial replication in target organs and its temporal association with expression of IL-12, TNF-α, and IFN-γ during primary infection and reinfection in the skin were monitored.
MATERIALS AND METHODS
Animals.
Female 5- to 6-week-old BALB/cJBom mice were purchased from Bomholtgård, Ry, Denmark, and used in experiments at approximately 8 weeks of age. The mice were housed at the Animal Facility, Defence Research Establishment, Umeå, Sweden, under conventional conditions and given food and water ad libitum.
Bacteria.
The vaccine strain F. tularensis LVS (ATCC 29684) was supplied by the U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, Frederick, Md. Bacteria were grown on modified Thayer-Martin agar (21) at 37°C to the logarithmic phase, suspended at a density of 3 × 109 organisms per ml in saline with the addition of 10% (wt/vol) glycerol, and stored in 200-μl aliquots at −70°C. For each experiment, inocula were prepared from frozen stocks and bacteria were diluted in sterile saline to the required concentration. Bacterial counts were retrospectively assessed after injection.
Inoculation and enumeration of bacteria.
Approximately 2 cm2 of the skin of the upper thorax was shaved 2 days before inoculation. Mice were challenged with an intradermal inoculation of 3.8 × 105 F. tularensis LVS and killed by decapitation after 1, 3, 5, 8, and 15 days of infection. One square centimeter of the skin at the site of inoculation was excised and put into tubes with saline after a brief wash in 70% ethanol. Spleen, liver, and draining lymph nodes were also collected. Samples from each of the four organs were homogenized, and the number of F. tularensis LVS was calculated by plating 10-fold serial dilutions. Mice that received a secondary challenge had been given a subcutaneous inoculation at another dermal site of 104 CFU of F. tularensis LVS 5 to 6 weeks earlier. Colonies were counted after 3 days of incubation at 37°C.
mRNA preparation and cDNA synthesis.
Mice were intradermally inoculated with 3 × 105 F. tularensis LVS and killed at 1, 6, 12, 24, 48, and 72 h postinfection. One square centimeter of the skin, including the site of inoculation in the center, was excised and immediately frozen in liquid nitrogen and stored at −70°C. RNA was isolated from frozen samples of skin, lymph nodes, spleens, and livers by using a guanidine isothiocyanate-phenol-chloroform single-step method (2). The optical density at 260 nm was used to estimate the concentration of total mRNA, yields being in the order of 10 to 100 μg per sample.
For cDNA synthesis, approximately 5 μg of total mRNA was incubated with 2.5 μg of random hexamers (Promega, Madison, Wis.) for 5 min at 94°C. After the solution was cooled on ice, the total volume was adjusted to 50 μl in reverse transcriptase buffer (Gibco BRL, Grand Island, N.Y.) with the addition of 0.6 mM of each deoxynucleoside triphosphatase (Pharmacia, Uppsala, Sweden), 40 U of RNasin (Promega), and 20 U of Superscript (Gibco BRL). After incubation at 37°C for 60 min, 42°C for 30 min, and 70°C for 5 min, a 1.0-μl portion of each sample was subjected to PCR amplification. Bands were visualized by ethidium bromide staining of agarose gels after electrophoresis.
PCR procedure.
One microliter of cDNA was added to a PCR mix containing (at a final concentration) 200 μM deoxynucleoside triphosphate mix, Taq reaction buffer (Advanced Biotechnologies, London, United Kingdom), 0.4 μM each primer, 1.5 mM MgCl2, and 1 U of thermostable Taq polymerase (Advanced Biotechnologies) in a total reaction volume of 25 μl. The reaction mixtures were subjected to 25 cycles (β2-microglobulin), 35 cycles (TNF-α, IFN-γ), or 40 cycles (IL-12p40) of amplification in a DNA thermal cycler 4800 (Perkin-Elmer, Norwalk, Conn.). An amplification cycle consisted of denaturation for 30 s at 94°C, primer annealing to the template at 65°C for 60 s, and primer extension at 72°C for 45 s. After amplification, 5 μl of each reaction mixture was subjected to electrophoresis in a 2% agarose gel and the amplified gene products were visualized by UV light after ethidium bromide staining. A 1-kb ladder (Gibco BRL) was used as a size marker.
PCR primers for TNF-α (354 bp) and IFN-γ (365 bp) were purchased from Clontech (Palo Alto, Calif.). The primers for murine β2-microglobulin (300 bp) and IL-12p40 (396 bp) have been published elsewhere (5, 31).
Competitive PCR.
To quantify the cDNA levels for β-actin, TNF-α, and IFN-γ, a competitive PCR from Clontech was used (12). IL-12p40 mRNA was quantitated by use of a competitive fragment purified from the plasmid pMUS (13).
An initial PCR with 10-fold serial dilutions of the β-actin, TNF-α, IL-12p40, or IFN-γ competitor fragments was followed by a PCR with twofold serial dilutions. The PCR procedures for β-actin, IFN-γ, and TNF-α were as previously described (12). The amount of cDNA was determined by identifying the dilution of the competitor fragment showing the same intensity after amplification as that of the amplicon resulting from the sample cDNA. The number of cycles was chosen such that amplicons accumulated in a constant ratio, although their original concentrations varied up to 100-fold. The samples were subjected to 35 cycles of amplification, each consisting of denaturation for 30 s at 94°C, primer annealing to the template at 60°C for 30 s, and primer extension at 72°C for 45 s.
Immunohistochemistry.
Skin biopsy specimens excised from the thorax of infected mice and draining lymph nodes were prepared for immunocytochemical staining by snap freezing in liquid propane. Tissues were placed in OCT compound (Tissue Tek), and samples were stored at −70°C until sectioned.
Immunohistochemistry for cytokine expression in infectious foci was performed as previously described (25). Rat anti-mouse IL-12 (10 μg/ml), TNF-α (15 μg/ml), and IFN-γ (5 μg/ml) were all from Pharmingen, San Diego, Calif. Secondary antibody was biotinylated rabbit anti-rat IgG (adsorbed against mouse antiserum) from Vector Lab Inc., Berlingame, Calif. Primary antibody was visualized with a peroxidase-labeled antibody. Microscopy was performed with a Leitz DRMBE microscope. No staining was visualized after incubation with isotype-matched irrelevant antibodies (rat IgG1 κ clone R3-34, rat IgG2a κ clone R35-95). To determine the frequency of cytokine-expressing cells, the number of peroxidase-stained epidermal cells per tissue section was determined, a commonly used method for quantitation (11, 19, 25). The slides were enumerated in a blinded fashion by two observers. The score was recorded as 1+ if 1 to 5 cells were stained in each visual field, 2+ for 5 to 10 cells, and 3+ for >10 cells. For each organ, the average score of 15 to 30 visual fields was calculated. The term moderate expression was used to denote mean arbitrary scores exceeding 0.5, and intense denoted scores exceeding 1.5.
RESULTS
Growth of F. tularensis LVS after primary infection and reinfection.
The growth of F. tularensis LVS was followed for 2 weeks after intradermal inoculation of a sublethal dose, 3.8 × 105 organisms, approximately 0.1 LD50. Immune mice had been immunized by an intradermal inoculation 5 weeks before reinfection. It was demonstrated in a previous publication that enhanced nonspecific antibacterial resistance had completely waned 3 weeks postinoculation (24). It was therefore assumed that resistance to reinfection at this time represented specific immunity.
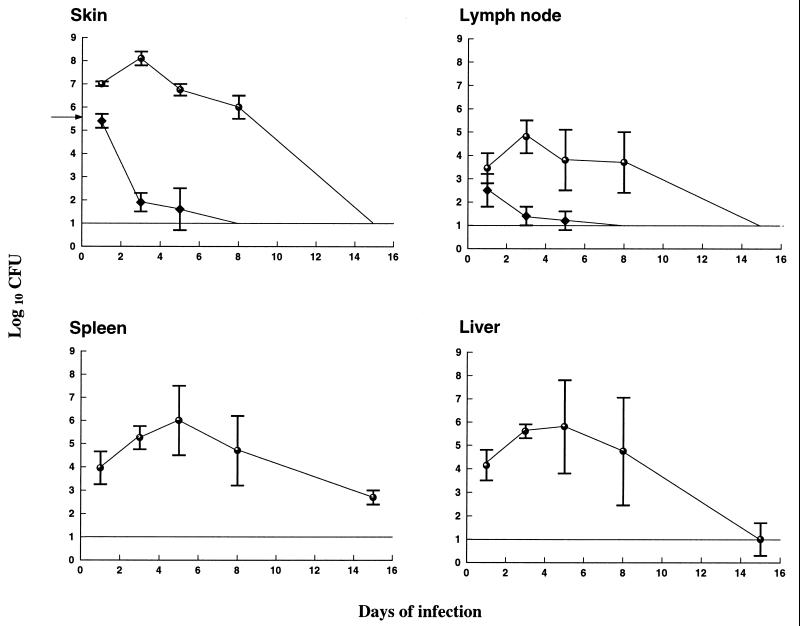
After primary infection, bacterial numbers increased during the first 2 days at the inoculation site and then started to decline but were not eradicated until 14 days after challenge (Fig. 1). By contrast, bacterial numbers declined within 1 day of infection at the inoculation site in immune mice and all bacteria were cleared within 8 days (Fig. 1). In draining lymph nodes, significantly lower bacterial numbers were detected in samples from immune mice throughout the course of infection (Fig. 1). No bacteria were present in the livers and spleens of immune mice. By contrast, bacteria replicated in the latter organs in nonimmune mice, commencing on the second day, and were not eradicated until 2 weeks postchallenge (Fig. 1). In other experiments, clearance was often complete at 2 weeks postinoculation and always complete at 3 weeks postinoculation. The kinetics of bacterial growth in the organs was similar in repeated experiments.
FIG. 1.
Growth curves of F. tularensis LVS in the skin, lymph nodes, livers, and spleens of immune (diamonds) and naive (open circles) mice. Mice received a sublethal intradermal inoculum, 3.8 × 105 CFU, of F. tularensis LVS, and bacterial numbers were determined over 15 days. Immune mice had received a sublethal intradermal inoculum 5 weeks before secondary challenge. The line represents the detection limit of the assay. The means for five mice per group and time point are shown.
To assess the longevity of this memory immune response, control of bacterial growth was followed after reinfection in mice immunized 1 and 3 months before rechallenge. As shown in Table 1, by contrast to naive mice, no group of immune mice exhibited dissemination of bacteria, and the immune mice had much lower bacterial numbers in skin—4.5 to 5.5 log10—than did the naive mice. Approximately 10-fold-lower bacterial numbers were present in skin samples of 1 month-immune mice compared to those in the 3 months-immune group (P < 0.05). The memory immune response was monitored up to 6 months in other experiments. On no occasion were bacteria observed in samples from liver and spleen. Although immunization in the reported experiments was intradermal, similar levels of resistance to reinfection were noted after intravenous immunization 1 month prior to intradermal reinfection (data not shown).
TABLE 1.
Longevity of immunity memory after immunization with F. tularensis LVS
| Organ | Mean ± SD CFU of F. tularensis LVS (log10) per sample at indicated time between rechallenge and immunizationa
|
||
|---|---|---|---|
| None | 1 mo | 3 mo | |
| Skin | 7.8 ± 0.1 | 2.3 ± 0.1 | 3.2 ± 0.5 |
| Spleen | 5.8 ± 1.1 | BDL | BDL |
The inoculum of the rechallenge was 5 × 105 CFU of F. tularensis LVS administered intradermally. The two groups were reinfected at the same time. Viable counts were determined 3 days after rechallenge for a group of four female, aged-matched mice. BDL, below detection limit (10 organisms/spleen).
Cytokine expression in skin.
Protein expression in skin lesions was determined by immunoperoxidase labeling of cryostat sections. In naive mice, staining for IL-12 at the three time points was weak, with scores of 0.15, 0.45, and 0.3 (Table 2). By contrast, IL-12 staining was already intense in the epidermal area at 24 h postchallenge, with a mean average arbitrary score of 2.2 out of a maximum of 3.0. Intense staining (mean score, 1.05) was also observed at 48 h but had disappeared at 72 h. Staining for TNF-α was weak or absent throughout the experiment in naive mice, whereas moderate staining for TNF-α (mean score, 0.6) was observed at 24 h postchallenge in immune mice. Later, the TNF-α staining faded, with mean scores of 0.5 and 0.2 at 48 and 72 h, respectively. No IFN-γ expression was visible at 24 or 48 h, and weak staining (mean score, 0.45) was observed in naive mice at 72 h postinoculation, whereas immune animals displayed moderate to intense staining (mean score, 1.6) at the latter time point. Virtually no staining of any cytokine was observed after the injection of saline. Representative examples of the immunohistochemistry are shown in Fig. 2 to 4. An identical experiment was performed, and onset of cytokine expression occurred in the two groups at the time points indicated above.
TABLE 2.
Immunohistochemical analyses of TNF-α, IFN-γ, and IL-12 expression in skin of mice infected with F. tularensis LVSa
| Time postchallenge | Immunohistochemical staining forb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TNF-α
|
IFN-γ
|
IL-12
|
|||||||
| Noninfected | Naive | Immune | Noninfected | Naive | Immune | Noninfected | Naive | Immune | |
| None | 0.0, 0.0 | 0.2, 0.0 | 0.1, 0.4 | ||||||
| 24 h | 0.0, 0.0 | 0.8, 0.4 | 0.3, 0.2 | 0.4, 0.2 | 0.3, 0.0 | 2.4, 2.1 | |||
| 48 h | 0.2, 0.0 | 0.6, 0.4 | 0.1, 0.1 | 0.4, 0.3 | 0.2, 0.7 | 1.3, 0.8 | |||
| 72 h | 0.0, 0.5 | 0.4, 0.0 | 0.3, 0.6 | 2.4, 0.8 | 0.1, 0.5 | 0.1, 0.2 | |||
Mice were challenged with an intradermal inoculation of saline (none) or with 3.8 × 105 F. tularensis LVS as a primary inoculum (naive mice) or secondary inoculum (immune mice) and killed after 1, 2, or 3 days of infection. One square centimeter of the skin at the site of inoculation was excised and snap frozen. Mice that received a secondary challenge had been given a subcutaneous inoculation of 104 CFU 5 weeks earlier.
Cytokine expression in infectious foci was determined by immunoperoxidase labeling of cryostat sections of biopsy samples with anticytokine antibodies. The number of peroxidase-stained cells in epidermis for each tissue section was determined by scoring the number of positive cells. The scoring system was as follows: 1+ if 1 to 5 cells were stained in each tissue section, 2+ for 5 to 10 cells, and 3+ for >10 cells. The average score for 15 to 30 tissue sections for each organ was calculated. Scores for two mice are given.
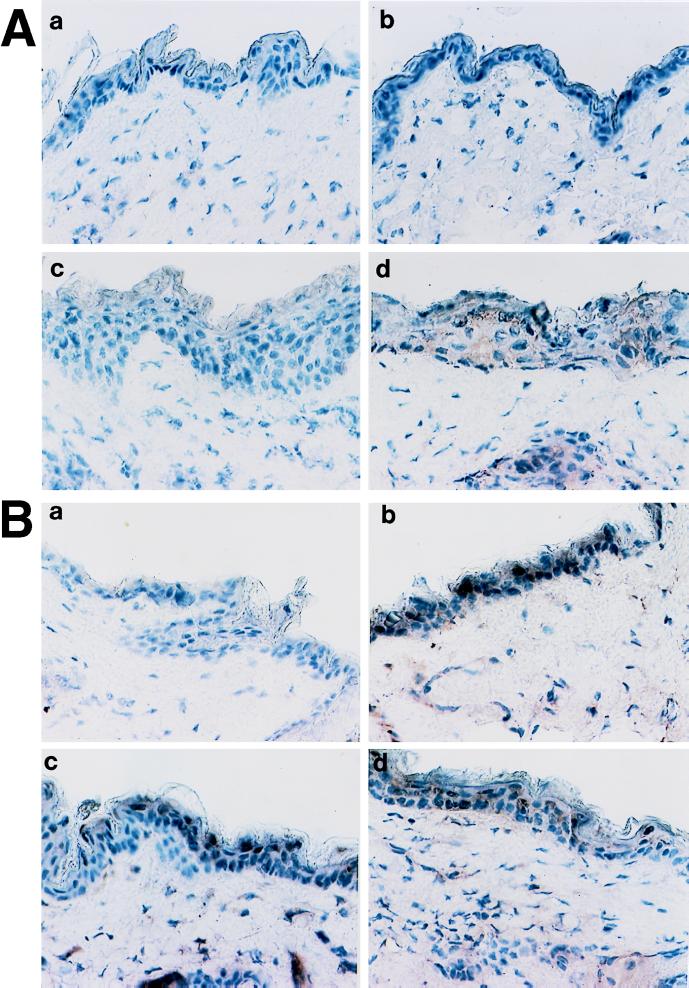
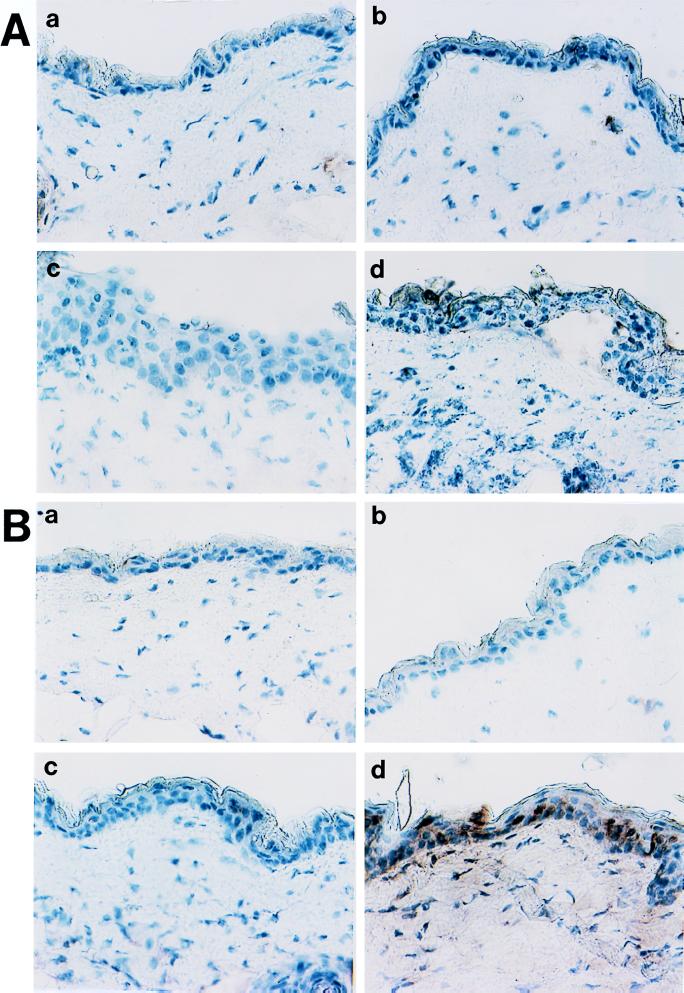
FIG. 2.
Expression of TNF-α in skin of infected mice. Samples are from naive (A) and immune (B) mice. For both types of samples, stained section from noninfected tissue (a), 24 h after inoculation (b), 48 h postchallenge (c), and 72 h postinoculation (d) are shown. Controls included staining with irrelevant primary antibodies and the absence of cross-reactivity of the secondary labeled antibodies with the primary antibodies of mismatched isotypes.
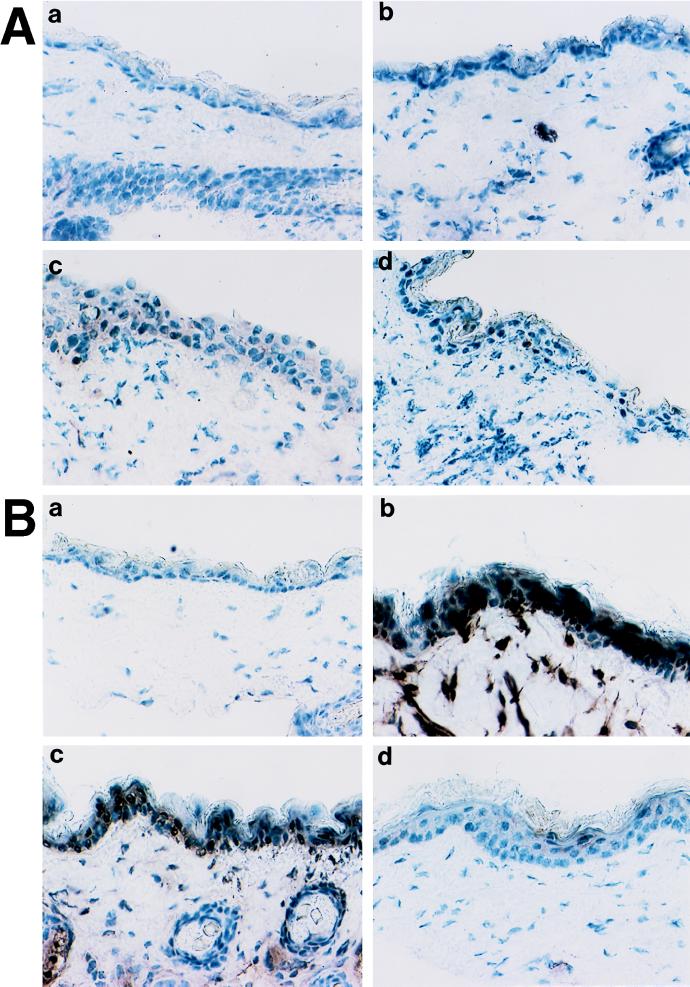
FIG. 4.
Expression of IL-12 in skin of infected mice. Samples are from naive (A) and immune (B) mice. For both samples, stained section from noninfected tissue (a), 24 h after inoculation (b), 48 h postchallenge (c), and 72 h postinoculation (d) are shown. Controls included staining with irrelevant primary antibodies and the absence of cross-reactivity of the secondary labeled antibodies with the primary antibodies of mismatched isotypes.
Kinetic analysis of cytokine gene expression in skin during primary and secondary tularemia.
PCR-assisted amplification of cDNA prepared before and after challenge with F. tularensis LVS was used to qualitatively assess the expression of cytokine mRNA and to correlate it to the observed cytokine expression. The number of cycles used for amplification was such that constitutive expression in skin was barely discernible or not discernible at all (data not shown).
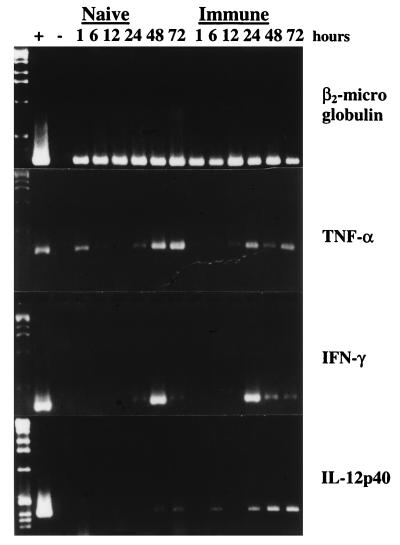
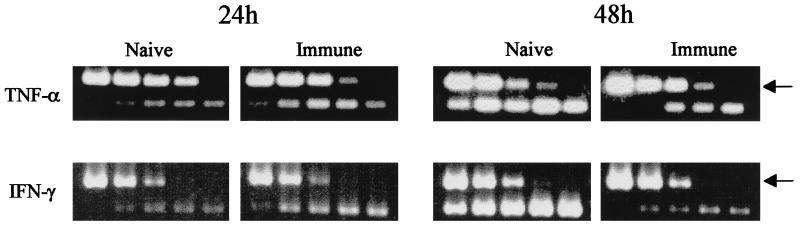
RT-PCR revealed the presence of TNF-α and IFN-γ mRNAs at 24 h and at all later time points after either primary infection or reinfection (Fig. 5). Weak expression of IL-12p40 was seen at 48 and 72 h in naive mice and at 6, 24, 48, and 72 h, but not at 12 h, in immune mice (Fig. 5). The experiment was performed three times, and similar results were observed.
FIG. 5.
PCR-assisted amplification of cytokine cDNA from skin samples after challenge with F. tularensis LVS. The number of cycles used for amplification was such that constitutive expression in skin was barely discernible or not discernible at all. Samples were taken at indicated time points after intradermal challenge of 5 × 105 CFU of F. tularensis LVS.
Competitive PCR for assessment of mRNA levels in skin after primary infection and reinfection.
The PCR analyses used in the initial experiments did not allow any definite conclusions about the mRNA levels expressed. To make a quantification, competitive PCR was used and cytokine cDNA was amplified in the presence of various dilutions of competitor DNA for IL-12p40, IFN-γ, TNF-α, or β-actin. The amounts of cDNA from the samples were equalized with β-actin competitor DNA.
The kinetic analysis had revealed that IFN-γ and TNF-α mRNAs were observed from 24 h on, and therefore samples from these time points were analyzed by competitive PCR. After determination of the relevant 10-fold dilution, a more exact quantitation was obtained by serial twofold dilutions in the appropriate range. The use of this competitive PCR indicated that TNF-α and IFN-γ mRNA levels were 5- and 30-fold higher, respectively, in immune mice than in naive mice at 24 h postinoculation. At 48 h, TNF-α mRNA levels were 10-fold higher and IFN-γ mRNA levels were 20-fold higher in naive mice. The 10-fold titrations of TNF-α and IFN-γ mRNAs are shown in Fig. 6. These results corroborated the kinetic PCR analysis which had indicated that higher mRNA levels of TNF-α and IFN-γ were present in immune mice at 24 h postinoculation and in naive mice at 48 h (Fig. 5). Similar differences were observed in two experiments. IL-12p40 mRNA levels were also quantitated. Although higher mRNA levels were observed in immune mice at 24 h in the experiment shown in Fig. 5, two repeated experiments showed slightly higher levels in naive mice at this time point. Thus, no consistent differences with regard to IL-12 mRNA levels could be determined between the two groups. Variations in the IL-12p40 mRNA levels can also be seen in Fig. 5. A visible amplicon was present at 6 and 24 h but not at 12 h.
FIG. 6.
Competitive PCR analysis of TNF-α and IFN-γ cDNA levels in skin samples taken at 24 and 48 h postchallenge. The concentration of cDNA was determined by identifying the dilution of the competitor fragment showing the same intensity after amplification as that of the amplicon of the sample cDNA. For each cytokine, the competitor fragment yielded a larger fragment (indicated with an arrow). The IFN-γ competitor DNA ranged, in 10-fold dilutions, from 10−19 to 10−23 mol, and the TNF-α DNA ranged from 10−18 to 10−22 mol.
DISCUSSION
We demonstrate here the capability of immune mice to respond rapidly to infection through the skin with expression of IL-12 and TNF-α, cytokines known to be crucial for host protection as well as effective in containing the spread of bacteria and in killing them. By contrast, naive mice could not mount rapid control of infection at the site of inoculation and did not prevent systemic dissemination. Moreover, TNF-α and IL-12 staining in skin samples from these naive mice were weak or not observed at all. The ability to prevent any spread of bacteria to liver and spleen appeared to be a hallmark of the acquired immune response and was still present 6 months after immunization. Notably, though, a slight but significant waning of memory immunity was observed in the skin in 3 month-immune mice compared to 1 month-immune mice.
The onset of IL-12 expression in immune mice occurred within 1 day. As recruitment of immune cells requires at least one day and as many as several days, it is possible that the source of IL-12 was a resident cell population. Indeed, several cell types present in the skin can produce cytokines, e.g., keratinocytes and dendritic cells. Dendritic cells have been identified as an important producer of IL-12 and can direct the development of Th1 T cells (16). The mechanism triggering the rapid onset of expression is unknown.
The rapid IL-12 secretion is probably beneficial for the host to protect against F. tularensis infection. For example, in an in vitro model of listeriosis, it has been shown that IL-12 together with TNF-α stimulates the release of the macrophage-activating agent IFN-γ by NK cells, enhancing bacterial killing (29). IL-12 also drives differentiation of Th1 T cells (10, 22). A previous communication showed that nonspecific killing of, e.g., Listeria monocytogenes, occurs after a challenge with F. tularensis (24). However, this mechanism was not operative after the first two weeks of infection (24). Therefore, it is not likely that such a nonspecific mechanism has any relation to the early induction of IL-12 that was operative 4 to 5 weeks postimmunization. It should be noted that virtually no staining for IL-12 was observed in samples from immune mice after the injection of saline.
There was a marked waning of IL-12 expression in immune mice between 1 and 3 days postinoculation. One explanation may be that the concentration of Francisella-specific, complement-activating antibodies increases at infectious foci after the first day of infection as part of the inflammatory response. Since a recent study showed that suppression of IL-12 secretion occurs after signaling via the CR3 receptor (17), such an increase can lead to activation of complement, binding to the CR3 receptor, and thereby to suppression of IL-12 secretion.
Expression of IFN-γ was not observed before the third day of reinfection. This lack of expression in immune mice during the first 2 days, when effective control of infection was observed, casts some doubt on its role in the memory immune response. In a previous study, it was found that neutralization of IFN-γ after reinfection affected bacterial killing only when the inocula of F. tularensis were very high, thereby demonstrating the possibility of IFN-γ-independent killing (24). In view of this, the late appearance of IFN-γ may not be critical for host protection. Alternatively, biological effects may be present below the levels detected by the immunohistochemical technique used.
The quantitative reverse transcriptase PCR indicated the presence of higher mRNA levels of TNF-α and IFN-γ in immune mice at 24 h postchallenge, reflected by higher expression of the two cytokines in these mice. However, the much-higher expression of IL-12 present in skin samples from the immune group was not been preceded by higher mRNA levels of IL-12p40. The reason for this discrepancy may be related to the complex transcriptional and translational control of the cytokine. It is possible that posttranscriptional or posttranslational events affect expression of the IL-12 dimer, hence explaining the observed discrepancy between mRNA levels of IL-12p40 and protein expression.
The skin is particularly relevant to immunity to tularemia, as the ulceroglandular form of the disease is the most common and inherent capability to control infection is much greater in the skin than in the internal organs. The present results demonstrate that potent mechanisms capable of expressing IL-12, IFN-γ, and TNF-α reside in the skin. This is of definite interest in relation to the rapid killing and containment of bacteria occurring in immune mice, since previous reports have documented a critical need for TNF-α and IFN-γ to protect against primary (14, 24) as well as secondary F. tularensis infection (24). As previously discussed, IL-12 is critically required for host resistance against infections caused by intracellular bacteria (10, 22, 28). Thus, each of the three cytokines probably plays an important role in the control of F. tularensis infection, and the early appearance of the cytokines at the site of inoculation is likely to benefit host protection.
Several experimental models of intracellular infection have provided evidence that the skin has a unique potential to effectively express protective immunity and to direct an immune response that subsequently affords systemic protection. For example, cutaneous infection with Leishmania donovani resulted in the development of a prominent local and systemic Th1 immune response and no detectable visceral parasitism, whereas intravenous inoculation resulted in a delayed Th1 immune response, as evidenced by minimal IL-12 mRNA expression and a progressive visceral parasite burden (18). In experimental schistosomiasis, the anamnestic immune response of the skin was already characterized by marked inflammation and increased tissue expression of ICAM-1 and mRNA for iNOS at 8 h after infection (20). In contrast, inflammation and expression of ICAM-1 and iNOS mRNA were minimal in naive controls up to 72 h postinfection. Together, these findings indicate that expression of memory immunity in the skin is characterized by rapid recruitment of inflammatory cells, early and prominent expression of Th1 cytokines, increased expression of adhesion molecules, and early activation of the pathway generating nitric oxide. The present finding, demonstrating early local production of IL-12, TNF-α, and IFN-γ, is an additional piece of evidence supporting the role of the skin as an essential barrier protecting against infection.
The cutaneous form of experimental L. donovani infection results in the generation of a Th1 cell response and no progression of disease. By contrast, intravenous inoculation of Leishmania is followed by progressive visceral disease (18). In experimental tularemia, effective protection results regardless of the route of immunization, and control of infection occurs with similar kinetics, regardless of whether inoculation is cutaneous or parenteral. Thus, in contrast to Leishmania infection, the control of F. tularensis infection is not dependent on the route of immunization. This result is probably related to the fact that F. tularensis without exception induces a Th1 immune response, whereas Leishmania infection and other parasitic diseases under certain circumstances result in the expansion of Th2 T cells and thereby disease progression.
Previous studies have focused mainly on the requirement of cytokines and cell subsets for host protection after parenteral routes of F. tularensis challenge. However, there is no direct evidence that these requirements are similar to those that are operative after cutaneous infection. The present study has provided evidence that helps elucidate which mechanisms are involved. To further clarify the process, study to identify the cellular source of the rapidly secreted IL-12, IFN-γ, and TNF-α is under way.
FIG. 3.
Expression of IFN-γ in skin of infected mice. Samples are from naive (A) and immune (B) mice. For both types of samples, stained section from noninfected tissue (a), 24 h after inoculation (b), 48 h postchallenge (c) and 72 h postinoculation (d) are shown. Controls included staining with irrelevant primary antibodies and the absence of cross-reactivity of the secondary labeled antibodies with the primary antibodies of mismatched isotypes.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (project no. 9485), Västerbotten Läns Landsting, and the Medical Faculty, Umeå University.
REFERENCES
- 1.Anthony L S, Ghadirian E, Nestel F P, Kongshavn P A. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- 2.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 3.Conlan J W, North R J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992;60:5164–5171. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlan J W, Sjöstedt A, North R J. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect Immun. 1994;62:5603–5607. doi: 10.1128/iai.62.12.5603-5607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehlers S, Mielke M E, Blankenstein T, Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992;149:3016–3022. [PubMed] [Google Scholar]
- 6.Elkins K L, Rhinehart-Jones T R, Culkin S J, Yee D, Winegar R K. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64:3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortier A H, Polsinelli T, Green S J, Nacy C A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60:817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortier A H, Slayter M V, Ziemba R, Meltzer M S, Nacy C A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991;59:2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golovliov I, Sandstrom G, Ericsson M, Sjostedt A, Tarnvik A. Cytokine expression in the liver during the early phase of murine tularemia. Infect Immun. 1995;63:534–538. doi: 10.1128/iai.63.2.534-538.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 11.Krajewski S, Zapata J M, Krajewska M, VanArsdale T, Shabaik A, Gascoyne R D, Reed J C. Immunohistochemical analysis of in vivo patterns of TRAF-3 expression, a member of the TNF receptor-associated factor family. J Immunol. 1997;159:5841–5852. [PubMed] [Google Scholar]
- 12.Larrick J W. Message amplification phenotyping (MAPPing)—principles, practice and potential. Trends Biotechnol. 1992;10:146–152. doi: 10.1016/0167-7799(92)90202-7. [DOI] [PubMed] [Google Scholar]
- 13.Legoux P, Minty C, Delpech B, Minty A J, Shire D. Simultaneous quantitation of cytokine mRNAs in interleukin-1 beta stimulated U373 human astrocytoma cells by a polymerisation chain reaction method involving co-amplification with an internal multi-specific control. Eur Cytokine Netw. 1992;3:553–563. [PubMed] [Google Scholar]
- 14.Leiby D A, Fortier A H, Crawford R M, Schreiber R D, Nacy C A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Kurlander R J. Analysis of the interrelationship between IL-12, TNF-alpha, and IFN-gamma production during murine listeriosis. Cell Immunol. 1995;163:260–267. doi: 10.1006/cimm.1995.1125. [DOI] [PubMed] [Google Scholar]
- 16.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 17.Marth T, Kelsall B L. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melby P C, Yang Y Z, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun. 1998;66:18–27. doi: 10.1128/iai.66.1.18-27.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paus R, van der Veen C, Eichmuller S, Kopp T, Hagen E, Muller-Rover S, Hofmann U. Generation and cyclic remodeling of the hair follicle immune system in mice. J Investig Dermatol. 1998;111:7–18. doi: 10.1046/j.1523-1747.1998.00243.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramaswamy K, He Y X, Salafsky B. ICAM-1 and iNOS expression increased in the skin of mice after vaccination with gamma-irradiated cercariae of Schistosoma mansoni. Exp Parasitol. 1997;86:118–132. doi: 10.1006/expr.1997.4178. [DOI] [PubMed] [Google Scholar]
- 21.Sandstrom G, Tarnvik A, Wolf-Watz H, Lofgren S. Antigen from Francisella tularensis: nonidentity between determinants participating in cell-mediated and humoral reactions. Infect Immun. 1984;45:101–106. doi: 10.1128/iai.45.1.101-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharton-Kersten T, Afonso L C, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 23.Sjostedt A, Conlan J W, North R J. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sjostedt A, North R J, Conlan J W. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology. 1996;142:1369–1374. doi: 10.1099/13500872-142-6-1369. [DOI] [PubMed] [Google Scholar]
- 25.Sunnemark D, Ulfgren A K, Orn A, Harris R A. Cytokine production in hearts of Trypanosoma cruzi-infected CBA mice: do cytokine patterns in chronic stage reflect the establishment of myocardial pathology? Scand J Immunol. 1996;44:421–429. doi: 10.1046/j.1365-3083.1996.d01-328.x. [DOI] [PubMed] [Google Scholar]
- 26.Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- 27.Tripp C S, Kanagawa O, Unanue E R. Secondary response to Listeria infection requires IFN-gamma but is partially independent of IL-12. J Immunol. 1995;155:3427–3432. [PubMed] [Google Scholar]
- 28.Tripp C S, Unanue E R. Macrophage production of IL12 is a critical link between the innate and specific immune responses to Listeria. Res Immunol. 1995;146:515–520. doi: 10.1016/0923-2494(96)83025-2. [DOI] [PubMed] [Google Scholar]
- 29.Tripp C S, Wolf S F, Unanue E R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yee D, Rhinehart-Jones T R, Elkins K L. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol. 1996;157:5042–5048. [PubMed] [Google Scholar]
- 31.Yoshida A, Koide Y, Uchijima M, Yoshida T O. IFN-gamma induces IL-12 mRNA expression by a murine macrophage cell line, J774. Biochem Biophys Res Commun. 1994;198:857–861. doi: 10.1006/bbrc.1994.1122. [DOI] [PubMed] [Google Scholar]